Abstract
Background:
Hypertensive disorders during pregnancy (HDP) are associated with the risk of long-term cardiovascular disease after pregnancy, but it has not yet been determined whether genetic predisposition for HDP can predict the risk for long-term cardiovascular disease.
Objective:
We aimed to evaluate the risk for long-term atherosclerotic cardiovascular disease (ASCVD) according to polygenic risk scores for HDP (HDP-PRS).
Study design:
Among UK Biobank participants, we included European-descent women (n=164,575) with at least one live birth. Subjects were divided according to genetic risk categorized by HDP-PRS (low risk, HDP-PRS ≤25th percentile; medium risk, HDP-PRS 25~75th percentile; high risk, HDP-PRS >75th percentile) and were evaluated for incident ASCVD, defined as the new occurrence of one of the followings: coronary artery disease, myocardial infarction, ischemic stroke, or peripheral artery disease.
Results:
Among the study population, 2,427 (1.5%) had a history of HDP, and 8,942 (5.6%) developed incident ASCVD after enrollment. Women with high genetic risk for HDP had a higher prevalence of hypertension at enrollment. After enrollment, women with high genetic risk for HDP had an increased risk for incident ASCVD, including coronary artery disease, myocardial infarction, and peripheral artery disease compared to those with low genetic risk, even after adjustment for HDP history.
Conclusions:
High genetic risk for HDP was associated with increased risk for ASCVD. This study provides evidence on the informative value of HDP-PRS in prediction of long-term cardiovascular outcomes later in life.
Keywords: hypertensive disease during pregnancy, preeclampsia, long-term outcomes, cardiovascular outcome, polygenic risk score
INTRODUCTION
Cardiovascular disease is one of the most common causes of mortality, and it is essential to identify high-risk populations and adapt preventive strategies in those populations1. As cardiovascular diseases are driven not only by environmental factors such as lifestyle but also by genetic factors, numerous studies have attempted to identify the genetic variants associated with cardiovascular disease2, 3. Data accumulated from genome-wide association studies (GWASs) with large sample sizes have allowed researchers to analyze polygenicity using multiple genetic variants4, 5. Polygenic risk scores (PRSs) are defined as estimated individual’s genetic predisposition to a specific disease. PRSs are calculated by a sum of significant single-nucleotide polymorphisms (SNPs) variants identified from GWAS, usually with a weighted sum in which the weights represent the estimated strength of association between those SNPs and a specific disease. PRSs have been used to categorize patients according to genetic burden. Recent studies have highlighted PRSs as an emerging technology in the field of disease risk prediction and have shown them to be correlated with disease incidence in several common diseases, such as coronary heart disease, type 2 diabetes, and breast cancer6-8. Risk prediction models that include genetic predisposition may improve the early identification of high-risk individuals; accordingly, the utility of PRSs for clinical care and disease prevention is an active area of investigation and discussion9-12.
Previous studies suggest that women with a history of hypertensive disorders during pregnancy (HDP) are at an increased risk not only for essential hypertension but also for long-term atherosclerotic cardiovascular disease (ASCVD) later in life.13-15 Evidence indicates that this increased risk after HDP is independent of chronic metabolic diseases, such as subsequent hypertension or diabetes. In addition, clinical guidelines recommend including HDP as an important female-specific factor in risk assessment for ASCVD.16 However, it is not clear whether the correlation between HDP and subsequent ASCVD can be attributed to certain genetic factors, common risk factors (such as obesity, smoking, or others), or the pathophysiologic impact of HDP itself. In addition, to the best of our knowledge, it has not yet been determined whether the genetic predisposition for HDP can predict the risk for subsequent cardiovascular disease.
In the current study, we developed polygenic risk scores for HDP (HDP-PRS) from GWAS data and evaluated the risk for long-term ASCVD according to HDP-PRS.
MATERIALS AND METHODS
Data source
The UK Biobank is a prospective cohort study that recruited more than 500,000 adult residents aged 40-69 years old at 22 assessment centers throughout the United Kingdom between 2006-201017, 18. Various data, including sociodemographic, lifestyle, and health information, were collected through questionnaires. Physical measures such as blood pressure or anthropometrics were also assessed, and blood samples were taken for genotyping. For this study, we included European-descent women with at least one live birth and available genetic data (Supplementary Figure 1). Subjects were divided according to genetic risk as categorized by HDP-PRS and were evaluated for incident ASCVD. The North West Multicenter Research Ethics Committee approved the UK Biobank19, and the current study was conducted after an approved application to use the UK Biobank resource (application number: 68416).
Hypertensive disease during pregnancy, comorbidities, and cardiovascular outcomes
At the baseline survey, female participants were asked about their reproductive history, including parity. HDP was defined as gestational hypertension, preeclampsia, eclampsia, or superimposed preeclampsia and was captured in the self-report at enrollment or by appropriate International Classification of Disease (ICD) codes, which were extracted from primary care or hospital records (Supplementary Table 1).
For the evaluation of HDP risk according to HDP-PRS, age at first pregnancy and the presence before pregnancy of a disease conferring high risk for HDP were selected as covariates, consistent with clinical guidelines for high-effect risk factors for HDP20, 21. According to those guidelines, high-HDP-risk diseases include hypertension, diabetes, renal disease, systemic lupus erythematosus, and antiphospholipid antibody syndrome. The presence of a high-HDP-risk disease before pregnancy was determined by either self-report or diagnosis with relevant ICD codes for each disease (Supplementary Table 1) that occurred before the first live birth.
For analysis of incident ASCVD and its association with HDP-PRS, participants with congenital heart disease were excluded to eliminate the possible association between congenital heart disease and cardiovascular outcomes (see Supplementary Table 1 for relevant diagnosis codes). Prevalent metabolic comorbidities, including hypertension, diabetes, and dyslipidemia, were used as adjusting covariates and were likewise ascertained either by self-report at enrollment or by ICD codes as described in Supplementary Table 1.
Incident ASCVD was defined as a diagnosis after enrollment in participants without -pre-existing cardiovascular disease and included coronary artery disease, myocardial infarction (MI), ischemic stroke, and peripheral artery disease. ICD codes for each cardiovascular disease are presented in Supplementary Table 1. In addition, myocardial infarction was defined algorithmically by the UK Biobank22. For each new-onset cardiovascular disease being considered, participants with the pre-existing disease at enrollment were excluded during analysis. For example, participants with pre-existing coronary artery disease at enrollment were excluded from the analysis for new-onset coronary artery disease, ensuring that recurrent coronary artery disease was not erroneously counted as new-onset.
Genotyping and polygenic risk score for hypertensive disease during pregnancy
The genotyping process and arrays used by the UK Biobank have been described elsewhere in detail18. 487,409 samples were genotyped using the Affymetrix UK BiLEVE axiom array and the Affymetrix UK Biobank axiom array. Samples with poor quality, as provided by the UK Biobank, were dropped. Samples related in the second degree or closer were excluded using a greedy algorithm to pick the least number of samples from among related pairs. Since the UK Biobank predominantly consists of European-descent samples, we only retained those with “white British” ancestry using UK Biobank showcase data field “Genetic ethnic grouping” which are samples who self-identified as ‘White British’ and have very similar genetic ancestry based on a principal components analysis of the genotypes. Samples having a mismatch between reported sex and genetically inferred sex were also excluded. For variant quality control, variants with an info score of < 0.3 and minor allele frequency (MAF) of < 0.01 were removed. Ultimately, a total of 377,909 samples and 9,505,768 variants passed the QC criteria.
PRS is a numerical value that estimates an individual’s genetic risk for a particular trait or disease using a combination of multiple genetic variants across the genome. Each of the variant is given a weight(beta) based on its association with the disease or trait. Usually, these weights can be obtained by publicly available GWAS summary statistics. These weights can then be used to calculate PRS by summation of weighted effect sizes of genetic variants. Though this is the basic way to calculate PRS there are several other methods that incorporate different strategies like PRSice2, LDPred and PRS-CS. PRSice2 prunes and filters genetic variants based on correlation thresholds and association p-values to calculate the PRS. In contrast, LDPred and PRS-CS use Bayesian methods to estimate the PRS. For the calculation of HDP-PRS, we only included unrelated European-descent women aged 40 to 69 (Supplementary Figure 1). Participants were excluded if they had not had any live birth as of the baseline study visit. The HDP-PRS was calculated by LDpred23 using summary statistics from FinnGen, another large-scale biobank24; specifically, we used the phecode of I9_HYPTENSPREG (‘Hypertension complicating pregnancy, childbirth, and the puerperium’) from a study population of 4,677 cases and 71,711 controls. LDpred was run using a Bayesian approach with multiple assumptions regarding the fraction of causal variants (1, 0.3, 0.1, 0.03, 0.01, 0.003, and 0.001). Additionally, pruning and thresholding were used to generate 24 additional polygenic risk scores using a variety of p value cutoffs (1, 0.5, 0.05, 5×10−4, 5×10−6, and 5×10−8) and r2 thresholds (0.2, 0.4, 0.6, and 0.8). The best polygenic risk score was selected based on area under the curve (AUC) values (Supplementary Table 2).
After calculating HDP-PRS, participants were divided according to categorical genetic risk for HDP; women were considered to have high genetic risk for HDP when HDP-PRS>75th percentile (highest quartile, Q4), medium genetic risk for HDP when HDP-PRS 25~75th percentile (medium quartiles, Q2~3), and low genetic risk for HDP when HDP-PRS≤25th percentile (lowest quartile, Q1) 25-27. Participants with low genetic risk (Q1) for HDP served as the reference group. Two groups of cases were defined, one in terms of the risk of HDP itself and the other based on the risk of incident ASCVD.
Genetic correlation between HDP and cardiovascular outcomes.
We calculated genetic correlations between HDP and risk of cardiovascular disease. The BOLT-REML 28 measures the genetic correlations among traits measured on same set of individuals. BOLT-REML provides an approximate REML method that leverages Monte Carlo techniques to scale up to larger sample sizes. We ran BOLT-REML on LD pruned UKBB genotype data to calculate pairwise genetic correlations, adjusting for all the covariates.
Validation of polygenic risk score for hypertensive disease during pregnancy in Penn Medicine Biobank
We aimed to validate any results obtained through the UK Biobank in an external, independent dataset. The Penn Medicine Biobank (PMBB) is a biobank that recruits participants who are part of the University of Pennsylvania Health System. Participants have their genetic data linked to electronic health record data containing detailed information on clinical lab measurements and ICD codes. Due to this biobank lacking questionnaires and self-reported information on participant pregnancies, we defined cases and controls based solely on ICD codes (Supplementary Table 1). Age at pregnancy, presence of high-HDP-risk diseases, and development of hypertension and atherosclerotic cardiovascular diseases after pregnancy were likewise extracted from ICD codes.
HDP-PRS was calculated with LDpred using the HapMap SNPs from African ancestry individuals in 1000 Genomes as an external reference panel and the same summary statistics from FinnGen as used in PRS calculation for the UK Biobank. We included only European ancestry participants from PMBB to match the summary statistics and prior analyses in the UK Biobank. Participants were then divided into high-, medium- and low-genetic-risk groups for HDP using the same definition as used for the UK Biobank. High genetic risk for HDP was defined as HDP-PRS>75p, and low genetic risk for HDP was defined as HDP-PRS≤25p. Participants were further divided based on their prior history of HDP, and we evaluated these groups for differences in incident hypertension and cardiovascular disease after pregnancy.
Statistical analysis
When comparing baseline characteristics among three groups, continuous variables were compared using analysis of variance (ANOVA) or Kruskal-Wallis test, and categorical variables were compared with the Pearson chi-square test or Fisher’s exact test, as appropriate. To determine the discriminative power of HDP-PRS for HDP, the area under the curve (AUC) was evaluated as a continuous variable, and the chi-square test for trend was used when considering HDP-PRS categories and the risk of HDP. To evaluate the risk of HDP according to HDP-PRS, logistic regression analysis was performed with covariates (age at first live birth and presence of high-risk disease for HDP before first live birth). To determine the association between HDP-PRS and new-onset cardiovascular outcomes, multivariable Cox regression analysis was performed with adjustment for history of HDP and other covariates, including age, body mass index (BMI), smoking, prevalent metabolic disease (hypertension, diabetes, and dyslipidemia), principal components (PCs), and genotype array. The proportional hazard assumption was examined with scaled Schoenfeld residuals29, and when it was violated, a stratified Cox model was applied. P values of less than 0.05 were considered statistically significant, and all analyses were carried out using R version 4.0.3.
RESULTS
Subject population
Among the 272,188 women aged 40-69 years who are enrolled in the UK Biobank, 51,787 were excluded due to having no history of live birth or a lack of information concerning parity. The case population with HDP was identified using self-report data and ICD codes, yielding 3,266 HDP cases and 217,135 controls. A further 7,091 women were excluded because of missing genotype data and another 47,985 due to the filtering of individuals of European descent and genotype QC, as described in the Methods section. After 750 cases with congenital heart disease, a total of 164,575 women (2,427 HDP cases and 162,148 controls) were included in the current analysis (Supplementary Figure 1).
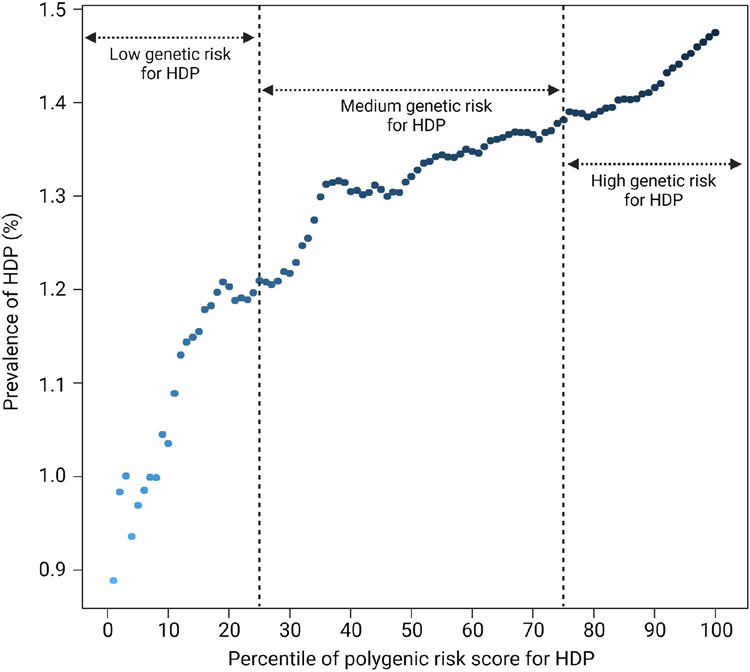
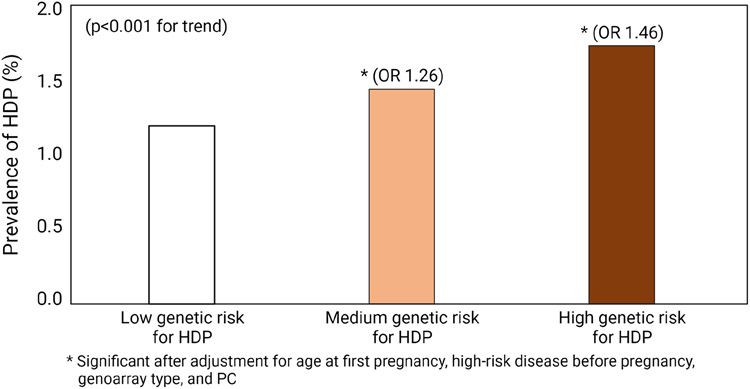
Figure 1(a) shows the risk of HDP during pregnancy according to HDP-PRS in the study population. As expected, risk increased with greater HDP-PRS, ranging from 0.889% in the lowest percentile to 1.475% in the highest percentile. In addition, women with high genetic risk for HDP (HDP-PRS>75th percentile) reported the highest frequency of HDP (p<0.001, chi-square for trend). Compared to those with low genetic risk (HDP-PRS≤25th percentile), women with high genetic risk had an increased risk for HDP with an adjusted odds ratio of 1.46 (Figure 1(b)).
Figure 1. Risk of hypertensive disorders during pregnancy (HDP) and polygenic risk scores for HDP (HDP-PRS) in UK Biobank.
(a) Prevalence of HDP according to the percentile of HDP-PRS
(b) Prevalence of HDP according to the stratified genetic risk for HDP
Abbreviations: HDP, hypertensive disease during pregnancy; PRS, polygenic risk score
Table 1 compares baseline clinical features and prevalent diseases in the study population according to genetic risk for HDP. Women with high genetic risk for HDP had higher BMI and blood pressure along with a higher prevalence of chronic metabolic disease (hypertension, diabetes, and dyslipidemia) compared to those with low genetic risk. At enrollment, a total of 41,984 out of 164,575 women (25.5%) had hypertension; women with high genetic risk for HDP had a higher unadjusted prevalence of hypertension than did those with low genetic risk (22.4% vs. 29.1%, p<0.001). The increased prevalence of hypertension in women with high genetic risk for HDP remained significant even after adjustment for history of HDP, age, BMI, smoking history, PCs, and genotype array (adjusted odds ratio 1.28, p<0.001 by logistic regression analysis). Supplementary Table 1 shows baseline characteristic in the study population according to the presence or absence of HDP. Women with history of HDP had higher BMI and blood pressure along with a higher prevalence of chronic metabolic disease (hypertension and diabetes) compared to those without history of HDP.
Table 1.
Baseline clinical features and prevalent diseases of the study population
| Characteristics | Group 1, Low genetic risk for HDP (HDP-PRS≤25th percentile, n = 41,177) |
Group 2, Medium genetic risk for HDP (HDP-PRS 25th~75th percentile, n = 82,275) |
Group 3, High genetic risk for HDP (HDP-PRS>75th percentile, n = 41,123) |
P-value |
|---|---|---|---|---|
| Baseline characteristics at enrollment | ||||
| Age (years) | 57.3 ± 7.6 | 57.2 ± 7.7 | 57.2 ± 7.6 | 0.551 |
| BMI | 26.9 ± 4.9 | 27.1 ± 5.0 | 27.3 ± 5.2 | <0.001 |
| Number of live births | 2.2 ± 0.9 | 2.2 ± 0.9 | 2.2 ± 0.9 | 0.868 |
| Mean duration between first birth and enrollment | 32.1 ± 9.5 | 32.2 ± 9.5 | 32.3 ± 9.5 | 0.049 |
| Ever smoking | 16,581 (40.3%) | 33,757 (41.0%) | 16,862 (41.0%) | 0.027 |
| Blood pressure at enrollment | ||||
| – Systolic blood pressure | 134.9 ± 19.2 | 136.2 ± 19.3 | 137.3 ± 19.3 | <0.001 |
| – Diastolic blood pressure | 80.1 ± 9.8 | 80.7 ± 9.9 | 81.4 ± 9.9 | <0.001 |
| Prevalent comorbidity at baseline | ||||
| Hypertension | 9,212 (22.4%) | 20,824 (25.3%) | 11,948 (29.1%) | <0.001 |
| Diabetes | 1,249 (3.0%) | 2,810 (3.4%) | 1,607 (3.9%) | <0.001 |
| Dyslipidemia | 4,619 (11.2%) | 9,793 (11.9%) | 5,256 (12.8%) | <0.001 |
Data are presented as proportion (%) or mean ± standard deviation.
Abbreviations: BMI, body mass index; PRS, polygenic risk score; Q, quartile
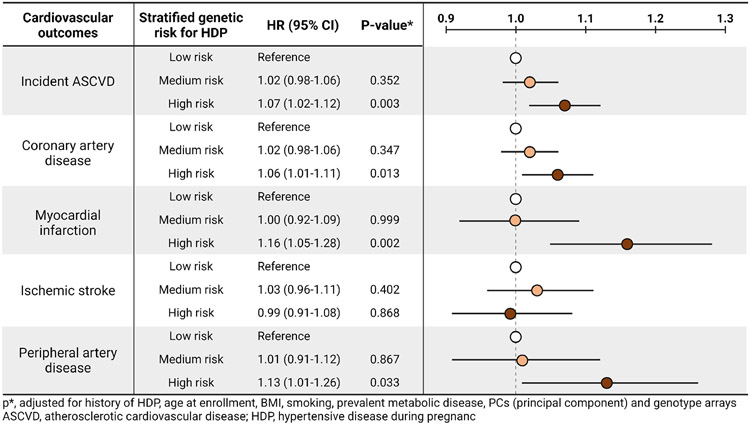
After enrollment, a total of 8,942 women developed new-onset ASCVD (4.55 occurrences per 1,000 women-years of follow-up). Women with high genetic risk for HDP had an increased risk for incident ASCVD with adjusted hazard ratio of 1.07 (95% CI, 1.09-1.12, Supplementary Table 3) compared to those with low genetic risk for HDP. Specifically, the risk for coronary artery disease, myocardial infarction, and peripheral artery disease was increased in women with high genetic risk for HDP, compared with those with low genetic risk for HDP (Table 2, Supplementary Figure 2). Figure 2 shows the hazard ratio of each cardiovascular outcome after adjustment for any history of HDP and other covariates, including age, BMI, smoking, prevalent metabolic disease, PCs, and genotype array.
Table 2.
Incident diagnosis of cardiovascular outcomes
| Number of new-onset occurrence per women | Number of new-onset occurrence per 1,000 women-years of follow-up |
Unadjusted p value |
Adjusted p value* |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | Group 1, Low genetic risk for HDP (n = 41,177) |
Group 2, Medium genetic risk for HDP (n = 82,275) |
Group 3, High genetic risk for HDP (n = 41,123) |
Group 1, Low genetic risk for HDP (n = 41,177) |
Group 2, Medium genetic risk for HDP (n = 82,275) |
Group 3, High genetic risk for HDP (n = 41,123) |
Group 1 vs. Group 2 |
Group 1 vs Group 3 |
Group 1 vs. Group 2 |
Group 1 vs Group 3 |
| Total ASCVD | 2,093 (5.1%) | 4,468 (5.4%) | 2,381 (5.8%) | 4.26 | 4.55 | 4.86 | 0.012 | <0.001 | 0.352 | 0.003 |
| Coronary artery disease | 1,619 (3.9%) | 3,437 (4.2%) | 1,880 (4.6%) | 3.35 | 3.56 | 3.91 | 0.035 | <0.001 | 0.347 | 0.013 |
| Myocardial infarction | 365 (0.9%) | 756 (0.9%) | 482 (1.2%) | 0.74 | 0.77 | 0.98 | 0.572 | <0.001 | 0.999 | 0.002 |
| Ischemic stroke | 494 (1.2%) | 1,054 (1.3%) | 516 (1.3%) | 1.01 | 1.08 | 1.06 | 0.218 | 0.445 | 0.402 | 0.868 |
| Peripheral artery disease | 278 (0.7%) | 594 (0.7%) | 362 (0.9%) | 0.57 | 0.61 | 0.74 | 0.351 | <0.001 | 0.867 | 0.033 |
Data are presented as proportion (%).
Abbreviations: ASCVD, atherosclerotic cardiovascular disease
adjusted for hypertensive disease during pregnancy, age, BMI, smoking, prevalent disease (hypertension, diabetes, or hypercholesterolemia), PCs (principal component) and genotype arrays by cox proportional hazards regression analysis
Figure 2. Hazard ratio of each cardiovascular outcomes according to the stratified genetic risk for HDP.
p*, adjusted for history of HDP, age at enrollment, BMI, smoking, prevalent metabolic disease, PCs (principal component) and genotype arrays
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; HDP, hypertensive disease during pregnancy
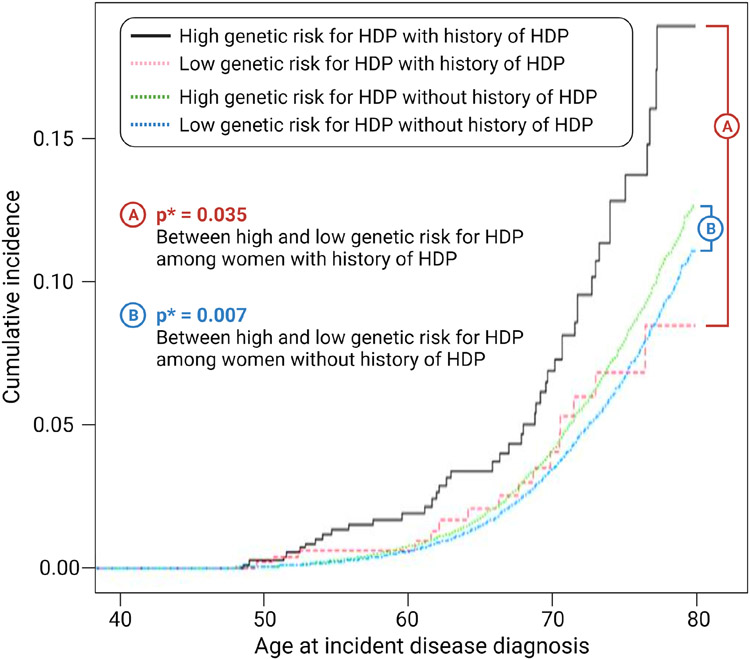
Overall, HDP-PRS was significantly associated with total atherosclerotic cardiovascular disease (p<0.001), coronary artery disease (p<0.001), myocardial infarction (p<0.001), and peripheral artery disease (p<0.001) (Supplementary Table 4). In the subgroup analysis according to the presence or absence of history for HDP, multivariable Cox regression analysis revealed that women with high genetic risk for HDP had an increased risk for incident ASCVD compared to those with low HDP-PRS both in women with history of HDP and in women without history of HDP (Figure 3 and Supplementary Table 5).
Figure 3. Survival analysis of total atherosclerotic cardiovascular outcome according to the history of HDP and stratified genetic risk for HDP.
p*, After adjustment for age, BMI, smoking, prevalent metabolic disease, PCs (principal component) and genotype array
In addition to high genetic risk (>75p) being associated with increased risk of cardiovascular disease, multivariable Cox regression analysis with adjustment for confounding variables revealed HDP-PRS itself (as a continuous variable) to also be associated with increased risk for total atherosclerotic cardiovascular disease (p<0.001), coronary artery disease (p<0.001), myocardial infarction (p<0.001), and peripheral artery disease (p=0.014).
Efficacy of the polygenic risk score for predicting hypertensive disease during pregnancy in the PMBB
HDP-PRS was calculated for the 21,969 women enrolled in the PMBB, and 1,144 European ancestry women (343 HDP cases and 801 controls) were included in subsequent analyses. Risk of HDP generally increased with greater HDP-PRS, and this increased risk remained significant after adjustment for age at pregnancy, prevalent metabolic diseases, and PCs (Supplementary Figure 3). The frequency of HDP was greater in women with high genetic risk for HDP (>75p) than in women with medium genetic risk (25~75p) and low genetic risk (≤25p) (Supplementary Figure 3, p<0.001 from chi-square for trend). In addition, compared to women with low genetic risk for HDP, women with high genetic risk for HDP had an increased risk for HDP with an adjusted odds ratio of 1.24 (Supplementary Figure 3). After delivery, 98 women developed new-onset hypertension (19.9 occurrences per 1,000 women-years of follow-up). Women with HDP had an increased risk for new-onset hypertension. Furthermore, women with high genetic risk for HDP (HDP-PRS>75p) had a higher prevalence of new-onset hypertension compared to those with low genetic risk for HDP for both women with and without HDP, although this difference was not significant (Supplementary Table 6). Notably, the relatively short-term follow-up period for individuals in the PMBB (mean follow-up period after pregnancy, 4.3 years) resulted in low sample sizes of women who developed new-onset cardiovascular disease and thus limited our ability to detect any differences in the risk of new-onset cardiovascular disease and any significant differences in the risk of developing new-onset hypertension
Genetic correlation between HDP and cardiovascular outcomes
The HDP exhibited a genetic correlation of 0.24 with ASCVD, 0.2 with coronary artery disease, 0.07 with myocardial infarction, 0.22 with ischemic stroke, and 0.56 with peripheral artery disease (Supplementary Table 7). We noticed that the standard errors were high, which is probably because of the limited number of cases (Supplementary Table 7). The highest genetic correlation was observed with peripheral artery disease and lowest genetic correlation was observed with myocardial infarction.
COMMENT
Principal finding
1) Women with high genetic risk for HDP-PRS had a higher frequency of HDP; 2) At enrollment, women with high genetic risk for HDP had a higher prevalence of hypertension than did those with low genetic risk; 3) After enrollment, women with high genetic risk for HDP had an increased risk for subsequent atherosclerotic cardiovascular diseases, including coronary artery disease, myocardial infarction, and peripheral artery disease compared to those with low genetic risk for HDP; 4) In an external validation with PMBB, HDP-PRS was also associated with the risk of HDP, and women with high genetic risk for HDP showed a tendency for an increased risk of new-onset hypertension when compared with those with low genetic risk for HDP.
Results
Several changes occur during pregnancy, such as increased circulatory volume, increased inflammatory factors, dyslipidemia, and insulin resistance30, that can pose physiological challenges to the cardiovascular system. While some pregnant women can tolerate these changes and challenges, others develop adverse pregnancy outcomes. As women with a history of complications during pregnancy are reported to be at increased risk for cardiovascular outcomes, pregnancy-related complications could be used to identify women at high risk for cardiovascular disease31-36. Accordingly, pregnancy is thought to be a key period for identifying women with long-term risk trajectories for heart disease37, and current international guidelines include pregnancy complications as risk factors for cardiovascular disease38-42.
Several studies have investigated the association of HDPs with an increased risk for subsequent cardiovascular disease and noted that this relationship is independent of further hypertension. From the literature to date, however, it is not clear whether this correlation between HDP and subsequent cardiovascular disease is mediated by genetic predisposition, by the impact of HDP itself, or by common environmental risk factors such as obesity and unhealthy lifestyle factors such as smoking43. We found HDP is genetically correlated with cardiovascular disease and that women who have a higher genetic risk for HDP are also at an increased risk for further cardiovascular disease. This increased risk remained significant even after adjustment for HDP itself and was independent of risk factors such as obesity and smoking, suggesting that the increased risk of cardiovascular disease in women with HDP might originate from a genetic predisposition.
The sharing of genetic predisposition between HPD and cardiovascular disease has been reported in previous studies. Steinthorsdottir et al. reported that variants associated with hypertension are also connected to HDP and found positive genetic correlations between HDP and hypertension, coronary artery disease, and diabetes44. Other studies have identified SNPs having simultaneous associations with both preeclampsia and cardiovascular disease or well-established risk factors for cardiovascular disease, such as triglyceride levels45. In addition, some studies have shown that the risk of developing preeclampsia is potentially affected by fetal variants near FLT1, a gene that regulates angiogenesis, suggesting fetal genomic role in the pathogenesis such as impaired trophoblast invasion or expression of anti-angiogenic factors44.
Clinical implications
In addition to evaluating genetic correlation, the current study tried to stratify patients according to HDP-PRS. Stratification of subjects into risk groups based on PRS values has been previously reported, representing the summation of thousands to tens of thousands of genetic variants, all of which have very minor effects. Specifically, several cardiovascular diseases can be predicted by disease specific PRSs, such as a score generated for coronary artery disease or atrial fibrillation6, 8. In addition to using a disease specific PRS, the current study is the first to report that a PRS for pregnancy complications can predict risk for subsequent cardiovascular disease.
Recently a study addressed the relationship between PRSs of cardiometabolic and hypertensive disease during pregnancy. Kivioja et al showed that PRSs for high blood pressure is associated with increased risk of preeclampsia, recurrent preeclampsia, and severe preeclampsia46. In addition to this previous study, we have further found that HDP-PRS itself is associated with further cardiovascular complications.
Nevertheless, relatively lower HR of HDP-PRS for subsequent ASCVD suggests that other modifiable risk factors such as BMI and smoking could be the first target of preventive strategies such as weight reduction or lifestyle modification. However, genetic risk factor (HDP-PRS) is unmodifiable but important factors for risk stratification. As patients at higher risk are more likely to benefit from preventive strategies, PRSs could be used to categorize patients and develop risk-based clinical strategies such as early screenings and interventions, including lifestyle modifications and medications10, 47-50. In addition, combination of genetic and environmental risk factors could be more helpful for risk stratification. Further studies are needed to determine whether HDP-PRS would be a useful consideration in preventive strategies.
Research implications
In the current study, we evaluated the application of HDP-PRS. Indeed, we first examined if PRS of pregnancy complications can identify the risk for long-term outcome after delivery, in addition to HDP itself. As a result, high genetic risk defined by HDP-PRS was independently associated with an increased risk for subsequent hypertension and incident ASCVD. The association between HDP-PRS and subsequent hypertension after delivery in the external validation with Penn Medicine Biobank also supports the usefulness of HDP-PRS. Further studies are needed to determine whether HDP-PRS would be a useful consideration even in other ethnicities.
In addition, we evaluated if HDP-PRS can predict long-term ASCVD after pregnancy in the current study. However, the PRS for ASCVD itself may also be helpful for prediction of incident ASCVD, and the evaluation of performance of other types of PRS’s could be another interesting topic. Furthermore, combination of multiple PRS’s could be next important research topics, although using multiple PRS’s is novel but not-confirmed methodology.
Strengths and limitations
To our knowledge, the current study is the first study to evaluate the application of HDP-PRS. Nevertheless, the current study has several limitations. In the current study, HDP-PRS was developed and evaluated in European women of UK Biobank. The application of HDP-PRS for other ethnic groups or the development of multi-ethnic HDP-PRS should be also evaluated in further studies. For clinical application in HDP-PRS in practice, some large scale studies are needed in the setting of preventive medicine with HDP-PRS. In addition, the combination of HDP-PRS and HDP specific biomarkers will be the next research interest for individual risk stratification, as there have been accumulating evidence regarding promising biomarkers on the cardiovascular risk after HDP51. Lastly, recent studies reported that not only maternal genotype but also fetal genotype could affect the development of pregnancy complications44, 52. However, we could not evaluate the impact of fetal genotype in the development of HDP-PRS and subsequent ASCVD, because UK Biobank does not have information regarding the fetal genotype. Further studies regarding the relationship between maternal and fetal genotype and the risk of pregnancy complications and subsequent CVD should be performed in further studies.
Conclusion
In conclusion, we have shown that high genetic risk for HDP could be helpful in prediction of long-term cardiovascular outcomes later in life. Adoption of HDP-PRS in era of personalized medicine might be the next important issue in further studies. In addition, population-specific analyses in other ethnicities are also needed, and datasets will likely be available in the near future.
Supplementary Material
AJOG at a Glance.
A. Why was the study conducted?
Previous studies suggest that hypertensive disorders during pregnancy (HDP) are associated with the risk of long-term cardiovascular disease later in life, and clinical guidelines recommend including HDP as an important female-specific factor in risk assessment. However, it has not yet been determined whether genetic predisposition for HDP contributes to the development of subsequent cardiovascular disease. In the current study, we developed polygenic risk scores for HDP (HDP-PRS) and evaluated its impact on long-term atherosclerotic cardiovascular disease (ASCVD).
B. What are the key findings?
1) As expected, women with high HDP-PRS more frequently reported HDP history than those with low HDP-PRS during pregnancy. 2) In addition, women with high HDP-PRS had a higher prevalence of hypertension at enrollment. 3) After enrollment, women with high HDP-PRS had increased risk for incident ASCVD, including coronary artery disease, myocardial infarction, and peripheral artery disease, even after adjustment for confounding variables (history of HDP and other covariates, including age, BMI, smoking, prevalent metabolic disease, and medication).
C. What does the study add to what is already known?
This study provides evidence on the informative value of HDP-PRS in the prediction of long-term cardiovascular outcomes later in life.
Condensation.
We evaluated the association between high genetic risk for hypertensive disorders during pregnancy (HDP) and incident atherosclerotic cardiovascular disease. Women with high genetic risk for HDP had increased risk not only for HDP itself but also for incident atherosclerotic cardiovascular disease, compared to those with low high genetic risk.
ACKNOWLEDGEMENTS
We want to acknowledge the participants and investigators of the FinnGen study.
Funding:
This work was supported by NIGMS R01 GM138597, National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2021R1F1A1046707) and the Seoul National University Hospital Research Fund (No 0320210230).
Nonstandard abbreviations and acronyms:
- ANOVA
analysis of variance
- ASCVD
atherosclerotic cardiovascular disease
- AUC
area under the curve
- BMI
body mass index
- HDP
hypertensive disorders during pregnancy
- HDP-PRS
polygenic risk scores for hypertensive disorders during pregnancy
- GWASs
genome-wide association studies
- ICD
International Classification of Disease
- MAF
minor allele frequency
- MI
myocardial infarction
- PCs
principal components
- PMBB
Penn Medicine Biobank
- PRS
polygenic risk score
- SNPs
single-nucleotide polymorphisms
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None to declare
DISCLOSURES
None to declare.
REFERENCES
- 1.Mortality GBD, Causes of Death C. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anene-Nzelu CG, Lee MCJ, Tan WLW, Dashi A, Foo RSY. Genomic enhancers in cardiac development and disease. Nat Rev Cardiol 2021. [DOI] [PubMed] [Google Scholar]
- 3.Kessler T, Schunkert H. Coronary Artery Disease Genetics Enlightened by Genome-Wide Association Studies. JACC Basic Transl Sci 2021;6:610–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng J, de Vlaming R, Wu Y, et al. Signatures of negative selection in the genetic architecture of human complex traits. Nat Genet 2018;50:746–53. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Qi G, Park JH, Chatterjee N. Estimation of complex effect-size distributions using summary-level statistics from genome-wide association studies across 32 complex traits. Nat Genet 2018;50:1318–26. [DOI] [PubMed] [Google Scholar]
- 6.Khera AV, Emdin CA, Drake I, et al. Genetic Risk, Adherence to a Healthy Lifestyle, and Coronary Disease. N Engl J Med 2016;375:2349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mavaddat N, Michailidou K, Dennis J, et al. Polygenic Risk Scores for Prediction of Breast Cancer and Breast Cancer Subtypes. Am J Hum Genet 2019;104:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khera AV, Chaffin M, Aragam KG, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet 2018;50:1219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson G On the utilization of polygenic risk scores for therapeutic targeting. PLoS Genet 2019;15:e1008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis CM, Vassos E. Prospects for using risk scores in polygenic medicine. Genome Med 2017;9:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet 2019;51:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torkamani A, Wineinger NE, Topol EJ. The personal and clinical utility of polygenic risk scores. Nat Rev Genet 2018;19:581–90. [DOI] [PubMed] [Google Scholar]
- 13.Honigberg MC, Zekavat SM, Aragam K, et al. Long-Term Cardiovascular Risk in Women With Hypertension During Pregnancy. J Am Coll Cardiol 2019;74:2743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ Cardiovasc Qual Outcomes 2017;10. [DOI] [PubMed] [Google Scholar]
- 15.Stuart JJ, Tanz LJ, Cook NR, et al. Hypertensive Disorders of Pregnancy and 10-Year Cardiovascular Risk Prediction. J Am Coll Cardiol 2018;72:1252–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:3168–209. [DOI] [PubMed] [Google Scholar]
- 17.SUDLOW C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS medicine 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018;562:203–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biobank UK . UK Biobank ethics and governance framework. Available at: https://prod.ukbiobank.ac.uk/media/0xsbmfmw/egf.pdf. Accessed February 08, 2021. [Google Scholar]
- 20.LeFevre ML, U.S. Preventive Services Task Force, Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;161:819–26. [DOI] [PubMed] [Google Scholar]
- 21.National Collaborating Centre for Women’s National Collaborating Centre for Women’s pregnancy: the management of hypertensive pregnancy: the management of hypertensive Press (2010).
- 22. https://www.ukbiobank.ac.uk/enable-your-research/about-our-data/health-related-outcomes-data .
- 23.Vilhjalmsson BJ, Yang J, Finucane HK, et al. Modeling Linkage Disequilibrium Increases Accuracy of Polygenic Risk Scores. Am J Hum Genet 2015;97:576–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FinnGen. (2020). FinnGen documentation of R2 release, https://finngen.gitbook.io/finngen-documentation/-LvQ4yR2YFUM5eFTjieO/.
- 25.Du Z, Lubmawa A, Gundell S, et al. Genetic risk of prostate cancer in Ugandan men. The Prostate 2018;78:370–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chami N, Preuss M, Walker RW, Moscati A, Loos RJF. The role of polygenic susceptibility to obesity among carriers of pathogenic mutations in MC4R in the UK Biobank population. PLoS medicine 2020;17:e1003196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pyun JM, Park YH, Lee KJ, et al. Predictability of polygenic risk score for progression to dementia and its interaction with APOE epsilon4 in mild cognitive impairment. Transl Neurodegener 2021;10:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loh P-R, Bhatia G, Gusev A, et al. Contrasting genetic architectures of schizophrenia and other complex diseases using fast variance-components analysis. Nature Genetics 2015;47:1385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994; 81 515–26. [Google Scholar]
- 30.Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ 2002;325:157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia 2019;62:905–14. [DOI] [PubMed] [Google Scholar]
- 32.Kessous R, Shoham-Vardi I, Pariente G, Sherf M, Sheiner E. An association between gestational diabetes mellitus and long-term maternal cardiovascular morbidity. Heart 2013;99:1118–21. [DOI] [PubMed] [Google Scholar]
- 33.Fadl H, Magnuson A, Ostlund I, Montgomery S, Hanson U, Schwarcz E. Gestational diabetes mellitus and later cardiovascular disease: a Swedish population based case-control study. BJOG 2014;121:1530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savitz DA, Danilack VA, Elston B, Lipkind HS. Pregnancy-induced hypertension and diabetes and the risk of cardiovascular disease, stroke, and diabetes hospitalization in the year following delivery. Am J Epidemiol 2014;180:41–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu P, Mamas MA, Gulati M. Pregnancy As a Predictor of Maternal Cardiovascular Disease: The Era of CardioObstetrics. Journal of women's health 2019;28:1037–50. [DOI] [PubMed] [Google Scholar]
- 36.Sondergaard MM, Hlatky MA, Stefanick ML, et al. Association of Adverse Pregnancy Outcomes With Risk of Atherosclerotic Cardiovascular Disease in Postmenopausal Women. JAMA Cardiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tooher J, Thornton C, Makris A, Ogle R, Korda A, Hennessy A. All Hypertensive Disorders of Pregnancy Increase the Risk of Future Cardiovascular Disease. Hypertension 2017;70:798–803. [DOI] [PubMed] [Google Scholar]
- 38.ACOG Committee Opinion No. 736: Optimizing Postpartum Care. Obstet Gynecol 2018;131:el40–e50. [DOI] [PubMed] [Google Scholar]
- 39.Bushnell C, McCullough LD, Awad IA, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:1545–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Authors/Task Force M, Piepoli MF, Hoes AW, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol 2016;23:NP1–NP96. [DOI] [PubMed] [Google Scholar]
- 41.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the american heart association. Circulation 2011;123:1243–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heida KY, Bots ML, de Groot CJ, et al. Cardiovascular risk management after reproductive and pregnancy-related disorders: A Dutch multidisciplinary evidence-based guideline. Eur J Prev Cardiol 2016;23:1863–79. [DOI] [PubMed] [Google Scholar]
- 43.Okoth K, Chandan JS, Marshall T, et al. Association between the reproductive health of young women and cardiovascular disease in later life: umbrella review. BMJ 2020;371:m3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinthorsdottir V, McGinnis R, Williams NO, et al. Genetic predisposition to hypertension is associated with preeclampsia in European and Central Asian women. Nat Commun 2020;11:5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spracklen CN, Saftlas AF, Triche EW, et al. Genetic Predisposition to Dyslipidemia and Risk of Preeclampsia. American journal of hypertension 2015;28:915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kivioja A, Toivonen E, Tyrmi J, et al. Increased Risk of Preeclampsia in Women With a Genetic Predisposition to Elevated Blood Pressure. Hypertension 2022;79:2008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chatterjee N, Shi J, Garcia-Closas M. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat Rev Genet 2016;17:392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mega JL, Stitziel NO, Smith JG, et al. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet 2015;385:2264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maas P, Barrdahl M, Joshi AD, et al. Breast Cancer Risk From Modifiable and Nonmodifiable Risk Factors Among White Women in the United States. JAMA Oncol 2016;2:1295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Natarajan P, Young R, Stitziel NO, et al. Polygenic Risk Score Identifies Subgroup With Higher Burden of Atherosclerosis and Greater Relative Benefit From Statin Therapy in the Primary Prevention Setting. Circulation 2017;135:2091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bovee EM, Gulati M, Maas AH. Novel Cardiovascular Biomarkers Associated with Increased Cardiovascular Risk in Women With Prior Preeclampsia/HELLP Syndrome: A Narrative Review. Eur Cardiol 2021;16:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gray KJ, Saxena R, Karumanchi SA. Genetic predisposition to preeclampsia is conferred by fetal DNA variants near FLT1, a gene involved in the regulation of angiogenesis. Am J Obstet Gynecol 2018;218:211–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.