Abstract
Immunotherapy that aims to boost the body’s immune responses against pathogens or diseased cells has achieved significant progress for treating different diseases over the past several decades, especially with the success of checkpoint blockades, chimeric antigen receptor T therapy, and cancer vaccines in clinical cancer treatment. Effective immunotherapy necessitates the generation of potent and persistent humoral and T-cell responses, which lies in the ability of modulating and guiding antigen-presenting cells to prime antigen-specific T and B cells in the lymphoid tissues, notably in the lymph nodes proximal to the disease site. To this end, various types of strategies have been developed to facilitate the delivery of immunomodulatory agents to immune cells (e.g. dendritic cells and T cells) in the lymph nodes. Among them, intranodal injection enables the direct exposure of immunomodulators to immune cells in lymph nodes, but is limited by the technical challenge and intrinsic invasiveness. To address, multiple passive and active lymph node-targeting technologies have been developed. In this review, we will provide an overview of different lymph node-targeting technologies developed to date, as well as the mechanism and merits of each approach.
Key words: lymph node targeting, materials, vaccine, immunotherapy, immune response
Highlights
-
•
Targeted modulation of immune cells in lymph nodes enable the development of enhanced immunotherapy.
-
•
Targeting of lymph nodes can be achieved via passive & active targeting technologies.
-
•
Passive targeting relies on lymphatic drainage of particulate systems.
-
•
Active targeting functions via antibody, albumin, immune cell homing materials, or external stimuli.
Introduction
Immunotherapy that aims to modulate the body’s adaptive immune system in a way to better control invading pathogens or diseased cells has reshaped the paradigm for clinical treatment of cancer and other diseases in the past decade.1, 2, 3 Among them, immune checkpoint blockades, chimeric antigen receptor T therapies, and cancer vaccines have been approved by the Food and Drug Administration for treating various types of cancers.4, 5, 6, 7, 8 Vaccines for preventing and treating various types of bacteria and viruses (e.g. severe acute respiratory syndrome coronavirus 2) have also achieved noticeable success.9,10 The effectiveness and robustness of immunotherapy often lies in its ability to modulate antigen-presenting cells (APCs) and amplify disease-specific humoral and T-cell responses. Dendritic cells (DCs), a prominent type of APCs in the body, can sample and present disease-specific antigens via major histocompatibility complexes (MHCs), and further prime antigen-specific T and B cells in the lymphoid tissues, notably lymph nodes proximal to the disease site.11,12 As the major type of lymphoid tissues, the bean-shaped lymph nodes are populated by various types of immune cells including T cells, B cells, DCs, macrophages, and neutrophils, and play a pivotal role in maintaining and regulating systemic adaptive immune responses.
Structurally, lymph nodes are composed of a group of lobules that are mechanically supported by fibrous tissues and encased by the subcapsular sinus (SCS) and capsule, and surrounded by afferent and efferent lymphatics that import and export lymph, respectively.13,14 Starting from the afferent end, lymph nodes can be divided into cortex that contains B-cell follicles, paracortex that contains T-cell follicles, and medulla. DCs that present specific antigens via MHCs can traffic to B- and T-cell follicles to prime antigen-specific T and B cells.15,16 Other immune cells such as lymphatic sinus-associated dendritic cells (LS-DCs) and SCS macrophages line next to the bottom layer of lymphatic endothelial cells.17,18 Within the highly compartmentalized lymph nodes, the migration of immune cells is often dictated by the gradient of chemokines and cytokines. The whole lymph node is also filled with ‘lymph’, the fluid that transports molecules and cells in and out of lymph nodes via afferent and efferent lymphatics. In addition to lymphatic drainage, antigens and immune cells can also enter lymph nodes via the high endothelial venules (HEVs).
As the primary location for T- and B-cell priming processes, lymph nodes have been a key tissue of target for developing potent vaccines and immunotherapies. Indeed, extensive effort has been made to improve the delivery of antigens and adjuvants, two main components of vaccines, to DCs in the lymph nodes, with a goal of amplifying the generation of antigen-presenting DCs and subsequent priming of antigen-specific T and B cells.19,20 The retention time of vaccines in the lymph nodes was also shown to correlate with the overall efficacy in the context of cancer, viral infection, and other diseases. For example, the delivery of antigens and adjuvants into lymph nodes in the form of nanoparticles or microparticles was able to induce a more persistent humoral and T-cell response than the bolus vaccine.21,22 Intranodal delivery of vaccines was also shown to improve the robustness of systemic humoral and T-cell responses in comparison with peripheral delivery.23,24 In addition to antigens and adjuvants, targeted delivery of cytokines to T cells or B cells in the lymph nodes has also been actively explored to orchestrate local and systemic humoral and T-cell responses.25 In the context of immunosuppression, inactivating or anergizing T and B cells in the lymph nodes is also a widely explored approach to achieving systemic immunosuppression.26,27 Among the various types of diseases related to the control of immune cells and immune responses in lymph nodes, cancer is the most extensively studied one to date. In a typical immune-oncology cycle, APCs including DCs sample tumor antigens from dying tumor cells and traffic to the lymph node where they further prime tumor antigen-specific T and B cells. The antigen-specific effector T cells can then find and kill cancer cells.28 Therefore, the targeted delivery of immunomodulatory agents including tumor antigens, adjuvants, and cytokines to immune cells in the lymph nodes has been a central goal for a variety of cancer immunotherapies.28,29
The most straightforward lymph node-targeting strategy is to directly inject immunomodulatory agents into the lymph nodes, as it can bypass the delivery challenges and enable the direct exposure of immunomodulators to immune cells such as DCs. This has been proved effective in inducing potent humoral and T-cell responses in the context of different diseases.30, 31, 32 However, intranodal administration is limited by the high invasiveness, difficulty in locating lymph nodes, and challenges in precise injection of agents or materials into the lymph node. To address this issue, various passive and active targeting strategies enabling improved lymph node delivery of immunomodulatory agents that are administered subcutaneously, intradermally, intramuscularly, peritoneally, or intravenously have also been developed (Figure 1). Among them, passive targeting strategies rely on the body’s lymphatic drainage system or immune cells to carry the administered immunomodulatory agents to the lymph nodes.33, 34, 35 For example, subcutaneously injected nanoscale materials that encapsulate immunomodulators can drain into the proximal lymph nodes via lymphatic vessels. Together with their enhanced in vivo stability, lymph node retention, and cell uptake efficiency, nanoscale materials enable the extended exposure of small-molecular-weight immunomodulators to immune cells in the lymph nodes, for improved antigen presentation and T- and B-cell priming.36, 37, 38, 39 Subcutaneously injected immunomodulatory materials, especially those with a large size, can also be taken up by immune cells that arrive at the injection site and become shuttled to lymph nodes.40, 41, 42 In contrast, active targeting strategies utilize rational molecular or material designs to facilitate the bounding or conjugation of immunomodulatory agents to specific immune cells in the lymph nodes, or external stimuli to facilitate the accumulation of immunomodulators into the lymph nodes (Figure 1). For example, the functionalization of immunomodulatory agents with a targeting ligand that can bind to the surface receptors of DCs or T cells can improve their retention and cellular uptake within the lymph nodes.43,44 Amphiphilic lipids that can bind to albumin which is an endogenous lymph node shuttle have also enabled the enhanced delivery of immunomodulatory agents into lymph nodes.45,46 Strategies to generate chemically tagged DCs in situ, taking advantage of chemokine-loaded macroporous material scaffolds, for subsequent targeted delivery of tumor antigens, adjuvants, and cytokines to DCs in the lymph nodes have also been developed.47,48 In this review, we will provide an overview of lymph node-targeting approaches developed to date, with a focus on the mechanism and merits of each approach instead of the composition or chemical structures of immunomaterials that have been extensively summarized in other reviews.37, 38, 39,42,47
Figure 1.
Targeted delivery of immunomodulatory agents to lymph nodes via passive and active targeting approaches. The administered immunomodulatory agents or materials can directly traffic to lymph nodes via lymphatic drainage or become shuttled by immune cells [e.g. dendritic cells (DCs) and macrophages] at the injection site. Immunomodulatory agents or materials can also be modified with targeting ligands that can bind to surface receptors of immune cells in the lymph nodes. Amphiphilic lipids have also been designed to efficiently bind to albumin in the serum and become shuttled by albumin to lymph nodes. In addition, immune cell homing materials can be utilized to generate chemically tagged immune cells in the draining lymph nodes, for subsequent targeted delivery of immunomodulators. External stimuli such as magnetic field also enable the enhanced accumulation of magnetic immunomaterials in the lymph node.
Intranodal delivery
Intranodal delivery, i.e. direct injection of molecules (e.g. DNA, messenger RNA, peptide, and protein) or materials (e.g. nanoparticles and microparticles) into the lymph node, enables the direct contact of immunomodulatory agents to immune cells in the lymph node while minimizing their exposure to other tissues. This approach was shown to enhance the overall humoral and T-cell response in the context of different diseases including cancer and infectious diseases. For example, intranodal injection of antigen-pulsed DCs was able to induce enhanced CD8 T-cell response than systemic or subcutaneous injection of DCs.49,50 Compared to subcutaneous injection, intranodally injected tumor lysates were also better processed by APCs in the lymph node, resulting in improved cytotoxic T-lymphocyte (CTL) response and antitumor efficacy.51 Intranodal injection of peptide or protein antigens and adjuvants has also been actively explored to enable their direct contact with target DCs in the lymph node for amplified antitumor efficacy. Cytokines can also be directly injected into lymph nodes to modulate the function and phenotypes of T and B cells. However, the benefits of intranodal injection in humoral and T-cell responses and overall efficacy, in comparison with other administration routes such as subcutaneous, intradermal, and intramuscular injection, could vary with the type of diseases and immunomodulatory agents. Also, although intranodal delivery could be beneficial for lymph node delivery of immunomodulatory agents that suffer from poor lymphatic drainage, systemic delivery and other administration routes may enable timely modulation of immune cells in the blood and peripheral tissues, which could lead to comparable or even improved adaptive immune responses and overall efficacy.51, 52, 53 Moreover, immunomodulatory agents that are directly injected into the lymph node may still experience poor retention and become rapidly cleared from the lymph node.
Despite the potential benefits in the direct modulation of immune cells in the lymph nodes, intranodal injection is also limited by its invasive nature and potential damage on lymph nodes and surrounding tissues, difficulty in locating the specific lymph node of interest, and challenge in precise injection of cargos into lymph nodes.32,54 These issues have motivated the development of strategies that can improve the lymphatic drainage of immunomodulatory agents that are administered subcutaneously, intradermally, intramuscularly, intraperitoneally, or intravenously. For example, extensive effort has been made to elucidate the impact of size, morphology, and surface chemistry on the lymphatic draining efficiency of nanoparticles or microparticles, and further optimize these parameters for the improved delivery of immunomodulatory agents to lymph nodes. Approaches that enable active targeting of agents to specific immune cells in the lymph node have also been actively pursued.
Passive lymph node targeting
Passive lymph node-targeting strategies can be classified into two categories: (i) direct lymphatic drainage of immunomodulatory agents or materials that are administered subcutaneously, intradermally, or intramuscularly to lymph nodes,36, 37, 38, 39 and (ii) sampling by immune cells at the injection site to be shuttled into lymph nodes (Figure 2A).40, 41, 42 The former is applicable to most types of molecules and materials. However, small-molecule immunomodulators typically retain poorly in the lymph nodes, despite their rapid lymphatic drainage from the injection site. They can also relatively easily leak into blood vessels to enter the systemic circulation and become distributed in various tissues. To this end, nanoparticles have been actively pursued to improve the lymphatic drainage and lymph node retention of immunomodulators. The impact of composition and physicochemical properties of nanoparticles on the lymphatic drainage and retention efficiency has also been extensively studied. At a larger size or higher hydrophobicity, the injected immunomodulatory agents or materials may retain at the injection site. Immune cells such as neutrophils, macrophages, and DCs that are at the injection site or become recruited to the injection site can take up the immunomodulators, a fraction of which will traffic to the draining lymph nodes to facilitate the T- and B-cell priming processes.
Figure 2.
Passive lymph node targeting. (A) Schematic illustration of common lymph node-targeting particulate systems and the dilemma in their design. (B) Effect of charge and particle size on lymph node retention and dendritic cell (DC) uptake. Adapted from Jiang et al.58 (C) Uptake of different sizes of fluorescein isothiocyanate (FITC)–nanoparticles (NPs) by DCs, as characterized by flow cytometry and confocal imaging. Scale bar: 10 μm. Adapted from Kim et al.56 APC, antigen-presenting cell; DLN, draining lymph node; PE, phosphorethanolamine; PEG, polyethylene glycol; PPM, Poly(phenylene methylene); PSA, polyethylenimine-stearic acid; PSAM, polyethylenimine-stearic acid micelle.
Direct lymphatic drainage of immunomodulators to lymph nodes
Upon subcutaneous, intradermal, intramuscular, or intraperitoneal injection, immunomodulatory agents can directly traffic to the draining lymph nodes via the afferent lymphatic vessels. The lymphatic drainage efficiency of immunomodulatory agents is dependent on the easiness of transportation by lymph, their interactions with molecules and cells within the lymphatic vessels, and likelihood of their leaking into the blood circulation, all of which are related to the composition and physicochemical properties (e.g. size, morphology, and hydrophobicity) of agents (Figure 2B and C).21,55,56 Size is undoubtedly one of the key parameters for affecting the lymphatic drainage efficiency. Small-molecule agents can be easily and efficiently transported by lymph, but are also rapidly cleared from the lymph node via efferent lymphatic vessels. They also have a higher chance of leaking into the blood vessels and become distributed to different tissues. Increasing the size of the immunomodulators by modifying them with polymers (e.g. polyethylene glycol) was shown to improve their retention and overall accumulation in the lymph nodes.57,58 Further, immunomodulatory agents are loaded into nanoscale materials (e.g. micelles, liposomes, and inorganic nanoparticles) for enhanced retention in lymph nodes and controlled release and exposure to immune cells within the lymph nodes. These polymeric conjugates and nanoparticles are also less likely to leak into the blood vessels. In addition, the tunable size, hydrophobicity, and surface chemistry of nanomaterials have enabled fine-tuning of lymphatic drainage efficiency and lymph node retention time. While the optimal size range varies with the type of nanomaterials,21,22,59, 60, 61 a diameter of 10-200 nm is generally considered suitable for drainage into the lymph nodes through the lymphatic vessels. With a diameter larger than 200 nm, nanoparticles possess limited diffusive and convection mobility and tend to remain in the peripheral tissue after injection. The dimension of the interstitial channel also limits their trafficking.59 As particles are traveling along the interstitial water channels, a hydrophilic surface often facilitates their lymphatic drainage.62,63 In terms of surface charge, negatively charged or neutral particles can reach the lymph node more easily compared with positively charged ones that are more likely to be trapped by the negatively charged extracellular matrix of interstitium.64 It is noteworthy that, with rational chemistry designs, nanomaterials also enable the tuning of the release kinetics of immunomodulatory agents once they enter the lymph node.
In addition to lymphatic drainage via the afferent lymphatic vessels, the entry of immunomodulatory agents or materials to lymph nodes via blood vessels can also be facilitated. For example, immunomodulatory agents can be functionalized with antibodies that can bind to HEVs, which can facilitate the penetration of agents across HEVs and enter lymph nodes by mimicking the multistep adhesion process of naïve B and T cells.65 Microparticles were also functionalized with MECA-79 antibody that can bind to peripheral node addressin of HEVs. Upon intravenous injection, anti-MECA-79-modified microparticles exhibited significantly enhanced accumulation in lymph nodes than the unmodified counterpart.66 This HEV-mediated delivery approach could be utilized to amplify the immunomodulatory effect of systemically administered agents, especially when the timely modulation of immune cells in the bloodstream or within tissues via intravenous administration of immunomodulators is crucial. Further understanding of the mechanism of blood capillary transport into lymph nodes as well as the careful evaluation of its potential influence on the distal sites such as liver and spleen are needed before fully exploiting this strategy.67
Lymphatic drainage via immune cells at the injection site
Upon the injection of the immunomodulatory agents, phagocytic cells such as macrophages and DCs at or near the injection site can also take up the agents and shuttle them to lymph nodes, taking advantage of their intrinsic ability to migrate to the lymph node through afferent lymphatic vessels or blood vessels under the guidance of cytokines, chemokines, as well as physical cues.65,68,69 This approach is especially useful for delivering large particles that cannot efficiently drain to lymph nodes by themselves. The injection of immunomodulators that can retain at the injection site is often accompanied by inflammation and the recruitment of immune cells such as neutrophils, macrophages, and DCs.70, 71, 72 These immune cells can take up the immunomodulators before they traffic to lymphatic tissues and pass the immunomodulators to other immune cells in the lymph node. Among them, macrophages and DCs, two major types of APCs, can also directly process and present antigens at the injection site, and migrate to lymph nodes to prime antigen-specific T and B cells. Both pathways could occur and the contribution from each pathway tends to be indistinguishable to some extent in the context of certain diseases. More sophisticated than the simple pick-up and transporting process is the modulation of immune cells at the injection site. For example, upon capturing and endocytosing the injected adjuvants or cytokines, immature DCs could mature into a less phagocytotic but more motile state, with the up-regulated expression of MHC, costimulatory receptors, as well as chemokine receptors on the cell surface.73,74 The mature and migratory DCs can then be attracted to lymphatic tissues by the chemokine gradients, where they interact with and prime T and B cells.75 The lymph node trafficking efficiency of immunomodulators mediated by immune cells in situ is undoubtedly dependent on the abundance and phagocytic and migratory properties of immune cells. The abundance of phagocytic cells at the injection site varies with the location of injection and the types of immunomodulators. For example, immunomodulators injected to the subcutaneous space tend to recruit a higher number of immune cells than those injected intramuscularly.76,77 Different types of immunomodulators also induce different levels of inflammation and thus recruit varied amounts of immune cells to the injection site. Among the same type of immune cells (e.g. DCs or macrophages), different cell subsets could also exhibit distinct phagocytic and migratory properties. All of these contribute to the lymph node trafficking efficiency.
Active lymph node targeting
Different from passive targeting that purely relies on the lymphatic drainage of particulate systems, active targeting aims to specifically deliver immunomodulatory agents to immune cells of interest within the lymph node. One approach is to modify immunomodulatory agents with targeting ligands that can specifically bind to surface receptors of immune cells. For example, nanoparticles encapsulating antigens and adjuvants can be modified with anti-DEC205 or anti-CD11c that can bind to DCs, for improved uptake and antigen presentation by DCs in the lymph node.58 Molecules or materials can also be functionalized with anti-CD3, anti-CD4, or anti-CD8 for T-cell targeting. In addition to targeting the endogenous receptors of resident immune cells, amphiphilic lipids that can bind to albumin and hijack the ability of albumin to target lymph nodes have also been developed. Immune cell homing macroporous biomaterials can also be utilized to generate chemically tagged DCs in the lymph nodes, for subsequent targeted conjugation of antigens, adjuvants, and cytokines via efficient and bioorthogonal click chemistry.
Antibody-mediated cell targeting
Functionalization of immunomodulatory agents or nanoparticles with antibodies that can specifically bind to the surface receptors of immune cells such as DCs, T cells, and B cells in the lymph nodes can improve their uptake by the target cells. Among them, DCs have been a frequent target for the delivery of antigens and adjuvants, especially in the context of cancer. For example, by modifying nanoparticles encapsulating tumor antigens and adjuvants with anti-DEC205 that can bind to DEC205-expressing DCs, the accumulation of antigens and adjuvants and exposure to DCs in the lymph nodes were improved, resulting in enhanced CTL response and antitumor efficacy.43,44,78 DEC205 is a type of endocytic C-type lectin receptors, and their binding can mediate a higher efficiency in antigen uptake and cross presentation by DCs.79 In the context of viral vaccines, it was also shown that conjugation of human immunodeficiency virus (HIV) gap protein with anti-DEC205 can significantly improve HIV-specific CD8+ T-cell response in comparison with protein without anti-DEC205 functionalization.80 In addition to DEC205, DCs that express various types of pattern recognition receptors can also bind to a variety of pathogen-associated molecular patterns such as mannose, lipopolysaccharide, fucose, peptidoglycans, and lipoproteins.81 For example, mannosylated antigens and adjuvants have been widely explored for DC-targeted delivery. Nanomaterial vaccines based on gold nanoparticle, dendrimer, and chitosan nanoparticles were also modified with mannoses for improved delivery into DCs in the lymph node, with a goal of improving antigen presentation and subsequent T- and B-cell priming processes.82, 83, 84, 85 To achieve the DC-targeting effect, extensive effort has also been made to modify vaccine components or nanomaterial vaccines with anti-CD11c that can bind to the CD11c lineage marker of DCs. While this approach was able to improve the uptake of vaccines by DCs in vitro and in vivo, its benefit in boosting the antigen presentation and subsequent T-cell responses has been inconsistent, casting a doubt on utilizing lineage markers to mediate the targeting effect.
Other types of immune cells in the lymph nodes, including T cells and SCS macrophages, have also been explored as the target. For example, nanosized exosomes coated with anti-cytotoxic T-lymphocyte-associated protein 4 and costimulatory molecules were utilized to target T cells. The engineered exosomes showed enhanced specificity toward T cells and facilitated tumor-specific T-cell response.86 Functionalization of immunomodulatory agents with antibodies against T-cell lineage markers such as anti-CD3, anti-CD4, and anti-CD8 has also been explored for selective modulation of T cells in the lymph nodes.87, 88, 89, 90 For the targeting of SCS macrophages, immunomodulatory nanogels functionalized with C-agarose, which can bind to Siglec-1 on the surface of macrophages, were able to exhibit enhanced uptake by SCS macrophages in lymph nodes and result in improved prevention of lymphatic metastasis in comparison with unmodified nanogels.91
Albumin-mediated targeting
Albumin has a natural abundance in the bloodstream and can bind water, cations, fatty acids, hormones, and pharmaceuticals. Albumins also tend to drain into the lymphatics where their concentration is lower than in the blood, making them an attractive endogenous vector for targeted delivery of cargos such as antigens and adjuvants into lymphatic tissues.92 To achieve lymph node targeting via albumins, the administered molecules need to be able to efficiently bind to albumins, and then become transported to the lymph node where they are passed on to DCs for subsequent antigen presentation and T- and B-cell priming processes.93 In view of the ability of albumin to bind to fatty acids and hydrophobic molecules, an albumin-binding domain composed of diacyl lipids was developed and utilized to conjugate CpG and peptide antigens. The amphiphilic conjugates are able to form nanosized micelles in aqueous solutions, effectively bind to albumin, and traffic to lymph nodes with the assistance from albumin, leading to improved accumulation in lymph nodes than antigens/adjuvants without the diacyl modification (Figure 3A-F). As a result, a significantly improved accumulation of peptide antigens and adjuvants in DCs in lymph nodes was achieved, as well as the enhanced CTL response and antitumor efficacy.94 The albumin-hitchhiking amphiphilic adjuvant was also shown to improve the humoral response toward protein antigens. In addition to antigens and adjuvants, the albumin-hitchhiking approach was also used to deliver azido-functionalzied 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-poly(ethylene glycol) (DSPE-PEG) to the lymph node to tag endothelial cells with azido groups, for subsequent targeted conjugation of dibenzocyclooctyne (DBCO)-bearing liposomes via efficient click chemistry.95 This approach improved the accumulation as well as cellular uptake of antigen/adjuvant-loaded liposomes by DCs in the lymph node, leading to a more robust and prolonged immune response compared with liposome alone.95
Figure 3.
Lymph node targeting via albumin-hitchhiking. (A) Design of amphiphilic adjuvant conjugate (amph-CpG) that can bind to albumin. (B) Size-exclusion chromatography (SEC) of CpGs alone or after incubation with fetal bovine serum (FBS) for 2 h (left), and percentage of CpG bounded to albumin (right). (C) In vivo imaging system (IVIS) images and fluorescence quantification of inguinal and axillary nodes at 24 h post-injection. (D) CpG accumulation in draining lymph nodes at different times. (E) Structure of amph-peptides. (F) Lymph node accumulation of subcutaneously injected free peptides (D-E7) or amph-peptides (amph-D-E7). ∗∗0.001<P <0.01; statistically significant. A-F are adapted from Liu et al.45
Immune cell homing material-based lymph node targeting
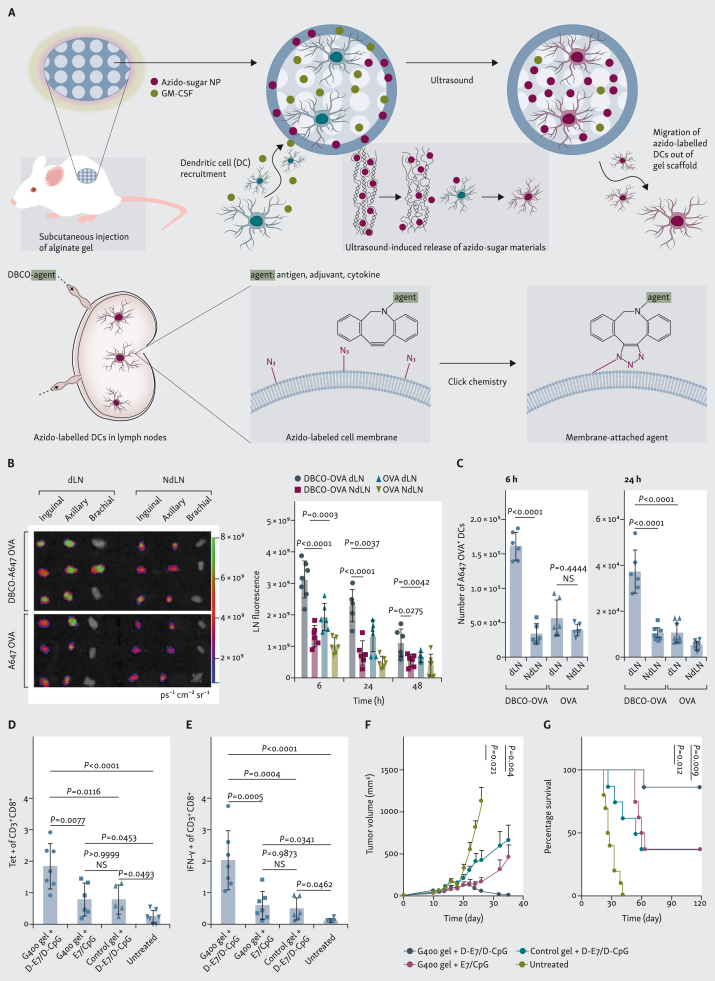
Instead of attempting to deliver immunomodulatory agents to immune cells in the lymph nodes, immune cell homing materials have been developed to actively recruit and program immune cells (e.g. DCs) in situ before they traffic to lymph nodes. In these designs, chemokines such as granulocyte–macrophage colony-stimulating factor (GM-CSF) are loaded in the macroporous biomaterial and gradually released to recruit DCs and other immune cells.96, 97, 98, 99 The recruited DCs can then become modulated within the biomaterial scaffold before they migrate out of the material and traffic to lymphatic tissues. By loading unnatural sugars [e.g. tetraacetyl-N-azidoacetylmannosamine (AAM)] into the GM-CSF-loaded macroporous scaffold, the recruited DCs can be metabolically labeled with azido groups via the metabolic glycoengineering process.47,48 These azido groups are expressed on DC membrane in the form of glycoproteins and glycolipids, and enable subsequent targeted conjugation of DBCO-bearing molecules via efficient and bioorthogonal click chemistry (Figure 4A). By subcutaneously injecting AAM- and GM-CSF-loaded macroporous gels into mice, a high number of azido-labeled DCs could be generated in situ, a fraction of which managed to migrate to the draining lymph nodes. Once within the lymph nodes, the azido-labeled DCs can covalently capture subsequently administered antigens, adjuvants, and cytokines for improved processing and presentation of antigens and enhanced CTL response and antitumor efficacy (Figure 4B-G).
Figure 4.
Immune cell homing material-enabled lymph node targeting. (A) Macroporous hydrogels loaded with granulocyte–macrophage colony-stimulating factor (GM-CSF) and azido-sugar nanoparticles (NPs) can recruit dendritic cells (DCs) and metabolically label DCs with azido groups in situ. Azido-labeled DCs can then migrate to lymph nodes for subsequent targeted conjugation of antigens, adjuvants, and cytokines via efficient click chemistry. (B and C) After injection of gels (day 0) and ultrasound treatment (day 3) to generate azido-labeled DCs in gels and lymph nodes, Alexa Fluor 647 (A647)-conjugated dibenzocyclooctyne (DBCO)-OVA or OVA was subcutaneously injected (day 6). (B) In vivo imaging system (IVIS) imaging of lymph nodes and quantification of A647 fluorescence signal in lymph nodes. (C) Total number of A647-OVA+ DCs in lymph nodes at 6 or 24 h post-injection of A647-conjugated DBCO-OVA or OVA. (D and E) Gels loaded with azido-sugar NPs and GM-CSF were subcutaneously injected (day 0), ultrasound was applied (day 3), and DBCO-E7 and DBCO-CpG were subcutaneously injected (days 6, 8, and 10). Percentage of (D) E7 tetramer+ cells and (E) interferon (IFN)-γ+ cells among CD8+ T cells in peripheral blood mononuclear cells (PBMCs) (day 16). (F and G) TC-1 tumors were inoculated (day 0), followed by subcutaneous injection of gels with azido-sugar NPs and GM-CSF (day 4), ultrasound treatment (day 7), and subcutaneous injection of DBCO-E7 and DBCO-CpG (days 10, 12, and 14). (F) Average tumor volumes over therapeutic study (statistical comparisons on day 35 given). (G) Kaplan–Meier plots. A-G are adapted from Wang et al.48
External stimuli-mediated lymph node targeting
External stimuli such as magnetic field can also be utilized to achieve lymph node targeting.100, 101, 102, 103, 104, 105, 106 For example, by locally applying magnetic field to the lymph node, the subcutaneously administered magnetic particles that traffic to the lymph nodes can retain better and become concentrated within the lymph node (Figure 5A and B). In the absence of the magnetic field, instead, magnetic particles that migrate to the lymph nodes are rapidly cleared via the efferent vessels. The magnet-assisted approach was able to prolong the retention of iron oxide nanoparticles loaded with CpG and coated with cancer cell membranes in the lymph node. A 5.99-fold enhancement in the retention half-life of the iron oxide nanoparticles in the lymph node was achieved at an optimized magnetic field.100 As a result, the exposure of the cancer antigens and adjuvants to DCs in the lymph node was extended, for improved CTL response and antitumor efficacy. Moreover, magnetic field can also be used to directly manipulate DC migration. By loading DCs with magnetic nanoparticles, the migratory property of DCs was improved in the presence of the magnetic field that can pull DCs though the lymph and extracellular matrix.102
Figure 5.
Magnetic field-mediated lymph node targeting.
(A) Fabrication and immunogencity of magnetic nanoclusters (MNCs) coated with cancer cell membrane and anti-CD205 and loaded with CpG. (B) Accumulation and retention of MNCs in the presence of external magnetic field. The cancer cell membrane coated on to MNCs was labeled with DiR. A and B are adapted from Li et al.100 CTL, cytotoxic T-lymphocyte; DC, dendritic cell; LN, lymph node; MF, membrane fragment; MRI, magnetic resonance imaging.
Summary and outlook
Targeted delivery of immunomodulatory agents including antigens, adjuvants, cytokines, and antibodies into immune cells in the lymph nodes is critical for achieving optimal humoral and T-cell responses in the context of cancer, infectious diseases, and other diseases. While intranodal delivery can directly expose immunomodulators to immune cells in the lymph nodes, the technical challenges and intrinsic invasiveness have limited its use. Alternatively, various passive and active lymph node-targeting strategies have been developed. These approaches have enabled the development of new immunotherapies with robust and persistent humoral and T-cell responses, and facilitated a better understanding of immune responses occurring within the lymph nodes, the communication between peripheral tissues and lymphatic tissues, and the critical role lymph nodes are playing in orchestrating systemic immune responses. Scientifically, much remains to be understood, including the synergistic effect of immune responses within the lymph nodes and the immune cascades at the peripheral tissues, especially at the injection site of immunomodulatory agents or materials. For example, subcutaneously antigen-loaded alum particles can form an inflammatory in situ node while releasing antigens to the draining lymph nodes, raising a question on the importance of immune responses at each site and the potential synergistic effect.
For passive targeting that is mediated by direct lymph drainage or migratory phagocytic cells, the contribution from each mechanism to the overall immunomodulatory effect has been elusive. While the physicochemical properties (e.g. size, surface charge, and hydrophobicity) of injected immunomodulatory materials can be adjusted to tune the lymph draining efficiency, scrutiny should be given to compare across different types of materials. Active targeting strategies can further improve the accumulation and retention of immunomodulatory agents in lymph nodes, and may also facilitate their delivery into immune cell types of interest. Nevertheless, significant room remains to further improve the targeting efficiency, especially toward DCs, T cells, and B cells in the lymph nodes. Translationally, various types of nanomaterial and biomaterial scaffold-based immunotherapies for the treatment of cancer and viral infections are under clinical trials. We expect more progress in the clinical translation of lymph node-targeted immunotherapies over the next decade. In addition to cancer and infectious diseases, lymph node-targeted delivery of immunomodulators also holds tremendous promise for the treatment of autoimmune diseases, inflammatory disorders, injured tissues, and other diseases, which hopefully will be more actively explored in the years to come.
Acknowledgments
Funding
The authors would like to acknowledge the financial support from NSF DMR 21-43673 CAR (H.W.), NIH R01CA274738 (H.W.), NIH R21CA270872 (H.W.), and start-up package (H.W.) from the Department of Materials Science and Engineering at the University of Illinois at Urbana-Champaign and the Cancer Center at Illinois (CCIL).
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Waldmann T.A. Immunotherapy: past, present and future. Nat Med. 2003;9:269–277. doi: 10.1038/nm0303-269. [DOI] [PubMed] [Google Scholar]
- 2.Couzin-Frankel J. Cancer immunothearpy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 3.Mellman I., Coukos G., Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Havel J.J., Chowell D., Chan T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19:133–150. doi: 10.1038/s41568-019-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.June C.H., O’Connor R.S., Kawalekar O.U., Ghassemi S., Milone M.C. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg S.A., Yang J.C., Restifo N.P. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahin U., Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359:1355–1360. doi: 10.1126/science.aar7112. [DOI] [PubMed] [Google Scholar]
- 9.Le T.T., Andreadakis Z., Kumar A., et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 10.Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20:615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mempel T.R., Henrickson S.E., Von Andrian U.H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 12.Bousso P., Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 13.Tadayon S., Dunkel J., Takeda A., et al. Lymphatic endothelial cell activation and dendritic cell transmigration is modified by genetic deletion of Clever-1. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.602122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gretz J.E., Kaldjian E.P., Anderson A.O., Shaw S. Sophisticated strategies for information encounter in the lymph node: the reticular network as a conduit of soluble information and a highway for cell traffic. J Immunol. 1996;157:495–499. [PubMed] [Google Scholar]
- 15.Victora G.D., Nussenzweig M.C. Germinal centers. Annu Rev Immunol. 2022;40:413–442. doi: 10.1146/annurev-immunol-120419-022408. [DOI] [PubMed] [Google Scholar]
- 16.Romani N., Ratzinger G., Pfaller K., et al. Migration of dendritic cells into lymphatics-the Langerhans cell example: routes, regulation, and relevance. Int Rev Cytol. 2001;207:237–270. doi: 10.1016/s0074-7696(01)07007-3. [DOI] [PubMed] [Google Scholar]
- 17.Gerner M.Y., Torabi-Parizi P., Germain R.N. Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity. 2015;42:172–185. doi: 10.1016/j.immuni.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Moran I., Grootveld A.K., Nguyen A., Phan T.G. Subcapsular sinus macrophages: the seat of innate and adaptive memory in murine lymph nodes. Trends Immunol. 2019;40:35–48. doi: 10.1016/j.it.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Dudziak D., Kamphorst A.O., Heidkamp G.F., et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 20.den Haan J.M., Lehar S.M., Bevan M.J. CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy S.T., van der Vlies A.J., Simeoni E., et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 22.Reddy S.T., Rehor A., Schmoekel H.G., Hubbell J.A., Swartz M.A. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J Control Release. 2006;112:26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Tagawa S.T., Lee P., Snively J., et al. Phase I study of intranodal delivery of a plasmid DNA vaccine for patients with Stage IV melanoma. Cancer. 2003;98:144–154. doi: 10.1002/cncr.11462. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi H., Kawamoto S., Bernardo M., Brechbiel M.W., Knopp M.V., Choyke P.L. Delivery of gadolinium-labeled nanoparticles to the sentinel lymph node: comparison of the sentinel node visualization and estimations of intra-nodal gadolinium concentration by the magnetic resonance imaging. J Control Release. 2006;111:343–351. doi: 10.1016/j.jconrel.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 25.St John A.L., Chan C.Y., Staats H.F., Leong K.W., Abraham S.N. Synthetic mast-cell granules as adjuvants to promote and polarize immunity in lymph nodes. Nat Mater. 2012;11:250–257. doi: 10.1038/nmat3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufman D.B., Shapiro R., Lucey M.R., Cherikh W.S., Bustami R.T., Dyke D.B. Immunosuppression: practice and trends. Am J Transplant. 2004;4:38–53. doi: 10.1111/j.1600-6135.2004.00397.x. [DOI] [PubMed] [Google Scholar]
- 27.Adams D.H., Sanchez-Fueyo A., Samuel D. From immunosuppression to tolerance. J Hepatol. 2015;62:S170–S185. doi: 10.1016/j.jhep.2015.02.042. [DOI] [PubMed] [Google Scholar]
- 28.Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura T., Harashima H. Dawn of lipid nanoparticles in lymph node targeting: potential in cancer immunotherapy. Adv Drug Deliv Rev. 2020;167:78–88. doi: 10.1016/j.addr.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Jewell C.M., Bustamante López S.C., Irvine D.J. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc Natl Acad Sci. 2011;108:15745–15750. doi: 10.1073/pnas.1105200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonuleit H., Giesecke-Tuettenberg A., Tüting T., et al. A comparison of two types of dendritic cell as adjuvants for the induction of melanoma-specific T-cell responses in humans following intranodal injection. Int J Cancer. 2001;93:243–251. doi: 10.1002/ijc.1323. [DOI] [PubMed] [Google Scholar]
- 32.Weber J., Boswell W., Smith J., et al. Phase 1 trial of intranodal injection of a Melan-A/MART-1 DNA plasmid vaccine in patients with stage IV melanoma. J Immunother. 2008;31:215–223. doi: 10.1097/CJI.0b013e3181611420. [DOI] [PubMed] [Google Scholar]
- 33.Esterházy D., Canesso M.C.C., Mesin L., et al. Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature. 2019;569:126–130. doi: 10.1038/s41586-019-1125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas S.N., Vokali E., Lund A.W., Hubbell J.A., Swartz M.A. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials. 2014;35:814–824. doi: 10.1016/j.biomaterials.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y., De Koker S., De Geest B.G. Engineering strategies for lymph node targeted immune activation. Acc Chem Res. 2020;53:2055–2067. doi: 10.1021/acs.accounts.0c00260. [DOI] [PubMed] [Google Scholar]
- 36.Zhu G., Zhang F., Ni Q., Niu G., Chen X. Efficient nanovaccine delivery in cancer immunotherapy. ACS Nano. 2017;11:2387–2392. doi: 10.1021/acsnano.7b00978. [DOI] [PubMed] [Google Scholar]
- 37.Irvine D.J. Materializing the future of vaccines and immunotherapy. Nat Rev Mater. 2016;1:1–2. [Google Scholar]
- 38.Irvine D.J., Dane E.L. Enhancing cancer immunotherapy with nanomedicine. Nat Rev Immunol. 2020;20:321–334. doi: 10.1038/s41577-019-0269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irvine D.J., Swartz M.A., Szeto G.L. Engineering synthetic vaccines using cues from natural immunity. Nat Mater. 2013;12:978–990. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Backer R., Schwandt T., Greuter M., et al. Effective collaboration between marginal metallophilic macrophages and CD8+ dendritic cells in the generation of cytotoxic T cells. Proc Natl Acad Sci. 2010;107:216–221. doi: 10.1073/pnas.0909541107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cyster J.G. B cell follicles and antigen encounters of the third kind. Nat Immunol. 2010;11:989–996. doi: 10.1038/ni.1946. [DOI] [PubMed] [Google Scholar]
- 42.Hubbell J.A., Thomas S.N., Swartz M.A. Materials engineering for immunomodulation. Nature. 2009;462:449–460. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- 43.Cheong C., Choi J.H., Vitale L., et al. Improved cellular and humoral immune responses in vivo following targeting of HIV Gag to dendritic cells within human anti–human DEC205 monoclonal antibody. Blood. 2010;116:3828–3838. doi: 10.1182/blood-2010-06-288068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macri C., Dumont C., Johnston A.P., Mintern J.D. Targeting dendritic cells: a promising strategy to improve vaccine effectiveness. Clin Transl Immunol. 2016;5:e66. doi: 10.1038/cti.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu H., Moynihan K.D., Zheng Y., et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tahir M.A., Guven Z.P., Arriaga L.R., et al. Calcium-triggered fusion of lipid membranes is enabled by amphiphilic nanoparticles. Proc Natl Acad Sci. 2020;117:18470–18476. doi: 10.1073/pnas.1902597117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H. Immune cell homing biomaterials for immunotherapy. Acc Mater Res. 2020;1:172–174. [Google Scholar]
- 48.Wang H., Sobral M.C., Zhang D.K.Y., et al. Metabolic labeling and targeted modulation of dendritic cells. Nat Mater. 2020;19:1244–1252. doi: 10.1038/s41563-020-0680-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nair S.K., Heiser A., Boczkowski D., et al. Induction of cytotoxic T cell responses and tumor immunity against unrelated tumors using telomerase reverse transcriptase RNA transfected dendritic cells. Nat Med. 2000;6:1011–1017. doi: 10.1038/79519. [DOI] [PubMed] [Google Scholar]
- 50.Okada H., Kalinski P., Ueda R., et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with alpha-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29:330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Senti G., Kundig T.M. Intralymphatic immunotherapy. World Allergy Organ J. 2015;8:9. doi: 10.1186/s40413-014-0047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohanan D., Slütter B., Henriksen-Lacey M., et al. Administration routes affect the quality of immune responses: a cross-sectional evaluation of particulate antigen-delivery systems. J Control Release. 2010;147:342–349. doi: 10.1016/j.jconrel.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 53.Lesterhuis W.J., de Vries I.J.M., Schreibelt G., et al. Route of administration modulates the induction of dendritic cell vaccine-induced antigen-specific T cells in advanced melanoma patients. Clin Cancer Res. 2011;17:5725–5735. doi: 10.1158/1078-0432.CCR-11-1261. [DOI] [PubMed] [Google Scholar]
- 54.Sukhbaatar A., Mori S., Kodama T. Intranodal delivery of modified docetaxel: innovative therapeutic method to inhibit tumor cell growth in lymph nodes. Cancer Sci. 2022;113:1125–1139. doi: 10.1111/cas.15283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cai S., Yang Q., Bagby T.R., Forrest M.L. Lymphatic drug delivery using engineered liposomes and solid lipid nanoparticles. Adv Drug Deliv Rev. 2011;63:901–908. doi: 10.1016/j.addr.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim H., Uto T., Akagi T., Baba M., Akashi M. Amphiphilic poly(amino acid) nanoparticles induce size-dependent dendritic cell maturation. Adv Funct Mater. 2010;20:3925–3931. [Google Scholar]
- 57.Kaminskas L.M., Kota J., McLeod V.M., Kelly B.D., Karellas P., Porter C.J. PEGylation of polylysine dendrimers improves absorption and lymphatic targeting following SC administration in rats. J Control Release. 2009;140:108–116. doi: 10.1016/j.jconrel.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Jiang H., Wang Q., Sun X. Lymph node targeting strategies to improve vaccination efficacy. J Control Release. 2017;267:47–56. doi: 10.1016/j.jconrel.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 59.Trevaskis N.L., Kaminskas L.M., Porter C.J. From sewer to saviour - targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discov. 2015;14:781–803. doi: 10.1038/nrd4608. [DOI] [PubMed] [Google Scholar]
- 60.Kaminskas L.M., Porter C.J. Targeting the lymphatics using dendritic polymers (dendrimers) Adv Drug Deliv Rev. 2011;63:890–900. doi: 10.1016/j.addr.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 61.Stylianopoulos T., Poh M.-Z., Insin N., et al. Diffusion of particles in the extracellular matrix: the effect of repulsive electrostatic interactions. Biophys J. 2010;99:1342–1349. doi: 10.1016/j.bpj.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rao D.A., Forrest M.L., Alani A.W., Kwon G.S., Robinson J.R. Biodegradable PLGA based nanoparticles for sustained regional lymphatic drug delivery. J Pharm Sci. 2010;99:2018–2031. doi: 10.1002/jps.21970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hong X., Zhong X., Du G., et al. The pore size of mesoporous silica nanoparticles regulates their antigen delivery efficiency. Sci Adv. 2020;6:eaaz4462. doi: 10.1126/sciadv.aaz4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ding Y., Li Z., Jaklenec A., Hu Q. Vaccine delivery systems toward lymph nodes. Adv Drug Deliv Rev. 2021;179 doi: 10.1016/j.addr.2021.113914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Girard J.P., Moussion C., Förster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12:762–773. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- 66.Azzi J., Yin Q., Uehara M., et al. Targeted delivery of immunomodulators to lymph nodes. Cell Rep. 2016;15:1202–1213. doi: 10.1016/j.celrep.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Irvine D.J., Aung A., Silva M. Controlling timing and location in vaccines. Adv Drug Deliv Rev. 2020;158:91–115. doi: 10.1016/j.addr.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.von Andrian U.H., Mempel T.R. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 69.Sallusto F., Lanzavecchia A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev. 2000;177:134–140. doi: 10.1034/j.1600-065x.2000.17717.x. [DOI] [PubMed] [Google Scholar]
- 70.Luster A.D., Alon R., von Andrian U.H. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 71.Calabro S., Tortoli M., Baudner B.C., et al. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine. 2011;29:1812–1823. doi: 10.1016/j.vaccine.2010.12.090. [DOI] [PubMed] [Google Scholar]
- 72.Oleszycka E., Moran H.B.T., Tynan G.A., et al. IL-1α and inflammasome-independent IL-1β promote neutrophil infiltration following alum vaccination. FEBS J. 2016;283:9–24. doi: 10.1111/febs.13546. [DOI] [PubMed] [Google Scholar]
- 73.Ohl L., Mohaupt M., Czeloth N., et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 74.Alvarez D., Vollmann E.H., von Andrian U.H. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schudel A., Francis D.M., Thomas S.N. Material design for lymph node drug delivery. Nat Rev Mater. 2019;4:415–428. doi: 10.1038/s41578-019-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang W.-W., Matlashewski G. Immunization with a toll-like receptor 7 and/or 8 agonist vaccine adjuvant increases protective immunity against leishmania major in BALB/c mice. Infect Immun. 2008;76:3777–3783. doi: 10.1128/IAI.01527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bal S.M., Ding Z., van Riet E., Jiskoot W., Bouwstra J.A. Advances in transcutaneous vaccine delivery: do all ways lead to Rome? J Control Release. 2010;148:266–282. doi: 10.1016/j.jconrel.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 78.Katakowski J.A., Mukherjee G., Wilner S.E., et al. Delivery of siRNAs to dendritic cells using DEC205-targeted lipid nanoparticles to inhibit immune responses. Mol Ther. 2016;24:146–155. doi: 10.1038/mt.2015.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Apostolopoulos V., Thalhammer T., Tzakos A.G., Stojanovska L. Targeting antigens to dendritic cell receptors for vaccine development. J Drug Deliv. 2013;2013 doi: 10.1155/2013/869718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bozzacco L., Trumpfheller C., Siegal F.P., et al. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc Natl Acad Sci U S A. 2007;104:1289–1294. doi: 10.1073/pnas.0610383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qian Y., Jin H., Qiao S., et al. Targeting dendritic cells in lymph node with an antigen peptide-based nanovaccine for cancer immunotherapy. Biomaterials. 2016;98:171–183. doi: 10.1016/j.biomaterials.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 82.Duinkerken S., Horrevorts S.K., Kalay H., et al. Glyco-dendrimers as intradermal anti-tumor vaccine targeting multiple skin DC subsets. Theranostics. 2019;9:5797–5809. doi: 10.7150/thno.35059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garcia-Vallejo J.J., Koning N., Ambrosini M., et al. Glycodendrimers prevent HIV transmission via DC-SIGN on dendritic cells. Int Immunol. 2013;25:221–233. doi: 10.1093/intimm/dxs115. [DOI] [PubMed] [Google Scholar]
- 84.Arnaiz B., Martinez-Avila O., Falcon-Perez J.M., Penades S. Cellular uptake of gold nanoparticles bearing HIV gp120 oligomannosides. Bioconjug Chem. 2012;23:814–825. doi: 10.1021/bc200663r. [DOI] [PubMed] [Google Scholar]
- 85.Renu S., Feliciano-Ruiz N., Patil V., et al. Immunity and protective efficacy of mannose conjugated chitosan-based influenza nanovaccine in maternal antibody positive pigs. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.584299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Phung C.D., Pham T.T., Nguyen H.T., et al. Anti-CTLA-4 antibody-functionalized dendritic cell-derived exosomes targeting tumor-draining lymph nodes for effective induction of antitumor T-cell responses. Acta Biomater. 2020;115:371–382. doi: 10.1016/j.actbio.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 87.McCormack E., Adams K.J., Hassan N.J., et al. Bi-specific TCR-anti CD3 redirected T-cell targeting of NY-ESO-1- and LAGE-1-positive tumors. Cancer Immunol Immunother. 2013;62:773–785. doi: 10.1007/s00262-012-1384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dinauer N., Adams K.J., Hassan N.J., et al. Selective targeting of antibody-conjugated nanoparticles to leukemic cells and primary T-lymphocytes. Biomaterials. 2005;26:5898–5906. doi: 10.1016/j.biomaterials.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 89.Canakci M., Singh K., Munkhbat O., et al. Targeting CD4+ cells with anti-CD4 conjugated mertansine-loaded nanogels. Biomacromolecules. 2020;21:2473–2481. doi: 10.1021/acs.biomac.0c00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ramishetti S., Kedmi R., Goldsmith M., et al. Systemic gene silencing in primary T lymphocytes using targeted lipid nanoparticles. ACS Nano. 2015;9:6706–6716. doi: 10.1021/acsnano.5b02796. [DOI] [PubMed] [Google Scholar]
- 91.Hu J., Xu J., Li M., et al. Targeting lymph node sinus macrophages to inhibit lymph node metastasis. Mol Ther Nucleic Acids. 2019;16:650–662. doi: 10.1016/j.omtn.2019.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoogenboezem E.N., Duvall C.L. Harnessing albumin as a carrier for cancer therapies. Adv Drug Deliv Rev. 2018;130:73–89. doi: 10.1016/j.addr.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abdallah M., Müllertz O.O., Styles I.K., et al. Lymphatic targeting by albumin-hitchhiking: applications and optimisation. J Control Release. 2020;327:117–128. doi: 10.1016/j.jconrel.2020.07.046. [DOI] [PubMed] [Google Scholar]
- 94.Qin H., Zhao R., Qin Y., et al. Development of a cancer vaccine using in vivo click-chemistry-mediated active lymph node accumulation for improved immunotherapy. Adv Mater. 2021;33(20) doi: 10.1002/adma.202006007. [DOI] [PubMed] [Google Scholar]
- 95.Famta P., Shah S., Jain N., et al. Albumin-hitchhiking: fostering the pharmacokinetics and anticancer therapeutics. J Control Release. 2022;353:166–185. doi: 10.1016/j.jconrel.2022.11.034. [DOI] [PubMed] [Google Scholar]
- 96.Wang H., Mooney D.J. Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat Mater. 2018;17:761–772. doi: 10.1038/s41563-018-0147-9. [DOI] [PubMed] [Google Scholar]
- 97.Ali O.A., Huebsch N., Cao L., Dranoff G., Mooney D.J. Infection-mimicking materials to program dendritic cells in situ. Nat Mater. 2009;8:151–158. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim J., Li W.A., Choi Y., et al. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat Biotechnol. 2015;33:64–72. doi: 10.1038/nbt.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ali O.A., Emerich D., Dranoff G., Mooney D.J. In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci Transl Med. 2009;1 doi: 10.1126/scitranslmed.3000359. 8ra19-18ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li F., Nie W., Zhang F., et al. Engineering magnetosomes for high-performance cancer vaccination. ACS Cent Sci. 2019;5:796–807. doi: 10.1021/acscentsci.9b00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jin L., Yang D., Song Y., et al. In situ programming of nanovaccines for lymph node-targeted delivery and cancer immunotherapy. ACS Nano. 2022;16:15226–15236. doi: 10.1021/acsnano.2c06560. [DOI] [PubMed] [Google Scholar]
- 102.Jin H., Qian Y., Dai Y., et al. Magnetic enrichment of dendritic cell vaccine in lymph node with fluorescent-magnetic nanoparticles enhanced cancer immunotherapy. Theranostics. 2016;6:2000–2014. doi: 10.7150/thno.15102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu L., Zhou Z., Mao H., Yang L. Magnetic nanoparticles for precision oncology: theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine (Lond) 2017;12:73–87. doi: 10.2217/nnm-2016-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tietze R., Zaloga J., Unterweger H., et al. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem Biophys Res Commun. 2015;468:463–470. doi: 10.1016/j.bbrc.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 105.Yang F., Jin C., Yang D., et al. Magnetic functionalised carbon nanotubes as drug vehicles for cancer lymph node metastasis treatment. Eur J Cancer. 2011;47:1873–1882. doi: 10.1016/j.ejca.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 106.Sanz-Ortega L., Rojas J.M., Marcos A., et al. T cells loaded with magnetic nanoparticles are retained in peripheral lymph nodes by the application of a magnetic field. J Nanobiotechnol. 2019;17:14. doi: 10.1186/s12951-019-0440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]