Abstract
BACKGROUND
Low birthweight resulting from preterm birth or fetal growth restriction is associated with poor neurocognitive development and child psychopathology affecting school performance and educational success. Prediction of developmental performance may therefore serve as a basis for early intervention strategies to improve educational success and mental health of our children in a timely manner.
OBJECTIVE
This study aimed to explore the predictive capacity of morphometric variables taken at birth and that of obstetrical risk factors to predict developmental performance at 4.3 (standard deviation, 0.8) years preschool age. We examined predicted Total psychomotor development score, predicted Developmental disability index, calculated Morphometric vitality index, and predicted Intelligence quotient, Maze test, and Neurologic examination optimality score in a large prospective screening (cranial ultrasound screening, n=5,301) and validation cohort (n=508,926).
STUDY DESIGN
In a single-center cohort observational study design (data collection done from 1984–1988, analysis done in 2022), a prospective cranial ultrasound screening study (1984–1988) was carried out on 5,301 live-born infants, including 571 (10.8%) preterm infants (≤36 weeks gestation), on the day of discharge of the mother at 5 to 8 days postpartum from a level 3 perinatal center. Predicted psychomotor development as assessed by predicted Total psychomotor development score, predicted Developmental disability index, calculated Morphometric vitality index, and predicted Intelligence quotient, Maze test, and Neurologic examination optimality score, was calculated. We related growth variables and obstetrical risk factors to Psychomotor development indices, and calculated Morphometric vitality index using odds ratios, receiver operating characteristics, analysis of variance, and multivariate analysis of variance.
RESULTS
The key result of our study is the observation that simple morphometric measures from newborns at birth like weight/head circumference ratio predict overall psychomotor development at 4.3 years (standard deviation, 0.8) of preschool age. Psychomotor development was assessed by predicted Total psychomotor development score, predicted Intelligence quotient, Maze test, and Neurologic examination optimality score, and related to weight/head circumference ratio in linear regression (P<.001) and ROC curve analyses (P<.001). Further, white matter damage strongly predicted adverse outcome in predicted Developmental disability index (P<.001). There was also a close correlation between calculated Morphometric vitality index and predicted Developmental disability index (P<.001). Finally, brain body weight ratio, weight/head circumference ratio, preterm birth, reduced Apgar at 10 minutes, weight/length ratio, and white matter damage yielded highest odds ratios for adverse outcome in predicted Total psychomotor development score and in predicted Developmental disability index (P<.001) and high effect sizes in reduced predicted Intelligence quotient, Maze test, and Neurologic examination optimality scores.
CONCLUSION
Simple morphometric data, birth variables, and obstetrical risk factors bear predictive capacity for neurocognitive performance in children at 4.3 years (standard deviation, 0.8) of age and hence provide a basis for parental consultation and early intervention to improve school performance, educational success, and mental health in developed and developing countries.
Key words: Apgar score, asymmetric growth restriction, birth asphyxia, cerebral palsy, disability, infantile brain dysfunction, intelligence quotient, intrauterine growth restriction, Maze test, Neurologic optimality score, parental consultation, preterm birth, weight/head circumference ratio, white matter damage
AJOG Global Reports at a Glance.
Why was this study conducted?
We explored the predictive capacity of morphometric variables taken at birth and that of obstetrical risk factors to predict developmental performance at 4.3(SD 0.8) years preschool age in a large prospective cranial ultrasound screening (CUS, n=5,301) and validation cohort (n=508,926).
Key findings
The key result of our study is the observation that simple morphometric measures from newborns at birth like weight/head circumference ratio (W/HC) and obstetrical risk factors predict overall Psychomotor development at 4.3(SD 0.8) years of preschool age.
What does this add to what is known?
Simple morphometric data, birth variables, and obstetrical risk factors bear predictive capacity for neurocognitive performance in preschool-aged children and hence provide a basis for parental consultation and early intervention to improve school performance, educational success, and mental health in developed and developing countries.
Introduction
In newborns, low birthweight resulting from preterm birth or fetal growth restriction is associated with poor neurocognitive development and child psychopathology that affect school performance and educational success.1, 2, 3, 4, 5, 6, 7 Timely support of these children who are at risk would profit from high plasticity of the human brain in early childhood to better overcome developmental shortcomings.8,9 Therefore, prediction of developmental trajectories is mandatory and may serve as a basis for effective early intervention.2,10
Taken together, the predictive capacity of simple growth and vitality variables available at birth may open up a new avenue for structured and individualised developmental support for children, for example, in social medical nurseries, and parental consultation, provided the results can be confirmed in a larger cohort.10,11 Therefore, we set out to validate the results from our matched pair study on 137 preschool infants by applying the results to all 5,301 newborns and their birth records contained in a prospective cranial ultrasound screening database over the full range of birth weights (350–5,370 g) and gestational ages (24–43 weeks).2,12
Materials and Methods
A prospective cranial ultrasound screening (CUS) study (1984–1988) was carried out on 5,301 live-born infants, including 571 (10.8%) preterms (≤36 weeks), on the day of discharge of the mother at 5–8 days postpartum (after excluding those 498 [8.6%] that left early, ie, at ≤4 days) from a level III perinatal centre at Giessen University, Germany.2,12,13 In a previous study (1982–86) from the same center, both cranial ultrasound screening results after birth and psychomotor development (PMD) were determined in 137(2.4%) children at 4.3 (standard deviation [SD], 0.8) years preschool age in a matched pair design, strictly controlling for confounders, for example, sex, socioeconomic status, maternal education, and brain damage.1,2,14 Intelligence quotient (IQ), Maze test (MT; adapted by Kramer et al, 1985),15 and Neurologic examination optimality score (NOS) were measured (m) and an average composite Total psychomotor development score (mTPMDS) for overall psychomotor development was formed (mTPMDS=[zIntelligence quotient IQ+zMaze test result+zNeurologic examination optimality score]/3).15, 16, 17, 18 These psychomotor development data were extrapolated to the whole ultrasound screening cohort (n=5,301) as follows. The measured psychomotor development testing results as assessed by the Total psychomotor development score were used to generate a prediction model with measured Total psychomotor development score as dependent variable by stepwise multiple regression analysis (pTPMDS=−17.87+0.00043 × weight−0.501 × WMD_present + 2.278 × Ph_umb.art+ 0.177 × mode of delivery; r=0.637, n=129, P<.001) that correlated well with the measured results (r=0.598, n=130, P<.001) and hence was used for extrapolation (n=5,301).1
Secondly, based on predicted (p) Intelligence quotient (pIQ=−153.61 − 1.545 × BBR+43.987 × Ph; r=0.459, n = 131, P<.001), predicted Maze Test (pMT = 541.20 + 0.14 × weight+23.176 × IUGR−12.064 × PIVH-1+2_present + 67.606 × Ph; r=0.516, n=133, P<.001), and predicted Neurologic examination optimality score (pzNOS= −14.03+ 0.30 × weight/length-ratio− 0.623 ×WMD_present–0.353 × PIVH-1 + 2_present+1.683 × Ph+0.326 × mode of delivery–0.366 × pathologic cardiotography; r=0.605; n=132, P<.001), a predicted Developmental disability index (DDI) was formed based on various degrees of Infantile brain dysfunction (IBD) and Cerebral palsy as described elsewhere.1 Briefly, “according to the achievements in IQ, MT, and NOS, the children were classified and grouped as unremarkable (“Control”, i.e., results from healthy term-born infants without obstetrical risk factors) or presenting IBD-0 (no obvious brain dysfunction, i.e., all tests passed with a minimum yield >mean – 1SD), mild IBD-1, moderate IBD-2, and Cerebral palsy (CP). Mild Infantile brain dysfunction (IBD-1) was defined as poor performance in one test, i.e., <mean -1SD, and moderate Infantile brain dysfunction (IBD-2) as poor performance in two tests, i.e., <mean -1SD. Cerebral palsy was defined as the composite of poor performance in Neurologic examination optimality score (<80%, i.e., <mean -1 SD) and inability to perform Maze test”.1 The predicted Developmental disability index (pDDI) was derived by stepwise multiple regression including all growth and obstetrical risk variables and cranial ultrasound results at birth using the grouped results of controls, Brain dysfunction IBD-0, IBD-1, IBD-2, and CP as dependent variable to predict the degree of Infantile brain dysfunction and CP (pDDI=25.218 −0.00057 × weight(g)+0.999 × WMD_present − 0.141 × Apgar_10−0.320 × mode of delivery− 2.934 × Ph_umb.art.; r=0.642, n=130, P<.001). Again, the predicted index pDDI correlated well with the measured Total psychomotor development score (pDDI=0.747−0.603×mTPMDS; r=0.598, n=130, P<.001).1
Finally, the calculated (c) Morphometric vitality index (MVI) (cMVI=[zWeight+zLength+zHeadCircumference+zWeight/length+zApgar_10)/5] was obtained from all 5,301 newborns that correlated well with predicted Total psychomotor development score (zpTPMDS=0.166+0.702 × cMVI; r = 0.844, n=5,191, P<.001).
To describe the effects of obstetrical risk factors on psychomotor development indices (pTPMDS, cMVI, pDDI) and measures (pIQ, pMT, pNOS), odds ratios (Table 1) and multivariate tests (MANOVA) (Table 2, Supplementary material) were calculated. The study was approved by the local institutional review board. This report follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for observational studies.
Table 1.
Odds ratios and 95% confidence intervals of predicted Total psychomotor development score (pTPMDS), calculated Morphometric vitality index (cMVI), and predicted Developmental disability index (pDDI) for obstetrical risk factors in 5,301 newborns (24–43 weeks gestation, 350–5,370 g birthweight) derived from a cranial ultrasound screening data base1,2,12
| Variable | Psychomotor Development (pTPMDS) |
Morphometric Vitality Index (cMVI) |
Developmental Disability Index (pDDI) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% confidence interval |
95% confidence interval | 95% confidence interval | |||||||||||||
| N | Odds ratio | Lower limit | Upper limit | P value | N | Odds ratio | Lower limit | Upper limit | P value | N | Odds ratio | Lower limit | Upper limit | P value | |
| Brain body weight ratio | 5,202 | 48.88 | 41.47 | 57.60 | .000 | 5,281 | 44.42 | 37.85 | 52.14 | .000 | 5,196 | 12.98 | 11.37 | 14.81 | .000 |
| Weight/Head circumference ratio | 5,202 | 48.87 | 41.47 | 57.60 | .000 | 5,281 | 44.72 | 38.09 | 52.50 | .000 | 5,196 | 13.04 | 11.42 | 14.88 | .000 |
| Preterm birth ≤36 wk | 5,202 | 42.73 | 25.90 | 70.48 | .000 | 5,281 | 116.70 | 52.10 | 261.42 | .000 | 5,198 | 13.86 | 10.16 | 18.90 | .000 |
| Weight/length ratio | 5,202 | 26.80 | 23.12 | 31.07 | .000 | 5,281 | 55.24 | 46.73 | 65.30 | .000 | 5,193 | 12.18 | 10.68 | 13.88 | .000 |
| IUGR | 5,202 | 19.78 | 10.15 | 38.55 | .000 | 5,281 | 187.79 | 26.33 | 1,339.08 | .000 | 5,202 | 17.70 | 9.38 | 33.39 | .000 |
| Multiples | 5,202 | 18.23 | 10.46 | 31.78 | .000 | 5,281 | 30.22 | 14.97 | 60.98 | .000 | 5,198 | 6.29 | 4.40 | 9.00 | .000 |
| Apgar 1 min, score < 9 | 5,195 | 3.57 | 3.11 | 4.10 | .000 | 5,280 | 2.82 | 2.47 | 3.23 | .000 | 5,197 | 2.39 | 2.09 | 2.73 | .000 |
| Apgar 1 min, score < 7 | 5,195 | 12.39 | 8.26 | 18.58 | .000 | 5,280 | 13.69 | 9.00 | 20.83 | .000 | 5,197 | 9.42 | 6.54 | 13.57 | .000 |
| Apgar 5 min, score < 10 | 5,194 | 4.67 | 3.95 | 5.51 | .000 | 5,278 | 4.42 | 3.75 | 5.20 | .000 | 5,195 | 3.51 | 3.00 | 4.11 | .000 |
| Apgar 5 min. score < 9 | 5,194 | 9.49 | 6.91 | 13.04 | .000 | 5,278 | 9.63 | 7.01 | 13.23 | .000 | 5,195 | 6.98 | 5.25 | 9.29 | .000 |
| Apgar 10 min. score < 10 | 5,191 | 11.01 | 8.05 | 15.05 | .000 | 5,281 | 24.62 | 15.99 | 37.91 | .000 | 5,198 | 13.40 | 9.57 | 18.76 | .000 |
| Apgar 10 min, score < 9 | 5,191 | 30.14 | 13.33 | 68.17 | .000 | 5,281 | 191.72 | 26.84 | 1,369.53 | .000 | 5,198 | 93.75 | 23.24 | 378.22 | .000 |
| pH umbilical artery <7.29 vs. ≥7.29 | 5,202 | 2.49 | 2.22 | 2.78 | .000 | 5,192 | 0.96 | 0.86 | 1.07 | .454 | 5,198 | 2.90 | 2.59 | 3.24 | .000 |
| PIVH grade 1+2 | 5,202 | 9.42 | 5.37 | 15.47 | .000 | 5,280 | 6.45 | 4.21 | 9.89 | .000 | 5,197 | 6.61 | 4.28 | 10.21 | .000 |
| PIVH grade 3 | 5,201 | 5.82 | 3.01 | 11.01 | .000 | 5,280 | 3.69 | 2.13 | 6.39 | .000 | 5,197 | 9.58 | 4.40 | 20.82 | .000 |
| PIVH grade 4 | 5,202 | 7.25 | 2.55 | 20.59 | .000 | 5,281 | 15.98 | 3.83 | 66.62 | .000 | 5,197 | 9.67 | 2.95 | 31.69 | .000 |
| PIVH present (all grades) | 5,202 | 6.42 | 5.46 | 13.79 | .000 | 5,281 | 4.52 | 3.25 | 6.28 | .000 | 5,198 | 5.88 | 4.01 | 8.47 | .000 |
| WMD present | 5,202 | 8.65 | 5.46 | 13.70 | .000 | 5,281 | 5.96 | 4.01 | 8.86 | .000 | 5,198 | 191.20 | 26.79 | 1361.86 | .000 |
| PIVH plus WMD vs PIVH only | 230 | 9.21 | 3.75 | 22.60 | .000 | 232 | 8.57 | 4.00 | 18.38 | .000 | 227 | 105.96 | 14.08 | 797.20 | .000 |
| PIVH without WMD | 5,050 | 2.41 | 1.48 | 3.91 | .000 | 5,050 | 1.61 | 1.02 | 2.54 | .052 | 5,048 | 1.50 | 0.95 | 2.37 | .085 |
| PIVH grade 1+2 (exclusive) | 4,973 | 1.82 | 0.98 | 3.38 | .065 | 5,049 | 0.93 | 0.51 | 1.69 | .879 | 4,970 | 0.94 | 0.52 | 1.72 | .879 |
| Breech presentation | 5,198 | 3.62 | 2.45 | 4.60 | .000 | 5,277 | 2.95 | 2.35 | 3.69 | .000 | 5,194 | 1.74 | 1.42 | 2.14 | .000 |
| Breech presentation, vag. delivery | 374 | 0.61 | 0.48 | 0.77 | .000 | 379 | 0.76 | 0.60 | 0.97 | .042 | 373 | 0.45 | 0.43 | 0.69 | .000 |
| Cardiotocography pathologic | 5,202 | 2.99 | 2.53 | 3.45 | .000 | 5,281 | 2.12 | 1.81 | 2.47 | .000 | 5,198 | 1.42 | 1.23 | 1.65 | .000 |
| sex | 5,196 | 1.10 | 1.04 | 1.16 | .001 | 5,275 | 1.27 | 1.20 | 1.35 | .000 | 5,192 | 1.16 | 1.10 | 1.23 | .000 |
| Amnion infection | 5,199 | 1.00 | 1.00 | 1.00 | .016 | 5,278 | 1.00 | 1.00 | 1.00 | .016 | 5,195 | 5.01 | 0.59 | 42.97 | .125 |
| Bleeding, vaginal | 5,199 | 1.98 | 1.49 | 2.63 | .000 | 5,278 | 1.62 | 1.23 | 2.13 | .001 | 5,195 | 1.44 | 1.09 | 1.89 | .009 |
| Hypertension | 5,185 | 1.66 | 1.19 | 2.31 | .003 | 5,264 | 1.25 | 0.91 | 1.72 | .099 | 5,181 | 1.40 | 1.01 | 1.94 | .049 |
| Prolonged or arrested labour | 5,202 | 1.65 | 1.39 | 1.97 | .000 | 5,281 | 2.03 | 1.70 | 2.43 | .000 | 5,198 | 5.31 | 4.27 | 6.59 | .000 |
| Primiparity | 5,201 | 1.64 | 1.47 | 1.84 | .000 | 5,280 | 1.65 | 1.48 | 1.84 | .000 | 5,197 | 1.40 | 1.25 | 1.56 | .000 |
| Maternal age <3% centile | 5,183 | 2.08 | 1.42 | 3.05 | .000 | 5,262 | 2.39 | 1.62 | 3.53 | .000 | 5,179 | 2.08 | 1.42 | 3.05 | .000 |
| Transfer to NICU | 2,655 | 1.70 | 1.39 | 2.08 | .000 | 2,669 | 1.54 | 1.26 | 1.88 | .000 | 2,651 | 1.20 | 1.51 | 2.32 | .000 |
| Malformation | 5,202 | 1.80 | 0.83 | 3.89 | .184 | 5,281 | 0.38 | 0.17 | 0.86 | .024 | 5,198 | 0.40 | 0.18 | 0.91 | .035 |
| Meconium stained amniotic fluid | 5,201 | 1.39 | 1.07 | 1.81 | .015 | 5,280 | 1.76 | 1.35 | 2.29 | .000 | 5,197 | 1.80 | 1.37 | 2.35 | .000 |
| PROM | 5,202 | 1.65 | 1.44 | 1.87 | .000 | 5,281 | 1.66 | 1.50 | 1.89 | .000 | 5,198 | 1.37 | 1.21 | 1.56 | .000 |
| EPH syndrome | 5,202 | 1.63 | 1.33 | 1.99 | .000 | 5,281 | 1.40 | 1.13 | 1.66 | .002 | 5,198 | 1.28 | 1.05 | 1.55 | .016 |
| Miscarrage | 5,201 | 1.22 | 1.06 | 1.40 | .005 | 5,280 | 1.15 | 1.00 | 1.32 | .045 | 5,197 | 1.16 | 1.01 | 1.33 | .037 |
| Maternal fever >38°C | 5,202 | 1.39 | 0.76 | 2.54 | .179 | 5,281 | 1.44 | 0.79 | 2.63 | .145 | 5,198 | 0.95 | 0.52 | 1.74 | .999 |
| Rh incompatibility | 5,202 | 1.40 | 0.62 | 3.15 | .270 | 5,281 | 0.67 | 0.30 | 1.49 | .423 | 5,198 | 0.60 | 0.26 | 1.37 | .306 |
| Diabetes mellitus | 5,201 | 1.10 | 0.67 | 1.81 | .706 | 5,280 | 1.13 | 0.70 | 1.84 | .706 | 5,197 | 1.07 | 0.65 | 1.76 | .800 |
| Maternal age >97% centile | 5,183 | 1.07 | 0.74 | 1.55 | .778 | 5,262 | 1.01 | 1.00 | 1.01 | .265 | 5,179 | 1.00 | 1.00 | 1.01 | .398 |
| Hypotension | 5,047 | 0.51 | 0.17 | 1.48 | .301 | 5,122 | 0.88 | 0.32 | 2.43 | .504 | 5,043 | 0.67 | 0.24 | 1.89 | .607 |
EPH, edema-proteinuria-hypertension; IUGR, intrauterine growth restriction; NICU, neonatal intensive care unit; PIVH, peri/-intraventricular
hemorrhage; PROM, premature rupture of membranes; WMD, white matter brain damage.
Jensen. Newborns’ growth, obstetrical risk, and psychomotor development. Am J Obstet Gynecol Glob Rep 2023.
Table 2.
Multivariate analysis variance, F test, and effect size of predicted Intelligence quotient (pIQ), predicted Maze test results (pMT), and predicted Neurologic examination optimality score (pNOS) for obstetrical risk factors in 5,301 newborns (24–43 weeks gestation, 350–5,370g birthweight) derived from a cranial ultrasound screening data base1,2,12
| Variable | Intelligence quotient | Maze test | Neurological examination optimality score | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (zpIQ) | (zpMT) | (pzNOS) | ||||||||||||
| Multivariate test | Multivariate test | Multivariate test | ||||||||||||
| N | df | n | F test | Effect size | P value | n | F test | Effect size | P value | n | F test | Effect size | P value | |
| Gestational age (centile) | 5,202 | 4 | 5,202 | 252.8 | 0.16 | .000 | 5,202 | 990.0 | 0.43 | .000 | 5,202 | 642.0 | 0.33 | .000 |
| Brain body weight ratio (centile) | 5,202 | 4 | 5,202 | 925.2 | 0.42 | .000 | 5,202 | 1525.8 | 0.54 | .000 | 5,202 | 1,142.0 | 0.47 | .000 |
| Preterm birth ≤36 weeks | 5,202 | 1 | 560 | 760.7 | 0.13 | .000 | 560 | 2629.1 | 0.34 | .000 | 560 | 1818.7 | 0.26 | .000 |
| Weight/length ratio (centile) | 5,202 | 4 | 5,202 | 616.5 | 0.32 | .000 | 5,202 | 1516.9 | 0.54 | .000 | 5,202 | 1,254.8 | 0.49 | .000 |
| IUGR | 5,202 | 1 | 187 | 375.9 | 0.07 | .000 | 187 | 26.2 | 0.01 | .000 | 187 | 402.1 | 0.07 | .000 |
| Multiples | 5,202 | 1 | 250 | 252.7 | 0.05 | .000 | 250 | 542.9 | 0.09 | .000 | 250 | 222.6 | 0.04 | .000 |
| Apgar 1 minute < 9 | 5,195 | 1 | 1,254 | 836.6 | 0.14 | .000 | 1,254 | 1151.6 | 0.18 | .000 | 1,254 | 532.7 | 0.09 | .000 |
| Apgar 5 minutes < 10 | 5,193 | 1 | 943 | 881.0 | 0.14 | .000 | 943 | 1326.2 | 0.20 | .000 | 943 | 774.5 | 0.13 | .000 |
| Apgar 10 minutes < 10 | 5,190 | 1 | 466 | 887.8 | 0.15 | .000 | 466 | 1768.0 | 0.25 | .000 | 466 | 1,266.9 | 0.20 | .000 |
| pH umbilical artery <7.29 vs.>=7.29 | 5,202 | 1 | 2,566 | 866.9 | 0.14 | .000 | 2,566 | 549.6 | 0.10 | .021 | 2,566 | 151.4 | 0.03 | .000 |
| PIVH grade 1+2 | 5,201 | 1 | 177 | 292.4 | 0.05 | .000 | 177 | 1339.6 | 0.20 | .000 | 177 | 1736.9 | 0.25 | .000 |
| PIVH grade 3 | 5,201 | 1 | 75 | 67.4 | 0.01 | .000 | 75 | 355.4 | 0.06 | .000 | 75 | 263.7 | 0.05 | .000 |
| PIVH grade 4 | 5,201 | 1 | 33 | 49.9 | 0.01 | .000 | 33 | 286.0 | 0.05 | .000 | 33 | 312.1 | 0.06 | .000 |
| PIVH present (all grades) | 5,202 | 1 | 230 | 272.5 | 0.05 | .000 | 230 | 1606.4 | 0.24 | .000 | 230 | 1,592.3 | 0.23 | .000 |
| WMD present | 5,201 | 1 | 193 | 317.4 | 0.06 | .000 | 193 | 1049.5 | 0.17 | .000 | 193 | 1,968.5 | 0.27 | .000 |
| PIVH without WMD | 5,050 | 1 | 78 | 9.8 | 0.00 | .002 | 78 | 275.0 | 0.05 | .000 | 78 | 79.9 | 0.02 | .000 |
| PIVH grade 1+2 (exclusive) | 4,973 | 1 | 43 | 1.0 | 0.00 | .309 | 43 | 62.6 | 0.01 | .000 | 43 | 37.3 | 0.01 | .000 |
| Breech presentation | 5,198 | 1 | 374 | 303.0 | 0.06 | .000 | 374 | 346.5 | 0.06 | .000 | 374 | 42.1 | 0.01 | .000 |
| Breech presentation, vaginal delivery | 374 | 1 | 154 | 7.2 | 0.02 | .007 | 154 | 27.0 | 0.07 | .000 | 154 | 75.0 | 0.17 | .000 |
| Cardiotocography pathologic | 5,202 | 1 | 655 | 471.1 | 0.08 | .000 | 655 | 405.9 | 0.07 | .000 | 655 | 775.5 | 0.13 | .000 |
| Amnion infection | 5,199 | 1 | 6 | 8.0 | 0.00 | .005 | 6 | 48.9 | 0.01 | .000 | 6 | 46.0 | 0.01 | .000 |
| Bleeding, vaginal | 5,199 | 1 | 222 | 12.3 | 0.00 | .000 | 222 | 48.7 | 0.01 | .000 | 222 | 18.0 | 0.00 | .000 |
| Hypertension | 5,185 | 1 | 153 | 36.0 | 0.01 | .000 | 153 | 18.6 | 0.00 | .000 | 153 | 28.7 | 0.01 | .000 |
| Prolonged or arrested labour | 5,202 | 1 | 597 | 3.8 | 0.00 | .051 | 597 | 11.1 | 0.00 | .000 | 597 | 466.9 | 0.08 | .000 |
| Primiparity | 5,199 | 1 | 2,539 | 84.7 | 0.02 | .000 | 2,539 | 100.6 | 0.02 | .000 | 2,539 | 10.3 | 0.00 | .001 |
| Maternal age <3 percentile | 5,183 | 1 | 123 | 0.6 | 0.00 | .432 | 123 | 5.1 | 0.00 | .024 | 123 | 12.3 | 0.00 | .000 |
| Transfer to NICU | 2,655 | 1 | 353 | 68.1 | 0.03 | .000 | 353 | 26.4 | 0.01 | .000 | 353 | 30.2 | 0.01 | .000 |
| Malformation | 5,202 | 1 | 28 | 18.9 | 0.00 | .000 | 28 | 14.3 | 0.00 | .000 | 28 | 18.1 | 0.00 | .000 |
| Meconium stained amniotic fluid | 5,201 | 1 | 242 | 3.2 | 0.00 | .073 | 242 | 16.9 | 0.00 | .000 | 242 | 4.0 | 0.00 | .046 |
| PROM | 5,202 | 1 | 829 | 19.9 | 0.00 | .000 | 829 | 97.0 | 0.02 | .000 | 829 | 34.4 | 0.01 | .000 |
| EPH syndrome | 5,202 | 1 | 378 | 93.4 | 0.02 | .000 | 378 | 47.1 | 0.01 | .000 | 378 | 61.8 | 0.01 | .000 |
| Miscarrage | 5,201 | 1 | 1,029 | 6.0 | 0.00 | .015 | 1,029 | 25.6 | 0.00 | .000 | 1,029 | 7.5 | 0.00 | .006 |
| sex | 5,196 | 1 | 2,529 | 10.2 | 0.00 | .001 | 2,529 | 20.3 | 0.00 | .000 | 2,529 | 16.4 | 0.00 | .000 |
| Maternal fever >38°C | 5,202 | 1 | 43 | 0.0 | 0.00 | .974 | 43 | 4.6 | 0.00 | .031 | 43 | 5.2 | 0.00 | .023 |
| Rh incompatibility | 5,202 | 1 | 24 | 0.1 | 0.00 | .821 | 24 | 1.3 | 0.00 | .257 | 24 | 5.7 | 0.00 | .017 |
| Diabetes mellitus | 5,201 | 1 | 63 | 1.6 | 0.00 | .205 | 63 | 5.1 | 0.00 | .024 | 63 | 0.1 | 0.00 | .745 |
| Maternal age >97 percentile | 5,183 | 1 | 116 | 1.1 | 0.00 | .287 | 116 | 0.4 | 0.00 | .543 | 116 | 0.5 | 0.00 | .914 |
| Hypotension | 5,047 | 1 | 15 | 0.3 | 0.00 | .582 | 15 | 0.3 | 0.00 | .565 | 15 | 0.0 | 0.00 | .938 |
EPH, edema-proteinuria-hypertension; IUGR, intrauterine growth restriction; NICU, neonatal intensive care unit; PIVH, peri/-intraventricular hemorrhage; PROM, premature rupture of membranes; WMD, white matter brain damage.
Jensen. Newborns’ growth, obstetrical risk, and psychomotor development. Am J Obstet Gynecol Glob Rep 2023.
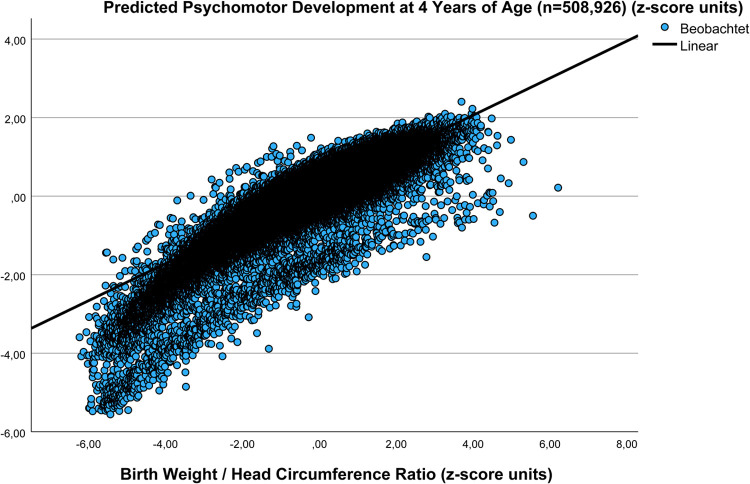
For validation purposes, the results of the correlation between W/HC and predicted Total psychomotor development score based on 5,301 newborns (1984–1988) has been confirmed in a large more recent data pool (1998–2000) on 508,926 records as part of a population based national perinatal survey (zpTPMDS=0.175+0.472 × zW/HC; r=0.878, SE estimate=0.256, n=502,993, P<.001, unpublished) (Figure 1) in that the MVI was calculated (n=502,993) to derive zpTotal psychomotor development score based on the above linear regression (zpTPMDS=0.166+0.702 × cMVI; r=0.844, SE estimate=0.387, n=5,191, P<.001). Interestingly, the intercepts of the two regressions were almost identical, while the slope was steeper in the Cranial Ultrasound Screening study (n=5,301), a fact attributable to the higher proportion of preterms (10, 8%) in the level 3 perinatal center cohort (selection bias) as compared with that in the normal population (6.4%) (Figure 2).
Figure 1.
Relation between pTPMDS at 4 years of age and W/HC at birth in a large validation cohort (n=508,926, 1998–2000)
For validation purposes, the results of the correlation between W/HC and pTPMDS in a large data pool of 508,926 records as part of a population based national perinatal survey (1998–2000) are depicted (zpTPMDS=0.175+0.472 × zW/HC; r=0.878, SE estimate=0.256 n=502,993, P<.001, unpublished).2 For extrapolation, cMVI was calculated (n=502,993) to derive zpTPMDS based on the linear regression (zpTPMDS=0.166+0.702 × cMVI; r=0.844, n=5,191; P<.001). The clear linear relation between variables in the large national perinatal survey cohort (n=502,993; 1998–2000) is comparable with that of the present study based on cranial ultrasound screening data (n=5,301; 1984–1988) (Figure 2). Interestingly, those cases presenting very low Apgar scores (score ≤3) at 5 and 10 minutes after birth (n=1,194 [0.24%]) form a visible subgroup of poor predicted Total Psychomotor Development Score performance below the bulk of data points (n=501,799 [99.76%]).
pTPMDS, predicted Total Psychomotor Development Score; W/HC, weight/head circumference ratio.
Figure 2.
Relation between pTPMDS at 4 years of age and W/HC at birth (n=5,301; 1984–1988)
The correlation between pTPMDS z-score units and W/HC (z-score units) in 5,301 newborns is depicted (zpTPMDS = 0.168+0.673 × zW/HC; r=0.931, SE estimate=0.265, n=5,201, P<.001). pTPMDS represents the average of predicted IQ, MT, and NOS at 4.3 years (standard deviation, 0.8) of age zpTPMDS=(zpIQ+zpMT result+zpNOS)/3) derived from stepwise multiple regression analyses from a previous study (pTPMDS=−17.87+0.00043 × weight−0.501 × WMD_present+2.278 × pH_umb.art+0.177 × mode of delivery; r=0.637, n=129, P<.001).1,2,12 The rational behind the extrapolation of pTPMDS from children in which psychomotor development was measured (n=130) to all 5,301 cases of the CUS resides in the fact that, first, these children underwent CUS in the same unit with identical obstetrical management. Secondly, the stepwise multiple regression bore a close relation between the variables (r=0.637) and, finally, the predicted pTPMDS was closely related to the summary z-score of the measured (m) results of IQ, MT, and NOS testing (mTPMDS) (r=0.598, n=130, P<.001).1,2 Of note, W/HC at birth allows for estimation of psychomotor development at preschool age. This is clinically relevant because a small W/HC is related to preterm birth as well as to asymmetric growth restriction, both risk factors yielding poor neurocognitive development demanding for early intervention strategies.
CUS, cranial ultrasound screening; IQ, intelligence quotient; MT, Maze test; NOS, Neurologic examination optimality score; pTPMDS, predicted Total Psychomotor Development Score; W/HC, weight/head circumference ratio.
Jensen. Newborns’ growth, obstetrical risk, and psychomotor development. Am J Obstet Gynecol Glob Rep 2023.
Statistical analysis
Results are presented as means and SD, apriori level of significance to reject null hypothesis being 2-alpha <0.05. We evaluated growth variables and obstetrical risk factors at birth in relation to z-score transformed (z) predicted psychomotor development indices and measures using parametric and nonparametric statistical procedures, ANOVA, and MANOVA where appropriate. Odds ratios were calculated for composite psychomotor development indices (pTPMDS, pDDI) based on predicted (p) Intelligence quotient (pIQ), Maze test (pMT), Neurologic examination optimality score (pNOS), and cMVI based on growth variables and Apgar Score at 10 mins.1 Receiver operating characteristics (ROC curve) were employed to test for sensitivity and specificity of weight/head circumference ratio (W/HC), weight/length (crown-heel) ratio, and white matter brain damage (WMD) of the newborns in predicting adverse outcome with regard to psychomotor development indices predicted Total psychomotor development score and predicted Developmental disability index at 4.3 (SD, 0.08) years of age. All procedures were performed using SPSS-28 (IBM Corporation, Armonk, NY), as statistical program. Deviations from the total number of participants are because of missing values.
Results
A total of 5,301 (91.4%) neonates (51.0% male) underwent cranial ultrasound screening (including twins) with no sex related differences in the overall rate of WMD (male 4.2% vs female 3.6 %, not significant), cerebral hemorrhage (male 4.8% vs female 4.2 %, not significant), Apgar scores at 1, 5, and 10 minutes, or umbilical arterial pH. There were small but statistically significant sex differences in predicted psychomotor development indices zpTotal psychomotor development score male 0.19 (SD, 0.74) vs female 0.14 (SD, 0.71), P<.001), predicted Developmental disability index (male, 0.17 [SD, 0.59] vs female, 0.24 [SD, 0.06]; P<.001), and in cMVI (zcMVI) (male, 0.08 [SD, 0.88) vs female, −0.07 [SD, 0.86]; P<.001). However, the indices are composite scores of zpIntelligence quotient (male, −0.04 [SD, 1.01] vs female, 0.05 [SD, 0.99]; P<.001) (ie, equivalent to pIQ [male, 125.33 (SD, 6.8) vs female, 125.93 (SD, 6.6); P<.001]), plus zpMaze Test (male, 0.09 [SD, 1.02] vs female, −0.10 [SD, 0.96], P<.001), plus zpNeurologic examination optimality score (male, 0.53 [SD, 0.49] vs female, 0.48 [SD, 0.46]; P<.001) divided by three, suggesting that the favourable female performance in zpIntelligence quotient is outweighed by favourable male performance in both zpMaze Test and zpNeurologic examination optimality score at 4 years of age. The sex differences in cMVI reside in larger morphometrics in male newborns.
The 5,301 newborns including 571 (10.8%) preterms (≤36 weeks) bore the following characteristics: mean gestational age, 39.2 weeks (SD, 2.6; range, 24–43), weight 3,231 g (SD, 686; range, 350–5,370), total body length 50.5 cm (SD, 3.8; range, 25–61), head circumference 34.4 cm (SD, 2.2; range, 21–43), Apgar score at 10 minutes <=9 (480/5,301; range, 2–9), and umbilical arterial pH 7.28 (SD, 0.07; range, 6.65–7.83). Mean zpTotal psychomotor development score was 0.17 (SD, 0.7; range, −4.0 to 2.3) and z weight/head circumference ratio was 0.00 (SD, 1.0; range, −4.7 to 3.5).
There was a close relation between weight/head circumference ratio (W/HC) and predicted Total psychomotor development score in that a smaller ratio, e.g., suggesting asymmetric growth restriction, was associated with poorer yields in the composite Total psychomotor development score (zpTPMDS=0.168+0.673 × zW/HC; r=0.931, SE estimate=0.265, n=5,201, P<.001) (Figure 2), predicted Intelligence quotient (zpIQ=−0.001+0.688 × zW/HC; r=0.688, SE estimate=0.726, n=5,206, P<.001) (Figure 3), predicted Maze test results (zpMT=0.000+0.981 × zW/HC; r=0.982, SE estimate=0.191, n=5,206, P<.001) (Figure 4), and predicted Neurologic examination optimality score (zpNOS=0.504+0.351 × zW/HC; r=0.739, SE estimate=0.320, n=5,201, P<.001) (Figure 5). Furthermore, cMVI, combining various growth variables with the Apgar score at 10 mins, was positively and negatively correlated to Total psychomotor development score (zpTPMDS=0.166+0702 × cMVI; r=0.844, SE estimate=0.387, n=5,190; P<.001) and to predicted Developmental disability index (pDDI=0.206−0.526 × cMVI; r=0.798, SE estimate=0.344, n=5,191, P<.001), respectively (Figure 6). These results underscore the significance of simple growth and vitality measures taken at birth for predicting developmental trajectories at 4 years of age.
Figure 3.
Relation between pIQ at 4 years of age and W/HC at birth (n=5,301, 1984-1988)
The correlation between pIQ z-score units and W/HC (z-score units) in 5,301 newborns is depicted (zpIQ =−0.001+0.688 × zW/HC; r=0.688, SE estimate=0.726, n=5,206, P<.001). The rational behind the extrapolation of pIQ from children in which psychomotor development was measured (m) (n=130) to all 5,301 cases of the CUS resides in the fact that, first, these children underwent CUS in the same unit with identical obstetrical management. Secondly, the stepwise multiple regression bore a close relation between the variables (pIQ=−153.61–1.545 × BBR+43.987 × pH; r=0.459, n=131, P<.001) and, finally, the predicted pIQ was closely related to the z-score of the measured (m) results of IQ (mIQ) (n=130, P<.001).1,2 Of note, W/HC at birth allows for estimation of predicted IQ at preschool age. This is clinically relevant because a small W/HC ratio is related to preterm birth as well as to asymmetric growth restriction, both risk factors yielding poor neurocognitive development making early intervention mandatory.
CUS, cranial ultrasound screening; IQ, intelligence quotienT; pIQ, predicted Intelligence Quotient; W/HC, weight/head circumference ratio.
Jensen. Newborns’ growth, obstetrical risk, and psychomotor development. Am J Obstet Gynecol Glob Rep 2023.
Figure 4.
Relation between pMT result at 4 years of age and W/HC at birth (n=5,301, 1984–1988)
The exceptionally close correlation between pMT z-score units and W/HC ratio (z-score units) in 5,301 newborns is depicted (zpMT=0.000+0.981 × zW/HC; r=0.982, SE estimate=0.191, n=5,206, P<.001). The rational behind the extrapolation of pMT from children in which psychomotor development was measured (m) (n=130) to all 5,301 cases of the CUS resides in the fact that, first, these children underwent CUS in the same unit with identical obstetrical management. Secondly, the stepwise multiple regression bore a close relation between the variables (pMT =−541.20+0.14 × weight+23.176 × IUGR−12.064 × PIVH_present+67.606 × pH_umb.art; r=0.516, n=133, P<.001) and, finally, the predicted pMT was closely related to the z-score of the measured (m) results of MT (mMT) (n=130, P<.001).1,2 Of note, W/HC at birth allows for estimation of pMT results at preschool age. This is clinically relevant because MT test domains are considered largely independent of standard IQ testing due to its untimed, configural, and problem-solving task. Furthermore, the Maze test is an uniquely sensitive measure of executive function ability, comprising the domains fine motor ability, dexterity, planning capacity, stability, and learning ability.1,2
CUS, cranial ultrasound screeninG; pMT, predicted Maze Test; W/HC, weight/head circumference ratio.
Jensen. Newborns’ growth, obstetrical risk, and psychomotor development. Am J Obstet Gynecol Glob Rep 2023.
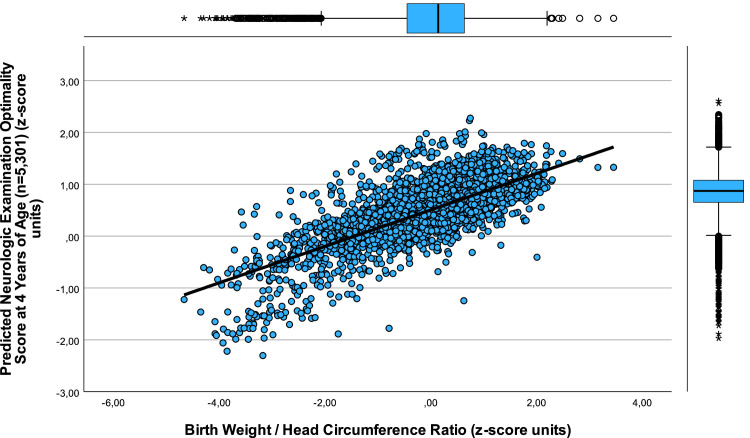
Figure 5.
Relation between pNOS at 4 years of age and W/HC at birth (n=5,301; 1984–1988)
The correlation between pNOS z-score units and W/HC (z-score units) in 5,301 newborns is depicted (pzNOS=0.504+0.351 × zW/HC; r=0.739, SE estimate=0.320, n=5,202, P<.001). The rational behind the extrapolation of pNOS from children in which PMD was measured (m) (n=132) to all 5,301 cases of the CUS resides in the fact that, first, these children underwent CUS in the same unit with identical obstetrical management. Secondly, the stepwise multiple regression bore a close relation between the variables (pzNOS=−14.03+0.30 × weight/length-ratio–0.623 × WMD_present – 0.353 × PIVH-1+2_present+1.683 × pH+0.326 × mode of delivery–0.366 × pathologic cardiotography; r=0.605; n=132, P<.001) and, finally, the predicted pNOS was closely related to the z-score of the measured (m) results of NOS (mNOS)) (n=132, P<.001).1,2 Of note, weight/head circumference ratio at birth allows for estimation of pNOS at preschool age. This is clinically relevant because a small W/HC ratio is related to preterm birth as well as to asymmetric growth restriction, both risk factors yielding poor neurocognitive development in general and neurologic deficits specifically, demanding for early intervention by neuro-rehabilitation.
CUS, cranial ultrasound screening; pNOS, predicted Neurologic Examination Optimality Score; W/HC, weight/head circumference ratio.
Jensen. Newborns’ growth, obstetrical risk, and psychomotor development. Am J Obstet Gynecol Glob Rep 2023.
Figure 6.
Relation between pDDI at 4 years of age and cMVI at birth
The cMVI at birth, combining various growth variables with the Apgar score at 10 minS (cMVI=[zWeight+zLength+zHeadCircumference+zWeight/Length+zApgar_10]/5), was negatively correlated to predicted DDI (pDDI=0.206−0.526 × cMVI; r=0.798, SE estimate=0.344, n=5,191, P<.001) in that smaller growth and Apgar values increase the pDDI. These results underscore the significance of simple growth and vitality measures taken at birth for predicting developmental trajectories at 4 years of age.
cMVI, calculated Morphometric Vitality index; pDDI, predicted Developmental Disability Index.
Jensen. Newborns’ growth, obstetrical risk, and psychomotor development. Am J Obstet Gynecol Glob Rep 2023.
Receiver operating characteristics (ROC curve) revealed that white matter brain damage (WMD vs pDDI, 97.0% sensitivity, 86.0% specificity, AUC 0.98, P<.001, PPV and NPV were 99.5% and 51.9%, respectively), weight/head circumference ratio of the newborn (W/HC vs pTPMDS, 93.1% sensitivity, 81.1% specificity, AUC 0.952, P<.001, PPV and NPV were 87.4% and 87.6%, respectively), and weight/length ratio (W/L vs pTPMDS, 86.4% sensitivity, 81.0% specificity, AUC 0.921, P<.001, PPV and NPV were 84.6% and 83%, respectively) have the highest sensitivity and specificity in predicting adverse outcome regarding predicted Developmental disability index and predicted Total psychomotor development score at 4 years of preschool age. Note, small weight/head circumference ratios (eg, mean −1 SD of zW/HC= −1.9 (SD, 0.8; n=695) result from preterm birth and/or growth restriction yielding poor psychomotor development (zpTPMDS= −1.1; SD, 0.7; n=683).
The odds ratios (OR) calculated for quantification of the association between growth variables and obstetrical risk factors with indices of psychomotor development predicted Total psychomotor development score, predicted Developmental disability index, and cMVI are given in Table 1. Among all obstetrical risk factors, Brain body weight ratio (BBR), weight/head circumference ratio, preterm birth ≤36 weeks gestation, reduced Apgar at 10 minutes, weight/length ratio, and white matter damage (WMD) present bore the strongest relation to poor performance in all three domains while white matter damage present, Peri/-intraventricular hemorrhage (PIVH) plus white matter damage, and reduced Apgar score at 10 mins particularly affected predicted Developmental disability index. In addition, with the exception of Peri/-intraventricular hemorrhage grade 1+2 (exclusive, ie, without white matter damage), maternal fever >38°C during delivery, Rh incompatibility, diabetes mellitus, maternal age >97% centile, and maternal hypotension during pregnancy, virtually all obstetrical risk factors significantly affected predicted Total psychomotor development score, predicted Developmental disability index, and cMVI (Table 1). Interestingly, small reductions in Apgar scores at 1, 5, and 10 minutes increase the odds ratios for adverse outcome substantially in all 3 domains.
A detailed multivariate analysis of predicted Intelligence quotient (zpIQ), Maze test (zpMT), and Neurologic examination optimality score (pzNOS) in relation to all obstetrical risk factors is given in Table 2 (Supplementary material). Again, with the exception of diabetes mellitus, maternal age >97% centile, and maternal hypotension during pregnancy, almost all obstetrical risk factors significantly affected the predicted psychomotor development testing results.
Discussion
Principal findings
This study confirms in a large prospective cohort of 5,301 complete obstetrical records of newborns previous observations that growth variables at birth bear predictive capacity for psychomotor development at preschool age.1, 2, 3 This is of clinical significance because neurocognitive development predicted at birth is forming a basis for parental consultation and further clinical assessments, eg, by imaging techniques like cranial ultrasound/MRI or neurologic examination, even if delivery was uneventful and the newborn seemingly healthy. This would pave the way for early intervention strategies, timely rehabilitation, or even cell therapies that have recently been developed.19 Furthermore, mental illnesses in childhood and adolescence, eg, male attention deficit hyperactivity disorders, and female depression and anxiety disorders, which are known to be related to both preterm birth and growth restriction, are likely to be prevented in part by timely intervention.4, 5, 6
Particularly close is the relationship between weight/head circumference ratio(W/HC) and psychomotor development as assessed by the predicted Total psychomotor development score(zpTPMDS) which is even closer than that between weight/length ratio and zpTPMDS from a previous account (r=0.931 vs r=0.892).2 The phenomenon that weight/head circumference is a psychomotor development index both for growth restriction and preterm birth is, first, related to the pathophysiology of circulatory centralisation with preferential head/brain perfusion when oxygen is at short supply and to preterm birth infants presenting relatively high head circumferences as compared to both weight and crown-heel length.2,20 Secondly, in newborns, the precision of head circumference measurement at the largest frontooccipital diameter is higher than that of the crown-heel length in hanging position.2 Thus, simple measures available directly after birth would allow for early risk assessment as a basis for further evaluation by neonatologists, radiologists, and neuropediatricians even if the infant is born with signs of unimpaired vitality.
Clinical Implications
Early prediction of psychomotor development by neurologic examination has proved to be difficult due to variability and instability of motor development “making a reasonable prediction of psychomotor performance of an individual child difficult if not impossible”.1,21,22 In the present study that is based on both cranial ultrasound screening and examinations of the children at 4.3 years (SD, 0.8), prediction is likely to be more reliable (Figures 2 to 6). This view is supported by the fact that previous results of cranial ultrasound were closely related to the predicted indices for psychomotor development, ie, predicted Total psychomotor development score and predicted Developmental disability index.1 This holds particularly true for WMD diagnosed in 3.6% (193/5,301) of the infants showing high odds ratios (OR, 191.2) for adverse outcome in the predicted Developmental disability index (pDDI, Table 1). Further support is provided by ROC analysis in which white matter damage shows extremely high sensitivity (97%) and specificity (86%) for adverse outcome in predicted Developmental disability index (AUC, 0.975; P<.001). Because WMD diagnosed by expert cranial ultrasound examination and measured weight, head circumference, and length, are hard facts derived from a large prospective cohort of newborns, our findings, along with the data from the national perinatal survey based on 508,926 records (Figure 1), lend further support to the validity of our psychomotor development prediction model.
Upon closer look, this model also has considerable differentiation capabilities as demonstrated for Apgar scores (Fig. 1) and various degrees of brain damage in that, eg, grade 1 and grade 2 peri/intraventricular hemorrhage in the absence of white matter damage did not show significant odds ratios for predicted psychomotor development indices (pTPMDS, pDDI) (Table 1). This is important for consulting the parents of affected newborns.
Another well-known risk factor used in the present study is the documented Apgar score at 10 minutes after birth that showed an average odds ratio as high as 93.75 (CI, 23.24–378.22) for poor performance in the predicted Developmental disability index (pDDI) when the score was < 9 (Table 1). Moreover, small reductions in Apgar scores at 1 and 5 mins after birth increase the odds ratios for poor developmental performance substantially, reminding us to employ an optimal prospective risk management in clinical obstetrics to prevent harm.12 Hence, the Apgar score at 10 minutes is part of the cMVI also comprising various growth variables important for prediction of development, ie, weight, length, head circumference, and weight/length ratio.13 Not surprisingly, the cMVI, which is readily available at birth, shows a particularly close relationship both to predicted Developmental disability index (r=0.798, n=5,191) (Figure 6) and to predicted Total psychomotor development score (r=0.844, n=5,190). Thus, the cMVI, encompassing growth variables along with Apgar scores taken at 10 minutes, allows for valid prediction of psychomotor development at 4.3 (SD, 0.8) years preschool age.
To account for medical care standards in rural areas and/or developing countries where cranial ultrasound may not be available, we propose to use weight/head circumference ratio, weight/length ratio, and/or cMVI to predict preschool psychomotor performance in individual children without access to cranial ultrasound results.1,2
The validity of clinical prediction models depends on a valid extrapolation of the original data onto a larger population. Ideally, the original data are part of the larger population to which the data are to be extrapolated. Moreover, it is advantageous if data have been collected at the same time under similar clinical management guidelines to avoid bias. All these conditions are fulfilled in the present single centre study, in which the psychomotor development was assessed in children that were part of the obstetrical population screened by cranial ultrasound (1982–1988) and extrapolated to the subset of five full screening vintages (1984–1988, n=5,301).12 However, like neonatal care, the improved technical equipment of cranial ultrasound in newborns, some of the obstetrical risk factors and their management, and the relation between more subtil brain damage and adverse psychomotor outcome might have changed significantly since data collection. Therefore, despite support by the validation cohort (1998–2000; n=508,926), the cranial ultrasound screening database (1984–1988), encompassing the full range of birthweights (350–5,370g) and gestational ages (24–43 weeks) of a level 3 perinatal center, is rather a valid source for the prediction of psychomotor trajectories among preschool-aged children within the boundaries of the data collection period.2
Strengths and limitations
The prediction model of psychomotor development based on growth variables and obstetrical risk factors at birth has been validated by large prospective cohorts and hence, within limits, allows for both parental consultation and early intervention in the clinical setting. A general limitation of this study is that the data (1) do not cover more recent populations, (2) lack stratification of those newborns at risk that might have received early rehabilitation efforts within the follow-up period and (3) are confined to preschool age. Specifically, the rate of diabetes is much lower in the present study cohort than today, the management of fetal growth restriction has undergone important changes as well as that of threatened preterm birth below 32 weeks’ gestation, of late preterm infants, or that of Rh-incompatibility. Moreover, there are some obstetrical risk factors with very low prevalence, thus, the data presented should be interpreted judiciously, also taking into account that over a 4 years lifespan, despite strictly controlling for confounders, there are many other factors that can condition psychomotor development.
Conclusions
It is to be hoped that in the future the prediction of psychomotor development trajectories based on simple growth and vitality variables determined at birth enter clinical procedures to pave the way for the development of early intervention strategies in a timely manner to provide individualized preschool support to improve developmental performance, educational success, and mental health in our children.
Acknowledgements
The authors are indebted to Dr Manfred Voigt, Sievershagen, Germany, for providing access to data of the National Perinatal Survey, 1998–2000.
Footnotes
The authors report no conflict of interest.
Patient consent was not required because no personal information or details are included.
A.J. had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Cite this article as: Jensen A, Neuhäuser G. Growth variables and obstetrical risk factors in newborns are associated with psychomotor development at preschool age. Am J Obstet Gynecol Glob Rep 2023;XX:x.ex–x.ex.
Dedicated to Professor Wayne R. Cohen, MD, University of Arizona College of Medicine, USA.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.xagr.2023.100219.
Appendix. Supplementary materials
References
- 1.Jensen A, Neuhäuser G, Jensen KO. Growth variables and brain damage at birth predict developmental disability at four years of age: a basis for individual preschool support. Ann Pediatr. 2019;2:1017. [Google Scholar]
- 2.Jensen A, Neuhäuser G. Association of weight-length ratio at birth with psychomotor trajectories among preschool-aged children., AJOG global reports. AJOG Glob Rep. 2022;2 doi: 10.1016/j.xagr.2022.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song IG, Kim EK, Cho H, Shin SH, Sohn JA, Kim HS. Differential effect of growth on development between AGA and SGA preterm infants. Int J Environ Res Public Health. 2020;17:3022. doi: 10.3390/ijerph17093022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dooley N, Healy C, Cotter D, Clarke M, Cannon M. The persistent effects of foetal growth on child and adolescent mental health: longitudinal evidence from a large populationbased cohort. Eur Child Adolesc Psychiatry. 2022 doi: 10.1007/s00787-022-02045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 6.Momany AM, Kamradt JM, Nikolas MA. A meta-analysis of the association between birth weight and attention deficit hyperactivity disorder. J Abnorm Child Psychol. 2018;46:1409–1426. doi: 10.1007/s10802-017-0371-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson BB, Dudovitz RN, Coker TR, et al. Predictors of poor school readiness in children without developmental delay at age 2. Pediatrics. 2016;138 doi: 10.1542/peds.2015-4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hadders-Algra M, Boxum AG, Hielkema T, Hamer EG. Effect of early intervention in infants at very high risk of cerebral palsy: a systematic review. Dev Med Child Neurol. 2017;59:246–258. doi: 10.1111/dmcn.13331. [DOI] [PubMed] [Google Scholar]
- 9.Jensen A. Pediatric stroke and cell-based treatment – pivotal role of brain plasticity. J Stem Cell Res Transplant. 2019;6:1029. [Google Scholar]
- 10.Vitrikas K, Savard D, Bucaj M. Developmental delay: when and how to screen. Am Fam Physician. 2017;96:36–43. [PubMed] [Google Scholar]
- 11.Fitzpatrick C, Boers E, Pagani LS. Kindergarten readiness, later health, and social costs. Pediatrics. 2020;146 doi: 10.1542/peds.2020-0978. [DOI] [PubMed] [Google Scholar]
- 12.Berger R, Bender S, Sefkow S, Klingmüller V, Künzel W, Jensen A. Peri/intraventricular haemorrhage: a cranial ultrasound study on 5286 neonates. Eur J Obstet Gynecol Reprod Biol. 1997;75:191–203. doi: 10.1016/s0301-2115(97)00135-8. [DOI] [PubMed] [Google Scholar]
- 13.Jensen A, Holmer B. White matter damage in 4,725 term-born infants is determined by head circumference at birth: the missing link. Obstet Gynecol Int. 2018;2018 doi: 10.1155/2018/2120835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patra K, Greene MM, Patel AL, Meier P. Maternal education level predicts cognitive, language, and motor outcome in preterm infants in the second year of life. Am J Perinatol. 2016;33:738–744. doi: 10.1055/s-0036-1572532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer J. Antonius-Verl; Solothurn (Swiss Confederation): 1985. Kramer-Test. 4. Anleitung zum labyrinth-test (Maze-Test) nach Porteus. Nachdr. d. 2. rev Aufl. von 1974.- 198–. - 15 S.: Ill., graph. Darst. [Google Scholar]
- 16.Porteus SD. Pacific Books; Palo Alto: 1965. Porteus maze test: fifty years’ application.http://worldcat.org/oclc/1006107 OCLC 1006107. Available at: [Google Scholar]
- 17.Kramer J. Antonius-verlag; Solothurn, Switzerland: 1972. Kramer Intelligenztest St. [Google Scholar]
- 18.Touwen BLC. Georg Thieme Verlag; Federal Republic of Germany: 1982. Die Untersuchung von Kindern mit geringen neurologischen Funktionsstörungen. [Google Scholar]
- 19.Jensen A. Cerebral palsy-brain repair with stem cells. J Perinat Med. 2022 doi: 10.1515/jpm-2022-0505. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Jensen A, Roman C, Rudolph AM. Effects of reducing uterine blood flow on fetal blood flow distribution and oxygen delivery. J Dev Physiol. 1991;15:309–323. [PubMed] [Google Scholar]
- 21.Neuhäuser G. Bewegungsentwicklung im Säuglingsalter. Variabilität und Varianten der frühkindlichen Motorik – Einzelartikel. Psychosozial. 1991;46:18–28. [Google Scholar]
- 22.Hadders-Algra M, Heineman KR, Bos AF, Middelburg KJ. The assessment of minor neurological dysfunction in infancy using the Touwen Infant Neurological Examination: strengths and limitations. Dev Med Child Neurol. 2010;52:87–92. doi: 10.1111/j.1469-8749.2009.03305.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.