Abstract
Rice blast, caused by Magnaporthe oryzae, is one of the most devastating diseases of rice. During infection, M. oryzae secretes effectors to facilitate blast development. Among these effectors, the avirulence factor AvrPi9 is recognized by Pi9, a broad-spectrum blast resistance protein that triggers Pi9-mediated resistance in rice. However, little is known about the interaction between AvrPi9 and Pi9 and how AvrPi9 exerts virulence to promote infection. In this study, we found that ectopic expression of AvrPi9 in the Pi9-lacking cultivar TP309 suppressed basal resistance against M. oryzae. Furthermore, we identified an AvrPi9-interacting protein in rice, which we named OsRGLG5, encoding a functional RING-type E3 ubiquitin ligase. During infection, AvrPi9 was ubiquitinated and degraded by OsRGLG5. Meanwhile, AvrPi9 affected the stability of OsRGLG5. Infection assays revealed that OsRGLG5 is a positive regulator of basal resistance against M. oryzae, but it is not essential for Pi9-mediated blast resistance in rice. In conclusion, our results revealed that OsRGLG5 is targeted by the M. oryzae effector AvrPi9 and positively regulates basal resistance against rice blast.
Key words: Oryza sativa, disease resistance, avirulence factor, rice–Magnaporthe oryzae interaction, plant defense
The Magnaporthe oryzae effector AvrPi9, which is recognized by the broad-spectrum blast resistance gene Pi9, suppresses basal resistance in rice. AvrPi9 targets the RING-type E3 ubiquitin ligase OsRGLG5. OsRGLG5 positively regulates basal resistance against blast but is not essential for Pi9-mediated resistance in rice.
Introduction
Plants have evolved a complicated and efficient immune system over a long period of time to resist pathogen invasion. The plant immune system includes Resistance gene (R gene)–mediated resistance and basal resistance (Peng et al., 2018). R gene–mediated resistance is strong but shows pathogen and species specificity. During infection, pathogens secrete a large number of effectors into the host to modulate the physiology of infected cells and the immune response. R gene–mediated resistance is elicited by avirulence (avr) factors, which are defined as effectors recognized directly or indirectly by particular R proteins of a plant. Basal resistance belongs to the first layer of plant natural immunity, which is relatively broad spectrum and not race specific (Wang et al., 2011; Holbein et al., 2016; Liang et al., 2020). Therefore, in recent years, increasing attention has been given to basal resistance against blast in rice (Li et al., 2017; Liu et al., 2019).
The rice–Magnaporthe oryzae pathosystem has emerged as an important model for understanding plant–pathogen interactions. To date, more than 38 rice R genes and 14 Avirulence genes of cognate rice R genes from the rice–M. oryzae pathosystem have been cloned (Devanna et al., 2022). Among these avirulence factors, Avr-Pita, Avr-Pik, and Avr-Pi54 interact with cognate R proteins directly to trigger the R protein–mediated hypersensitive response (Jia et al., 2000; Orbach et al., 2000; Kanzaki et al., 2012; Ribot et al., 2013; Ray et al., 2016). By contrast, some avirulence factors, including Avirulence Conferring Enzyme1 (ACE1) and AvrPiz-t, interact with cognate R proteins indirectly to initiate R protein–mediated disease resistance. Some avirulence factors play dual roles: they are recognized by host R proteins directly or indirectly and target host proteins to suppress basal resistance to promote infection. For instance, the avirulence factor Avr-Pita, a zinc metalloprotease, is recognized by Pi-ta to initiate Pi-ta-mediated resistance (Orbach et al., 2000). AvrPita suppresses host basal resistance by interfering with reactive oxygen species (ROS) metabolism (Han et al., 2021). The avirulence factor Avr-Pii triggers R gene Pii–mediated resistance (Yoshida et al., 2009; Fujisaki et al., 2015). AvrPii also targets the rice NADP-malic enzyme to regulate ROS accumulation and suppress basal resistance (Singh et al., 2016). AvrPiz-t induces R gene Piz-t–mediated resistance and suppresses basal resistance by targeting a variety of rice proteins (Li et al., 2009; Park et al., 2012, 2016; Tang et al., 2017; Shi et al., 2018; Zhang et al., 2020). In our previous study, we found that the avirulence factor AvrPi9 is recognized by the R protein Pi9 to trigger Pi9-mediated resistance (Qu et al., 2006; Wu et al., 2015). However, it is not clear whether and how AvrPi9 regulates basal resistance to promote M. oryzae infection.
The plant ubiquitin/26S proteasome system (UPS) is an important intracellular protein degradation system in which substrate protein is first ubiquitinated by ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2), and ubiquitin-ligating enzyme (E3). E2 and E3 catalyze the ubiquitination labeling of target proteins, which are then recognized and degraded by the 26S proteasome (Sharma et al., 2016; Copeland and Li, 2019). E3 ubiquitin ligases determine the specificity of target proteins. According to their mechanism of action and subunit composition, E3 ubiquitin ligases are classified into four types: HECT, RING, U-box, and CRL (cullin-RING) (Vierstra, 2009). The RGLG RING-type ligases are a class of proteins with a von Willebrand factor type A (vWA) structural domain at the amino terminus and a RING structural domain at the carboxyl terminus. There are five RGLG proteins in Arabidopsis, AtRGLG1 to 5 (Stone et al., 2005; Yin et al., 2007). AtRGLG1 and AtRGLG2 play important roles in growth factor–regulated apical dominance and responses to drought and iron-deficiency stress (Yin et al., 2007; Cheng et al., 2012; Pan et al., 2015). AtRGLG3 and AtRGLG4 are involved in jasmonate-mediated susceptibility to Pseudomonas syringae pv. tomato DC3000 and wound response as well as regulation of iron–sulfur protein degradation (Zhang et al., 2012; Durand et al., 2016). AtRGLG1 and AtRGLG5 function in the ABA signaling pathway through degradation of the phosphatase PP2CA (Wu et al., 2016; Belda-Palazon et al., 2019). However, there have been no functional studies of the RGLG gene family in rice, and the role of RGLG genes in the rice immune response remains unknown.
The broad-spectrum rice blast resistance gene Pi9, which encodes a nucleotide-binding site plus leucine-rich repeat (NBS–LRR) protein, was cloned by a map-based cloning strategy (Qu et al., 2006). In our previous study, the corresponding avirulence gene of Pi9, AvrPi9, was identified by comparative genomics analysis of strains derived from a sequential planting method (Wu et al., 2015). In the present study, we found that ectopic expression of AvrPi9 in the Pi9-lacking cultivar TP309 suppressed basal resistance against M. oryzae. AvrPi9 interacted with OsRGLG5, which then ubiquitinated and degraded AvrPi9. In addition, AvrPi9 affected the stability of OsRGLG5. Infection assays revealed that OsRGLG5 is a positive regulator of basal resistance against M. oryzae but is not essential for Pi9-mediated blast resistance in rice.
Results
Rice basal resistance against M. oryzae was compromised by ectopic expression of AvrPi9
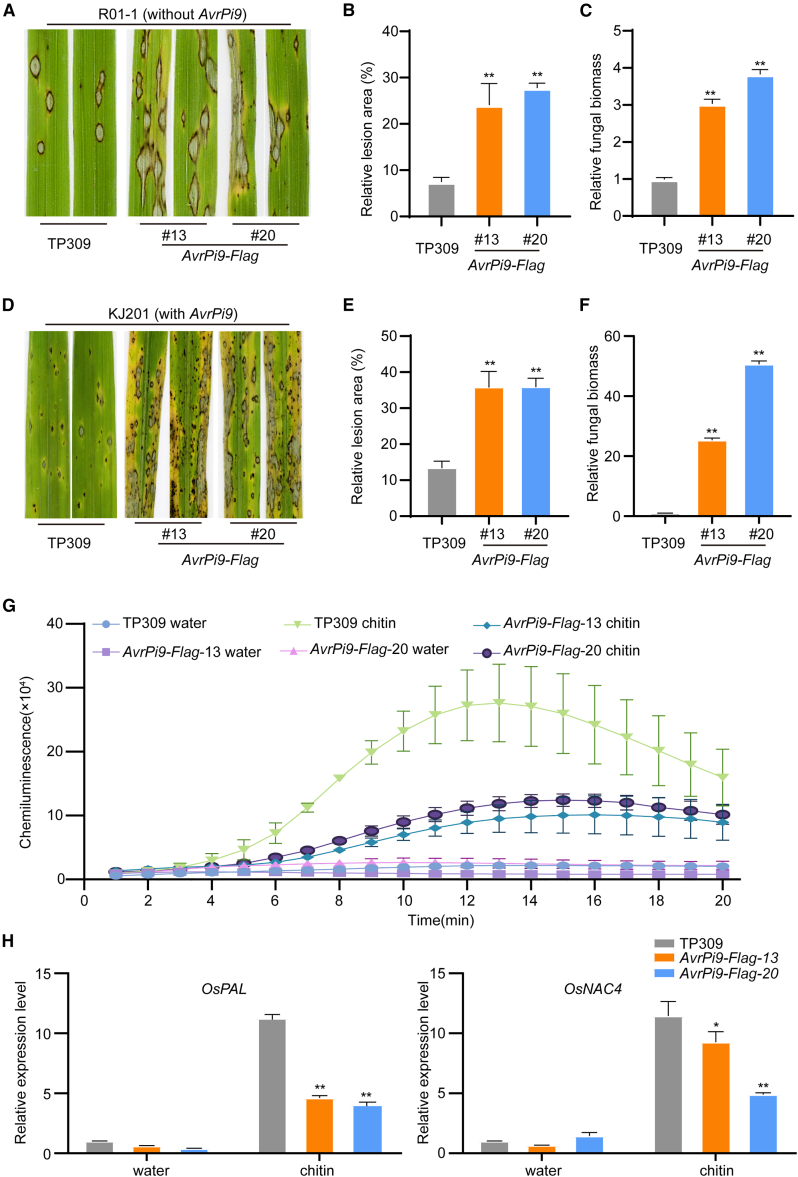
In our previous study, AvrPi9 was identified as the corresponding avirulence factor of the broad-spectrum resistance gene Pi9. During infection, AvrPi9 is delivered into rice cells (Wu et al., 2015). To reveal the function of AvrPi9 in rice plants, stable transgenic rice lines that expressed truncated AvrPi9 (lacking the N-terminal signal peptide) with the C terminus fused to a 3× Flag tag under the maize (Zea mays) ubiquitin promoter were generated in the Pi9-free cultivar TP309. Twenty-five AvrPi9-Flag transgenic lines were produced, and two positive independent homozygous T2 transgenic lines were used for the following experiments. To determine whether AvrPi9 is involved in modulating resistance against rice blast, punch and spray inoculation assays of the AvrPi9-Flag plants were performed with the M. oryzae strains R01-1 (without AvrPi9) and KJ201 (with AvrPi9). With spray inoculation, we found that the disease lesion areas caused by R01-1 and KJ201 were larger on the AvrPi9-Flag plants than on wild-type TP309 (Figure 1A, 1B, 1D, and 1E). Relative fungal biomass, which was determined using DNA-based qPCR to calculate the M. oryzae Pot2 gene value relative to the rice Ubiquitin gene value, was greater in the AvrPi9-Flag plants (Figure 1C and 1F). Consistent with the results of the spray inoculation assay, the AvrPi9-Flag transgenic rice plants were more susceptible to the pathogen than the wild type in the punch inoculation assay (Supplemental Figure 1A). Lesion length (Supplemental Figure 1B) and relative fungal biomass (Supplemental Figure 1C) were also greater in AvrPi9-Flag plants than in wild-type TP309. These results suggested that ectopic expression of AvrPi9 compromises resistance against M. oryzae.
Figure 1.
Ectopic expression of AvrPi9 impairs basal resistance to M. oryzae in rice.
(A and D) Disease symptoms of AvrPi9-Flag and TP309 seedlings after spray inoculation with M. oryzae strain R01-1 (without AvrPi9) in (A) and KJ201 (with AvrPi9) in (D). The images were photographed at 7 days post inoculation.
(B and C) Relative lesion areas and relative fungal biomass of M. oryzae-inoculated TP309 and AvrPi9-Flag plants in (A). Data are shown as the mean ± SEM (n = 3). Asterisks represent significant differences (Student’s t-test, ∗P < 0.05, ∗∗P < 0.01).
(E and F) Relative lesion areas and relative fungal biomass of TP309 and AvrPi9-Flag plants in (D). The relative fungal biomass of M. oryzae was determined by DNA-based qPCR of the M. oryzae Pot2 gene and the rice Ubiquitin gene. Data are shown as the mean ± SEM (n = 3). Asterisks represent significant differences (Student’s t-test, ∗P < 0.05, ∗∗P < 0.01).
(G) Chitin-induced ROS burst in AvrPi9-Flag and wild-type TP309 plants. Leaf discs were treated with water and 8 nM chitin (hexa-N-acetyl-chitohexaose), and ROS were detected with a luminol assay. Data are shown as the mean ± SEM (n = 3).
(H) Relative expression levels of the defense-related genes OsPAL and OsNAC4. The leaf samples for qPCR assays were incubated in water and chitin for 3 h. Data are shown as the mean ± SEM (n = 3). Asterisks represent significant differences (Student’s t-test, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001).
A variety of plant pathogen effectors are known to suppress basal resistance (Wang et al., 2022b). To investigate whether expression of AvrPi9 affects basal resistance in rice, leaves of AvrPi9-Flag plants and corresponding wild-type plants were used to measure ROS production induced by the elicitor chitin. There was less ROS accumulation in the AvrPi9-Flag plants than in the wild-type plants (Figure 1G). Relative expression levels of the defense-related genes OsPAL and OsNAC4, common marker genes of basal resistance (Park et al., 2012; Fan et al., 2018), were lower in AvrPi9-Flag plants than in wild-type plants when plants were treated with chitin (Figure 1H). Taken together, these results suggested that the ectopic expression of AvrPi9 impairs basal resistance in rice.
AvrPi9 interacts with the RING-type E3 ubiquitin ligase OsRGLG5
To investigate the molecular mechanism by which AvrPi9 mediates suppression of basal resistance, a yeast two-hybrid (Y2H) assay was performed to identify the potential host target protein of AvrPi9. Because the AvrPi9 signal peptide is cleaved in the mature protein, AvrPi919-91 (without the signal peptide) was used to construct the vector and perform the following analysis. By screening a rice cDNA library with AvrPi919-91, a RING-type E3 ubiquitin ligase was identified as a candidate (Figure 2A). Blast analysis (https://blast.ncbi.nlm.nih.gov/Blast.cgi) showed that this candidate shared the highest similarity with AtRGLG5 from Arabidopsis thaliana, and we therefore named it OsRGLG5. There are six RGLG homologs in rice (Supplemental Figure 2B). The OsRGLG5 gene consists of a 1344-bp open reading frame that encodes a protein of 448 amino acids (Supplemental Figure 2A). The OsRGLG5 protein, which has two predicted conserved domains, the vWA domain (67–317 aa) and a C3HC4-type RING-finger domain (403–447 aa), belongs to the ubiquitous and conserved RING-finger protein family found in eukaryotes.
Figure 2.
AvrPi9 interacts with OsRGLG5 in vitro and in vivo.
(A) Y2H assay to detect the interaction among AvrPi9, OsRGLG5, and Pi9. AvrPi919-91 (without the signal peptide) and Pi9 were fused to pGBKT7 (BD). OsRGLG5 was fused to pGADT7 (AD). The positive and negative controls were pGADT7-T + pGBKT7-53 and pGADT7-T + pGBKT7-lam. SD (−WL) indicates selective medium lacking leucine and tryptophan. SD (−WLHA) indicates selective medium lacking leucine, tryptophan, adenine, and histidine.
(B) Split-luciferase complementation assays to test the interaction between AvrPi9 and OsRGLG5 in N. benthamiana leaves. AvrPi919–91 (without the signal peptide) was fused to the C-terminal portion of LUC, and OsRGLG5 was fused to the N-terminal portion of LUC. Agrobacterium EHA105 strains carrying the indicated vectors were co-infiltrated into leaves of N. benthamiana. Images were captured 48 h post infiltration using a CCD imaging apparatus.
(C) Co-immunoprecipitation assay with AvrPi9-GFP and OsRGLG5-Flag. At 3 days post infiltration, total proteins were extracted from N. benthamiana leaves for western blotting. GFP served as a negative control.
(D) Subcellular localization and co-localization of AvrPi9 and OsRGLG5 in rice protoplasts. Scale bars, 10 μm. All experiments were repeated three times with similar results.
To identify the fragment of the OsRGLG5 protein that interacts with AvrPi9, a bait vector was constructed with truncated OsRGLG5 and used to perform a Y2H assay. The results showed that OsRGLG5 physically interacts with AvrPi9 through the vWA domain in yeast (Supplemental Figure 3). Because AvrPi9 is the corresponding avirulence factor of Pi9 (Wu et al., 2015), we tested whether OsRGLG5 interacts with Pi9. The Y2H assay and split-LUC assay showed that OsRGLG5 is unlikely to interact with Pi9 in yeast (Supplemental Figure 2C) or in planta (Supplemental Figure 2D).
To further confirm the interaction between AvrPi9 and OsRGLG5, a split-LUC assay was performed in Nicotiana benthamiana leaves by the agroinfiltration method (Figure 2B). As shown in Figure 2B, strong fluorescence was observed in the area of N. benthamiana leaves that co-expressed cLUC-AvrPi9 and OsRGLG5-nLUC. By contrast, almost no fluorescence was detected in the control combinations (cLUC + nLUC, cLUC-AvrPi9 + nLUC, and cLUC + nLUC-OsRGLG5). Another five RGLG homologs were also found to interact with AvrPi9 in yeast (Supplemental Figure 2C) and in planta (Supplemental Figure 2D). Next, coimmunoprecipitation (coIP) assays were performed by transiently expressing OsRGLG5-Flag and AvrPi9-green fluorescent protein (GFP) in N. benthamiana leaves. When the GFP-tagged protein AvrPi9 was precipitated by the anti-GFP antibody, the OsRGLG5-Flag protein was coprecipitated and detected by the anti-Flag antibody. By contrast, no signal was detected in the N. benthamiana leaves that coexpressed GFP and the OsRGLG5-Flag protein (Figure 2C), suggesting that AvrPi9 interacts specifically with OsRGLG5 in planta. In addition, a subcellular localization assay showed that AvrPi9 colocalized with OsRGLG5 in rice protoplasts (Figure 2D). These results indicated that the avirulence factor AvrPi9 interacts with the RING-type E3 ubiquitin ligase OsRGLG5 in vitro and in vivo.
OsRGLG5 encodes a functional E3 ubiquitin ligase
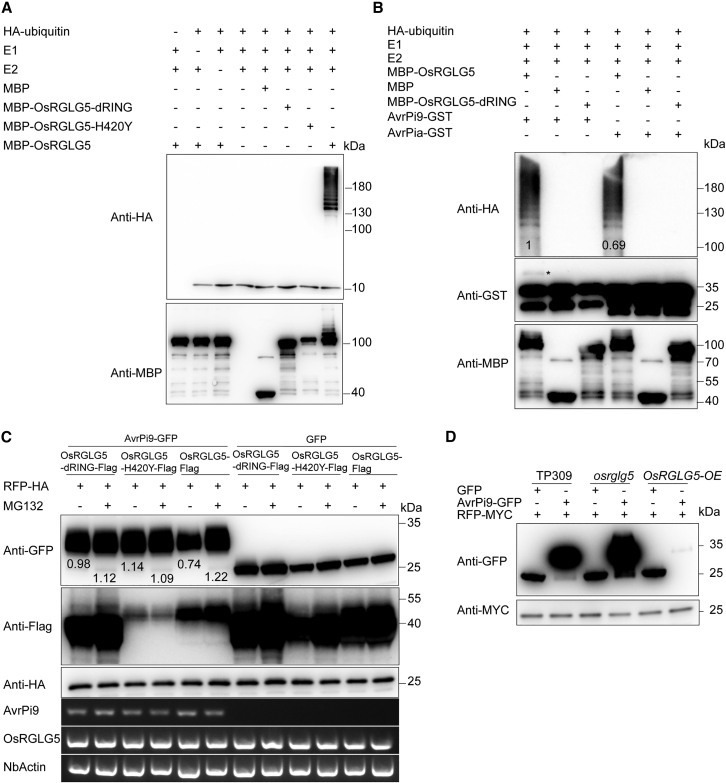
On the basis of sequence analysis, OsRGLG5 is a RING-type ligase. To determine whether OsRGLG5 possesses E3 ubiquitin ligase activity, an in vitro E3 ubiquitin ligase assay was performed, including negative controls that lacked E1, E2, or ubiquitin. An maltose binding protein (MBP)-tagged full-length OsRGLG5 fusion protein (MBP-OsRGLG5) was expressed in Escherichia coli. The MBP-OsRGLG5 protein was purified and incubated with human E1 (UBE1), human E2 (UBE2D3), and hemagglutinin (HA)-tagged ubiquitin for the E3 ligase activity assay. In the presence of ubiquitin, E1, and E2, MBP-OsRGLG5 could carry out self-ubiquitination, but no clear protein ubiquitination was detected in the absence of E1 or E2 enzymes or MBP-OsRGLG5 (Figure 3A). These results suggested that OsRGLG5 is a functional E3 ligase.
Figure 3.
OsRGLG5 targets AvrPi9 for ubiquitination and degradation.
(A) Self-ubiquitination assay of OsRGLG5. Ubiquitin-bound proteins were detected by western blotting with anti-HA antibody and anti-MBP antibody.
(B) OsRGLG5 ubiquitinates AvPi9 in vitro. An in vitro ubiquitination assay was performed with GST-AvrPi9 and MBP-OsRGLG5 fusion proteins. Ubiquitination of GST-AvrPi9 was detected by immunoblotting with an anti-GST antibody. GST-AvrPia was used as a negative control. The asterisk represents the ubiquitinated GST-AvrPi9 protein. The experiments were performed at least three times with similar results.
(C) Degradation of AvrPi9 by OsRGLG5 in N. benthamiana. OsRGLG5-Flag, OsRGLG5-dRING-Flag, and OsRGLG5-H420Y-Flag were co-expressed with AvrPi9-GFP or GFP in N. benthamiana by Agrobacterium-mediated infiltration. RFP-HA served as an internal control. The protein levels were detected at 3 days post infiltration by immunoblotting with anti-HA antibody. MG132 (50 μM) was infiltrated 18 h before sampling, and water was used as a control. The expression level of each gene was determined by semi-quantitative PCR.
(D) Degradation of AvrPi9 by OsRGLG5 in rice protoplasts. AvrPi9-GFP or GFP was co-expressed with RFP-MYC in rice protoplasts of wild-type TP309, osrglg5 mutant, and OsRGLG5 overexpression lines. Rice cells were collected after transient transformation for about 20 h for protein extraction and western blotting. RFP-MYC served as an internal control.
To further analyze the function of OsRGLG5, ubiquitination experiments were performed using mutated OsRGLG5 proteins in which the RING domain (OsRGLG5-dRING) was deleted or the 420th histidine was replaced with tyrosine (OsRGLG5-H420Y). The results showed that the mutated OsRGLG5 did not have ubiquitination function, indicating that the 420th histidine of the RING domain had an important role in the activity of OsRGLG5 E3 ubiquitin ligase (Figure 3A).
OsRGLG5 targets AvrPi9 for ubiquitination and degradation
OsRGLG5 interacts with AvrPi9 and functions as an E3 ligase; thus, we speculated that AvrPi9 may be a substrate of OsRGLG5. To test this hypothesis, we performed an in vitro ubiquitination experiment, similar to the assay for OsRGLG5 E3 ubiquitin ligase activity, by adding purified AvrPi9-glutathione S-transferase (GST) or AvrPia-GST (negative control) protein to the reaction mixture (Figure 3B). Western blotting revealed that AvrPi9-GST was ubiquitinated by OsRGLG5, as indicated by the asterisk (Figure 3B, second panel in lane 1). By contrast, no such band was found in the presence of the M. oryzae effector fusion protein AvrPia-GST (Figure 3B, second panel in lane 4). These results demonstrated that AvrPi9 is a substrate for the E3 ubiquitin ligase activity of OsRGLG5 in vitro.
To further investigate the ubiquitination of AvrPi9 by OsRGLG5, we performed an in vivo ubiquitin assay in which E3 ubiquitin ligase and substrate were co-expressed in N. benthamiana leaves by agroinfiltration (Liu et al., 2010). Western blotting showed that the abundance of AvrPi9-GFP was significantly lower in leaves expressing OsRGLG5-Flag than in leaves expressing OsRGLG5-dRING-Flag or OsRGLG5-H420Y-Flag (Figure 3C). These results indicated that OsRGLG5 promotes degradation of AvrPi9 in N. benthamiana leaves. Moreover, pretreatment of leaves with the 26S proteasome inhibitor MG132 significantly inhibited the degradation of AvrPi9-GFP by OsRGLG5-Flag (Figure 3C). By contrast, the negative controls OsRGLG5-dRING-Flag and OsRGLG5-H420Y-Flag, in which the E3 ubiquitin ligase activity of OsRGLG5 was inactivated, did not change the protein abundance of AvrPi9-GFP, with or without MG132 pretreatment (Figure 3C). To further investigate the degradation of AvrPi9 by OsRGLG5, we detected AvrPi9-GFP protein levels in protoplasts of osrglg5 mutant and OsRGLG5-overexpressing plants (OsRGLG5-OE). Western blotting showed that AvrPi9-GFP was almost undetectable in protoplasts of OsRGLG5-OE, whereas high levels of AvrPi9-GFP accumulated in protoplasts of the osrglg5 mutant (Figure 3D). These results suggested that OsRGLG5 targets AvrPi9 for ubiquitin-dependent degradation through the 26S proteasome system in planta.
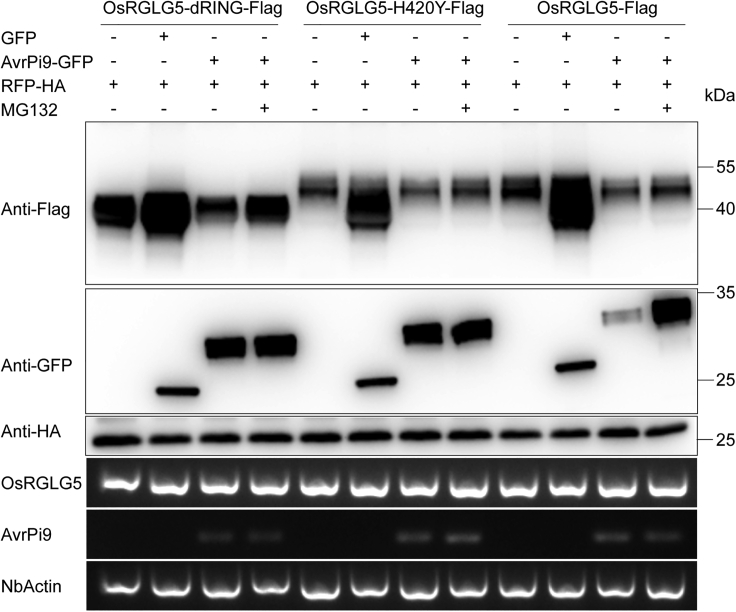
AvrPi9 affects the stability of OsRGLG5
During infection, some effectors target and change the stability of host E3 ligases to promote invasive growth (Park et al., 2012, 2016). To test whether AvrPi9 affects the stability of OsRGLG5, accumulation of OsRGLG5 protein was measured in plants that expressed AvrPi9-GFP. The results showed that, when co-expressed with AvrPi9-GFP, OsRGLG5-Flag was present at lower levels (Figure 4) compared with the GFP negative control (Figure 4 and Supplemental Figure 4). Protein levels of OsRGLG5-H420Y-Flag and OsRGLG5-dRING-Flag showed a pattern similar to that of pretreatment with MG132 (Figure 4). These results provide evidence that AvrPi9 may affect the stability of OsRGLG5 through the 26S proteasome pathway in rice.
Figure 4.
AvrPi9 affects the stability of OsRGLG5.
Agrobacteria carrying OsRGLG5-Flag, OsRGLG5-dRING-Flag, and OsRGLG5-H420Y-Flag plasmids were co-infiltrated into N. benthamiana with GFP or AvrPi9-GFP (without signal peptide). RFP-HA served as an internal control. The leaves were harvested 3 days post infiltration. MG132 (50 μM) was infiltrated 18 h before sampling, and water served as a control. RNA expression levels of the genes were determined by semi-quantitative PCR.
OsRGLG5 is a positive regulator of basal resistance against M. oryzae in rice
Ectopic expression of AvrPi9 impaired basal resistance in rice (Figure 1). It is possible that OsRGLG5 may also be involved in basal resistance. To explore the contribution of OsRGLG5 to basal resistance, rice cultivars NIL75-1-127 (with Pi9) and IR31917 (without Pi9) were infected with an AvrPi9-containing M. oryzae strain. Expression of OsRGLG5 was induced by M. oryzae in both NIL75-1-127 and IR31917 compared with the mock control plants (Supplemental Figure 5). To further investigate the role of OsRGLG5 in resistance against M. oryzae, OsRGLG5 knockout mutants were generated in the Pi9-lacking cultivar TP309 using the CRISPR–Cas9 system (Supplemental Figure 6). The resultant osrglg5 mutants were used for blast inoculation assays with M. oryzae strains KJ201 and R01-1. A punch inoculation assay showed that the osrglg5 mutants formed larger lesions with higher relative fungal biomass than wild-type TP309 (Supplemental Figure 7). Consistent with results from the punch inoculation assay, spray inoculation with M. oryzae strains KJ201 and R01-1 showed that relative fungal biomass was greater in the osrglg5 mutants than in wild-type TP309 (Figure 5A–5D). These results indicated that the OsRGLG5 knockout mutants were more susceptible to M. oryzae than the wild type. Next, we measured ROS production upon chitin elicitor treatment and found that chitin-induced ROS accumulation was significantly reduced in the osrglg5 mutants compared with the wild-type (Figure 5I). Knockout of the OsRGLG5 gene also suppressed induction of the defense-related genes OsPAL and OsNAC4 in response to M. oryzae inoculation (Figure 5K).
Figure 5.
OsRGLG5 positively regulates the basal resistance of rice against M. oryzae.
(A and C) Disease symptoms of osrglg5 and TP309 seedlings after spray inoculation with M. oryzae strain R01-1 (without AvrPi9) (A) and KJ201 (with AvrPi9) (C). The images were photographed at 7 days post inoculation. Experiments were repeated three times with similar results.
(B and D) Relative fungal biomass of the osrglg5 mutants and TP309 in (A) and (C). The relative fungal biomass was determined by DNA-based quantitative PCR (qPCR) using the threshold cycle (CT) values of theM. oryzae transposable element MoPot2 gene and the rice Ubiquitin gene.
(E and G) Spray inoculation of OsRGLG5 overexpression plants and wild-type TP309 with the M. oryzae strains R01-1 (E) and KJ201 (G). Data are shown as mean ± SEM (n = 3). Asterisks represent significant differences. (Student’s t-test, ∗P < 0.05, ∗∗P < 0.01).
(F and H) Relative fungal biomass of OsRGLG5 overexpression plants and TP309 in (E) and (G). Data are shown as mean ± SEM (n = 3). Asterisks represent significant differences. (Student’s t-test, ∗∗P < 0.01, ∗∗∗P < 0.001).
(I and J) Chitin-induced ROS burst in osrglg5 and wild-type TP309 plants (I) and OsRGLG5 overexpression plants and wild-type TP309 (J). Leaf disks were treated with water and 8 nM chitin, and ROS accumulation was measured by luminol assay. Data are shown as mean ± SEM (n = 3).
(K and L) Relative expression levels of the defense-related genes OsPAL and OsNAC4 in wild-type TP309 and osrglg5 mutants (K) and OsRGLG5 overexpression plants and wild-type TP309 (L) inoculated with M. oryzae strain KJ201. Samples were harvested at 48 h post inoculation for qRT–PCR assay. Data are shown as mean ± SEM (n = 3). Asterisks represent significant differences (Student’s t-test, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001).
To further analyze the function of OsRGLG5, OsRGLG5-overexpressing transgenic lines were generated, and T2 plants were used for further analyses. Punch inoculation assays showed that leaf lesions on OsRGLG5-OE plants were smaller than those on wild-type TP309 (Supplemental Figure 8A and 8B), and relative fungal biomass was lower (Supplemental Figure 8C). Spray inoculation assays with KJ201 and R01-1 strains showed that OsRGLG5-OE plants were more resistant to rice blast (Figure 5E and 5G). Relative fungal biomass (Figure 5F and 5H) was lower in OsRGLG5-OE plants than in wild-type plants. In contrast to the osrglg5 mutants, OsRGLG5-OE plants showed greater chitin-induced ROS generation than the wild type (Figure 5J). Moreover, after inoculation with M. oryzae strain KJ201, expression levels of the defense-related genes OsPAL and OsNAC4 were higher in OsRGLG5-OE plants than in wild-type plants (Figure 5L). Taken together, these results suggest that OsRGLG5 plays a positive role in basal resistance against M. oryzae in rice. It is also possible that AvrPi9 suppresses basal resistance by affecting the stability of OsRGLG5.
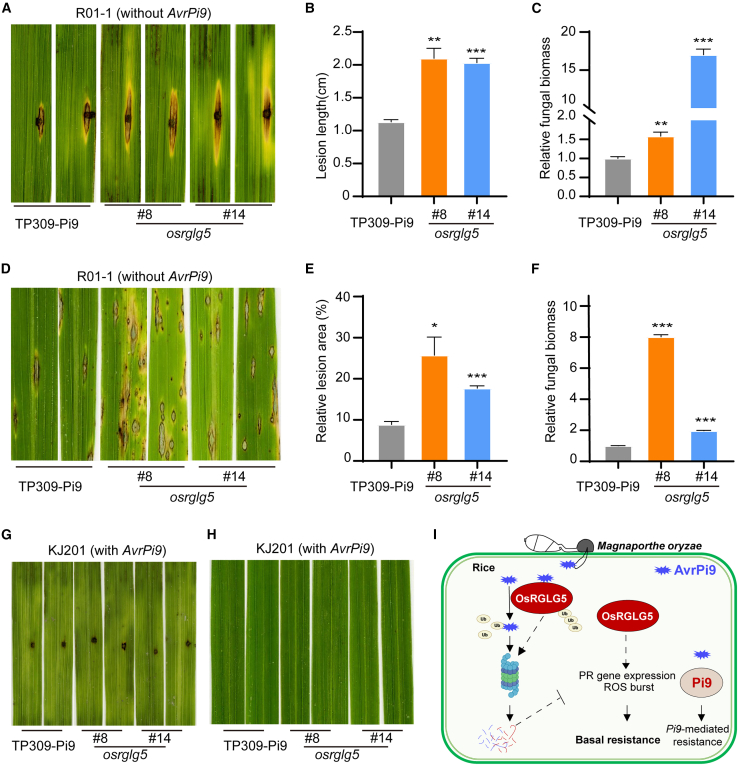
OsRGLG5 is not essential for Pi9-mediated blast resistance
AvrPi9, which corresponds to the M. oryzae avirulence factor Pi9, targets OsRGLG5 in rice. We therefore asked whether OsRGLG5 participates in the Pi9-mediated immunity response. To answer this question, OsRGLG5 knockout mutants were generated in the TP309-Pi9 background, which contains the Pi9 gene. Punch and spray inoculation assays of osrglg5 (TP309-Pi9) mutants were then performed using M. oryzae strains KJ201 (with AvrPi9) and R01-1 (without AvrPi9). The punch inoculation assay showed that lesion length (Figure 6A and 6B) and relative fungal biomass (Figure 6C) were greater in the osrglg5 (TP309-Pi9) mutants than in wild-type TP309-Pi9. The spray inoculation assay showed that wild-type TP309-Pi9 and osrglg5 (TP309-Pi9) mutants both developed typical rice blast susceptibility symptoms (Figure 6D–6F). Moreover, osrglg5 (TP309-Pi9) mutants developed larger lesions than TP309-Pi9 (Figure 6A), consistent with results of the punch and spray inoculation assays of osrglg5 mutants in the TP309 background (Figure 5A). These results indicated that OsRGLG5 plays a positive role in resistance against virulent M. oryzae in the TP309-Pi9 background. However, both punch (Figure 6G) and spray inoculation (Figure 6H) with strain KJ201 revealed that osrglg5 (TP309-Pi9) mutants were avirulent to M. oryzae strain KJ201 (with AvrPi9), suggesting that OsRGLG5 is not essential for Pi9-mediated blast resistance in rice.
Figure 6.
OsRGLG5 is not essential for Pi9-mediated blast resistance.
(A–F) Punch (A–C) and spray inoculation (D–F) assays of osrglg5 mutants in the TP309-Pi9 background. Approximately 4-week-old rice plant leaves were inoculated with M. oryzae strain R01-1, which does not harbor AvrPi9. The images were taken at 7 dpi. Experiments were repeated three times with similar results. Data shown are mean ± SEM (n = 3). Student’s t-test, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
(G and H) Punch (G) and spray inoculation (H) of TP309-Pi9 and OsRGLG5 mutants in the TP309-Pi9 background with M. oryzae strain KJ201, which harbors AvrPi9. The images were taken at 7 days post inoculation.
(I) Working model of OsRGLG5-mediated basal resistance against M. oryzae in rice. During infection, AvrPi9 is secreted into rice cells by M. oryzae. AvrPi9 targets and destabilizes the E3 ubiquitin ligase OsRGLG5 to inhibit basal resistance in rice. In return, OsRGLG5 adds ubiquitin to AvrPi9 and subsequently degrades AvrPi9 through the 26S proteasome pathway. Meanwhile, the expression of defense-related genes and the ROS burst are induced by OsRGLG5, which positively regulates basal resistance against M. oryzae in rice.
Discussion
Pathogens secrete effectors to interfere with the host defense response and promote infection. In this study, our results showed that when AvrPi9 was secreted into rice cells by M. oryzae, it targeted and destabilized the E3 ubiquitin ligase OsRGLG5 to suppress basal resistance in rice. In response, OsRGLG5 ubiquitinated and subsequently degraded AvrPi9 through the 26S proteasome pathway while upregulating expression of defense-related genes and increasing ROS accumulation. Furthermore, we found that OsRGLG5 is not essential for Pi9-mediated blast resistance (Figure 6I).
The arms race between pathogens and plants is a dynamic coevolutionary process, and the molecular interaction mechanisms are different between Avirulence genes and R genes. Three models have been proposed for R–Avirulence factor interactions: the direct interaction model, the guard/decoy model, and the integrated decoy model (Jones et al., 2016; Deng et al., 2020). In the rice–M. oryzae pathosystem, the interactions between Avr-Pita and Pita, Avr-PiK and PiK, and Avr-Pi54 and Pi54 trigger R protein-mediated disease resistance according to the direct interaction model (Jia et al., 2000; Orbach et al., 2000; Kanzaki et al., 2012; Ray et al., 2016). Avr-Pii and Pii, AvrPib and Pib, and AvrPiz-t and Piz-t appear to act according to the guard/decoy model (Fujisaki et al., 2015; Park et al., 2016; Zhang et al., 2018, 2020). Rice NLRs RGA4 and RGA5 and their cognate M. oryzae avirulence factors Avr1-CO39 and Avr-Pia fit the integrated decoy model (Cesari et al., 2013; Jones et al., 2016; Deng et al., 2020). Recently, excellent research showed that AvrPi9 stabilizes ANIP1 to participate in the Pi9-mediated immunity response (Shi et al., 2023). In our study, no direct interaction between Avr-Pi9 and Pi9 was observed in a Y2H assay (Figure 2A), suggesting that there may be an indirect recognition mechanism between AvrPi9 and Pi9. Furthermore, we found that AvrPi9 interacts with OsRGLG5 both in vitro and in vivo, but OsRGLG5 did not interact with Pi9 in a Y2H assay (Figure 2A), indicating that OsRGLG5 may not be a guardee or decoy in the Pi9–AvrPi9 interaction. Consistent with these results, an M. oryzae infection assay showed that OsRGLG5 is not essential for Pi9-mediated blast resistance in rice (Figure 6). How Pi9 recognizes AvrPi9 and activates downstream defense immunity requires further research.
In addition to being recognized by R proteins, M. oryzae avirulence factors also suppress basal resistance by targeting various rice proteins. In particular, relatively systematic and in-depth research has been performed on the avirulence factor AvrPiz-t. AvrPiz-t targets the rice ubiquitin proteasome system, including the E3 ubiquitin ligases APIP6 and APIP10, to suppress basal defense in rice plants (Park et al., 2012, 2016). AvrPiz-t also targets the nucleoporin protein Nup98 homolog APIP12 and the rice plasma membrane–localized K+ channel protein OsAKT1 to suppress basal resistance to rice blast (Tang et al., 2017; Shi et al., 2018). A recent study showed that AvrPiz-t structurally mimics ROD1 and activates the same ROS-scavenging cascade to suppress host immunity and promote virulence (Gao et al., 2021). AvrPii, the avirulence factor of Pii, targets rice NADP-malic enzymes to regulate ROS accumulation and suppress basal resistance (Singh et al., 2016). AvrPita interacts with the mitochondrial electron transport chain (METC) cytochrome c oxidase (COX) assembly protein OsCOX11 in rice mitochondria, thereby enhancing the activity of complex IV of METC, leading to reduced ROS accumulation and decreased innate immunity in rice (Han et al., 2021). The broad-spectrum blast R protein Pi9 recognizes avirulence factor AvrPi9 to trigger Pi9-mediated resistance (Wu et al., 2015). In this study, we found that ectopic expression of AvrPi9 in susceptible wild-type TP309 compromises basal resistance against M. oryzae. Consistent with these results, ectopic expression of AvrPi9 in the NIL-Pigm background led to enhanced susceptibility with decreased expression of pathogen-related genes and suppressed basal resistance (Zhai et al., 2022). However, there is little information about the target protein of AvrPi9 in rice, and it remains unclear how AvrPi9 exerts virulence to promote infection. Recently, an excellent study showed that AvrPi9 targets and degrades the deubiquitinase PICI1 to dampen basal resistance (Zhai et al., 2022). In this study, we found that AvrPi9 targets and destabilizes the E3 ubiquitin ligase OsRGLG5 to suppress basal resistance against M. oryzae without affecting other agronomic traits (Supplemental Figure 9), suggesting that AvrPi9 can regulate the host immune response to promote infection through a variety of mechanisms (Supplemental Figure 11).
E3 ubiquitin ligases play crucial roles in disease resistance in rice. For instance, the RING-type E3 ligases OsBBI, EBR1, and MEL offer broad-spectrum disease resistance in rice (Li et al., 2011; You et al., 2016; Fu et al., 2022). OsCUL3a interacts with OsRBX1a and OsRBX1b to form a CUL-RING-like E3 ubiquitin ligase complex and negatively regulates cell death and immunity by degrading OsNPR1 in rice (Liu et al., 2017). The RING-type E3 ligase APIP6 can degrade the rice catalase OsCATC to regulate rice immunity (You et al., 2022). The U-box-type ubiquitin ligase PUB44 mediates PBI1 degradation, which subsequently leads to activation of WRKY45 (Ichimaru et al., 2022). OsFBK16, a CRL-type E3 ligase, interacts with and degrades OsPALs to negatively regulate disease resistance to M. oryzae (Wang et al., 2022a). In this study, a new RING-type E3 ligase, OsRGLG5, which harbors a vWA structural domain at the amino terminus and a RING structural domain at the carboxyl terminus, was identified as a target of avirulence factor AvrPi9 and plays a positive role in disease resistance. OsRGLG5 ubiquitinates and subsequently degrades AvrPi9 through the 26S proteasome pathway while upregulating the expression of defense-related genes and increasing ROS accumulation in rice.
In A. thaliana, the RGLG E3 ubiquitin ligases play vital roles in growth and development. Among five RGLGs in A. thaliana, AtRGLG1 and AtRGLG5 act as important modulators of ABA signaling by controlling PP2CA half-life (Wu et al., 2016). AtRGLG2 mediates the transcriptional activity of AtEFR53 and thus negatively regulates the drought stress response (Cheng et al., 2012). AtRGLG3 and AtRGLG4 regulate the response to coronatine and susceptibility to P. syringae pv. tomato DC3000 (Zhang et al., 2012). AtRGLG3 and AtRGLG4 also act as essential coordinators of the response to mycotoxin fumonisin B1 (FB1), which hijacks the jasmonic acid (JA) pathway to initiate programmed cell death (Zhang et al., 2015). However, little is known about the function of RGLG genes in rice. In our study, OsRGLG5 was identified as a functional E3 ubiquitin ligase. We revealed a new function of RGLG, which positively regulates basal resistance against M. oryzae in rice without compromising other agronomic traits (Figures 3C, 6, and S10). In rice, plant hormones, including salicylic acid (SA), JA, abscisic acid (ABA), and ethylene, play important roles in blast resistance. On the basis of Arabidopsis RGLG studies, we speculated that OsRGLG5 may regulate hormone signaling pathways, such as those of JA and ABA, to confer blast resistance in rice. Further research will be needed to identify the substrates of OsRGLG5 and investigate how OsRGLG5 activates basal resistance in rice.
In conclusion, our results revealed a novel mechanism by which the M. oryzae avirulence factor AvrPi9 targets the rice RING-type E3 ubiquitin ligase OsRGLG5 to suppress host basal resistance and promote infection. OsRGLG5 degrades AvrPi9 through the 26S proteasome pathway but is not required for Pi9-mediated blast resistance in rice.
Experimental procedures
Plant materials and growth conditions
The rice cultivar TP309 (Oryza sativa L. japonica) was used as the wild type in this study. TP309-Pi9 was a kind gift from Prof. Bo Zhou (Qu et al., 2006). The osrglg5 mutants were generated using the CRISPR-Cas9 system as described previously (Ma et al., 2015). The primers used in this study are listed in Supplemental Table 1. The wild type and all transgenic plants were grown in greenhouses or protected paddy fields in Hangzhou, Zhejiang Province.
The N. benthamiana plants were grown in a greenhouse at 24°C under a long-day photoperiod of 16-h light and 8-h dark.
Fungal strains and infection assay
The M. oryzae strains KJ201 (with AvrPi9) and R01-1 (without AvrPi9) were grown on prune agar medium with 2 days dark and 5 days light at 28°C to collect conidia for infection assays (Kou et al., 2017).
For inoculation assays, the concentration of the conidial suspension was adjusted to 5 × 105 per mL with 0.1% gelatin. Four-week-old rice seedlings were used for punch or spray inoculation assays. Symptoms were measured at 7 days post inoculation using a ruler or ImageJ software (https://imagej.nih.gov/ij/). To determine relative fungal biomass, DNA-based quantitative PCR (qPCR) was performed to calculate relative fungal growth using the threshold cycle value (CT) of the M. oryzae transposable element MoPot2 relative to that of the rice Ubiquitin gene (Wang et al., 2021). The primers used in this study are listed in Supplemental Table 1.
Measurement of ROS
Luminol-based ROS measurements were performed as described previously (Park et al., 2012). In brief, the top second leaves of 4-week-old rice seedlings were used to measure the ROS burst. Leaf discs approximately 4 mm in diameter were cut using a puncher and soaked overnight in sterilized water in 2.0-ml microcentrifuge tubes. Three leaf discs from each sample were then placed in a 1.5-ml microcentrifuge tube with 100 μl luminol (Bio-Rad), 1 μl horseradish peroxidase (Jackson ImmunoResearch), and 1 μl chitin (8 nM hexa-N-acetylchitohexaose) elicitor or water (control). A GloMax 20/20 luminometer (Promega) was used to measure luminescence at 10-s intervals for 20 min. Three biological replicates were used.
RNA isolation and qRT–PCR
Total RNA was extracted from rice seedlings with the RNAiso reagent according to the manufacturer’s instructions (TaKaRa). One microgram of RNA was reverse transcribed into cDNA with the PrimeScript RT reagent kit (TaKaRa). Quantitative real-time RT‒PCR (qRT‒PCR) was performed using TB Green Premix Ex Taq (TaKaRa) on a CFX Connect Real-Time System (Bio-Rad). Three biological replicates were performed for each gene. The rice Ubiquitin gene (LOC_Os03g13170) was used as the internal control for all analyses. The qRT‒PCR primers are listed in Supplemental Table 1.
Y2H analysis
The Matchmaker two-hybrid system (Clontech) was used according to the manufacturer’s instructions to screen the AvrPi9 interacting protein. The coding sequence (CDS) region of AvrPi9 (without the signal peptide) was cloned into the bait vector pGBKT7, which was linearized by EcoRI and BamHI. pGBKT7-AvrPi9 and the cDNA library, which was cloned into the prey vector pGADT7 using mRNA isolated from seedlings of the indica rice line CO39 infected with M. oryzae strain B157, were co-transformed into the Y2HGold yeast strain. Primers used to generate the plasmids are listed in Supplemental Table 1. After sequencing, the candidate preys were cloned into pGADT7 linearized by EcoRI and BamHI. The negative control pair was pGADT7-T and pGBKT7-lam, and the positive control pair was pGADT7-T and pGBKT7-p53. Transformants were grown on basic medium lacking leucine and tryptophan for 2 days at 30°C. Then the yeast clones were transferred to a more stringently selective medium that lacked leucine, tryptophan, adenine, and histidine for about 3–6 days.
Split-LUC assay in N. benthamiana
The split-LUC assay was performed as described previously (Chen et al., 2008). In brief, the coding sequences of AvrPi9 and OsRGLG5 were amplified and cloned into pCAMBIA-35S-cLuc and pCAMBIA-35S-nLuc, respectively. The primers used are listed in Supplemental Table 1. After sequencing, the plasmids were transformed into Agrobacterium tumefaciens strain EHA105. Leaves of 4- to 6-week-old N. benthamiana were infiltrated with Agrobacterium EHA105 carrying nLUC or cLUC fusion plasmids, which were equally mixed using a 1-ml needleless syringe. Three days after infiltration, the leaves were injected with 0.1 mM luciferin and kept in the dark for 10 min to quench autofluorescence. Images were captured using a Lumazone PyLoN1300B system and processed using ImageJ software.
CoIP assay and immunoblotting
The coIP assay was performed as described previously with some modifications (Wang et al., 2020; Shi et al., 2021). The full-length CDS of AvrPi9 (without the signal peptide) was cloned into the pYBA1132 vector digested by EcoRI and HindIII, and the full-length OsRGLG5 was cloned into the pHY35S-Flag vector digested by KpnI and SalI. The primers used are listed in Supplemental Table 1. OsRGLG5-Flag was transiently co-expressed with AvrPi9-GFP or empty GFP in N. benthamiana leaves through Agrobacterium-mediated infiltration (Wang et al., 2020). Total proteins were extracted with protein extraction buffer (150 mM NaCl, 25 mM Tris–HCl [pH 7.4], 1 mM EDTA, 5% glycerol, 1% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, and 1× Roche protease inhibitor cocktail). After centrifugation at 12 000 g for 10 min, the supernatant was precipitated with GFP-Trap (Chromotek, GTMA-20) according to the manufacturer’s instructions. The immunoprecipitated proteins and input control were detected by western blotting with primary antibodies, including anti-GFP antibody (HUABIO) and anti-Flag (Sigma, A8592), and secondary antibodies (Beyotime, A0208). Individual bands were detected with the ChemDoc Touch Imaging System (Bio-Rad).
Rice protoplast transient expression and subcellular localization assay
Rice protoplast transient expression assays were performed as described previously (He et al., 2016). In brief, etiolated rice seedlings grown on nutrient soil without light for approximately 10 days were used for the assays. The rice seedling sheaths were cut into 0.5-mm strips and immersed in enzyme solution (0.6 M mannitol, 0.5% Macerozyme R-10, 1.0% Cellulase RS, 1 mM CaCl2, 10 mM 2-(N-Morpholino)ethanesulfonic acid (MES), and 5 mM β-mercaptoethanol, pH 5.7) for approximately 4–5 h with gentle shaking (40–60 rpm, 28°C) in the dark. After digestion, the strips were washed with W5 solution (125 mM CaCl2, 154 mM NaCl, 2 mM MES, 5 mM KCl, and 0.5% glucose, pH 5.7) two times and filtered through 40-mm nylon mesh. After centrifugation at 1000 g for 3 min, the rice protoplasts were washed with W5 three times and finally resuspended in W5 solution with an adjusted concentration of 2 × 106 cells/ml.
After 5 μg of plasmid DNA was mixed with 100 μl of rice protoplasts in 2.0-ml centrifuge tubes, the same volume of polyethylene glycol (PEG) solution containing 40% (w/v) PEG 4000, 0.1 M CaCl2, and 0.2 M mannitol was added. The tube contents were gently mixed by inversion several times, then incubated for 20 min at room temperature without light. Finally, a double volume of W5 solution was added to the mixture and mixed gently to terminate the PEG-mediated transfection. After approximately 14–20 h of incubation at 28°C in the dark, the rice cells were collected by centrifugation at 1000 g for 3 min and resuspended in 100 μl of W5 solution. The GFP and mCherry fluorescence signals were detected using a Zeiss LSM 710 confocal laser scanning microscope. The primers used are listed in Supplemental Table 1.
In vitro ubiquitination assays
The full-length CDS of OsRGLG5 or OsRGLG5-dRING was amplified and cloned into the pMAL-C5X vector digested by EcoRI and PstI. The full-length CDS of OsRGLG5-H420Y was amplified by introducing point mutations in the primers and cloned into the pMAL-C5X vector through homologous recombination. The full-length CDS of AvrPi9 (without the signal peptide) was cloned into the pGEX-4T-1 vector digested with BamHI and EcoRI. The full-length CDS of AvrPia was cloned into pGEX-4T-1 digested with EcoRI. The primers used are listed in Supplemental Table 1. All the plasmids that were sequenced correctly were transformed into the E. coli BL21 strain to induce recombinant protein expression. Protein purification with GST (Yeasen, 20507ES10) and MBP (Yeasen, 20507ES10) tags was performed according to the manufacturer’s instructions.
The in vitro ubiquitination assays were performed as described previously with some modifications (Liao et al., 2017). The 30-μl reaction system contained 0.25 μg of human E1 (UBE1, Boston Biochem), 0.5 μg of human E2 (UBE2D3, Boston Biochem), 1.25 μg of HA-tagged ubiquitin (Boston Biochem), 1 μg of purified AvrPi9-GST or AvrPi9-GST as the substrate protein, and 1 μg of purified MBP-OsRGLG5 or MBP-OsRGLG5-dRING protein as E3 in the reaction buffer (25 mM MgCl2, 0.1 M Tris–HCl [pH 7.5], 10 mM ATP, and 2.5 mM dithiothreitol). After gentle inversion mixing and rapid centrifugation, the reaction mixtures were incubated in a 30°C water bath for 2 h. The reaction was stopped by adding 5× SDS protein loading buffer, and the samples were boiled at 95°C for 5 min. The samples were separated by 10% SDS‒PAGE. Polyubiquitin bands were detected by immunoblotting with anti-HA antibody (Transgen), followed by chemiluminescence detection with Super ECL Detection Reagent (Yeasen).
In vivo degradation assay
For the in vivo degradation assay, Agrobacterium EHA105 carrying AvrPi9-GFP or GFP plasmids and Agrobacterium EHA105 carrying OsRGLG5-dRING-Flag, OsRGLG5-H420Y-Flag, or OsRGLG5-Flag plasmids were cotransfected into N. benthamiana. The empty vector pYBA1133, which expresses RFP-HA protein, was cotransfected as an internal control. N. benthamiana leaves were harvested at 3 days post infiltration, and 50 μM MG132 or water (control) was injected into the corresponding leaves 16 h before sampling (Park et al., 2012, 2016). For the in vivo degradation assay in rice protoplasts, AvrPi9-pRTVc-GFP or pRTVc-GFP plasmids were co-transformed with RFP-pRTVc-MYC into protoplasts extracted from ∼10-day-old yellow seedlings of rice. Protoplasts were collected after transient transformation for about 20 h to extract total proteins for western blotting analysis. RFP-MYC served as an internal control. ImageJ software was used to calculate the band intensity. The primers used for vector construction are listed in Supplemental Table 1.
Phylogenetic analysis
A phylogenetic tree of OsRGLG5 family proteins in rice and Arabidopsis was generated using MEGA X (Kumar et al., 2018) with the neighbor-joining method and 1000 bootstrap replicates.
Funding
This project was supported by the National Natural Science Foundation of China (32171944 to Y.K.), the Chinese Academy of Agricultural Sciences under the Elite Youth program and the Agricultural Science and Technology Innovation Program, Youth Innovation Program of Chinese Academy of Agricultural Sciences (Y2023QC22), and the Key Projects of Zhejiang Provincial Natural Science Foundation (LZ23C130002).
Author contributions
Y.K. and J.Q. designed this work. Z.L. and J.Q. performed most of the experiments with support from Z.S., C.W., N.J., and H.S. Y.K. and Z.L. contributed to manuscript writing. J.Q., H.S., and N.J. contributed to manuscript editing. All authors read and approved the final manuscript.
Acknowledgments
We thank the Rice–Pathogen Interaction group of China National Rice Research Institute for helpful discussion and suggestions. We thank Prof. Yan Liang (Zhejiang University) for providing the pMAL-C5X vector and in vitro ubiquitination assay protocol. We thank Prof. Lei Yao (Beijing Agro-Biotechnology Research Center) for providing the pYBA1133 vector. We thank Prof. Zonghua Wang from Fujian Agriculture and Forestry University for useful discussion and suggestions. We thank Prof. Dongping Lu from Shanghai Jiao Tong University for useful discussion and suggestions.No conflict of interest is declared.
Published: May 11, 2023
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Supplemental information is available at Plant Communications Online.
Accession numbers
Gene sequences mentioned in this article can be found at The Rice Genome Project website (http://rice.uga.edu/) or The Arabidopsis Information Resource (TAIR, https://www.arabidopsis.org/) under the following accession numbers: OsRGLG1, LOC_Os01g68060; OsRGLG2, LOC_Os12g19030; OsRGLG3, LOC_Os01g73000; OsRGLG4, LOC_Os08g04130; OsRGLG5, LOC_Os06g40650; OsRGLG6, LOC_Os08g38600; AtRGLG1, AT3G01650; AtRGLG2, AT5G14420; AtRGLG3, AT5G63970; AtRGLG4, AT1G79380; AtRGLG5, AT1G67800.
Supplemental information
Data availability statement
All data generated or analyzed during the present study can be found within the manuscript and its supporting materials.
References
- Belda-Palazon B., Julian J., Coego A., Wu Q., Zhang X., Batistic O., Alquraishi S.A., Kudla J., An C., Rodriguez P.L. ABA inhibits myristoylation and induces shuttling of the RGLG1 E3 ligase to promote nuclear degradation of PP2CA. Plant J. 2019;98:813–825. doi: 10.1111/tpj.14274. [DOI] [PubMed] [Google Scholar]
- Cesari S., Thilliez G., Ribot C., Chalvon V., Michel C., Jauneau A., Rivas S., Alaux L., Kanzaki H., Okuyama Y., et al. The rice resistance protein pair RGA4/RGA5 recognizes the Magnaporthe oryzae effectors AVR-Pia and AVR1-CO39 by direct binding. Plant Cell. 2013;25:1463–1481. doi: 10.1105/tpc.112.107201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Zou Y., Shang Y., Lin H., Wang Y., Cai R., Tang X., Zhou J.M. Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol. 2008;146:368–376. doi: 10.1104/pp.107.111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M.C., Hsieh E.J., Chen J.H., Chen H.Y., Lin T.P. Arabidopsis RGLG2, functioning as a RING E3 ligase, interacts with AtERF53 and negatively regulates the plant drought stress response. Plant Physiol. 2012;158:363–375. doi: 10.1104/pp.111.189738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland C., Li X. Regulation of plant immunity by the proteasome. Int Rev Cel Mol Bio. 2019;343:37–63. doi: 10.1016/bs.ircmb.2018.06.004. [DOI] [PubMed] [Google Scholar]
- Deng Y., Ning Y., Yang D.L., Zhai K., Wang G.L., He Z. Molecular basis of disease resistance and perspectives on breeding strategies for resistance improvement in crops. Mol. Plant. 2020;13:1402–1419. doi: 10.1016/j.molp.2020.09.018. [DOI] [PubMed] [Google Scholar]
- Devanna B.N., Jain P., Solanke A.U., Das A., Thakur S., Singh P.K., Kumari M., Dubey H., Jaswal R., Pawar D., et al. Understanding the dynamics of blast resistance in rice-Magnaporthe oryzae interactions. J. Fungi. 2022;8:584. doi: 10.3390/jof8060584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagels Durand A., Iñigo S., Ritter A., Iniesto E., De Clercq R., Staes A., Van Leene J., Rubio V., Gevaert K., De Jaeger G., et al. The Arabidopsis iron-sulfur protein GRXS17 is a target of the ubiquitin E3 ligases RGLG3 and RGLG4. Plant Cell Physiol. 2016;57:1801–1813. doi: 10.1093/pcp/pcw122. [DOI] [PubMed] [Google Scholar]
- Fan J., Bai P., Ning Y., Wang J., Shi X., Xiong Y., Zhang K., He F., Zhang C., Wang R., et al. The monocot-specific receptor-like kinase SDS2 controls cell death and immunity in rice. Cell Host Microbe. 2018;23:498–510.e5. doi: 10.1016/j.chom.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S., Wang K., Ma T., Liang Y., Ma Z., Wu J., Xu Y., Zhou X. An evolutionarily conserved C4HC3-type E3 ligase regulates plant broad-spectrum resistance against pathogens. Plant Cell. 2022;34:1822–1843. doi: 10.1093/plcell/koac055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki K., Abe Y., Ito A., Saitoh H., Yoshida K., Kanzaki H., Kanzaki E., Utsushi H., Yamashita T., Kamoun S., Terauchi R. Rice Exo70 interacts with a fungal effector, AVR-Pii, and is required for AVR-Pii-triggered immunity. Plant J. 2015;83:875–887. doi: 10.1111/tpj.12934. [DOI] [PubMed] [Google Scholar]
- Gao M., He Y., Yin X., Zhong X., Yan B., Wu Y., Chen J., Li X., Zhai K., Huang Y., et al. Ca(2+) sensor-mediated ROS scavenging suppresses rice immunity and is exploited by a fungal effector. Cell. 2021;184:5391–5404.e17. doi: 10.1016/j.cell.2021.09.009. [DOI] [PubMed] [Google Scholar]
- Han J., Wang X., Wang F., Zhao Z., Li G., Zhu X., Su J., Chen L. The fungal effector Avr-Pita suppresses innate immunity by increasing COX activity in rice mitochondria. Rice. 2021;14 doi: 10.1186/s12284-021-00453-4. 12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F., Chen S., Ning Y., Wang G.L. Rice (Oryza sativa) protoplast isolation and its application for transient expression analysis. Curr. Protoc. Plant Biol. 2016;1:373–383. doi: 10.1002/cppb.20026. [DOI] [PubMed] [Google Scholar]
- Holbein J., Grundler F.M.W., Siddique S. Plant basal resistance to nematodes: an update. J. Exp. Bot. 2016;67:2049–2061. doi: 10.1093/jxb/erw005. [DOI] [PubMed] [Google Scholar]
- Ichimaru K., Yamaguchi K., Harada K., Nishio Y., Hori M., Ishikawa K., Inoue H., Shigeta S., Inoue K., Shimada K., et al. Cooperative regulation of PBI1 and MAPKs controls WRKY45 transcription factor in rice immunity. Nat. Commun. 2022;13:2397–2416. doi: 10.1038/s41467-022-30131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y., McAdams S.A., Bryan G.T., Hershey H.P., Valent B. Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J. 2000;19:4004–4014. doi: 10.1093/emboj/19.15.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.D.G., Vance R.E., Dangl J.L. Intracellular innate immune surveillance devices in plants and animals. Science. 2016;354:aaf6395. doi: 10.1126/science.aaf6395. [DOI] [PubMed] [Google Scholar]
- Kanzaki H., Yoshida K., Saitoh H., Fujisaki K., Hirabuchi A., Alaux L., Fournier E., Tharreau D., Terauchi R. Arms race co-evolution of Magnaporthe oryzae AVR-Pik and rice Pik genes driven by their physical interactions. Plant J. 2012;72:894–907. doi: 10.1111/j.1365-313X.2012.05110.x. [DOI] [PubMed] [Google Scholar]
- Kou Y., Tan Y.H., Ramanujam R., Naqvi N.I. Structure-function analyses of the Pth11 receptor reveal an important role for CFEM motif and redox regulation in rice blast. New Phytol. 2017;214:330–342. doi: 10.1111/nph.14347. [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. Mega X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Zhong S., Li G., Li Q., Mao B., Deng Y., Zhang H., Zeng L., Song F., He Z. Rice RING protein OsBBI1 with E3 ligase activity confers broad-spectrum resistance against Magnaporthe oryzae by modifying the cell wall defence. Cell Res. 2011;21:835–848. doi: 10.1038/cr.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Wang B., Wu J., Lu G., Hu Y., Zhang X., Zhang Z., Zhao Q., Feng Q., Zhang H., et al. The Magnaporthe oryzae avirulence gene AvrPiz-t encodes a predicted secreted protein that triggers the immunity in rice mediated by the blast resistance gene Piz-t. Mol Plant Microbe In. 2009;22:411–420. doi: 10.1094/Mpmi-22-4-0411. [DOI] [PubMed] [Google Scholar]
- Li W., Zhu Z., Chern M., Yin J., Yang C., Ran L., Cheng M., He M., Wang K., Wang J., et al. A natural allele of a transcription factor in rice confers broad-spectrum blast resistance. Cell. 2017;170:114–126.e15. doi: 10.1016/j.cell.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Liang T., Chi W., Huang L., Qu M., Zhang S., Chen Z.Q., Chen Z.J., Tian D., Gui Y., Chen X., et al. Bulked segregant analysis coupled with whole-genome sequencing (BSA-Seq) mapping identifies a novel pi21 haplotype conferring basal resistance to rice blast disease. Int. J. Mol. Sci. 2020;21:2162–2213. doi: 10.3390/ijms21062162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D., Cao Y., Sun X., Espinoza C., Nguyen C.T., Liang Y., Stacey G. Arabidopsis E3 ubiquitin ligase PLANT U-BOX13 (PUB13) regulates chitin receptor LYSIN MOTIF RECEPTOR KINASE5 (LYK5) protein abundance. New Phytol. 2017;214:1646–1656. doi: 10.1111/nph.14472. [DOI] [PubMed] [Google Scholar]
- Liu L., Zhang Y., Tang S., Zhao Q., Zhang Z., Zhang H., Dong L., Guo H., Xie Q. An efficient system to detect protein ubiquitination by agroinfiltration in Nicotiana benthamiana. Plant J. 2010;61:893–903. doi: 10.1111/j.1365-313X.2009.04109.x. [DOI] [PubMed] [Google Scholar]
- Liu M., Zhang S., Hu J., Sun W., Padilla J., He Y., Li Y., Yin Z., Liu X., Wang W., et al. Phosphorylation-guarded light-harvesting complex II contributes to broad-spectrum blast resistance in rice. Proc. Natl. Acad. Sci. USA. 2019;116:17572–17577. doi: 10.1073/pnas.1905123116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Ning Y., Zhang Y., Yu N., Zhao C., Zhan X., Wu W., Chen D., Wei X., Wang G.L., et al. OsCUL3a negatively regulates cell death and immunity by degrading OsNPR1 in rice. Plant Cell. 2017;29:345–359. doi: 10.1105/tpc.16.00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Zhang Q., Zhu Q., Liu W., Chen Y., Qiu R., Wang B., Yang Z., Li H., Lin Y., et al. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant. 2015;8:1274–1284. doi: 10.1016/j.molp.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Orbach M.J., Farrall L., Sweigard J.A., Chumley F.G., Valent B. A telomeric avirulence gene determines efficacy for the rice blast resistance gene Pi-ta. Plant Cell. 2000;12:2019–2032. doi: 10.1105/tpc.12.11.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan I.C., Tsai H.H., Cheng Y.T., Wen T.N., Buckhout T.J., Schmidt W. Post-transcriptional coordination of the Arabidopsis iron deficiency response is partially dependent on the E3 ligases RING DOMAIN LIGASE1 (RGLG1) and RING DOMAIN LIGASE2 (RGLG2) Mol. Cell. Proteomics. 2015;14:2733–2752. doi: 10.1074/mcp.M115.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.H., Chen S., Shirsekar G., Zhou B., Khang C.H., Songkumarn P., Afzal A.J., Ning Y., Wang R., Bellizzi M., et al. The Magnaporthe oryzae effector AvrPiz-t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen-associated molecular pattern-triggered immunity in rice. Plant Cell. 2012;24:4748–4762. doi: 10.1105/tpc.112.105429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.H., Shirsekar G., Bellizzi M., Chen S., Songkumarn P., Xie X., Shi X., Ning Y., Zhou B., Suttiviriya P., et al. The E3 ligase APIP10 connects the effector AvrPiz-t to the NLR receptor Piz-t in rice. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., van Wersch R., Zhang Y. Convergent and divergent signaling in PAMP-triggered Immunity and effector-triggered immunity. Mol Plant Microbe In. 2018;31:403–409. doi: 10.1094/Mpmi-06-17-0145-Cr. [DOI] [PubMed] [Google Scholar]
- Qu S., Liu G., Zhou B., Bellizzi M., Zeng L., Dai L., Han B., Wang G.L. The broad-spectrum blast resistance gene Pi9 encodes a nucleotide-binding site-leucine-rich repeat protein and is a member of a multigene family in rice. Genetics. 2006;172:1901–1914. doi: 10.1534/genetics.105.044891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S., Singh P.K., Gupta D.K., Mahato A.K., Sarkar C., Rathour R., Singh N.K., Sharma T.R. Analysis of Magnaporthe oryzae genome reveals a fungal effector, which is able to induce resistance response in transgenic rice line containing resistance gene, Pi54. Front. Plant Sci. 2016;7:1140–1216. doi: 10.3389/fpls.2016.01140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot C., Césari S., Abidi I., Chalvon V., Bournaud C., Vallet J., Lebrun M.H., Morel J.B., Kroj T. The Magnaporthe oryzae effector AVR1CO39 is translocated into rice cells independently of a fungal-derived machinery. Plant J. 2013;74:1–12. doi: 10.1111/tpj.12099. [DOI] [PubMed] [Google Scholar]
- Sharma B., Joshi D., Yadav P.K., Gupta A.K., Bhatt T.K. Role of ubiquitin-mediated degradation system in plant biology. Front. Plant Sci. 2016;7:806–808. doi: 10.3389/fpls.2016.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Meng S., Qiu J., Wang C., Shu Y., Luo C., Kou Y. MoWhi2 regulates appressorium formation and pathogenicity via the MoTor signalling pathway in Magnaporthe oryzae. Mol. Plant Pathol. 2021;22:969–983. doi: 10.1111/mpp.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Xiong Y., Zhang K., Zhang Y., Zhang J., Zhang L., Xiao Y., Wang G.L., Liu W. The ANIP1-OsWRKY62 module regulates both basal defense and Pi9-mediated immunity against Magnaporthe oryzae in rice. Mol. Plant. 2023;16:739–755. doi: 10.1016/j.molp.2023.03.001. [DOI] [PubMed] [Google Scholar]
- Shi X., Long Y., He F., Zhang C., Wang R., Zhang T., Wu W., Hao Z., Wang Y., Wang G.L., Ning Y. The fungal pathogen Magnaporthe oryzae suppresses innate immunity by modulating a host potassium channel. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Dangol S., Chen Y., Choi J., Cho Y.S., Lee J.E., Choi M.O., Jwa N.S. Magnaporthe oryzae effector AVR-Pii helps to establish compatibility by inhibition of the rice NADP-malic enzyme resulting in disruption of oxidative burst and host innate immunity. Mol. Cells. 2016;39:426–438. doi: 10.14348/molcells.2016.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone S.L., Hauksdóttir H., Troy A., Herschleb J., Kraft E., Callis J. Functional analysis of the RING-type ubiquitin ligase family of Arabidopsis. Plant Physiol. 2005;137:13–30. doi: 10.1104/pp.104.052423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M., Ning Y., Shu X., Dong B., Zhang H., Wu D., Wang H., Wang G.L., Zhou B. The Nup98 homolog APIP12 targeted by the effector AvrPiz-t is involved in rice basal resistance against Magnaporthe oryzae. Rice. 2017;10:5–11. doi: 10.1186/s12284-017-0144-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra R.D. The ubiquitin-26S proteasome system at the nexus of plant biology. Nat Rev Mol Cell Bio. 2009;10:385–397. doi: 10.1038/nrm2688. [DOI] [PubMed] [Google Scholar]
- Wang J., Wang R., Fang H., Zhang C., Zhang F., Hao Z., You X., Shi X., Park C.H., Hua K., et al. Two VOZ transcription factors link an E3 ligase and an NLR immune receptor to modulate immunity in rice. Mol. Plant. 2021;14:253–266. doi: 10.1016/j.molp.2020.11.005. [DOI] [PubMed] [Google Scholar]
- Wang R., You X., Zhang C., Fang H., Wang M., Zhang F., Kang H., Xu X., Liu Z., Wang J., et al. An ORFeome of rice E3 ubiquitin ligases for global analysis of the ubiquitination interactome. Genome Biol. 2022;23:154–221. doi: 10.1186/s13059-022-02717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Barnaby J.Y., Tada Y., Li H., Tör M., Caldelari D., Lee D.U., Fu X.D., Dong X. Timing of plant immune responses by a central circadian regulator. Nature. 2011;470:110–114. doi: 10.1038/nature09766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Pruitt R.N., Nürnberger T., Wang Y. Evasion of plant immunity by microbial pathogens. Nat. Rev. Microbiol. 2022;20:449–464. doi: 10.1038/s41579-022-00710-3. [DOI] [PubMed] [Google Scholar]
- Wang Y., Hou Y., Qiu J., Wang H., Wang S., Tang L., Tong X., Zhang J. Abscisic acid promotes jasmonic acid biosynthesis via a 'SAPK10-bZIP72-AOC' pathway to synergistically inhibit seed germination in rice (Oryza sativa) New Phytol. 2020;228:1336–1353. doi: 10.1111/nph.16774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Kou Y., Bao J., Li Y., Tang M., Zhu X., Ponaya A., Xiao G., Li J., Li C., et al. Comparative genomics identifies the Magnaporthe oryzae avirulence effector AvrPi9 that triggers Pi9-mediated blast resistance in rice. New Phytol. 2015;206:1463–1475. doi: 10.1111/nph.13310. [DOI] [PubMed] [Google Scholar]
- Wu Q., Zhang X., Peirats-Llobet M., Belda-Palazon B., Wang X., Cui S., Yu X., Rodriguez P.L., An C. Ubiquitin ligases RGLG1 and RGLG5 regulate abscisic acid signaling by controlling the turnover of phosphatase PP2CA. Plant Cell. 2016;28:2178–2196. doi: 10.1105/tpc.16.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X.J., Volk S., Ljung K., Mehlmer N., Dolezal K., Ditengou F., Hanano S., Davis S.J., Schmelzer E., Sandberg G., et al. Ubiquitin lysine 63 chain forming ligases regulate apical dominance in Arabidopsis. Plant Cell. 2007;19:1898–1911. doi: 10.1105/tpc.107.052035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Saitoh H., Fujisawa S., Kanzaki H., Matsumura H., Yoshida K., Tosa Y., Chuma I., Takano Y., Win J., et al. Association genetics reveals three novel avirulence genes from the rice blast fungal pathogen Magnaporthe oryzae. Plant Cell. 2009;21:1573–1591. doi: 10.1105/tpc.109.066324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Q., Zhai K., Yang D., Yang W., Wu J., Liu J., Pan W., Wang J., Zhu X., Jian Y., et al. An E3 ubiquitin ligase-BAG protein module controls plant innate immunity and broad-spectrum disease resistance. Cell Host Microbe. 2016;20:758–769. doi: 10.1016/j.chom.2016.10.023. [DOI] [PubMed] [Google Scholar]
- You X., Zhang F., Liu Z., Wang M., Xu X., He F., Wang D., Wang R., Wang Y., Wang G., et al. Rice catalase OsCATC is degraded by E3 ligase APIP6 to negatively regulate immunity. Plant Physiol. 2022;190:1095–1099. doi: 10.1093/plphys/kiac317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai K., Liang D., Li H., Jiao F., Yan B., Liu J., Lei Z., Huang L., Gong X., Wang X., et al. NLRs guard metabolism to coordinate pattern- and effector-triggered immunity. Nature. 2022;601:245–251. doi: 10.1038/s41586-021-04219-2. [DOI] [PubMed] [Google Scholar]
- Zhang C., Fang H., Shi X., He F., Wang R., Fan J., Bai P., Wang J., Park C.H., Bellizzi M., et al. A fungal effector and a rice NLR protein have antagonistic effects on a Bowman-Birk trypsin inhibitor. Plant Biotechnol. J. 2020;18:2354–2363. doi: 10.1111/pbi.13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., He D., Zhao Y., Cheng X., Zhao W., Taylor I.A., Yang J., Liu J., Peng Y.L. A positive-charged patch and stabilized hydrophobic core are essential for avirulence function of AvrPib in the rice blast fungus. Plant J. 2018;96:133–146. doi: 10.1111/tpj.14023. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wu Q., Ren J., Qian W., He S., Huang K., Yu X., Gao Y., Huang P., An C. Two novel RING-type ubiquitin ligases, RGLG3 and RGLG4, are essential for jasmonate-mediated responses in Arabidopsis. Plant Physiol. 2012;160:808–822. doi: 10.1104/pp.112.203422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Wu Q., Cui S., Ren J., Qian W., Yang Y., He S., Chu J., Sun X., Yan C., et al. Hijacking of the jasmonate pathway by the mycotoxin fumonisin B1 (FB1) to initiate programmed cell death in Arabidopsis is modulated by RGLG3 and RGLG4. J. Exp. Bot. 2015;66:2709–2721. doi: 10.1093/jxb/erv068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during the present study can be found within the manuscript and its supporting materials.