Abstract
Taxus leaves provide the raw industrial materials for taxol, a natural antineoplastic drug widely used in the treatment of various cancers. However, the precise distribution, biosynthesis, and transcriptional regulation of taxoids and other active components in Taxus leaves remain unknown. Matrix-assisted laser desorption/ionization–mass spectrometry imaging analysis was used to visualize various secondary metabolites in leaf sections of Taxus mairei, confirming the tissue-specific accumulation of different active metabolites. Single-cell sequencing was used to produce expression profiles of 8846 cells, with a median of 2352 genes per cell. Based on a series of cluster-specific markers, cells were grouped into 15 clusters, suggesting a high degree of cell heterogeneity in T. mairei leaves. Our data were used to create the first Taxus leaf metabolic single-cell atlas and to reveal spatial and temporal expression patterns of several secondary metabolic pathways. According to the cell-type annotation, most taxol biosynthesis genes are expressed mainly in leaf mesophyll cells; phenolic acid and flavonoid biosynthesis genes are highly expressed in leaf epidermal cells (including the stomatal complex and guard cells); and terpenoid and steroid biosynthesis genes are expressed specifically in leaf mesophyll cells. A number of novel and cell-specific transcription factors involved in secondary metabolite biosynthesis were identified, including MYB17, WRKY12, WRKY31, ERF13, GT_2, and bHLH46. Our research establishes the transcriptional landscape of major cell types in T. mairei leaves at a single-cell resolution and provides valuable resources for studying the basic principles of cell-type-specific regulation of secondary metabolism.
Key words: scRNA-seq, taxol biosynthesis, Taxus mairei, secondary metabolism, transcription regulation
This study reports the visualization of various secondary metabolites in T. mairei leaf sections using matrix-assisted laser desorption/ionization–mass spectrometry imaging analysis and shows that most taxol biosynthesis genes are expressed mainly in leaf mesophyll cells though single-cell sequencing of the Taxus leaf. A number of novel and cell-specific transcription factors were also identified, providing valuable resources for studying the cell-type-specific regulation of secondary metabolism.
Introduction
Taxol, a tetracyclic diterpenoid first isolated from Taxus brevifolia, is reported to be one of the most effective antitumor drugs (Wani et al., 1971). However, the slow growth of wild yew and the low taxol content of plant tissues cause shortages of taxol (Dang et al., 2017). Although the highest levels of taxoids are found in the stem bark, bark stripping is a destructive production method. As the commercial value of taxol increases, so does the destruction of Taxus trees (Yu et al., 2018). Taxus wallichiana var. mairei (T. mairei) is an evergreen tree from the family Taxaceae that is widely distributed in Southeast Asia (Wang et al., 2019b). Leaves and twigs of T. mairei are used for industrial extraction of taxol because they have sufficient biomass and are renewable.
The production of taxol involves a complex, 20-step biosynthetic pathway that begins with a diterpenoid precursor (Croteau et al., 2006; Yu et al., 2018). The first discrete process in the biogenesis of taxol is the construction of the taxane skeleton by taxadiene synthase (TS), which is a key, but not rate-limiting, enzyme in the pathway (Kui et al., 2022). Baccatin III is produced during the second part of the pathway and involves a series of cytochrome P450 taxoid hydroxylases, including 2α-, 5α-, 7β-, 9α-, 10β-, 13α-, and 14β-hydroxylases and 10-deacetylbaccatin III-10-O-acetyltransferase (DBAT) (Kaspera and Croteau, 2006). Then, baccatin III-3-amino,3-phenylpropanoyltransferase (BAPT) catalyzes the connection of a phenylalanine-derived C13 side chain to baccatin III, producing 3′-N-debenzoyl-2′-deoxytaxol. Finally, 3′-N-debenzoyl-2′-deoxytaxol is converted to paclitaxel by 3′-N-debenzoyl-2′-deoxytaxol-N-benzoyl transferase (DBTNBT) in the last step of taxol biosynthesis (Long et al., 2008).
Transcription factors (TFs) play important roles in the transcriptional regulation of taxol biosynthesis. Several MYB (TmMYB39, TcMYB29a, and TmMYB3), WRKY (TcWKRY33, TcWKRY8, and TcWKRY47), ERF (TcERF12 and TcERF15), and MYC (TcMYC2a) family members and their taxol biosynthesis–related target genes have been functionally identified in various Taxus species (Zhang et al., 2015, 2018; Yu et al., 2020, 2022; Chen et al., 2021; Cao et al., 2022). Mining novel TFs is therefore an effective way to accelerate the genetic improvement of Taxus trees.
During the past decade, a number of taxoids have been isolated and identified from Taxus leaves. New taxane diterpenoids such as 9,13-diacetyltaxumairol W, 10,13-dibenzoyltaxacustin, 7,13-diacetylwallifoliol, 7,13-dibenzoylwallifoliol, and 7,9-dibenzoyltaxumairol P, have been isolated from Taxus sumatrana leaves (Shen et al., 2002). Three novel taxanes, namely baccatin VIII, baccatin IX, and baccatin X, were isolated from the leaves of Taxus yunnanensis (Hai et al., 2014). Seven taxanes, taxinine, taxinine A, taxinine B, 2-deacetoxytaxinine B, taxacin, taxchinin B, and taxol, were isolated as antiplatelet components from the leaves of Taxus cuspidata (Kim and Yun-Choi, 2010).
Recently, the spatial distribution of active components in medicinal plants has been a research focus. For example, the spatial distribution of alkaloid components of sacred lotus leaves has been investigated (Liu et al., 2021b). In Arabidopsis, the distributions of flavonol glycosides, fatty acids, fatty acid esters, galactolipids, and glycosphingolipids vary significantly between different leaf substructures (Hieta et al., 2021). The spatial distribution of active alkaloids, coumarins, sugars, and organic acids has been visualized in Clausena lansium leaves (Tang et al., 2021). Data on the spatial distribution of secondary metabolites provide guidance for sampling and help to reveal the roles of metabolites in plant leaves.
In vascular plants, leaves, the primary sites of light acquisition and carbon fixation, harbor a number of cell types organized into different tissues (Kalve et al., 2014). Because different cell types carry nearly identical genetic information, variations in metabolism are controlled by transcriptional regulation during the process of cell differentiation (Wang et al., 2021). Newly developed single-cell RNA-sequencing (scRNA-seq) techniques enable the transcriptome profiles of individual cells to be characterized. In plants, the application of scRNA-seq has been limited to a few plant species, depending on the protoplast preparation and availability of the genome sequence (Liu et al., 2022). scRNA-seq provides a transcriptomic atlas, defining various developmental processes, including inflorescence development, mesophyll cell development, and xylem formation (Li et al., 2021a; Tao et al., 2022; Zong et al., 2022). Recently, scRNA-seq techniques have been used to uncover novel secondary metabolite biosynthetic pathways. For example, scRNA-seq revealed the biosynthetic pathway of a floral scent in Nicotiana attenuata corolla cells and a novel metabolic pathway of catechin esters in tea leaves (Wang et al., 2022a; Kang et al., 2022). Thus, scRNA-seq can be used extensively to reveal distinct gene expression patterns within heterogeneous cell populations.
Mass spectrometry (MS) imaging and scRNA-seq were performed in this study to clarify the cell-type specificity of secondary metabolite biosynthesis and accumulation. Matrix-assisted laser desorption/ionization (MALDI) imaging MS analysis was used to visualize various secondary metabolites in leaf sections, confirming the tissue-specific accumulation of different active metabolites. scRNA-seq analysis can provide a metabolic profile of T. mairei leaves at the single-cell level. The identification of cell-type-specific TFs may strengthen our understanding of the transcriptional regulation of secondary metabolism in T. mairei leaves. Our study provides novel insights into the tissue-specific accumulation and regulation of secondary metabolites in medicinal plants.
Results
Overview of leaf data from timsTOF fleX MALDI-2
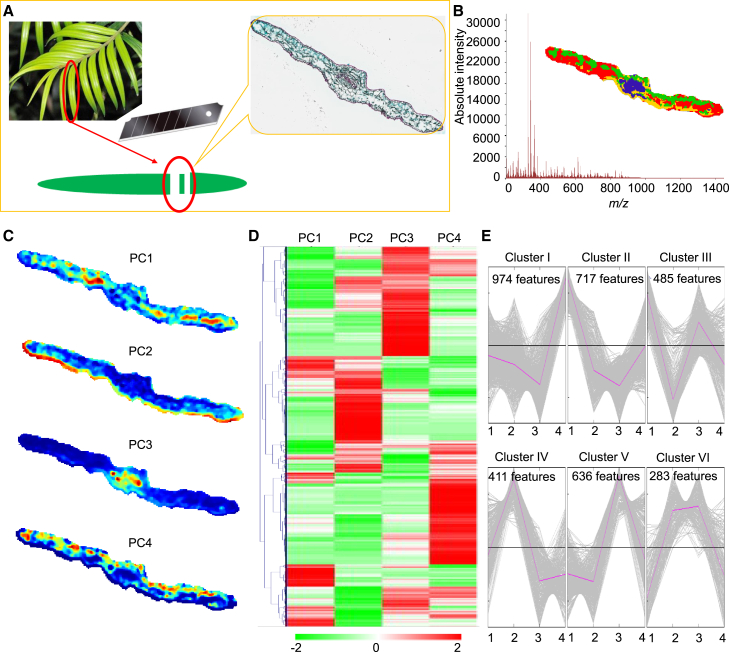
timsTOF fleX MALDI-2 was performed to detect the accumulation pattern of active ingredients in T. mairei leaves (Figure 1A and Supplemental Figure 1). The raw MS imaging data were imported into a SCiLS Lab workstation for MS feature processing, including baseline deduction, peak alignment, data smoothing, and normalization (Supplemental Table 1). Segmentation analysis showed that all detected features were located on a colored leaf map with 3510 points. The map separated the features into different locations, suggesting that the active ingredients accumulated in tissue-specific patterns (Figure 1B). All detected features were further separated into four principal components (PCs) associated with different known leaf tissues: phloem and spongy mesophyll cells (SM, PC1), epidermal cells (EP, PC2), bundle sheath cells (BS, PC3), and palisade mesophyll cells (PM, PC4) (Figure 1C).
Figure 1.
Analysis of data from timsTOF fleX MALDI-2.
(A) Sampling and sectioning strategy for spatial metabolomic analysis.
(B) Segmentation analysis produced a colored map with different data points.
(C) PCA of all detected metabolites in T. mairei leaves. Four PCs indicate different accumulation patterns of detected metabolites in leaves.
(D) Heatmap showing the relative accumulation levels of each detected metabolite in four PCs. The heatmap scale ranges from −2 to +2 on a log2 scale.
(E) Clustering analysis grouped all the detected MS features into six clusters.
The loading intensities of all features were displayed using a heatmap (Figure 1D and Supplemental Table 2). MeV clustering analysis grouped all features into six clusters. Cluster I contained 974 features highly accumulated in PM cells; Clusters II and III contained 717 and 485 features significantly accumulated in SM cells; Cluster IV contained 411 features mainly accumulated in EP cells; Cluster V contained 636 features predominantly accumulated in BS cells; and Cluster VI contained 283 features highly accumulated in both EP and BS cells (Figure 1E).
Visualization of active ingredient locations in the T. mairei leaf
We detected numerous active ingredients, including heterocycles, steroids, coumarins, phenolics, terpenes, glycosides, and taxoids, in the leaves of T. mairei. Four typical active compounds from each major class were visualized with color maps (Figure 2A). The flavonoids ginkgetin, quercetin, and rutin were mainly distributed in EP cells, and (R)-glabridin accumulated uniformly in the four tissue groups. The steroids crustecdysone, cortisol, and ponasterone A were mainly distributed in BS cells, and schottenol accumulated to a high level in BS and PM cells. Four selected coumarin metabolites, bergaptol, coumestan, coumesterol, and dicumarol, were detected at high levels in EP cells but rarely in BS and PM cells. The phenolics (−)-gossypol and theaflavin were detected only in BS cells; procyanidin accumulated to high levels in SM and BS cells, and xenognosin A accumulated to significant levels in SM cells. The terpenes carnosol, kahweol, and lactaroviolin were mainly distributed in SM cells, whereas squalene was mostly distributed in PM cells. The glycosides chrysoeriol 7-O-neohesperidoside and glyvenol accumulated to high levels in PM cells, and digitalin and pseudojervine accumulated evenly in BS, SM, and PM cells. The taxoids 10-deacetyl cephalomannine, 10-deacetyl paclitaxel, baccatin III, and 10-deacetyl baccatin III accumulated mainly in SM cells (Figure 2B). Detailed location information for selected active ingredients in the T. mairei leaf is provided in Supplemental Table 3.
Figure 2.
Visualization of taxoid accumulation in T. mairei leaves.
(A) The tissue-specific distribution of various typical metabolites, including four flavonoids, four steroids, four coumarins, four phenolics, four terpenes, four glycosides, and four taxoids, was detected by MALDI–imaging MS analysis. The color scale ranges from 0% to 100%.
(B) The proportions of various typical metabolites in different leaf tissues, including spongy mesophyll cells, epidermal cells, bundle sheath cells, and palisade mesophyll cells.
scRNA-seq of T. mairei leaves
Leaf protoplasts were isolated to produce single cells (Figure 3A). Approximately 1500 protoplasts/μl were isolated from the leaves of T. mairei. The basic experimental procedures for library construction and scRNA-seq are shown in Figure 3B. The purified protoplasts were processed using a commercial 10× Chromium platform. The number of effective cells was evaluated using barcodes and unique molecular identifier (UMI) counts (Figure 3C). scRNA-seq data were produced from a pool of 8846 cells with an average of 35 874 reads and 2352 genes per cell (Figure 3D). t-distributed stochastic neighbor embedding (t-SNE) projections of cells are shown as colored UMI counts (Figure 3E). Expression data for 36 808 genes were imported into Seurat Loupe software, and a total of 15 552 cluster-enriched genes were identified (Supplemental Table 4). On the basis of cluster-enriched genes, all cells were grouped into 15 clusters with uneven cell numbers (Supplemental Figure 2A). Cluster 0 contained the largest number of cells (1180 cells), and Cluster 14 contained the fewest (90 cells) (Supplemental Figure 2B). The t-SNE projection of cells from each cluster is shown in Supplemental Figure 2C. Three representative cluster-enriched genes for each cell cluster are shown in Figure 3F.
Figure 3.
scRNA-seq and cell clustering of T. mairei leaves.
(A) Isolation of protoplasts from leaf tissues.
(B) Workflow for scRNA-seq of T. mairei leaves. Protoplasts isolated from leaves were loaded into a 10× Genomics Chromium controller.
(C) The number of effective cells was quantified using barcodes and UMI counts.
(D) Numbers of cells, reads, and genes.
(E) t-SNE projection of cells colored by UMI counts.
(F) Expression patterns of three representative cluster-enriched genes from each cluster. Dot diameter represents the proportion of cluster cells that expressed a given gene.
(G) Expression patterns of 11 leaf tissue–specific marker genes in 15 different clusters.
(H) Visualization of 15 cell clusters using the t-SNE method. Dots indicate individual cells, and colors indicate different cell clusters.
Cell-type markers and cluster annotation of leaf cells
A number of cell-type marker genes for the leaf have been identified in model plants (Liu et al., 2022). By sequence alignment, several Arabidopsis leaf cell-type marker genes were used to annotate the cell types (Supplemental Table 5). Here, 11 cell-type marker genes were used to annotate the T. mairei leaf cell types: 1-AMINO-CYCLOPROPANE-1-CARBOXYLIC ACID OXIDASE 3 (ACO3, ctg2007-gene.6), expressed specifically in BS and vein cells, was enriched in Cluster 3; LIGHT HARVESTING COMPLEX PHOTOSYSTEM II SUBUNIT 6 (LHCB6, ctg18492_gene.1) and CHLOROPHYLL A/B BINDING PROTEIN 3 (CAB3, ctg8753_gene.1), expressed specifically in leaf mesophyll cells, were enriched in Clusters 6 and 7; FOUR LIPS (FLP, ctg9019_gene.2) and GLABRA 2 (GL2, ctg9664_gene.1), expressed specifically in leaf stomatal complex cells, were enriched in Clusters 8 and 12; PHLOEM INTERCALATED WITH XYLEM (PXY, ctg3593_gene.5), expressed specifically in vascular/procambium cells, was enriched in Cluster 9; ALTERED PHLOEM DEVELOPMENT (APL, ctg8447_gene.3), expressed specifically in phloem cells, was enriched in Cluster 14; MAP KINASE 9 (MAPK9, ctg4435_gene.11), expressed specifically in leaf guard cells, was enriched in Clusters 8 and 12; SMALLER TRICHOMES WITH VARIABLE BRANCHES 2 (SVB2, ctg4709_gene.1) and ECERIFERUM 3 (CER3, ctg560_gene.1), expressed specifically in leaf epidermal cells, were enriched in Cluster 12; and RIBULOSE BISPHOSPHATE CARBOXYLASE SMALL CHAIN 1A (RBCS1A, ctg596-gene.10), expressed specifically in leaf pavement cells, was enriched in Cluster 13 (Figure 3G). Our data revealed the high degree of cell heterogeneity in T. mairei leaves.
On the basis of the cell-type marker genes, most of the cell clusters were annotated. Cluster 3 contained BS cells and vein cells; Clusters 6 and 7 contained leaf mesophyll cells; Clusters 8 and 12 contained leaf stomatal complex cells, guard cells, and EP cells; Cluster 9 contained vascular cells and procambium cells; and Cluster 13 contained leaf pavement cells (Figure 3H).
Cell-type specificity of taxol biosynthesis in T. mairei leaves
A number of key enzymes are reported to participate in the taxol biosynthesis pathway (Croteau et al., 2006). The scRNA-seq analysis detected many taxol biosynthesis–related genes, including three TS genes, four T5OH genes, four T13OH genes, seven TAT genes, four T10OH genes, five TBT genes, six DBBT genes, two BAPT genes, one DBAT gene, three DBTNBT genes, three PAL genes, three T7OH genes, and one T14OH gene (Figure 4A). The cell coverage of gene expression was calculated to identify the major genes. Of the TS genes, ctg6088_gene.1 displayed the largest cell coverage (7.85%); of the T5OH genes, ctg7747_gene.2 was expressed in more than 5% of cells (8.17%); of the T13OH genes, ctg593_gene.6 had the largest cell coverage (5.37%); of the TAT genes, ctg195_gene.31 showed the largest cell coverage (13.48%); of the T10OH genes, ctg2120_gene.5 displayed the largest cell coverage (5.51%); of the TBT genes, ctg6463_gene.2 showed the largest cell coverage (6.67%); of the DBBT genes, ctg12512_gene.2 had the largest cell coverage (12.47%); of the BAPT genes, ctg5183_gene.4 displayed the largest cell coverage (3.96%); of the DBTNBT genes, ctg887_gene.16 showed the largest cell coverage (22.48%); of the PAL genes, ctg965_gene.9 showed the largest cell coverage (15.0%); and of the T7OH genes, ctg2120_gene.2 showed the largest cell coverage (4.79%) (Figure 4B).
Figure 4.
Cell-type specificity of taxol biosynthesis in T. mairei leaves.
(A) Overview of the paclitaxel biosynthesis pathway, including enzymes and metabolites.
(B) Cell coverage of taxol biosynthesis pathway–related genes. Each key enzyme is encoded by at least one gene.
(C) Cell-specific expression analysis of taxol biosynthesis–related genes. Clusters 6 and 7 contain mesophyll cells.
(D) Successive differentiation trajectory during the process of mesophyll tissue development.
(E) Heatmap showing expression levels of taxol-related genes during the mesophyll tissue development process. The heatmap scale ranges from +3 to −3 on a log2 scale.
(F) All leaf mesophyll cells were re-clustered into four subclasses, A to D. The red color indicates the expression level, and the dot size indicates the cell percentage.
Expression analysis showed that most of the taxol biosynthesis–related genes were expressed in the leaf mesophyll cells (Clusters 6 and 7). However, expression of T10OH (ctg2120_gene.5), TBT (ctg6463_gene.2), DBBT (ctg4356_gene.1), and DBTNB (ctg887_gene.16) was detected in the BS and vein cells (Cluster 3) (Figure 4C). Uniform manifold approximation and projection (UMAP) visualization of the expression patterns of 12 major taxol-related genes is shown in Supplemental Figure 3. The successive differentiation trajectory was calculated to reveal the gene expression pattern during the development of leaf mesophyll tissue. The pseudotime trajectory has five branchpoints, dividing all cells into six branches (Figure 4D). Our data suggested that most of the taxol biosynthesis pathway–related genes, such as DBAT (ctg10533_gene.2), T10OH (ctg2120_gene.10 and ctg5026_gene.32), TAT (ctg195_gene.31), and TS (ctg6088_gene.1), were expressed in mature leaf mesophyll cells. Only one gene, T14OH (ctg8816_gene.2), was expressed in young leaf mesophyll cells (Figure 4E).
Main population of cells involved in taxol biosynthesis
Because most taxol biosynthesis-related genes were expressed in the leaf mesophyll cells (Clusters 6 and 7) on the basis of specifically expressed marker genes, all of the leaf mesophyll cells were re-clustered into four subclasses, A to D (Supplemental Figure 4A). There were 168 cells in subclass A, 109 in subclass B, 103 in subclass C, and 79 in subclass D (Supplemental Figure 4B). UMAP visualization of the expression patterns of genes in subclasses A–D is shown in Supplemental Figure 4C. Interestingly, most of the taxol biosynthesis pathway genes were expressed in cells from subclasses C and D (Figure 4F). Cells in subclasses A and B were associated with young leaf mesophyll tissue and those in subclasses C and D with mature leaf mesophyll tissue. Thus, scRNA data indicated that taxol biosynthesis occurred in a mature population of mesophyll cells, subclasses C and D, in Clusters 6/7.
Enrichment analysis grouped the genes specifically expressed in Clusters 6/7 into different Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) terms. Two taxol biosynthesis–related terms, “paclitaxel metabolic process” (GO: 0042616, P = 1.75E−34) and “taxoid 14-beta-hydroxylase activity” (GO: 0036203, P = 3.04E−32), were identified as significantly enriched GO terms (Supplemental Figure 4D). One term related to terpenoid precursor biosynthesis, “terpenoid backbone biosynthesis” (map00900, P = 2.80E−7), was identified as a significantly enriched KEGG term (Supplemental Figure 4E).
Cell-specific expression pattern of the CYP superfamily
As a part of the classic taxol biosynthesis pathway, CYP-mediated oxygenation is required for modification of the taxane skeleton (Jennewein et al., 2003). On the basis of the public T. mairei genome, 487 CYP genes were identified, including 74 from the CYP725 subfamily (Supplemental Table 6). Notably, the CYP725 subfamily genes were enriched in Clusters 6/7 (Figure 5A). Re-clustering analysis showed that most of the CYP725 subfamily genes were expressed in cells from subclasses C and D of Clusters 6/7. The expression pattern of CYP725 subfamily genes showed high similarity to that of taxol biosynthesis–related genes at the single-cell level (Figure 5B). For example, ctg7747_gene.4, ctg7598_gene.6, ctg7598_gene.5, ctg11883_gene.1, ctg6028_gene.1, and ctg7747_gene.3 are Cluster 7–specific CYP725 genes, providing several candidate genes for improving the taxol synthesis pathway (Figure 5C and 5D). A pseudotime trajectory analysis showed that most of the CYP725 genes were expressed in the later stage of leaf mesophyll tissue development (Figure 5E).
Figure 5.
Expression patterns of the CYP superfamily.
(A) Cell-specific expression analysis of CYP family genes at the single-cell level by scRNA-seq. The blue box indicates that the CYP725 subfamily members were enriched in cells from Clusters 6 and 7.
(B) All leaf mesophyll cells were re-clustered into four subclasses, A to D. The red color indicates expression level, and the dot size indicates cell percentage.
(C) UMAP visualization of expression patterns of six selected CYP725 family members in T. mairei leaves.
(D) Violin plot of expression patterns of six selected CYP725 family members in T. mairei leaves.
(E) KEGG enrichment analysis of genes significantly expressed in Clusters 6 and 7.
Cell-type specificity for terpenoid and steroid metabolism
For terpenoid and steroid metabolism, three DXS genes, two DXR genes, one CMK gene, three MDS genes, two HDS genes, four GPPS genes, six GGPPS genes, one SQLE gene, two CYP51 genes, one EBP gene, and three DWF1 genes were identified by scRNA-seq (Supplemental Figure 5A and Supplemental Table 7). Expression of ctg7293_gene.1 (DXS), ctg895_gene.1 (DXR), ctg10290_gene.1 and ctg739_gene.2 (MDSs), ctg1206_gene.2 (GPPS), ctg1119_gene.5 (CYP51), and ctg5554_gene.1 (DWF1) was detected in more than 10% of cells (Supplemental Figure 5B). Most of the terpenoid and steroid biosynthesis–related genes were detected in Cluster 7 cells (Supplemental Figure 5C).
Expression analysis showed that most of the terpenoid and steroid biosynthesis pathway genes were expressed in cells from Clusters 6 and 7. According to the annotation information, terpenoid and steroid biosynthesis–related genes might be expressed in leaf mesophyll cells. The successive differentiation trajectory during the development of leaf mesophyll tissue is shown in Supplemental Figure 5D and 5E. Some terpenoid and steroid biosynthesis–related genes, such as ctg10290_gene.1 and ctg1119_gene.7, were expressed in young leaf mesophyll tissue, whereas others, such as ctg2545_gene.5 and ctg6176_gene.1, were expressed in mature leaf mesophyll tissue (Supplemental Figure 5F).
Cell-type specificity of phenolic acid and flavonoid metabolism
For phenolic acid metabolism, scRNA-seq detected several genes encoding three key enzymes, including three C4H genes, nine C3H genes, and six COMT genes (Supplemental Table 8). The cell coverage was calculated to identify the major genes involved in phenolic acid metabolism. Of the C4H genes, ctg2905_gene.4 displayed the largest cell coverage (14.84%); of the C3H genes, ctg6560_gene.3 showed the largest cell coverage (2.78%); and of the COMT genes, ctg15240_gene.2 showed the largest cell coverage (8.70%). For flavonoid metabolism, scRNA-seq detected three 4CL genes, eight CHS genes, one CHI gene, two F3H genes, and six FLS genes. Of the CHS genes, ctg3517_gene.14 had the largest cell coverage (4.66%); of the F3H genes, ctg17092_gene.1 displayed the largest cell coverage (4.51%); and of the FLS genes, ctg2147_gene.1 showed the largest cell coverage (6.01%) (Supplemental Figure 6A).
Expression analysis showed that most of the phenolic acid and flavonoid biosynthesis pathway genes were expressed in cells from Clusters 8, 11, and 12. According to their annotation information, phenolic acid and flavonoid biosynthesis–related genes may be expressed in leaf EP cells, stomatal complex cells, and guard cells (Supplemental Figure 6B).
Discovery of cell-specific TFs involved in the regulation of secondary metabolism
Although a number of TFs have been identified in Taxus species, information on the transcriptional regulation of secondary metabolism at the single-cell level is limited. The expression levels of all TF genes are listed in Supplemental Table 9. Because mesophyll tissue and leaf EP tissue are the main sites of secondary metabolism, several cell-cluster-specific TFs, including six MYB genes, five bHLH genes, four GT_2 genes, five ERF genes, and seven WRKY genes, were selected using the criteria log2(target cluster/other clusters) > 0.26 and P < 0.01 (Supplemental Table 10). The expression patterns of the above TF genes in all annotated clusters are shown in Figure 6A. To identify potential TFs involved in secondary metabolism, the 2000-bp promoter regions of 24 taxoid biosynthesis–related genes, 6 phenolic acid biosynthesis–related genes, 6 flavonoid metabolism–related genes, 13 terpenoid biosynthesis–related genes, and 5 steroid biosynthesis–related genes were extracted from the public T. mairei genome. Distributions of various cis-elements, such as MBE, GT element, W box, ABRE, and GCC box, in all selected promoters are shown in Figure 6B.
Figure 6.
Discovery of cell-specific TFs involved in secondary metabolism in T. mairei leaves.
(A) Cell-specific expression analysis of TFs at the single-cell level by scRNA-seq. The proportions of the expression levels of each TF in eight metabolism-related clusters are shown.
(B) Identification of TF-binding elements in the promoter regions of secondary metabolism–related genes. Triangles indicate the different TF-binding elements, including MBE (light blue), GT-element (pink), GCC-box (red), W-box (yellow), and ABRE (purple).
(C) Prediction of transcriptional regulation networks according to the cell-type-specific expression pattern.
Two criteria, similar expression patterns and matched binding elements, were used to screen the cell-specific TFs. For taxol biosynthesis, two MYB genes (ctg21726_gene.1 and ctg2891_gene.5), four GT_2 genes (ctg2396_gene.1, ctg3435_gene.2, ctg3885_gene.7, and ctg21853_gene.2), three bHLH genes (ctg18566_gene.1, ctg458_gene.1, and ctg183_gene.1), five WRKY genes (ctg3845_gene.3, ctg8684_gene.3, ctg3000_gene.1, and ctg1880_gene.5), and three ERF genes (ctg996_gene.4, ctg4461_gene.2, and ctg505_gene.15) were identified as potential regulators. Furthermore, a number of TFs were predicted to participate in various secondary metabolism pathways, and the regulatory network is shown in Figure 6C.
Verification of the binding of TFs to their potential targets
Six TFs, including MYB17 (ctg21726_gene.1), WRKY12 (ctg8684_gene.3), WRKY31 (ctg3845_gene.3), ERF13 (ctg996_gene.4), GT_2 (ctg3435_gene.2), and bHLH46 (ctg18566_gene.1), were randomly selected to verify the binding of cell-specific TFs to their target sites. The full-length sequences of these six TF genes were cloned and inserted into vectors. Electrophoretic mobility shift assay (EMSA) results showed that MYB17 bound directly to the MBE element in the TS promoter, WRKY12 bound directly to the W box in the DBAT promoter, WRKY31 bound directly to the W box in the SQE promoter, ERF13 bound directly to the GCC box in the C3H promoter, GT_2 bound directly to the GT element in the CHS promoter, and bHLH46 bound directly to the ABRE element in the GGPPS promoter (Figure 7A–7F).
Figure 7.
Verification of the binding of TFs to their potential targets.
(A–F) The GST-only or TF–GST fusion protein was incubated with probes containing the binding element derived from the promoters. − and + represent absence and presence, respectively, and 20× or 200× show increasing amounts of probes for competition.
(G) Overview of constructs prepared for dual-luciferase reporter assays. The promoter fragments were ligated to the pGreenII 0800-LUC vector to produce the reporters. The effectors were produced by inserting the TF genes into the pGreenII-62-SK vector.
(H) Dual-luciferase assays in tobacco leaves showed that co-transformation of TFs affected the target promoters.
(I) The cell-type locations in T. mairei leaves.
(J) The location of taxol biosynthesis–related genes.
(K) The location of terpenoid and steroid metabolism–related genes.
(L) The location of phenolic acid and flavonoid metabolism–related genes.
To determine the transcriptional activities of MYB17, WRKY12, WRKY31, ERF13, GT_2, and bHLH46, a dual-luciferase (LUC) reporter assay was performed using a tobacco transient expression system (Figure 7G). The results showed that WRKY12, WRKY31, GT_2, and ERF13 significantly activated the expression of their downstream target genes (DBAT, SQE, CHS, and C3H, respectively), whereas MYB17 and bHLH46 significantly inhibited the expression of their downstream target genes (TS and GGPPS, respectively) (Figure 7H).
Discussion
The complexity of biochemical processes in plant tissue is not only programmed by genetic information but also affected by the chemical’s localization. Thousands of secondary metabolites, including flavonoids, taxoids, polysaccharides, and abietane diterpenoids, have been detected in T. mairei leaves (Monacelli et al., 2002; Yang et al., 2016; Hao et al., 2017; Wang et al., 2019a). However, the precise locations of different types of secondary metabolites in T. mairei leaves are largely unknown.
High-throughput MALDI-2 imaging is a recently developed tool used to explore the spatial distribution patterns of chemicals in plant tissues (Bien et al., 2022). Mesophyll tissue is the main site of photosynthesis, which provides energy and raw materials for the biosynthesis of primary and secondary metabolites (Allahverdiyeva et al., 2015). Statistical results showed that the largest numbers of metabolites were enriched in SM (1202 metabolites) and PM (947 metabolites), suggesting that T. mairei mesophyll cell–related tissues are the main sites of active compound biosynthesis. Because of their different biological functions, various types of metabolites preferentially accumulated in specific tissues. For example, three classic flavonoids, ginkgetin, quercetin, and rutin, preferentially accumulated in EP cells, consistent with their roles in protecting plants against exposure to UV radiation and high light conditions (Ferreyra et al., 2021). MALDI-2 imaging further showed that several coumarins, including bergaptol and coumestan, accumulated at high levels in leaf EP cells. EP cell–accumulated coumarins play an important role in the response to nutrient-deficient conditions (Robe et al., 2021). Furthermore, most of the detected steroids, phenolics, and terpenes accumulated at high levels in SM cells, and most of the detected glycosides and taxoids accumulated in SM and PM cells. Our data provide detailed and precise location information for different types of active compounds to promote the comprehensive utilization of Taxus leaves.
T. mairei leaves are an important, renewable resource for the industrial extraction of active ingredients, especially taxoids (Li et al., 2021b). MALDI-2 imaging results confirmed distinct accumulation patterns of active ingredients in different cell types of the T. mairei leaf. The revolutionary application of 10× Genomics technology in plant scRNA-seq has enhanced the ability to analyze heterogeneity of gene expression at the single-cell level (Liu et al., 2022). To date, leaf single-cell transcriptional profiles have been reported for several plants, including Nepeta tenuifolia, Arabidopsis, rice, peanut, Brassica rapa, and tea (Liu et al., 2021a; Lopez-Anido et al., 2021; Wang et al., 2021, 2022a; Zhou et al., 2022a). Although the number of captured cells varied among these different experiments, the median number of genes per cell was less than 2000. In the present study, the median number of genes per cell was 2352, suggesting that sequencing depth was sufficient to screen valuable genes.
All T. mairei leaf cells were grouped into a number of cell clusters. Cell cluster annotation is difficult in medicinal plants owing to a lack of marker genes. In model plants, many cell-type marker genes have been confirmed by in situ hybridization and reporter systems. For example, in situ hybridization confirmed that OsDEF7 (LOC_Os02g41904) is highly expressed in inner mesophyll cells and OsENOD93 (LOC_Os06g05000) is highly expressed in mature mesophyll cells (Wang et al., 2021); a reporter system confirmed that PbZIP9 is expressed specifically in leaf vasculature cells (Kim et al., 2021); and a GFP fluorescence system confirmed that AtMYS1 (AT2G38300) is specifically expressed in guard cells (Zhang et al., 2021). According to known markers (PCMDB database), 11 cluster-specific genes were used to annotate cell types covering the major tissues in T. mairei leaves. Because of a lack of reliable marker genes, several large clusters could not be annotated with known marker genes. Establishing a rapid genetic transformation system and in situ hybridization system will be essential for improving cell annotation in T. mairei in the future.
The biosynthesis of endogenous active components involves a number of rate-limiting enzymes, which are encoded by multiple genes (Zhou et al., 2022b). In the present study, differences in the cell coverage of different encoding genes were carefully evaluated. TS catalyzes the formation of taxadiene, the first committed precursor of taxol (Croteau et al., 2006). Among the three TS genes, ctg5306_gene.4 and ctg7747_gene.1 were silent in most leaf cells. The important taxol rate-limiting enzyme BAPT is encoded by two genes (ctg5183_gene.3 and ctg5183_gene.4). Interestingly, the cell coverage of ctg5183_gene.4 was about 50-fold that of ctg5183_gene.3. TBT, which transforms the debenzoyl intermediate to 10-DAB, is another important rate-limiting enzyme involved in chemical modification of the taxoid core structure (Walker and Croteau, 2000). Among the five TBT genes, only ctg6463_gene.2 was expressed in more than 5% of leaf cells. Similarly, numerous silenced genes involved in the terpenoid, steroid, phenolic acid, and flavonoid metabolic pathways were identified. Increasing the cell coverage of the silenced genes is a potential way to promote efficiency in active compound biosynthesis.
Genetic improvement of medicinal plants requires the spatial screening of key metabolic genes (Wang et al., 2022b). Most taxol biosynthesis–related genes were expressed in the leaf mesophyll cells, and re-clustering analysis focused them into subclasses C and D of Clusters 6/7, suggesting that there is a specific cell group for taxol biosynthesis within the mesophyll cells. The terpenoid biosynthesis pathway provides the precursor GGPP for taxol synthesis in Taxus species (Croteau et al., 2006). The main site of terpenoid biosynthetic pathway gene expression is also the leaf mesophyll cells, guaranteeing a supply of precursors for taxol synthesis (Soliman and Tang, 2015). Carbon flux to steroid biosynthesis is a competitive pathway with terpenoid biosynthesis (Syklowska-Baranek et al., 2022). Clustering analysis showed that the main expression site for steroid biosynthesis–related genes was similar to that for terpenoid biosynthesis genes. Downregulation of terpenoid biosynthesis genes has been shown to be a useful approach to improving the production of taxol (Oksman-Caldentey and Inze, 2004). Flavonoids and phenolic acid derivatives are important photo-protective compounds and antioxidants (Casanova et al., 2020). Significantly, phenolic acid and flavonoid metabolism–related genes are specifically expressed in leaf EP cells and stomatal complex cells. The accumulation of phenolic acids and flavonoids in leaf EP cells might play a role in protection against the invasion of pathogenic microorganisms and damaging UV light on the front line (Schafer and Wink, 2009).
Plant secondary metabolism is coordinated by a complex TF network (Chen et al., 2020; Yu et al., 2020, 2021, 2022; Zhan et al., 2022; Feng et al., 2023). To date, several TFs, including stimulus-responsive TFs (such as TcERF12 and TcERF15), tissue-specific TFs (such as TmMYB3), and species-specific TFs (such as TmMYB39), have been functionally identified in various Taxus species (Zhang et al., 2015; Zhou et al., 2019; Yu et al., 2020, 2022). After a search of the T. mairei genome, a large number of TFs were annotated in the scRNA-seq dataset (Xiong et al., 2021). To screen novel TFs at a single-cell resolution, we proposed two screening criteria: cis-element-based potential physical binding capability and matched spatiotemporal expression patterns between the TF and its targets.
On the basis of these criteria, six MYBs, five bHLHs, four GT_2s, five ERFs, and seven WRKYs were predicted to participate in a regulatory network of secondary metabolism in T. mairei leaves. Our previous studies identified two MYB family members and their target genes. TmMYB39, a predominantly female-expressed TF, regulates the taxol biosynthesis pathway by targeting T10OH, T13OH, and TBT genes (Yu et al., 2022). TmMYB3, a phloem-specific TF, enhances taxol biosynthesis by binding to the promoters of TBT, DBTNBT, and TS (Yu et al., 2020). Our results suggested a new TcMYB17–TS pair, enriching our understanding of the role of the MYB family in taxol biosynthesis. In T. mairei, jasmonate-induced WRKY26 and salicylic acid–induced WRKY33 activated the expression of a DBAT gene by binding to the W box in its promoter region (Chen et al., 2021, 2022). In our study, WRKY12 was also identified as a regulator of DBAT expression, indicating that WRKY12 might be an important target of phytohormone-induced taxol biosynthesis.
In Taxus species, TFs involved in secondary metabolism other than taxol biosynthesis are largely unknown. SQE acts as a rate-limiting enzyme in the biosynthesis of steroidal compounds. Recently, two Asparagus racemosus bZIP family TFs (TGA1 and TGA2) and two Panax notoginseng AP2/ERF family TFs (ERF2 and ERF3) were considered to participate in the regulation of SQE expression (Lin et al., 2020; Upadhyay et al., 2020). In T. mairei, SQE (ctg680_gene.8) is highly expressed in mesophyll and stomatal complex cells, and its promoter contains multiple cis-elements, such as MBE, ABRE, and W box. Thus, mesophyll and stomatal complex cell–specific MYB, bHLH, and WRKY family TFs are potential regulators of the steroid biosynthesis pathway. In bean, the silencer region of the CHS15 promoter contains multiple binding sites for SBF1, a GT_1 like factor (Harrison et al., 1991). In T. mairei, the promoter of the CHS gene (ctg13079_gene.1) contains multiple GT_2 binding sites. In vitro and in vivo assays confirmed the role of epidermis-specific GT_2 in the activation of CHS expression, leading to the accumulation of flavonoids in EPs. The gene expression patterns were largely consistent with the location of compounds from each category.
Our research establishes a transcriptional landscape of the major cell types in T. mairei leaves at a single-cell resolution and provides a valuable resource for studying the basic principles of cell-type-specific regulation of secondary metabolism (Figure 7I–7K).
Methods
Plant materials and genome data
Five-year-old T. mairei trees were grown in an open field in the Cangqian campus of Hangzhou Normal University, Hangzhou, China. Leaves of young twigs were harvested for microsection and protoplasting. The T. mairei reference genome was downloaded from the Genome Sequence Archive at the National Genomics Data Center (Xiong et al., 2021).
Sample preparation and MS imaging
Young leaves of T. mairei were frozen and cut into 20-μm sections using a Leica CM1950 freezing microtome. The sections were transferred onto the MALDI-2 special conductive glass slide, vacuum dried under a stable stream of nitrogen, and transferred to −80°C for sealed storage. MALDI-2 special matrix solution containing 2,5-dihydroxybenzoic acid and 2,5-dihydroxyacetophenone was evenly sprayed on the slide with the leaf sections.
The coated conductive slide was placed on the target plate of the timsTOF fleX MALDI-2 system, and the detection area was screened using Bruker data imaging software. After setting the imaging resolution, the detection area was divided into several two-dimensional points according to the section size. The leaf section was scanned by laser radiation to detect the released ionized molecules at the target site. Details of the experimental setup and data processing are as follows: detection range of metabolite charge ratio, 150–1200 Da; ion detection mode, positive; MADLI-2, on; ion mobility, on; rise time, 300 ms; 1/K0 range, 0.6–1.7 V s/cm2; imaging resolution, 20 μm; laser frequency, 1000 Hz; laser energy, 44%; and acquisition times per pixel, 50.
MS imaging data processing
The MS data were loaded into SCiLS Lab MVS software (v.2021a Pro; Bruker Daltonics) to generate MS images. A reduced mass list of all peaks was stored in imzML format for further processing. The imaging pixels in the target area were clustered using the unsupervised spatial clustering analysis function of SCiLS software. Regions with similar accumulation patterns were marked with the same color to intuitively classify the slices from the molecular level. For metabolite annotation, MS spectrum data were extracted and quantified with a Bruker Metaboscape workstation. The resulting data were searched against the Bruker Library MS Metabase 3.0 database (https://www.bruker.com/en/products-and-solutions/mass-spectrometry/ms-software/metabolomics-spectral-libraries.html). Molecular mass error was set to <10 ppm. Principal-component analysis (PCA) was used to analyze the distribution of ion features on the imaging.
Preparation of Taxus leaf protoplasts
About 20 tender leaves of T. mairei were harvested and cut into small pieces. The leaf samples were treated with enzymolysis solution (cellulase R-10, isolation enzyme P, mannitol, KCl, and MES [pH 5.7]) in a triangular flask and vacuum infiltrated for 10 min. The leaf samples were incubated in darkness for 4–5 h at 28°C to isolate protoplasts. After reaching a certain number of protoplasts, the enzymolysis solution was removed. The isolated protoplasts were washed with 8% mannitol solution three times and filtered with a 40-μm cell sifter. Protoplast cell activity was detected by trypan blue staining. The dead cells were removed using a Miltenyi Dead Cell Removal Kit (MACS 130-090-101).
scRNA-seq library construction and raw data processing
For library construction, single-cell suspensions were loaded onto 10× Chromium according to the manufacturer’s instructions of the 10× Genomics Chromium Single-Cell 3 kit (V3). Libraries were sequenced on the Illumina NovaSeq 6000 sequencing system by LC-BioTechnology (Hangzhou, China) with a minimum depth of 20 000 reads per cell. The paired-end multiplexing run was set to 150 bp.
Sequencing results were downloaded and converted to FASTQ format using Illumina bcl2fastq software (v.2.20). Sample demultiplexing, barcode processing, and single-cell 3′ gene counting were performed with the Cell Ranger pipeline (v.6.1.1). scRNA-seq data were aligned to the T. mairei reference genome. Quality control parameters such as Q30, median UMI, and gene number per cell were determined using Cell Ranger (v.3.0.0) software and the Seurat software package (v.2.3.4). To remove low-quality cells, the data were filtered according to the average number of genes detected in a single cell. Specifically, cells with detected genes <500 and >5000, mitochondrial reads >5%, and mitochondrial reads >20% were considered to be doublets and were removed with DoubletFinder software (v.2.0.2).
Cell visualization and clustering
To visualize the data, the dimensionality of all remaining cells was reduced into 2D space using Seurat software. The expression levels of genes in each cell were calculated using the “Normalization” function in Seurat. The normalized expression values were used for PCA, and the top 10 PCs were used for clustering and t-SNE analysis with a weighted graph-based clustering method. The marker genes for each cell cluster were selected using the “FindAllMarkers” function in Seurat software with the Wilcoxon rank-sum test and likelihood-ratio test.
Tissue-specific marker genes and leaf cell-type identification
Orthologous gene alignments of reported leaf-related marker genes from the model plant Arabidopsis were used to identify cell-type-specific markers. The detailed sequences of Arabidopsis marker genes were downloaded from the Plant Cell Marker DataBase (http://www.tobaccodb.org/pcmdb/) (Jin et al., 2022). Using the Arabidopsis marker genes as query sequences, homologous T. mairei genes were identified using the BLASTP function in TBtools software. The top scoring hits were selected and annotated as corresponding T. mairei cell-type-specific genes.
Pseudotime trajectory analysis
To visualize the trajectory in a reduced dimensional space, all cells were ordered using Monocle software (v.3.0) with a matrix of cells and gene expression profiles. For pseudotime analysis, the sequence count was first converted into the “CellDataSet” with the “importCDS” function in Monocle 2. Then, we used the “estimateSizeFactors” and “estimateDispersions” functions to precalculate several parameters. The “Differential GeneTest” module in Monocle software was used to identify differentially expressed genes between different groups of cells. Dimensional reduction clustering analysis was performed using the “reduceDimension” function with default parameters. Then, gene expression was plotted to track changes during pseudotime according to a previously published method (Liu et al., 2022).
Electrophoretic mobility shift assay
The 2000-bp promoter sequences of various secondary metabolism–related genes were extracted from the T. mairei genome (Xiong et al., 2021). The promoter sequences were uploaded and scanned using the PlantCARE program (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
The cDNAs of MYB17, WRKY12, WRKY31, ERF13, GT_2, and bHLH46 were cloned and inserted into the pGEX4-T vector. The resulting vector was transformed into Escherichia coli (DE3) cells to produce recombinant TF-His tag protein. The recombinant protein induced by 1 mM isopropyl-beta-D-thiogalactopyranoside (IPTG) was purified with His60 Ni Superflow resin (Clontech, Beijing, China). The purified His fusion proteins were isolated by 12% SDS–PAGE and used for the EMSA experiment.
EMSA was performed according to the protocol of the Light Shift Chemiluminescent EMSA kit (Beyotime, Shanghai, China) (Yu et al., 2020). Two MYB binding elements (CAGTTG and CAACTG), two bHLH binding elements (CACGT and CACGTG), one WKRY binding element (TTGACC), two GT_2 binding elements (GGTAATT and GGTAAAT), and two ERF binding elements (GCCGCC and CCGAC) were used to design probes. Potential TF binding elements derived from the downstream promoters were labeled with 5′ 6-FAM fluorescent dye. Unlabeled sequences were used as competitive probes, and sequences with CCCGGG were used as mutated probes.
Dual-luciferase reporter assays
The cDNAs of several selected TF genes were cloned into the pGreenII62-SK vector as effectors, and the downstream promoter sequences were cloned into the pGreenII0800-LUC vector as reporters. The completed constructs were co-expressed in tobacco leaves via Agrobacterium tumefaciens GV3101–mediated transient transformation. The binding activities of TFs to the selected promoters were calculated from the LUC/REN ratio, which was determined using a dual-LUC assay kit (Promega, Beijing, China).
Bioinformatics and statistical analysis
GO and KEGG enrichment of genes with cell-specific expression was analyzed using the Blast2GO program (https://www.blast2go.com/). Statistical analysis was performed using SPSS software v.19.0 (SPSS, Chicago, IL, USA). Significance was determined at P < 0.05 by analysis of variance followed by Duncan’s least significant difference test. For hierarchical clustering and visualization, all data were Z-score normalized by Euclidean distance using MeV software. Two biological replicates were used for the MS imaging analysis, three technical replicates for the EMSA experiment, and three biological replicates for the dual-LUC assay.
Funding
This work was funded by the National Natural Science Foundation of China (32271905 and 32270382); the Zhejiang Provincial Natural Science Foundation of China under grants LY23C160001, LY18C050005, LY19C150005, and LY19C160001; the Opening Project of Zhejiang Provincial Key Laboratory of Forest Aromatic Plant-Based Healthcare Functions (2022E10008); the Open Foundation of State Key Laboratory of Subtropical Silviculture, Zhejiang A&F University (KF201708); the Major Increase or Decrease Program in The Central Finance Level (grant 2060302); and Zhejiang Provincial Key Research & Development Project grants (2017C02011, 2018C02030).
Author contributions
X.Z., S.F., H. Zeng, and C.S. conceptualized the initial study; C.S., H. Zhang, M.W., and Q.W. were involved in the experimental layout; H. Zhang, Q.W., T.Q., K.H., Z.W., C.C., X.W., X.L., and X.-l.L. performed the lab experiments; S.F., H.W., C.Y., and C.S. drafted the initial article; all authors discussed the results, reviewed the article, and approved the final article.
Acknowledgments
We are grateful to LC Sciences Co. (Hangzhou, China) for transcriptomic analysis. We are also grateful to Professor Jianbin Yan (Agricultural Genomics Institute at Shenzhen, Chinese Academy of Agricultural Sciences, Shenzhen, China) for genomic analysis. No conflict of interest is declared.
Published: May 25, 2023
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Supplemental information is available at Plant Communications Online.
Contributor Information
Huizhong Wang, Email: whz62@163.com.
Chenjia Shen, Email: shencj@hznu.edu.cn.
Supplemental information
Data availability
The T. mairei reference genome was downloaded from the NCBI database (NCBI: PRJNA730337). scRNA-seq data are available at the NCBI database (BioProject: PRJNA909435). The datasets generated and analyzed in the current study are available at Baidu Netdisk (https://pan.baidu.com/s/1hXL8X9bCF5ZLi4UGKXRlHQ [password: tjhg]).
References
- Allahverdiyeva Y., Suorsa M., Tikkanen M., Aro E.M. Photoprotection of photosystems in fluctuating light intensities. J. Exp. Bot. 2015;66:2427–2436. doi: 10.1093/jxb/eru463. [DOI] [PubMed] [Google Scholar]
- Bien T., Koerfer K., Schwenzfeier J., Dreisewerd K., Soltwisch J. Mass spectrometry imaging to explore molecular heterogeneity in cell culture. Proceedings of the National Academy of Sciences of the USA. 2022;119 doi: 10.1073/pnas.2114365119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Xu L., Li L., Wan W., Jiang J. TcMYB29a, an ABA-responsive R2R3-MYB transcriptional factor, upregulates Taxol biosynthesis in Taxus chinensis. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.804593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J.M., Dos Santos Nascimento L.B., Casanova L.M., Leal-Costa M.V., Costa S.S., Tavares E.S. Differential distribution of flavonoids and phenolic acids in leaves of kalanchoe delagoensis Ecklon & Zeyher (Crassulaceae) Microsc. Microanal. 2020;26:1061–1068. doi: 10.1017/S1431927620024344. [DOI] [PubMed] [Google Scholar]
- Chen L., Wu L., Yang L., Yu H., Huang P., Wang Y., Yao R., Zhang M. TcJAV3-TcWRKY26 cascade is a missing link in the jasmonate-activated expression of Taxol biosynthesis gene DBAT in Taxus chinensis. Int. J. Mol. Sci. 2022;23:13194. doi: 10.3390/ijms232113194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhang M., Jin X., Tao H., Wang Y., Peng B., Fu C., Yu L. Transcriptional reprogramming strategies and miRNA-mediated regulation networks of Taxus media induced into callus cells from tissues. BMC Genom. 2020;21:168. doi: 10.1186/s12864-020-6576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhang H., Zhang M., Zhang W., Ou Z., Peng Z., Fu C., Zhao C., Yu L. Salicylic acid-responsive factor TcWRKY33 positively regulates Taxol biosynthesis in Taxus chinensis in direct and indirect ways. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.697476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R., Ketchum R.E.B., Long R.M., Kaspera R., Wildung M.R. Taxol biosynthesis and molecular genetics. Phytochem. Rev. 2006;5:75–97. doi: 10.1007/s11101-005-3748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang P.H., Nguyen H.X., Duong T.T.T., Tran T.K.T., Nguyen P.T., Vu T.K.T., Vuong H.C., Phan N.H.T., Nguyen M.T.T., Nguyen N.T., Awale S. alpha-Glucosidase inhibitory and cytotoxic taxane diterpenoids from the stem bark of Taxus wallichiana. J. Nat. Prod. 2017;80:1087–1095. doi: 10.1021/acs.jnatprod.7b00006. [DOI] [PubMed] [Google Scholar]
- Feng S., Kailin H., Zhang H., Chen C., Huang J., Wu Q., Zhang Z., Gao Y., Wu X., Wang H., Shen C. Investigation of the role of TmMYB16/123 and their targets (TmMTP1/11) in the tolerance of Taxus media to cadmium. Tree Physiol. 2023 doi: 10.1093/treephys/tpad019. [DOI] [PubMed] [Google Scholar]
- Ferreyra M.L.F., Serra P., Casati P. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. Physiol. Plant. 2021;173:736–749. doi: 10.1111/ppl.13543. [DOI] [PubMed] [Google Scholar]
- Hai P., Wen S.Z., Li Y., Gao Y., Jiang X.J., Wang F. New taxane diterpenoids from Taxus yunnanensis. Nat. Prod. Bioprospect. 2014;4:47–51. doi: 10.1007/s13659-014-0003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J., Guo H., Shi X., Wang Y., Wan Q., Song Y.B., Zhang L., Dong M., Shen C. Comparative proteomic analyses of two Taxus species (Taxus × media and Taxus mairei) reveals variations in the metabolisms associated with paclitaxel and other metabolites. Plant Cell Physiol. 2017;58:1878–1890. doi: 10.1093/pcp/pcx128. [DOI] [PubMed] [Google Scholar]
- Harrison M.J., Lawton M.A., Lamb C.J., Dixon R.A. Characterization of a nuclear protein that binds to three elements within the silencer region of a bean chalcone synthase gene promoter. Proc. Natl. Acad. Sci. USA. 1991;88:2515–2519. doi: 10.1073/pnas.88.6.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieta J.P., Sipari N., Räikkönen H., Keinänen M., Kostiainen R. Mass spectrometry imaging of Arabidopsis thaliana leaves at the single-cell level by infrared laser ablation atmospheric pressure photoionization (LAAPPI) J. Am. Soc. Mass Spectrom. 2021;32:2895–2903. doi: 10.1021/jasms.1c00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennewein S., Rithner C.D., Williams R.M., Croteau R. Taxoid metabolism: taxoid 14beta-hydroxylase is a cytochrome P450-dependent monooxygenase. Arch. Biochem. Biophys. 2003;413:262–270. doi: 10.1016/s0003-9861(03)00090-0. [DOI] [PubMed] [Google Scholar]
- Jin J., Lu P., Xu Y., Tao J., Li Z., Wang S., Yu S., Wang C., Xie X., Gao J., et al. PCMDB: a curated and comprehensive resource of plant cell markers. Nucleic Acids Res. 2022;50:D1448–D1455. doi: 10.1093/nar/gkab949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalve S., De Vos D., Beemster G.T.S. Leaf development: a cellular perspective. Front. Plant Sci. 2014;5:362. doi: 10.3389/fpls.2014.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M., Choi Y., Kim H., Kim S.G. Single-cell RNA-sequencing of Nicotiana attenuata corolla cells reveals the biosynthetic pathway of a floral scent. New Phytol. 2022;234:527–544. doi: 10.1111/nph.17992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspera R., Croteau R. Cytochrome P450 oxygenases of Taxol biosynthesis. Phytochemistry reviews. Phytochem. Rev. 2006;5:433–444. doi: 10.1007/s11101-006-9006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.Y., Symeonidi E., Pang T.Y., Denyer T., Weidauer D., Bezrutczyk M., Miras M., Zöllner N., Hartwig T., Wudick M.M., et al. Distinct identities of leaf phloem cells revealed by single cell transcriptomics. Plant Cell. 2021;33:511–530. doi: 10.1093/plcell/koaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.Y., Yun-Choi H.S. A comparative optical aggregometry study of antiplatelet activity of taxanes from Taxus cuspidata. Thromb. Res. 2010;125:e281–e284. doi: 10.1016/j.thromres.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Kui L., Majeed A., Dong Y. Reference-grade Taxus genome unleashes its pharmacological potential. Trends Plant Sci. 2022;27:10–12. doi: 10.1016/j.tplants.2021.10.010. [DOI] [PubMed] [Google Scholar]
- Li H., Dai X., Huang X., Xu M., Wang Q., Yan X., Sederoff R.R., Li Q. Single-cell RNA sequencing reveals a high-resolution cell atlas of xylem in Populus. J. Integr. Plant Biol. 2021;63:1906–1921. doi: 10.1111/jipb.13159. [DOI] [PubMed] [Google Scholar]
- Li L., Chen Y., Ma Y., Wang Z., Wang T., Xie Y. Optimization of Taxol extraction process using response surface methodology and investigation of temporal and spatial distribution of Taxol in Taxus mairei. Molecules. 2021;26:5485. doi: 10.3390/molecules26185485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T., Du J., Zheng X., Zhou P., Li P., Lu X. Comparative transcriptome analysis of MeJA-responsive AP2/ERF transcription factors involved in notoginsenosides biosynthesis. 3 Biotech. 2020;10:290. doi: 10.1007/s13205-020-02246-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Hu D., Du P., Wang L., Liang X., Li H., Lu Q., Li S., Liu H., Chen X., et al. Single-cell RNA-seq describes the transcriptome landscape and identifies critical transcription factors in the leaf blade of the allotetraploid peanut (Arachis hypogaea L.) Plant Biotechnol. J. 2021;19:2261–2276. doi: 10.1111/pbi.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Shi K., Shi J., Feng Y., Hao C., Peng J., Chen S. A simple strategy to monitor the temporal and spatial distribution of alkaloids in sacred lotus leaves. Biosci. Biotechnol. Biochem. 2021;85:1332–1340. doi: 10.1093/bbb/zbab038. [DOI] [PubMed] [Google Scholar]
- Liu Z., Yu X., Qin A., Zhao Z., Liu Y., Sun S., Liu H., Guo C., Wu R., Yang J., et al. Research strategies for single-cell transcriptome analysis in plant leaves. Plant J. 2022;112:27–37. doi: 10.1111/tpj.15927. [DOI] [PubMed] [Google Scholar]
- Long R.M., Lagisetti C., Coates R.M., Croteau R.B. Specificity of the N-benzoyl transferase responsible for the last step of Taxol biosynthesis. Arch. Biochem. Biophys. 2008;477:384–389. doi: 10.1016/j.abb.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Anido C.B., Vatén A., Smoot N.K., Sharma N., Guo V., Gong Y., Anleu Gil M.X., Weimer A.K., Bergmann D.C. Single-cell resolution of lineage trajectories in the Arabidopsis stomatal lineage and developing leaf. Dev. Cell. 2021;56:1043–1055.e4. doi: 10.1016/j.devcel.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monacelli B., Pasqua G., Botta B., Vinciguerra V., Gacs-Baitz E., Monache G.D. Abietane diterpenoids from callus cultures of Taxus baccata. Planta Med. 2002;68:764–766. doi: 10.1055/s-2002-33802. [DOI] [PubMed] [Google Scholar]
- Oksman-Caldentey K.M., Inzé D. Plant cell factories in the post-genomic era: new ways to produce designer secondary metabolites. Trends Plant Sci. 2004;9:433–440. doi: 10.1016/j.tplants.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Robe K., Conejero G., Gao F., Lefebvre-Legendre L., Sylvestre-Gonon E., Rofidal V., Hem S., Rouhier N., Barberon M., Hecker A., et al. Coumarin accumulation and trafficking in Arabidopsis thaliana: a complex and dynamic process. New Phytol. 2021;229:2062–2079. doi: 10.1111/nph.17090. [DOI] [PubMed] [Google Scholar]
- Schäfer H., Wink M. Medicinally important secondary metabolites in recombinant microorganisms or plants: progress in alkaloid biosynthesis. Biotechnol. J. 2009;4:1684–1703. doi: 10.1002/biot.200900229. [DOI] [PubMed] [Google Scholar]
- Shen Y.C., Wang S.S., Pan Y.L., Lo K.L., Chakraborty R., Chien C.T., Kuo Y.H., Lin Y.C. New taxane diterpenoids from the leaves and twigs of Taxus sumatrana. J. Nat. Prod. 2002;65:1848–1852. doi: 10.1021/np0202273. [DOI] [PubMed] [Google Scholar]
- Soliman S., Tang Y. Natural and engineered production of taxadiene with taxadiene synthase. Biotechnol. Bioeng. 2015;112:229–235. doi: 10.1002/bit.25468. [DOI] [PubMed] [Google Scholar]
- Syklowska-Baranek K., Kaminska M., Paczkowski C., Pietrosiuk A., Szakiel A. Metabolic modifications in terpenoid and steroid pathways triggered by methyl jasmonate in Taxus × media hairy roots. Plants. 2022;11 doi: 10.3390/plants11091120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Zhao M., Chen Z., Huang J., Chen Y., Wang F., Wan K. Visualizing the spatial distribution of metabolites in Clausena lansium (Lour.) skeels using matrix-assisted laser desorption/ionization mass spectrometry imaging. Phytochemistry. 2021;192 doi: 10.1016/j.phytochem.2021.112930. [DOI] [PubMed] [Google Scholar]
- Tao S., Liu P., Shi Y., Feng Y., Gao J., Chen L., Zhang A., Cheng X., Wei H., Zhang T., Zhang W. Single-cell transcriptome and network analyses unveil key transcription factors regulating mesophyll cell development in Maize. Genes. 2022;13 doi: 10.3390/genes13020374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay S., Jeena G.S., Kumar S., Shukla R.K. Asparagus racemosus bZIP transcription factor-regulated squalene epoxidase (ArSQE) promotes germination and abiotic stress tolerance in transgenic tobacco. Plant Sci. 2020;290 doi: 10.1016/j.plantsci.2019.110291. [DOI] [PubMed] [Google Scholar]
- Walker K., Croteau R. Taxol biosynthesis: molecular cloning of a benzoyl-CoA:taxane 2alpha-O-benzoyltransferase cDNA from Taxus and functional expression in Escherichia coli. Proc. Natl. Acad. Sci. USA. 2000;97:13591–13596. doi: 10.1073/pnas.250491997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Wu Y., Peng A., Cui J., Zhao M., Pan Y., Zhang M., Tian K., Schwab W., Song C. Single-cell transcriptome atlas reveals developmental trajectories and a novel metabolic pathway of catechin esters in tea leaves. Plant Biotechnol. J. 2022;20:2089–2106. doi: 10.1111/pbi.13891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Zhang F., Zhuang W., Shu X., Wang Z. Metabolic variations of flavonoids in leaves of T. media and T. mairei obtained by UPLC-ESI-MS/MS. Molecules. 2019;24 doi: 10.3390/molecules24183323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Chen Y., Zhuang W., Zhang F., Shu X., Wang Z., Yang Q. Transcriptome sequencing reveals regulatory mechanisms of Taxol synthesis in Taxus wallichiana var. mairei. Int. J. Genomics. 2019;2019 doi: 10.1155/2019/1596895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Huan Q., Li K., Qian W. Single-cell transcriptome atlas of the leaf and root of rice seedlings. J Genet Genomics. 2021;48:881–898. doi: 10.1016/j.jgg.2021.06.001. [DOI] [PubMed] [Google Scholar]
- Wang Y., Gui C., Wu J., Gao X., Huang T., Cui F., Liu H., Sethupathy S. Spatio-temporal modification of lignin biosynthesis in plants: a promising strategy for lignocellulose improvement and lignin valorization. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.917459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani M.C., Taylor H.L., Wall M.E., Coggon P., McPhail A.T. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- Xiong X., Gou J., Liao Q., Li Y., Zhou Q., Bi G., Li C., Du R., Wang X., Sun T., et al. The Taxus genome provides insights into paclitaxel biosynthesis. Nat. Plants. 2021;7:1026–1036. doi: 10.1038/s41477-021-00963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Zheng Z.S., Cheng F., Ruan X., Jiang D.A., Pan C.D., Wang Q. Seasonal dynamics of metabolites in needles of Taxus wallichiana var. mairei. Molecules. 2016;21:1403. doi: 10.3390/molecules21101403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Luo X., Zhan X., Hao J., Zhang L., L Song Y.B., Shen C., Dong M. Comparative metabolomics reveals the metabolic variations between two endangered Taxus species (T. fuana and T. yunnanensis) in the Himalayas. BMC Plant Biol. 2018;18:197. doi: 10.1186/s12870-018-1412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Zhang C., Xu X., Huang J., Chen Y., Luo X., Wang H., Shen C. Omic analysis of the endangered Taxaceae species Pseudotaxus chienii revealed the differences in taxol biosynthesis pathway between Pseudotaxus and Taxus yunnanensis trees. BMC Plant Biol. 2021;21:104. doi: 10.1186/s12870-021-02883-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Huang J., Wu Q., Zhang C., Li X.L., Xu X., Feng S., Zhan X., Chen Z., Wang H., Shen C. Role of female-predominant MYB39-bHLH13 complex in sexually dimorphic accumulation of taxol in Taxus media. Hortic. Res. 2022;9:uhac062. doi: 10.1093/hr/uhac062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Luo X., Zhang C., Xu X., Huang J., Chen Y., Feng S., Zhan X., Zhang L., Yuan H., et al. Tissue-specific study across the stem of Taxus media identifies a phloem-specific TmMYB3 involved in the transcriptional regulation of paclitaxel biosynthesis. Plant J. 2020;103:95–110. doi: 10.1111/tpj.14710. [DOI] [PubMed] [Google Scholar]
- Zhan X., Chen Z., Chen R., Shen C. Environmental and genetic factors involved in plant protection-associated secondary metabolite biosynthesis pathways. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.877304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Li S., Nie L., Chen Q., Xu X., Yu L., Fu C. Two jasmonate-responsive factors, TcERF12 and TcERF15, respectively act as repressor and activator of tasy gene of taxol biosynthesis in Taxus chinensis. Plant Mol. Biol. 2015;89:463–473. doi: 10.1007/s11103-015-0382-2. [DOI] [PubMed] [Google Scholar]
- Zhang M., Jin X., Chen Y., Wei M., Liao W., Zhao S., Fu C., Yu L. TcMYC2a, a basic helix-loop-helix transcription factor, transduces JA-signals and regulates taxol biosynthesis in Taxus chinensis. Front. Plant Sci. 2018;9:863. doi: 10.3389/fpls.2018.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.Q., Chen Y., Wang J.W. A single-cell analysis of the Arabidopsis vegetative shoot apex. Dev. Cell. 2021;56:1056–1074.e8. doi: 10.1016/j.devcel.2021.02.021. [DOI] [PubMed] [Google Scholar]
- Zhou P., Chen H., Dang J., Shi Z., Shao Y., Liu C., Fan L., Wu Q. Single-cell transcriptome of Nepeta tenuifolia leaves reveal differentiation trajectories in glandular trichomes. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.988594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T., Luo X., Yu C., Zhang C., Zhang L., Song Y.B., Dong M., Shen C. Transcriptome analyses provide insights into the expression pattern and sequence similarity of several taxol biosynthesis-related genes in three Taxus species. BMC Plant Biol. 2019;19:33. doi: 10.1186/s12870-019-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Rao S., Wrightstone E., Sun T., Lui A.C.W., Welsch R., Li L. Phytoene synthase: the key rate-limiting enzyme of carotenoid biosynthesis in plants. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.884720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong J., Wang L., Zhu L., Bian L., Zhang B., Chen X., Huang G., Zhang X., Fan J., Cao L., et al. A rice single cell transcriptomic atlas defines the developmental trajectories of rice floret and inflorescence meristems. New Phytol. 2022;234:494–512. doi: 10.1111/nph.18008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The T. mairei reference genome was downloaded from the NCBI database (NCBI: PRJNA730337). scRNA-seq data are available at the NCBI database (BioProject: PRJNA909435). The datasets generated and analyzed in the current study are available at Baidu Netdisk (https://pan.baidu.com/s/1hXL8X9bCF5ZLi4UGKXRlHQ [password: tjhg]).