Abstract
Background
The acute cardiovascular and pulmonary effects of contemporary electronic nicotine delivery systems (ENDS) in long-term users are not known.
Research Question
What are the cardiovascular and pulmonary responses to an acute 15-min product use challenge with ENDS and combustible cigarettes in regular nicotine-containing product users compared with control participants who do not use tobacco or vape?
Study Design and Methods
Observational challenge study before and after nicotine-containing product use of 395 individuals who used ENDS exclusively (n = 164; exhaled carbon monoxide level, < 5 parts per million [ppm]; positive urine NicCheck I [Mossman Associates] results, 82%; fourth-generation ENDS), participants who smoked cigarettes exclusively (n = 117; carbon monoxide level, > 5 ppm; positive urine NicCheck I results), and control participants (n = 114; carbon monoxide level, < 5 ppm; negative urine NicCheck I results).
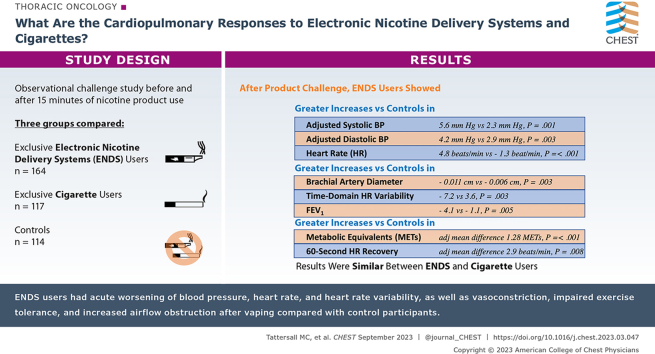
Results
During the 15-min product challenge, cigarette users took a median of 14.0 puffs (interquartile range [IQR], 9.3 puffs); ENDS users took 9.0 puffs (IQR, 7.5 puffs; P < .001). After product challenge, compared with control participants, ENDS users showed greater increases in adjusted mean differences in systolic BP (5.6 mm Hg [95% CI, 4.4-6.8 mm Hg] vs 2.3 mm Hg [95% CI, 0.8-3.8 mm Hg]; P = .001), diastolic BP (4.2 mm Hg [95% CI, 3.3-5.0 mm Hg] vs 2.0 mm Hg [95% CI, 1.1-3.0 mm Hg; P = .003), and heart rate (4.8 beats/min [95% CI, 4.0-5.6 beats/min] vs –1.3 beats/min [95% CI, –2.2 to –0.3 beats/min]; P < .001) and greater reductions in brachial artery diameter (–0.011 cm [95% CI, –0.013 to 0.009 cm] vs –0.006 cm [95% CI, –0.004 to –0.009 cm]; P = .003), time-domain heart rate variability (–7.2 ms [95% CI, –10.5 to –3.7 ms] vs 3.6 ms [95% CI, 1.6-9.3 ms]; P = .001), and FEV1 (ENDS: –4.1 [95% CI, –5.4 to –2.8] vs control participants: –1.1 [95% CI, –2.7 to 0.6]; P = .005) with values similar to those of cigarette users. ENDS users performed worse than control participants on all exercise parameters, notably metabolic equivalents (METs; adjusted mean difference, 1.28 METs [95% CI, 0.73-1.83 METs]; P < .001) and 60-s heart rate recovery (adjusted mean difference, 2.9 beats/min [95% CI, 0.7-5.0 beats/min]; P = .008).
Interpretation
ENDS users had acute worsening of blood pressure, heart rate, and heart rate variability, as well as vasoconstriction, impaired exercise tolerance, and increased airflow obstruction after vaping, compared to control participants.
Trial Registry
ClinicalTrials.gov; No.: NCT03863509; URL: www.clinicaltrials.gov
Key Words: autonomic function, cardiovascular risk, exercise stress testing, nicotine, pulmonary disease, risk factors, smoking, spirometry, vaping
Graphical Abstract
Take-home Points.
Study Question: What are the cardiovascular and pulmonary responses to an acute nicotine-containing product use challenge with electronic nicotine delivery systems (ENDS) and combustible cigarettes in regular users compared with control participants who neither smoke nor vape?
Results: Compared with control participants (n = 114), ENDS users (n = 164) showed acute, clinically relevant worsening of BP, heart rate, and heart rate variability, as well as vasoconstriction, impaired exercise tolerance, and increased airflow obstruction after using ENDS.
Interpretation: One episode of ENDS use is associated with acute worsening of cardiovascular and pulmonary health indexes among long-term ENDS users.
Electronic nicotine delivery system (ENDS) use in the United States is increasing rapidly, especially among youth and adults under age 25 years.1,2 In 2018, > 20% of high school-aged youths reported current ENDS use, and prevalence of ENDS use is highest among those 18 to 24 years of age.1,3 Furthermore, contemporary devices can achieve higher temperatures, which may increase delivery of nicotine and other toxins, potentially producing greater dependence and health risks.4,5 Despite the potential public health burden of ENDS use, human data defining the acute cardiovascular and pulmonary effects of ENDS use in a community-based population of long-term contemporary ENDS users are limited. Discovering the health effects of contemporary ENDS use is vital, given their high use prevalence among youth and young adults and the long latency of cardiovascular and pulmonary disease development. The Cardiac and Lung E-cig Smoking Study is a large, before-and-after nicotine-containing product challenge study designed to characterize comprehensively the cardiovascular and pulmonary effects of acute nicotine-containing product use among long-term product users. We evaluated differences in cardiovascular, pulmonary, and exercise stress test responses after use of ENDS by long-term ENDS users, use of combustible cigarettes by long-term cigarette users, and no product use among control participants who never used ENDS or combustible cigarettes.
Study Design and Methods
Design and Participants
The Cardiac and Lung E-cig Smoking Study (ClinicalTrials.gov Identifier: NCT03863509) is an observational, before-and-after nicotine-containing product challenge study that was approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board. Detailed recruiting procedures and inclusion and exclusion criteria are in e-Appendix 1. Three groups of participants were recruited from the community: exclusive ENDS users (exhaled carbon monoxide [CO] < 5 parts per million [ppm], positive urine nicotine results [NicCheck I; Mossman Associates]), exclusive combustible cigarette users (CO > 5 ppm, positive urine nicotine results), and control participants who neither vape nor smoke (CO < 5 ppm, negative urine nicotine results).
At visit 1, urine nicotine and cotinine and exhaled CO concentrations were measured to verify product use group, and demographic and anthropometric information was obtained. Product-use intensity was quantified for cigarette users in pack-years and for ENDS users in vape-years. Vape-years was calculated as the product of the number of uses of ENDS per day and the number of years divided by 10, which has been described as the average number of ENDS use episodes by ENDS users in 1 day.6 Eligible participants completed a second in-person visit approximately 7 days later, in the morning, having fasted and refrained from nicotine-containing product use for ≥ 8 h. This visit included repeat exhaled CO testing, laboratory testing, cardiovascular and pulmonary testing, and a nicotine-containing product challenge. Participants underwent the following sequential procedures: spirometry, measurement of fractional exhaled nitric oxide (Feno), measurement of exhaled CO, assessment of heart rate variability (HRV), arterial pulse wave analysis, carotid artery ultrasonography, brachial artery reactivity testing, assessment of standing HRV, and placement of an IV catheter for blood testing. Participants subsequently completed a maximum 15-min ad libitum product use challenge in a dedicated room with external ventilation, under remote supervision by two trained observers. ENDS users used their ENDS, cigarette users used their combustible cigarettes, and control participants rested. Immediately afterward, the following assessments were performed in this sequence: postchallenge questionnaire, spirometry, Feno, exhaled CO, blood draw for postchallenge plasma nicotine and cotinine levels, BP measurement, resting HRV simultaneous with brachial artery reactivity testing, and standing HRV. All tests were completed within a mean ± SD of 23.5 ± 2.7 min of the product use session. Participants then completed a treadmill stress test a mean ± SD of 91.3 ± 16.2 min after the product use challenge. ENDS generation was determined by a single reviewer using the Centers for Disease Control classification.7
Cardiovascular, Pulmonary, and Laboratory Testing
Details of all cardiovascular, pulmonary, and laboratory testing procedures are in e-Appendix 2. Carotid and brachial artery ultrasound were performed by a certified sonographer.8, 9, 10, 11, 12, 13, 14 Pulse wave analysis used radial artery tonometry to determine aortic augmentation index adjusted for an HR of 75 beats/min.15 Automated upper arm sphygmomanometry was performed in triplicate on an oscillometric system after a 5-min rest.10 HRV was measured in time and frequency domains.16 Spirometry followed American Thoracic Society guidelines.17, 18, 19 Feno was measured in on-line breath condensate using a rapid-response chemiluminescent analyzer.20, 21, 22 Exhaled CO was measured with an electrochemical sensor. The maximum exercise treadmill stress test used a modified Balke protocol according to American College of Sports Medicine Standards.23, 24, 25, 26 Peak metabolic equivalents (METs), rate-pressure product (peak heart rate [HR] × peak systolic BP), HR increase, HR reserve (maximum achieved HR / [220 – age in years]; expressed as %), and 60-s HR recovery (maximum HR minus HR at 60 s) were calculated. Fasting laboratory samples were collected as described previously.27
Statistical Methods
The analysis sample comprised participants attending both in-person visits (n = 395). All variables were summarized as mean ± SD or median (interquartile range [IQR]). Skewed variables were log-transformed before analyses. Baseline differences between groups in demographic and product use characteristics (Table 1)28 were tested using one-way analysis of variance or t tests for continuous variables and χ 2 tests for categorical measures.
Table 1.
Participant Characteristics
| Variable | Control Participants (n = 114) | ENDS Users (n = 164) | Cigarette Users (n = 117) | P Valuea (Group Effect) | Pairwise Comparisons |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, y | 30.8 ± 11.9 | 27.4 ± 10.6 | 42.8 ± 13.8 | < .001 | S > C > E |
| Female sex | 57 (50.0) | 64 (39.0) | 52 (44.4) | .190 | NA |
| Race or ethnicity | < .001 | ||||
| Black | 5 (4.4) | 4 (2.4) | 47 (11.9) | S > C, E | |
| White | 79 (69.3) | 141 (86.0) | 65 (55.6) | E > C > S | |
| Other | 30 (26.3) | 19 (11.6) | 14 (12.0) | C > E, S | |
| Nicotine-containing product use measurements | |||||
| Exhaled carbon monoxide, ppm | 0.00 (1.00) | 0.00 (1.00) | 18.00 (17.00) | < .001 | S > C, E |
| Serum cotinine, ng/mLb | 1.99 (0.00) | 179.00 (166.00) | 213.50 (157.00) | < .001 | S, E > C |
| Serum nicotine, ng/mLb | 1.99 (0.00) | 1.99 (1.01) | 1.99 (2.76) | < .001 | S > E > C |
| Urinary nicotine, ng/mL | 0.00 (0.00) | 4.00 (4.00) | 5.00 (4.00) | < .001 | S > E > C |
| Smoking history | |||||
| Years | 0.00 (0.00) | 0.00 (5.00) | 21.00 (24.00) | < .001 | S > E > C |
| Pack-y | 0.00 (0.00) | 0.00 (5.25) | 24.00 (25.00) | < .001 | S > E > C |
| Vaping history | |||||
| Years | 0.00 (0.00) | 4.00 (3.00) | 0.00 (0.00) | < .001 | E > S, C |
| Vaping-y | 0.00 (0.00) | 9.00 (11.00) | 0.00 (0.00) | < .001 | E > S, C |
| ENDS type | |||||
| Third generation | NA | 29 (18) | NA | NA | NA |
| Fourth generation | NA | 135 (82) | NA | NA | NA |
Data are presented as No. (%), mean ± SD, or median (interquartile range), unless otherwise indicated. For baseline measurements, we constructed a series of analyses of covariance models adjusted for age, sex, and race or ethnicity. To constrain the false discovery rate at 0.05, the Benjamini and Hochberg procedure was applied to the set of 24 tests of between-group differences before the product challenge in Table 2.28 C = control participants; E = electronic nicotine delivery system users; ENDS = electronic nicotine delivery system; NA = not available; ppm = parts per million; S = cigarette users.
Based on Kruskal-Wallis overall test for group differences in distribution for quantitative measures and χ2 tests for categorical measurements.
For these assays, the minimum value was coded as 1.99 when the concentration was less than the threshold of detection (< 2 ng/mL).
Group differences surviving false discovery rate correction were followed by pairwise group comparisons. To compare within-group and between-group responses to the product challenge, we created linear mixed models to predict outcome measures from group, time, and group × time with age, sex, and race or ethnicity as covariates. Treadmill stress test responses (after challenge only) were evaluated using analyses of covariance models adjusted for age, sex, and race or ethnicity. Separate false discovery rate correction was applied to the set of 17 product challenge tests (13 group × time interactions) (Fig 1, e-Tables 1-20) and four treadmill test group main effects (Fig 2). All nominally significant effects that survived false discovery rate correction were followed by specific group contrasts. All statistical tests were two-sided with P < .05 indicating nominal statistical significance and multiple comparison corrections, as stated previously. Detailed findings from covariate-adjusted statistical tests for group differences are in e-Appendix 3 and e-Table 4. Sensitivity analyses, most notably the effects of prior tobacco use and tobacco use burden on cardiovascular measurements in ENDS users, are in e-Appendix 4 and e-Tables 12, 18, and 20. Statistical analyses used SPSS version 27 software (IBM).
Figure 1.
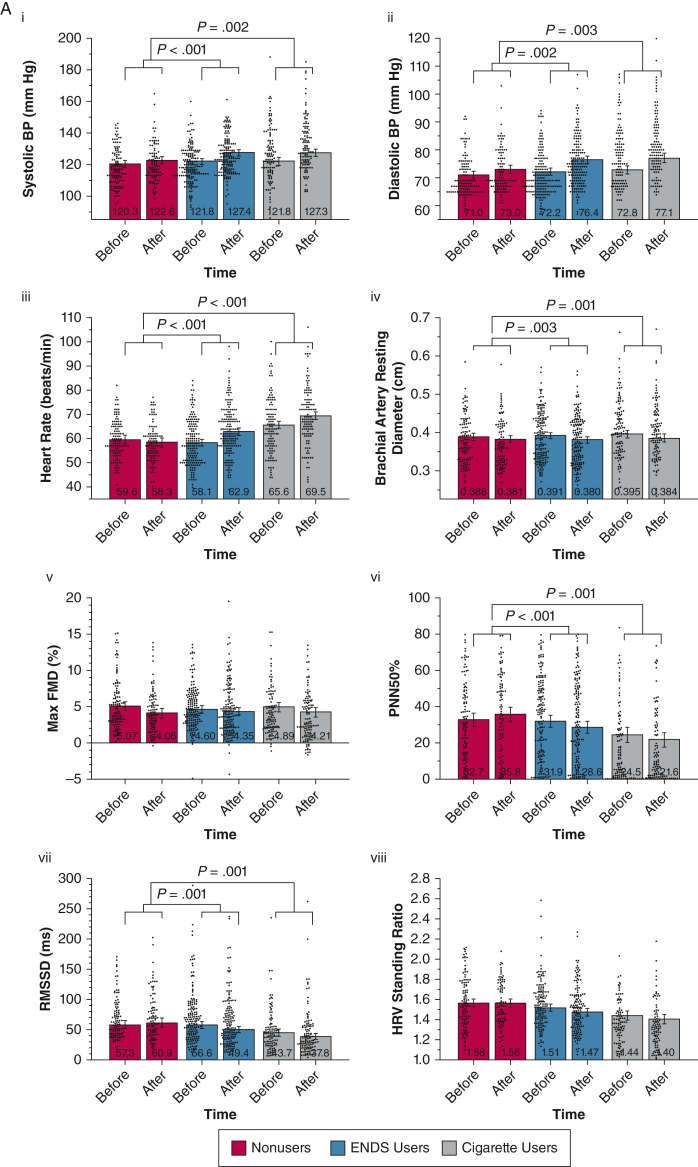
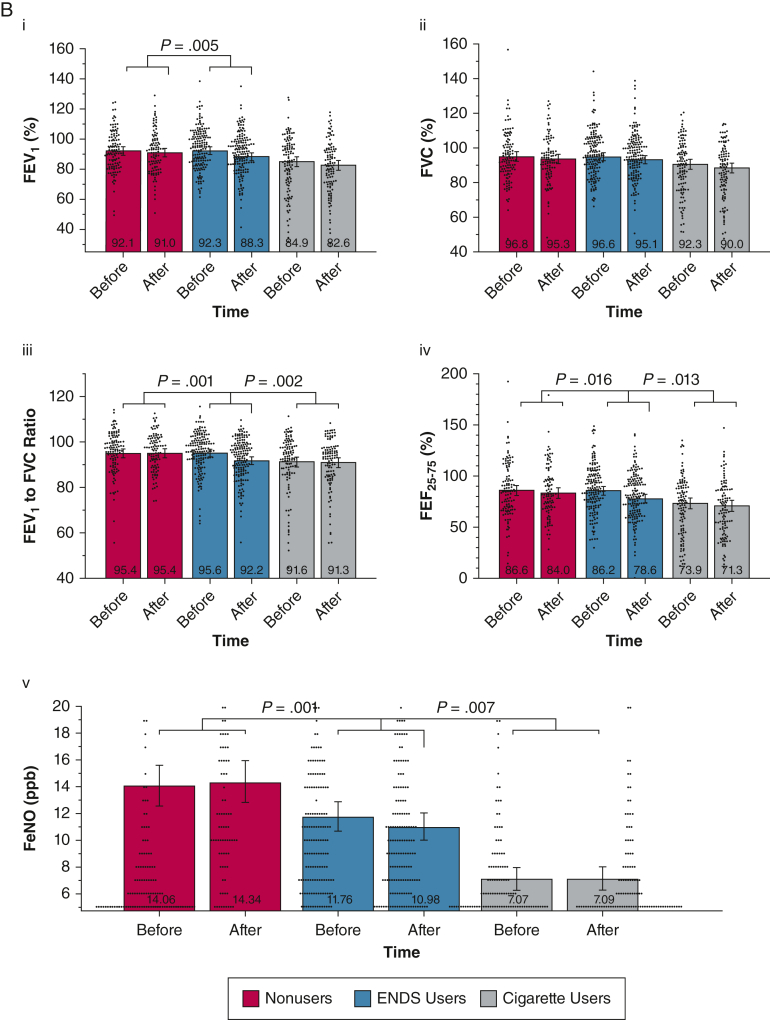
A, B, Combined violin and bar plots showing cardiovascular and pulmonary measurements obtained before and after the nicotine-containing product challenge: cardiovascular measures (A) and pulmonary measures (B). Linear mixed models adjusted for age, sex, and race or ethnicity compared the within-group and between-group responses after product use. Violin plots show distributions of observed outcomes for within each group and time point. Bar plots depict model-estimated marginal means and associated 95% CIs with covariates fixed at their mean values. P value annotations reflect effects associated with specific group × time contrasts (e-Tables 1-20). False discovery rate correction using the Benjamini and Hochberg procedure was applied to all 17 cardiovascular and pulmonary product challenge tests. All nominally significant effects in this set survived false discovery rate correction and were followed by specific group contrasts. ENDS = electronic nicotine delivery system; Feno, fractional exhaled nitric oxide; FMD = flow-mediated dilation; HRV = heart rate variability; PNN50% = percentage of successive R-R intervals that differ by > 50 ms; RMSSD = root mean square differences in successive normal intervals.
Figure 2.
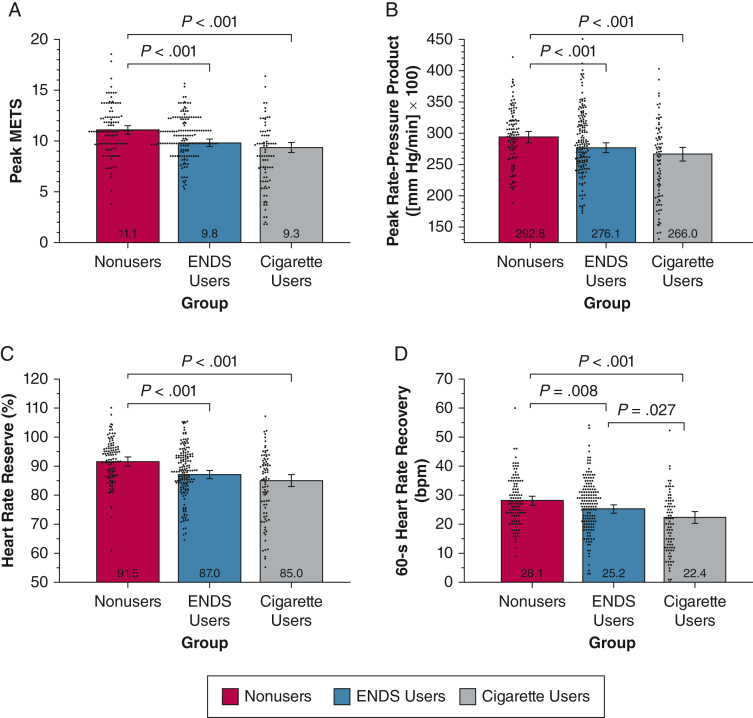
A-D, Combined violin and bar plots showing treadmill stress testing measurements after nicotine-containing product challenge: peak metabolic equivalents (A), peak rate-pressure product (B), heart rate reserve (C), and 60-s heart rate recovery (D). Age-, sex-, and race- or ethnicity-adjusted analysis of covariance tests to compare the within-group and between-group differences after product challenge. Violin plots show distributions of observed outcomes for within each group. Bar plots depict model-estimated marginal means and associated 95% CIs with covariates fixed at their mean values. P value annotations reflect pairwise group comparisons following significant omnibus main effects. False discovery rate correction using the Benjamini and Hochberg procedure was applied to all four tests. All nominally significant effects in this set survived false discovery rate correction and were followed by specific group contrasts. ENDS = electronic nicotine delivery system; MET = metabolic equivalent.
Results
Participant Characteristics
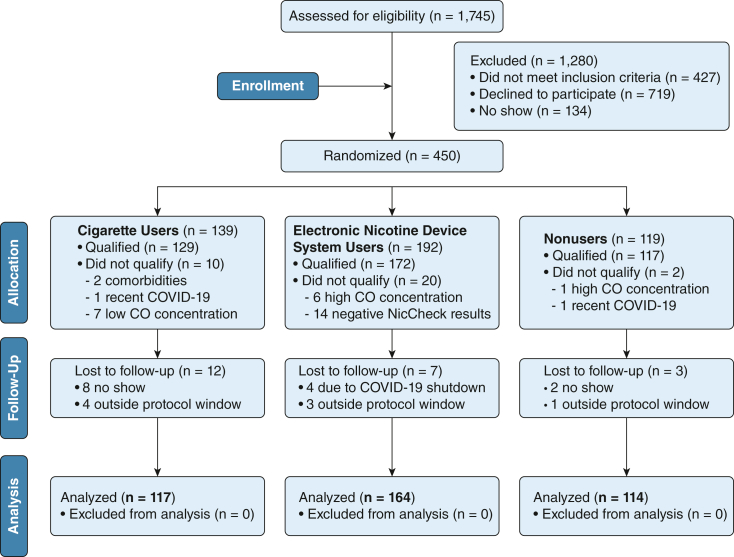
Of 1,745 individuals screened, 450 participants were randomized (Fig 3). Characteristics of the 395 participants who completed both in-person visits are in Table 1. As expected, the use groups differed on several characteristics including age, race, and product use measurements (Table 1). The 114 control participants had a mean ± SD age of 30.8 ± 11.9 years and were 50% female and 69% White. The 164 ENDS users had a mean ± SD age of 27.4 ± 10.6 years and were 39% female and 86% White. The median duration of ENDS use in the ENDS participants was 4.0 years (IQR, 3.0 years); 82% used fourth-generation ENDS (e-Appendix 5). The 117 combustible cigarette users were a mean ± SD age of 42.8 ± 13.8 years and were 44% female and 65% White. Cigarette users did so for a median of 21.0 years (IQR, 24.0 years). Median exhaled CO levels were higher in cigarette users (18.0 ppm; IQR, 17.0 ppm) than in ENDS users (0.0 ppm; IQR, 1.0 ppm) and control participants (0.0 ppm; IQR, 1.0 ppm). Consistent with eligibility criteria, baseline urinary nicotine levels were detectable in ENDS and cigarette users and were undetectable in control participants, as confirmed by serum nicotine and cotinine levels. Use of relevant medication was uncommon: statins were used by two control participants, three ENDS users, and nine cigarette users; β-blockers were used by two control participants, five ENDS users, and 11 cigarette users; vasodilators were used by three control participants, four ENDS users, and 12 cigarette users. During the 15-min product challenge, cigarette users self-administered a median of 14.0 puffs (IQR, 9.3 puffs); ENDS administered a median of 9.0 puffs (IQR, 7.5 puffs; P < .001). Details of the ENDS used by participants are in e-Appendix 5.
Figure 3.
Strengthening the Reporting of Observational Studies in Epidemiology Participant flow diagram for the study. CO = carbon monoxide.
Baseline Cardiovascular and Pulmonary Measurements
Baseline cardiovascular and pulmonary measurements by long-term product use group are shown in Table 2. All cardiovascular and autonomic function measurements obtained before product exposure were similar between groups after adjusting for differences in baseline age, sex, and race or ethnicity, with the exceptions of HR, aortic augmentation index adjusted for an HR of 75 beats/min, and HRV standing ratio, each of which was significantly worse among cigarette users than among ENDS users and control participants. ENDS users and control participants did not differ on these measurements except that ENDS users showed higher aortic augmentation index adjusted for an HR of 75 beats/min than control participants, a difference that persisted in sensitivity analyses (e-Appendix 4).
Table 2.
Cardiovascular and Pulmonary Measurements (Before Product Use Challenge, n = 395)
| Variable | Control Participants (n = 114) | ENDS Users (n = 164) | Cigarette Users (n = 117) | Adjusted Model |
|
|---|---|---|---|---|---|
| P Valuea (Group Effect) | Post Hoc Tests | ||||
| Cardiovascular measurements | |||||
| BMI, kg/m2 | 24.8 ± 4.7 | 25.7 ± 5.7 | 28.3 ± 7.5 | .091 | NA |
| BP, mm Hg | |||||
| Systolic | 118.4 ± 11.7 | 119.7 ± 11.2 | 126.4 ± 16.7 | .549 | NA |
| Diastolic | 70.4 ± 7.1 | 71.1 ± 7.5 | 76.6 ± 10.7 | .199 | NA |
| Heart rate, beats/min | 59.6 ± 8.2 | 58.3 ± 9.2 | 65.3 ± 11.7 | < .001b | S > C, Ec |
| Carotid artery intima-media thickness, mm | 0.54 ± 0.10 | 0.54 ± 0.10 | 0.67 ± 0.18 | .074 | NA |
| Carotid artery plaque score, log-transformed | 0.38 ± 0.96 | 0.34 ± 1.01 | 1.84 ± 2.64 | .255 | NA |
| Carotid artery distensibility, mm Hg–1 × 10–3) | 5.26 ± 1.81 | 5.61 ± 2.00 | 4.06 ± 1.54 | .112 | NA |
| Brachial artery diameter, cm | 0.38 ± 0.06 | 0.39 ± 0.64 | 0.41 ± 0.73 | .483 | NA |
| Brachial artery flow-mediation dilation, % | 5.31 ± 3.43 | 4.89 ± 2.92 | 4.25 ± 3.17 | .494 | NA |
| Aortic augmentation index at 75 beats/min, % | 1.0 (20.1) | 0.5 (20.5) | 19.5 (21.8) | < .001b | S > E > Cc |
| Autonomic measurements | |||||
| PNN50% | 31.0 (32.8) | 37.1 (37.0) | 11.4 (25.7) | .035 | . . . |
| RMSSD, ms | 58.3 (41.4) | 63.3 (48.9) | 43.6 (30.3) | .150 | . . . |
| Low- to high-frequency power ratio, log-transformed | 1.52 ± 1.86 | 1.58 ± 1.73 | 1.97 ± 1.88 | .323 | . . . |
| Heart rate variability standing ratio | 1.57 ± 0.26 | 1.55 ± 0.23 | 1.38 ± 0.21 | .001b | C, E > Sc |
| Pulmonary measurements | |||||
| FEV1, % | 92.0 ± 13.4 | 93.8 ± 13.3 | 82.9 ± 18.9 | .001b | C, E > Sc |
| FVC, % | 96.5 ± 15.1 | 98.3 ± 13.5 | 90.3 ± 16.0 | .022 | . . . |
| FEV1 to FVC, % | 95.4 ± 9.6 | 95.7 ± 8.9 | 91.2 ± 12.6 | .047 | . . . |
| FEF25-75, % | 86.9 ± 27.3 | 87.9 ± 23.5 | 71.1 ± 31.1 | .003 | C, E > Sc |
| Feno, log-transformed, ppb | 16.2 ± 11.0 | 14.5 ± 14.7 | 9.7 ± 12.3 | < .001b | C>E>Sc |
| Laboratory measurements | |||||
| Hemoglobin, g/dL | 14.1 ± 1.4 | 14.2 ± 1.38 | 14.1 ± 1.4 | .185 | . . . |
| WBC count, K/μL | 5.0 ± 1.7 | 6.1 ± 1.5 | 7.3 ± 2.3 | < .001b | S > C, Ec |
| C-reactive protein, log-transformed, mg/L | 1.7 ± 2.4 | 2.0 ± 6.0 | 5.7 ± 8.4 | < .001b | S > C, Ec |
| Non-high-density lipoprotein cholesterol, mg/dL | 121.3 ± 32.2 | 115.7 ± 36.4 | 130.2 ± 39.3 | .313 | . . . |
| F2-isoprostane to creatinine ratio, ng/mg | 0.56 ± 0.19 | 0.54 ± 0.29 | 0.78 ± 0.42 | < .001b | S > C, Ec |
Data are presented as mean ± SD or median (interquartile range), unless otherwise indicated. C = control participants; E = electronic nicotine device system user; ENDS = electronic nicotine delivery system; Feno = fractional exhaled nitric oxide; FEF25-75 = forced expiratory flow at 25% to 75% of FVC; NA = not available; PNN50% = percentage of successive R-R intervals that differ by > 50 ms; RMSSD = root mean square differences in successive normal intervals; S = cigarette user.
Adjusted for age, sex, and race.
P < .05 after Benjamini and Hochberg adjustment to control false discovery rate across the 24 tests in the table.
Group pairwise comparisons with P < .05 with Bonferroni multiple comparisons correction.
Pulmonary testing showed that cigarette users showed significantly worse FEV1 and forced midexpiratory flow at 25% to 75% of FVC (FEF25-75) than ENDS users and control participants, with a trend toward lower FEV1 to FVC ratio than ENDS users and control participants. Spirometry results for ENDS users and control participants did not differ significantly. Cigarette users showed lower Feno than ENDS users (P < .001), but ENDS users demonstrated lower values than control participants (P = .013). Cigarette users also showed higher WBC counts, C-reactive protein levels, and urine F2-isoprostane to creatinine ratios.
Cardiovascular Measurements Before and After the Product Challenge
Figure 1A and e-Appendix 3 show cardiovascular measurements before and after the product challenge. Systolic BP (Fig 1Ai), diastolic BP (Fig 1Aii), and HR (Fig 1Aiii) increased after challenge in all three groups; however, increases were higher in ENDS users (P ≤ .002 for all) and cigarette users (P ≤ .003 for all) than in control participants. Increases in systolic BP, diastolic BP, and HR did not differ between ENDS and cigarette users. Similarly, brachial artery diameters (Fig 1Aiv) decreased more in ENDS users (P = .003) and cigarette users (P = .001) than in control participants; however, brachial artery flow-mediated dialtion (FMD) responses in ENDS users, cigarette users, and control participants were similar (P = .175 for group × time interaction), even after adjusting for changes in brachial artery diameter (P = .222 for group × time interaction), medication use, or using absolute diameter change (Fig 1Aiv). ENDS and cigarette users showed significant worsening in percentage of successive R-R intervals that differ by > 50 ms (Fig 1Avi) and root mean square differences in successive normal intervals (Fig 1Avii) that were more than in control participants (P ≤ .001 for all). No significant between-groups change effects were found for standing HRV (P = .182 for group × time interaction) (Fig 1Aviii). These before and after effects of product use on the cardiovascular measurements were similar among ENDS users with and without a history of cigarette use. Neither nicotine nor CO boost correlated strongly with changes in any cardiovascular measurements (e-Figs 1-3).
Pulmonary Measures Before and After the Product Challenge
Figure 1B and e-Appendix 3 show pulmonary measurements before and after the product challenge period. FEV1 declined in all groups, significantly more so in ENDS users than in control participants (P = .005), but similar to that of cigarette users (Fig 1Bi). The decline in FEV1 to FVC ratio (Fig 1Biii) was greater in ENDS users than in both control participants (P < .001) and cigarette users (P = .002), with similar results for FEF25-75 (Fig 1Biv), which declined more in ENDS users than in control participants (P = .016) and cigarette users (P = .013). Feno decreased significantly after the challenge in ENDS users (P < .001), but not in control participants or cigarette users (Fig 1Bv). These before and after effects of product use on the pulmonary measurements were similar among ENDS users with and without a history of cigarette smoking, except for a weak interaction whereby ENDS users with a history of smoking of at least 10 pack-years showed a smaller decline in FEF25-75 (P = .047). Other than weak, inverse correlations between nicotine boost and Feno (r = –0.20 to –0.29), neither nicotine nor CO boost correlated strongly with changes in any pulmonary measurements (e-Figs 1, 2).
Symptom-limited Maximum Stress Test Measurements
The results of symptom-limited maximum treadmill stress tests performed approximately 90 min after the product challenge (Fig 2) showed that ENDS users consistently performed worse on all four exercise measurements than control participants with intermediate values that were not statistically different from those of cigarette users with the exception of 60-s HR recovery (Fig 2iv). These results were robust in sensitivity tests for current use of cardiovascular medications, history of reported cigarette smoking among ENDS users, and pack-years smoked among ENDS users (e-Appendix 3).
Discussion
This large, before-and-after nicotine product challenge study revealed multiple cardiovascular and pulmonary changes after the product challenge. Some of these changes provide new evidence of cardiovascular and pulmonary effects of ENDS use among long-term ENDS users that could have meaningful health consequences. Before the product challenge, ENDS users and control participants showed similar values on cardiovascular and pulmonary measurements. After the product challenge, ENDS users showed statistically significant worsening on several cardiovascular and pulmonary measurements compared with control participants, with changes that were similar to those observed in cigarette users. The pathophysiologic changes after ENDS use were consistent across the cardiovascular and pulmonary measures and seem to reflect increased sympathetic tone and airway obstruction. These changes included acute increases in systolic and diastolic BP, increases in HR, decreases in HRV, brachial artery constriction, and impaired HR recovery after exercise, effects consistent with increased sympathetic tone. ENDS users also showed reduced exercise capacity and peak achieved cardiac workload approximately 90 min after ENDS use. The spirometry changes reflected airway obstruction with reductions in FEV1, FEV1 to FVC ratio, and FEF25-75 with a decline in Feno. These data revealed acute and short-term cardiovascular and pulmonary changes after use of contemporary ENDS products among ENDS users and suggest a potential for health harms.
Previous product challenge studies with ENDS have been small, focused on either cardiovascular or pulmonary outcomes, used older-generation ENDS, and yielded mixed patterns of physiologic results.29, 30, 31, 32 Herein, we report the results of comprehensive cardiovascular and pulmonary testing in the largest product use challenge study performed to date, to the best of our knowledge. We examined acute ENDS effects in individuals who used ENDS exclusively rather than in healthy control participants or dual-product users.31,32 Baseline screening protocols excluded current users of multiple nicotine products from the use groups. Participants used their personal ENDS ad libitum after a period of deprivation in an attempt to capture the effects of real-world ENDS use. The ENDS used by participants in The Cardiac and Lung E-cig Smoking Study primarily were fourth-generation devices that differ significantly from earlier generation ENDS in design, size, battery power, nicotine, and toxin delivery.4,5 Our results show that use of these contemporary devices yielded nicotine levels after challenge similar to those of cigarette users and exceeded levels reported in prior acute challenge studies using ENDS.32
The magnitude of the changes observed with ENDS use after the product challenge could be clinically relevant. After ENDS use, an approximately 6-mm Hg increase in systolic BP was found (adjusted for age, sex, and race or ethnicity). Prior studies demonstrated a strong, graded association of even small, chronic systolic BP increases with cardiovascular disease (CVD) events among healthy individuals with normal baseline BP.33 Furthermore, a large analysis of randomized, controlled pharmacologic BP-lowering trials described a 10% decrease in CVD events for each 5-mm Hg reduction in systolic BP, even in the normal systolic BP range.34 ENDS users also demonstrated a significant rise in HR after product use. Tonic resting HR is an independent predictor of cardiovascular and all-cause mortality with continuous increases in risk at > 60 beats/min.35,36 After product use, ENDS users showed impaired exercise tolerance compared with control participants, achieving only 9.8 METs (adjusted for age, sex, and race or ethnicity), which is substantially lower than the average age-predicted functional capacity (11.7 METs for men; 11.1 METs for women).37, 38, 39 Lower functional capacity on an exercise stress test is associated with increased CVD and all-cause mortality risk.38,40 ENDS users also showed worse 60-s HR recovery, an independent predictor of CVD death, compared with control participants.41 A 60-s heart rate recovery of < 25 beats has been associated with a 2.2-fold higher risk of sudden death.41 Despite their comparatively younger age and healthier baseline cardiovascular profiles, ENDS users demonstrated an adjusted recovery of only 25.2 beats/min. Furthermore, ENDS users in our study showed an adjusted, acute mean 4% reduction in FEV1 after a single ENDS use session. Pharmacologic trials of long-term treatments for COPD have shown that FEV1 changes of ≥ 5% are clinically significant.42
The long-term cardiovascular and pulmonary effects of repeated ENDS use are not known, and it is unclear if the acute changes observed in this study might translate into tonic levels of these physiologic parameters. Although it is reassuring that baseline levels of cardiovascular and pulmonary measurements were similar between ENDS users and control participants, it is concerning that one episode of ENDS use was associated with the acute worsening of cardiovascular and pulmonary indexes of future health risks among long-term ENDS users.
The cardiovascular and pulmonary responses observed after the ENDS challenge may reflect nicotine-dependent and nicotine-independent effects. Inhaled nicotine’s pulmonary effects may be mediated partially through binding of lung tissue-specific nicotinic receptors and promotion of collagen deposition and inflammation propagation via processes mediated by IL-1β.43,44 Nicotine also has been reported to drive a systemic sympathomimetic response on cardiovascular hemodynamics.32 However, these nicotine-dependent cardiopulmonary effects have not been observed consistently across studies.29 Production of e-vapors from thermal degradation of e-liquids yields numerous chemicals including carbonyls, organic compounds, nitrosamines, metals, and ultrafine particulate matter; these products are associated with adverse cardiopulmonary effects including airway irritation, sympathomimetic responses, and deleterious changes in the cardiovascular system.5,45, 46, 47 The mechanisms underlying the effects of ENDS on cardiovascular and pulmonary physiology require further study.
Limitations
Our study was observational, so the changes observed after the product challenge are not proof of the causal effects of ENDS use. Despite statistical adjustment for confounders, the groups differed on several variables such as age, race or ethnicity, and length of product use; these and other differences may have affected the results. We aimed to investigate the cardiovascular and pulmonary effects of real-world nicotine product use; therefore, participants self-administered their own products, simulating real-world use. Differing products, use patterns, and dosing in the ENDS and cigarette user groups could have increased error and reduced detection of product effects. Prior combustible cigarette use among ENDS users may have affected their cardiopulmonary outcomes; however, only 75 ENDS users (45.7%) previously used combustible cigarettes, and the median smoking history in the group was 0.0 pack-years (IQR, 5.3 pack-years). The cardiovascular and pulmonary effects we observed in ENDS users were robust regarding any prior cigarette use or ≥ 10-pack-years use. Only a weak interaction in FEF25-75 was observed. We observed acute changes among ENDS users in several cardiopulmonary measurements that exceeded those found in cigarette smokers. Of note, cigarette smokers demonstrated worse baseline cardiopulmonary measures, so a ceiling effect on change in these cardiopulmonary measurements cannot be excluded.
Our study was not designed to identify the mechanistic effects or toxicology of the various e-liquid compositions. Also, it is unclear why FMD did not differ between groups at baseline or after the product use challenge. This is anomalous because the cigarette users were older and showed less favorable CVD risk profiles than the other groups. Baseline FMD values on average were low in all groups, possibly reflecting the lower arm occlusion technique, and differential changes in brachial artery diameter (the denominator of the FMD calculation) within groups was found, likely decreasing statistical power. Further, our results may not be generalizable to dual-product users or to some racial and ethnic groups because the ENDS users mostly were White.
Interpretation
In this large, before-and-after product use nicotine-containing product challenge study using contemporary ENDS, ENDS users showed acute, clinically relevant worsening of several cardiovascular and pulmonary measurements after using END Scompared with control participants who did not use a nicotine-containing product. ENDS users showed acute worsening of BP, HR, and HRV, as well as vasoconstriction, impaired exercise tolerance, and increased airflow obstruction after using ENDS compared with control participants. These findings raise concerns about the potential harms of contemporary ENDS.
Funding/Support
This research was funded by the National Heart, Lung, and Blood Institute, [Grant R01 HL139331]. C. M. H. was supported by the National Heart, Lung, and Blood Institute [Grant T32 HL007936].
Financial/Nonfinancial Disclosures
The authors have reported to CHEST the following: T. B. B. discloses that he is the Glaxo Wellcome Chair for the Study of Tobacco Dependence at the University of Wisconsin. J. H. S. discloses that he is a consultant (DMC Chair) for Lilly and Silence Therapeutics and that he received royalties for a book chapter from UpToDate Medicine. None declared (M. C. T., C. M. H., T. M. P., C. E. K., K. M. H., N. R. O., N. S., M. C. F.).
Acknowledgments
Author contributions: J. H. S. is responsible for all content of the manuscript. M. C. T. contributed to data interpretation, manuscript draft, and critical revision and final approval of manuscript. C. M. H. contributed to data interpretation, manuscript draft, and critical revision and final approval of manuscript. T. M. P. contributed to data analysis, data interpretation, manuscript draft, and critical revision and final approval of manuscript. C. E. K. contributed to conception and design, data acquisition, data interpretation, data analysis, and critical revision and final approval of manuscript. K. M. H. contributed to data acquisition, data interpretation, and critical revision and final approval of manuscript. N. R. O. contributed to data acquisition, data interpretation, and final approval of manuscript. N. S. contributed to data interpretation, data analysis, and final approval of manuscript. M. C. F. contributed to data acquisition, data interpretation, and critical revision and final approval of manuscript. T. B. B. contributed to conception and design, data interpretation, manuscript draft, and critical revision and final approval of manuscript. J. H. S. contributed to conception and design, data interpretation, data analysis, manuscript draft, and critical revision and final approval of manuscript.
Role of sponsors: The sponsors had no role in the design, conduct, or analysis of the study.
Additional information: The e-Appendixes, e-Figures, and e-Tables are available online under “Supplementary Data.”
Supplementary Data
References
- 1.Creamer M.R., Wang T.W., Babb S., et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(45):1013–1019. doi: 10.15585/mmwr.mm6845a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villarroel M.A., Cha A.E., Vahratian A. Electronic cigarette use among U.S. adults, 2018. NCHS Data Brief. 2020;(365):1–8. [PubMed] [Google Scholar]
- 3.Gentzke A.S., Creamer M., Cullen K.A., et al. Vital signs: tobacco product use among middle and high school students—United States, 2011-2018. MMWR Morb Mortal Wkly Rep. 2019;68(6):157–164. doi: 10.15585/mmwr.mm6806e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandon T.H., Goniewicz M.L., Hanna N.H., et al. Electronic nicotine delivery systems: a policy statement from the American Association for Cancer Research and the American Society of Clinical Oncology. J Clin Oncol. 2015;33(8):952–963. doi: 10.1200/JCO.2014.59.4465. [DOI] [PubMed] [Google Scholar]
- 5.Qasim H., Karim Z.A., Rivera J.O., Khasawneh F.T., Alshbool F.Z. Impact of electronic cigarettes on the cardiovascular system. J Am Heart Assoc. 2017;6(9) doi: 10.1161/JAHA.117.006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yingst J., Foulds J., Veldheer S., et al. Measurement of electronic cigarette frequency of use among smokers participating in a randomized controlled trial. Nicotine Tob Res. 2020;22(5):699–704. doi: 10.1093/ntr/nty233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.E-cigarette, or Vaping . Products Visual Dictionary. United States Department of Health and Human Services, Centers for Disease Control and Prevention; 2019. pp. 1–25.https://www.cdc.gov/tobacco/basic_information/e-cigarettes/pdfs/ecigarette-or-vaping-products-visual-dictionary-508.pdf [Google Scholar]
- 8.Tattersall M.C., Gassett A., Korcarz C.E., et al. Predictors of carotid thickness and plaque progression during a decade: the Multi-Ethnic Study of Atherosclerosis. Stroke. 2014;45(11):3257–3262. doi: 10.1161/STROKEAHA.114.005669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein J.H., Korcarz C.E., Mays M.E., et al. A semiautomated ultrasound border detection program that facilitates clinical measurement of ultrasound carotid intima-media thickness. J Am Soc Echocardiogr. 2005;18(3):244–251. doi: 10.1016/j.echo.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Gepner A.D., Korcarz C.E., Colangelo L.A., et al. Longitudinal effects of a decade of aging on carotid artery stiffness: the multiethnic study of atherosclerosis. Stroke. 2014;45(1):48–53. doi: 10.1161/STROKEAHA.113.002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson H.M., Gossett L.K., Piper M.E., et al. Effects of smoking and smoking cessation on endothelial function: 1-year outcomes from a randomized clinical trial. J Am Coll Cardiol. 2010;55(18):1988–1995. doi: 10.1016/j.jacc.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torriani F.J., Komarow L., Parker R.A., et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: the ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52(7):569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein J.H., Brown T.T., Ribaudo H.J., et al. Ultrasonographic measures of cardiovascular disease risk in antiretroviral treatment-naive individuals with HIV infection. AIDS. 2013;27(6):929–937. doi: 10.1097/QAD.0b013e32835ce27e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein J.H., Keevil J.G., Wiebe D.A., Aeschlimann S., Folts J.D. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation. 1999;100(10):1050–1055. doi: 10.1161/01.cir.100.10.1050. [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson I.B., Mohammad N.H., Tyrrell S., et al. Heart rate dependency of pulse pressure amplification and arterial stiffness. Am J Hypertens. 2002;15(1 Pt 1):24–30. doi: 10.1016/s0895-7061(01)02252-x. [DOI] [PubMed] [Google Scholar]
- 16.Camm A.J., Pratt C.M., Schwartz P.J., et al. Mortality in patients after a recent myocardial infarction: a randomized, placebo-controlled trial of azimilide using heart rate variability for risk stratification. Circulation. 2004;109(8):990–996. doi: 10.1161/01.CIR.0000117090.01718.2A. [DOI] [PubMed] [Google Scholar]
- 17.Miller M.R., Crapo R., Hankinson J., et al. General considerations for lung function testing. Eur Respir J. 2005;26(1):153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 18.Miller M.R., Hankinson J., Brusasco V., et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 19.Baker T.B., Piper M.E., Stein J.H., et al. Effects of nicotine patch vs varenicline vs combination nicotine replacement therapy on smoking cessation at 26 weeks: a randomized clinical trial. JAMA. 2016;315(4):371–379. doi: 10.1001/jama.2015.19284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dweik R.A., Boggs P.B., Erzurum S.C., et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boot J.D., de Ridder L., de Kam M.L., Calderon C., Mascelli M.A., Diamant Z. Comparison of exhaled nitric oxide measurements between NIOX MINO electrochemical and Ecomedics chemiluminescence analyzer. Respir Med. 2008;102(11):1667–1671. doi: 10.1016/j.rmed.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 22.American Thoracic Society European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med. 2005;171(8):912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 23.Asthana A., Piper M.E., McBride P.E., et al. Long-term effects of smoking and smoking cessation on exercise stress testing: three-year outcomes from a randomized clinical trial. Am Heart J. 2012;163(1):81–87.e1. doi: 10.1016/j.ahj.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balke B., Ware R.W. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J. 1959;10(6):675–688. [PubMed] [Google Scholar]
- 25.Riebe D., Ehrman J.K., Liguori G., Magal M. eds. ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Wolters Kluwer. 2018 [Google Scholar]
- 26.Cole C.R., Blackstone E.H., Pashkow F.J., Snader C.E., Lauer M.S. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341(18):1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 27.King C.C., Piper M.E., Gepner A.D., Fiore M.C., Baker T.B., Stein J.H. Longitudinal impact of smoking and smoking cessation on inflammatory markers of cardiovascular disease risk. Arterioscler Thromb Vasc Biol. 2017;37(2):374–379. doi: 10.1161/ATVBAHA.116.308728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol. 1995;57(1):289–300. [Google Scholar]
- 29.Vansickel A.R., Cobb C.O., Weaver M.F., Eissenberg T.E. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1945–1953. doi: 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flouris A.D., Chorti M.S., Poulianiti K.P., et al. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhal Toxicol. 2013;25(2):91–101. doi: 10.3109/08958378.2012.758197. [DOI] [PubMed] [Google Scholar]
- 31.Antoniewicz L., Brynedal A., Hedman L., Lundbäck M., Bosson J.A. Acute effects of electronic cigarette inhalation on the vasculature and the conducting airways. Cardiovasc Toxicol. 2019;19(5):441–450. doi: 10.1007/s12012-019-09516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaumont M., de Becker B., Zaher W., et al. Differential effects of e-cigarette on microvascular endothelial function, arterial stiffness and oxidative stress: a randomized crossover trial. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-28723-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whelton S.P., McEvoy J.W., Shaw L., et al. Association of normal systolic blood pressure level with cardiovascular disease in the absence of risk factors. JAMA Cardiol. 2020;5(9):1011–1018. doi: 10.1001/jamacardio.2020.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blood Pressure Lowering Treatment Trialists’ Collaboration Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet. 2021;397(10285):1625–1636. doi: 10.1016/S0140-6736(21)00590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox K., Borer J.S., Camm A.J., et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50(9):823–830. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 36.Diaz A., Bourassa M.G., Guertin M.C., Tardif J.C. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J. 2005;26(10):967–974. doi: 10.1093/eurheartj/ehi190. [DOI] [PubMed] [Google Scholar]
- 37.Kligfield P., Lauer M.S. Exercise electrocardiogram testing: beyond the ST segment. Circulation. 2006;114(19):2070–2082. doi: 10.1161/CIRCULATIONAHA.105.561944. [DOI] [PubMed] [Google Scholar]
- 38.Peterson P.N., Magid D.J., Ross C., et al. Association of exercise capacity on treadmill with future cardiac events in patients referred for exercise testing. Arch Intern Med. 2008;168(2):174–179. doi: 10.1001/archinternmed.2007.68. [DOI] [PubMed] [Google Scholar]
- 39.Shetler K., Marcus R., Froelicher V.F., et al. Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol. 2001;38(7):1980–1987. doi: 10.1016/s0735-1097(01)01652-7. [DOI] [PubMed] [Google Scholar]
- 40.Gulati M., Black H.R., Shaw L.J., et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med. 2005;353(5):468–475. doi: 10.1056/NEJMoa044154. [DOI] [PubMed] [Google Scholar]
- 41.Jouven X., Empana J.P., Schwartz P.J., Desnos M., Courbon D., Ducimetière P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352(19):1951–1958. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 42.Cazzola M., MacNee W., Martinez F.J., et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31(2):416–469. doi: 10.1183/09031936.00099306. [DOI] [PubMed] [Google Scholar]
- 43.Vicary G.W., Ritzenthaler J.D., Panchabhai T.S., Torres-González E., Roman J. Nicotine stimulates collagen type I expression in lung via α7 nicotinic acetylcholine receptors. Respir Res. 2017;18(1):115. doi: 10.1186/s12931-017-0596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kummer W., Lips K.S., Pfeil U. The epithelial cholinergic system of the airways. Histochem Cell Biol. 2008;130(2):219–234. doi: 10.1007/s00418-008-0455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Künzli N., Jerrett M., Mack W.J., et al. Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect. 2005;113(2):201–206. doi: 10.1289/ehp.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Egle J.L. Effects of inhaled acetaldehyde and propionaldehyde on blood pressure and heart rate. Toxicol Appl Pharmacol. 1972;23(1):131–135. doi: 10.1016/0041-008x(72)90212-8. [DOI] [PubMed] [Google Scholar]
- 47.Henning R.J., Johnson G.T., Coyle J.P., Harbison R.D. Acrolein can cause cardiovascular disease: a review. Cardiovasc Toxicol. 2017;17(3):227–236. doi: 10.1007/s12012-016-9396-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.