Abstract
Topic Importance
Right ventricular dysfunction in pulmonary hypertension (PH) contributes to reduced exercise capacity, morbidity, and mortality. Exercise can unmask right ventricular dysfunction not apparent at rest, with negative implications for prognosis.
Review Findings
Among patients with pulmonary vascular disease, right ventricular afterload may increase during exercise out of proportion to increases observed among healthy individuals. Right ventricular contractility must increase to match the demands of increased afterload to maintain ventricular-arterial coupling (the relationship between contractility and afterload) and ultimately cardiac output. Impaired right ventricular contractile reserve leads to ventricular-arterial uncoupling, preventing cardiac output from increasing during exercise and limiting exercise capacity. Abnormal pulmonary vascular response to exercise can signify early pulmonary vascular disease and is associated with increased mortality. Impaired right ventricular contractile reserve similarly predicts poor outcomes, including reduced exercise capacity and death. Exercise provocation can be used to assess pulmonary vascular response to exercise and right ventricular contractile reserve. Noninvasive techniques (including cardiopulmonary exercise testing, transthoracic echocardiography, and cardiac MRI) as well as invasive techniques (including right heart catheterization and pressure-volume analysis) may be applied selectively to the screening, diagnosis, and risk stratification of patients with suspected or established PH. Further research is required to determine the role of exercise stress testing in the management of pulmonary vascular disease.
Summary
This review describes the current understanding of clinical applications of exercise testing in the risk assessment of patients with suspected or established PH.
Key Words: chronic obstructive exercise, physical aerobic exercise, pulmonary arterial hypertension, pulmonary disease, pulmonary hypertension, right ventricular dysfunction
Pulmonary hypertension (PH) reduces exercise capacity and increases hospitalizations and mortality through impaired right ventricular (RV) function.1, 2, 3, 4 Gaps remain in our ability to diagnose and manage RV dysfunction.5 In particular, supine resting assessments do not adequately characterize abnormalities that occur during activities of daily living,6, 7, 8 and RV dysfunction unmasked by exercise carries negative functional and prognostic implications.8, 9, 10 Exercise stress testing identifies abnormal pulmonary vascular function contributing to increased pulmonary artery pressure and RV afterload, deficits in RV contractile reserve or ability to augment contractility, and ventricular-arterial uncoupling; that is, failure of RV contractility to match afterload.11, 12, 13 Among patients at risk for PH, exercise testing can be applied in clinical practice to complement commonly used techniques including resting transthoracic echocardiography (TTE) in screening for early pulmonary vascular disease.2 Additionally, among patients with established pulmonary vascular disease, exercise testing can be used to describe RV functional adaptations, to identify comorbid left-sided heart dysfunction, and to predict prognosis.2,12,14 Finally, exercise testing can differentiate among severity and subtype of pulmonary vascular disease.15 Further investigation is needed to understand how it may inform the management of patients with PH.3 Herein, we provide a state-of-the-art review of clinical applications of exercise stress testing for the assessment of RV function. In particular, this review describes noninvasive methods (including cardiopulmonary exercise testing [CPET], TTE, and cardiac MRI [CMR]) and invasive methods (including right heart catheterization [RHC] and pressure-volume analysis), emphasizes how exertional RV function informs clinical outcomes, and outlines an approach to the use of exercise testing in screening patients at risk of PH.
Literature Search
PubMed was searched using terms such as right ventricle, exercise, pulmonary hypertension, pulmonary arterial hypertension, chronic thromboembolic pulmonary hypertension, chronic obstructive pulmonary disease, interstitial lung disease, restrictive lung disease, scleroderma, human immunodeficiency virus, sickle cell disease, BMPR2, portopulmonary hypertension, pulmonary embolism, obstructive sleep apnea, obesity hypoventilation syndrome, tricuspid regurgitation, and valvular heart disease. Peer-reviewed original research and reviews were screened by abstract and then full text when appropriate. References in relevant articles were cross-checked.
Evidence Review
Exertional RV Function in Health and Pulmonary Vascular Disease
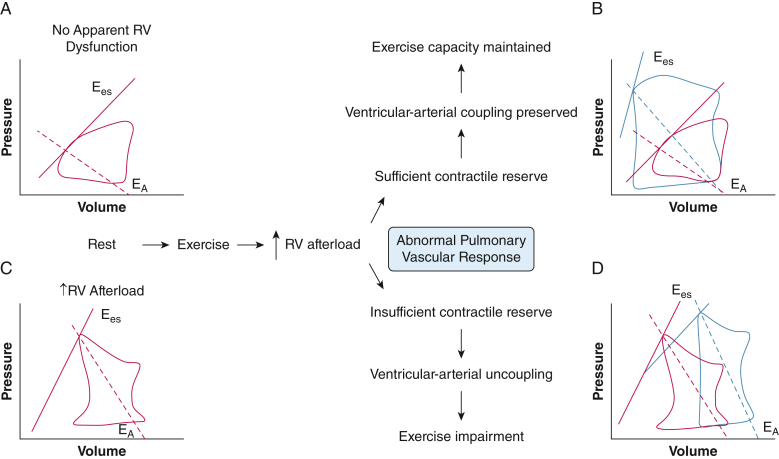
Cardiac output (Qc) is a composite of preload (venous return), afterload (pulmonary vascular resistance [PVR] to blood flow), and contractility. RV afterload increases during exercise.6 Although exertional pulmonary vasodilation and capillary recruitment reduce PVR, increased Qc and pulmonary artery wedge pressure (PAWP) lead to a net increase in mean pulmonary artery pressure (mPAP; mPAP = PVR × Qc + PAWP). In healthy individuals, mPAP increases by approximately 0.8 to 1.5 mm Hg per 1-L/min increase in Qc.16 Ventricular-arterial coupling characterizes the relationship between contractility and afterload and is preserved so long as RV contractile reserve (ie, ability to augment contractility) is sufficient to match the increased afterload.6,13 Ventricular-arterial coupling is quantified by the quotient of end-systolic elastance (Ees), defined by the slope of the end-systolic pressure-volume relationship) and effective arterial elastance (EA), determined by the quotient of end-systolic pressure and stroke volume).13,17 During peak exercise, the healthy RV increases contractility up to threefold to fivefold and diastolic relaxation by up to threefold.6 As a result, ventricular-arterial coupling is preserved (Fig 1).
Figure 1.
A-D, Diagram showing RV pressure-volume analysis in patients with abnormal pulmonary vascular response to exercise with vs without contractile reserve. A, Schematic of RV pressure-volume loop in a patient without apparent RV dysfunction at rest. B, Schematic of RV pressure-volume response to exercise in a patient with RV contractile reserve in the setting of increased afterload during exercise. Red indicates rest and blue indicates exercise. Contractility (Ees) increases such that even with increased afterload (EA), ventricular-arterial coupling is maintained. C, Schematic of RV pressure-volume loop in a patient with increased RV afterload apparent at rest. Relative to the patient without apparent RV dysfunction (A), contractility (Ees) is increased to maintain ventricular-arterial coupling in the face of increased afterload (EA). D, Schematic of RV pressure-volume response from rest (red) to exercise (blue) in a patient with insufficient RV contractile reserve in the setting of increased afterload during exercise. Contractility (Ees) fails to increase in response to increased afterload (EA), resulting in reduced ventricular-arterial coupling. Increased RV end-systolic and end-diastolic volumes are observed. EA = arterial elastance, a measure of RV afterload determined by the slope between end-systolic coordinates and the end-diastole volume-axis intercept, or the quotient of end-systolic pressure and stroke volume13,17; Ees = end-systolic elastance, a measure of RV contractility defined by the slope of the end-systolic pressure-volume relationship; RV = right ventricular.
Among patients with pulmonary vascular disease, RV afterload at rest and during exercise may be much higher than among healthy individuals because of the effects of pulmonary vascular remodeling, hypoxic pulmonary vasoconstriction, local capillary destruction, or a combination thereof.8,9,18, 19, 20, 21, 22, 23, 24, 25 Comorbid left-sided heart dysfunction also may contribute to RV afterload through an exaggerated increase in PAWP with exercise.16 Total pulmonary resistance, calculated as the quotient of mPAP and Qc at a point in time, encompasses both the left heart and pulmonary vascular contributions to RV afterload. The "pressure-flow" relationship, determined by mPAP vs Qc slope from rest through increasing intensity of exercise, may also be used to describe RV afterload. It describes the change in pulmonary artery pressure with increasing Qc and is abnormal when it is > 3 mm Hg/L/min.16 Increased afterload increases RV stroke work,8 and stroke volume is augmented through RV dilatation.12 If contractile reserve is insufficient to match the increased afterload, ventricular-arterial uncoupling (quantified by Ees/EA< 0.65-0.80) is observed.12,26,27
Exercise Testing in Risk Assessment of PH
The use of exercise testing to diagnose pulmonary vascular disease has been revisited over the years. The 6th World Symposium on Pulmonary Hypertension recommended against defining a clinical syndrome of “exercise PH” until further investigation better delineated normal vs abnormal physiologic features.28 However, understanding RV function during exercise is acknowledged as a critical component of understanding RV function in PH,3 and the 2022 joint European Society of Cardiology and European Respiratory Society guidelines suggest a role for exercise testing in screening symptomatic patients for PH and risk-stratifying patients who are at risk of pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH).2 Exercise provocation can unmask abnormalities in RV afterload, contractile reserve, and ventricular-arterial coupling that are not apparent at rest and thus may help to identify early pulmonary vascular disease.1,12,18,29 The practical application of exercise testing requires consideration of assessment technique and exercise method. Noninvasive methods including CPET, TTE, and CMR and invasive methods including RHC and pressure-volume analysis are described herein, along with examples of their clinical applications.
Noninvasive Methods of Exercise Testing
Cardiopulmonary Exercise Testing
CPET may be used to screen and risk-stratify patients with suspected and established PH. Radial artery catheterization should be used when possible to characterize arterial oxygen saturation accurately.30,31 Features suggesting PH include low end-tidal partial pressure of CO2, high ventilatory equivalent for CO2, blunted reduction in dead space fraction (dead space volume to tidal volume ratio), low oxygen pulse (oxygen consumption to heart rate ratio), and reduced maximum oxygen uptake (VO2max), when compared to healthy controls.2,30,32, 33, 34 For example, when comparing 15 patients with CTEPH with 15 patients with chronic thromboembolic disease and 10 sedentary control subjects, peak exercise dead space fraction of > 45% demonstrated a sensitivity of 92% and specificity of 83% in predicting CTEPH.35
Transthoracic Echocardiography
Two-dimensional TTE is the most accessible form of RV imaging and may be used with exercise to screen and risk-stratify patients with or at risk of PH.36,37 However, visualization of the RV is technically challenging because of its anatomic features, and the time constraints imposed by obtaining images during peak heart rate adds to this challenge.7 Moreover, measuring pressures by TTE is imprecise, particularly among patients with lung disease.38 Calculations of pulmonary artery systolic pressure (PASP) are based on tricuspid regurgitant jet velocity and right atrial pressure. Right atrial pressure typically is assumed to be 5 to 10 mm Hg, although this may result in underestimation of PASP particularly when moderate or severe tricuspid regurgitation is present.39,40 The presence of diastolic dysfunction or left-sided valvular disease may increase exercise PASP and, although potentially relevant for management, may not indicate underlying pulmonary vascular disease.39 Additionally, suboptimal Doppler tracings may result in overestimation of PASP if so-called Doppler noise, rather than the true maximum, is measured.39 In a research setting, calculation of exercise PASP was possible among 88% of 25 healthy volunteers with intraobserver variability of 8.2% at peak exercise.41 One might expect greater variability in routine clinical practice,41 and the potential for significant false-negative and false-positive findings is one of the predominant limitations of exercise TTE for risk assessment of PH.
When reliable measurements are obtained, exercise TTE can be used to assess surrogates of afterload and contractile reserve. The simplest metric of afterload is PASP. A tricuspid regurgitant jet velocity of > 3.1 m/s, corresponding with PASP of > 38 mm Hg plus right atrial pressure, is considered abnormal.39 Afterload also may be estimated by calculations of mPAP vs Qc slope, where mPAP is calculated as 0.6 × PASP + 2 and Qc is calculated from the aortic velocity-time integral.39,41,42
Among 61 individuals (28 healthy participants, eight asymptomatic carriers of BMPR2 mutation, five symptomatic patients post-pulmonary embolism, and 20 patients with CTEPH), mPAP vs Qc slope was able to be determined by TTE in 87% of patients.43 mPAP vs Qc slope obtained by TTE was greater than mPAP vs Qc slope obtained via simultaneous CMR and RHC (+0.9 mm Hg/L/min [95% CI, –3.6 to 5.4 mm Hg/L/min]).43 Contractile reserve may be inferred via PASP augmentation with a blunted increase of < 30 mm Hg during exercise among patients with baseline elevated PASP, suggesting impaired contractile reserve.36 Alternatively, a blunted increase in longitudinal RV strain also suggests reduced contractile reserve.44,45
Cardiac MRI
CMR is the gold standard method of noninvasive assessment of resting RV volume and function7 and, when coupled with exercise, may be used in screening and risk stratification of PH.46,47 However, careful attention must be to paid to temporal and spatial resolution to ensure accuracy, and interpretation can be time-consuming.47,48 Moreover, CMR is not available universally and may be contraindicated in some patients because of claustrophobia or indwelling devices such as pacemakers or defibrillators.7
Exercise CMR is useful for characterizing contractile reserve and ventricular-arterial coupling through RV ejection fraction (RVEF).7,20,48 Among 35 patients with known or suspected PH (mean ± SD age, 57.3 ± 13.9 years; 86% women; mean ± SD mPAP, 34 ± 2 mm Hg), lack of contractile reserve (defined as failure to augment Ees with submaximal [25 W] exercise) was associated with significantly decreased RVEF (P < .05).12 Moreover, exertional RVEF of < 38% was superior to resting RVEF in predicting occult ventricular-arterial uncoupling, detecting resting Ees/EA < 1 with 85% sensitivity and 77% specificity.12
Invasive Methods of Exercise Testing
Exercise RHC
Exercise RHC is the gold standard clinical method to diagnose abnormal pulmonary vascular response to exercise.2 Repeated hemodynamic measurements including heart rate, systolic BP, mPAP, PAWP, and Qc should be performed allowing for calculation of PVR, cardiac index, mPAP vs Qc slope, and PAWP vs Qc slope.2 Right atrial pressure, mixed venous oxygen saturation, and arterial oxygen saturation should be measured at rest and at submaximal and peak exercise.2,12 It should be noted that hemodynamic measurements derived from patients with pulmonary disease such as COPD may be confounded by dynamic hyperinflation and elevated intrinsic positive end-expiratory pressure with concomitant increases in intrathoracic pressures.49 These issues may be minimized by averaging pressure measurements over the respiratory cycle.4
Exercise RHC may be used to characterize afterload, contractile reserve, and ventricular-arterial coupling. Metrics of afterload include mPAP vs Qc slope, PAWP vs Qc slope (for left heart contribution to afterload), and PVR.2,8 As noted previously, an mPAP vs Qc slope of > 3 mm Hg/L/min indicates increased RV afterload.2 PAWP vs Qc slope of > 2 mm Hg/L/min, particularly when resting PAWP is borderline elevated (12-15 mm Hg), suggests left heart contribution to increased afterload.2,16 It should be noted that mPAP vs Qc slope and PAWP vs Qc slope normally increase with age, although still should be < 3 mm Hg/L/min and < 2 mm Hg/L/min, respectively.2,50 Contractile reserve and ventricular-arterial coupling may be inferred based on changes in stroke volume, Qc, and cardiac index.17,51 For example, among nine patients with PAH (mean ± SD age, 63 ± 12 years; 66% women; median [interquartile range] mPAP, 35 [30-42] mm Hg) compared with 20 control participants (mean ± SD age, 63 ± 9 years; 40% women; median [interquartile range] mPAP, 13 [12-15] mm Hg), patients with PAH did not augment Ees with exercise and also demonstrated blunted stroke volume index augmentation (rest to peak exercise among patients with PAH: 36 ± 6 mL/m2 to 36.1 ± 5.6 mL/m2 vs controls: 34 ± 7 mL/m2 to 48.9 ± 8.1 mL/m2; P < .01).52
Exercise Pressure-Volume Analysis
Exercise pressure-volume analysis through a conductance catheter inserted to the RV is the gold standard method to determine RV function for research purposes.7 The conductance catheter measures simultaneous pressure and volume to generate load-independent metrics of RV contractility, diastolic function, ventricular-arterial coupling, and myocardial energetics.13 Pressure-volume analysis can define contractile reserve (Fig 1) and can detect occult RV dysfunction.12,53
However, the use of conductance catheters requires technical expertise and infrastructure that is not widely available in clinical settings. Alternatively, pressure-volume analysis may be accomplished through RHC with simultaneous CMR or gas-exchange analysis (ie, CPET) to determine relevant volume metrics. Through these techniques, metrics of contractility can be determined, including end-systolic pressure-volume relationship (mPAP to RV end-systolic volume ratio) or Ees ([maximum isovolumic pressure – mPAP] / stroke volume index).17,29,54 Additionally, afterload (EA = mPAP to stroke volume index ratio) and ventricular-arterial coupling (the quotient of Ees and EA) may be calculated.17 Like pressure-volume analysis via a conductance catheter, this technique requires specific equipment capabilities and technical expertise that are not widely available in clinical settings, but in research settings provide significant insight into RV adaptation to PH.3,6,17,29,54
Exercise Methods
The most common methods of exercise for stress testing are treadmill and bicycle.55 Relative to treadmill testing, bicycle testing provides more stable body position, facilitating accurate measurements during exercise. Both treadmill and bicycle testing may be combined with gas-exchange analysis (ie, CPET) providing a more complete assessment of physiologic limitations to exercise, which may be of particular importance in patients with lung disease.56 Maximum exercise protocols provide the greatest information, including prognosticators such as VO2max,31,36,57 but submaximal exercise combined with imaging or hemodynamics has been used across multiple clinical settings and can provide useful screening, diagnostic, and risk stratification information.12,29 An alternative to treadmill and bicycle testing is the 6-min walk test, which is well established as a prognostic marker,42,58,59 although less able to be standardized for exercise intensity. Although a standalone 6-min walk test is advantageous inasmuch as it is easy and inexpensive, it is limited in that it provides less physiologic information about mechanisms limiting functional capacity. Resistance exercise has been described in limited settings,16,60 but given anticipated intrathoracic pressure, variation cannot be recommended at this time for functional RV assessment.
In addition to type of exercise, an important consideration is exercise position. Supine or semisupine positioning facilitates TTE and CMR image acquisition, but alters systemic and pulmonary hemodynamics because of the effects on gravitational and sympathetic tone.39,61 During upright vs supine exercise, higher mPAP, PVR, and mPAP vs Qc slope are observed.61 Supine exertional assessment thus likely underestimates exertional pulmonary vascular abnormalities experienced during upright activities of daily living.
Fluid Challenge and Passive Leg Raise
Exercise testing may be combined with other provocative tests including fluid challenge and passive leg raise to delineate pulmonary vascular characteristics further. For example, an increase in PAWP to ≥ 18 mm Hg in response to a fluid challenge of 500 mL saline administered over 5 min has been used to identify left-sided heart disease.62 Among 85 patients (mean ± SD age, 62 ± 12 years; 53% women) with suspected or established PH, a strong correlation was found in PAWP response to submaximal supine exercise and 500-mL fluid challenge (Spearman correlation coefficient, 0.82; P < .01) and a moderate correlation was found between passive leg raise to 45° for 1 min and fluid challenge (Spearman correlation coefficient, 0.53; P < .01).63 Among patients without evident postcapillary PH at baseline, fluid challenge vs passive leg raise identified a postcapillary response (mPAP > 20 mm Hg and PAWP > 18 mm Hg) in 21% vs 11% of patients, whereas exercise challenge identified a postcapillary response (PAWP vs Qc slope > 2 mmHg/L/min) in 48% of patients.63 Like exercise, fluid challenge has been demonstrated to unmask ventricular-arterial uncoupling with relevance to clinical outcomes.64 Among 32 patients with PAH or CTEPH (median [interquartile range] mPAP, 42 [36-52] mm Hg), a 200-mL fluid challenge caused ventricular-arterial uncoupling (baseline to fluid challenge median [interquartile range] ventricular-arterial coupling, 0.89 [0.69-1.23] to 0.16 [0.12-0.34]; P < .01), with greater uncoupling associated with clinical worsening over a median follow-up of 13.5 months (hazard ratio [HR] 0.96; 95% CI, 0.93-1.00; P < .05).64 Thus, passive leg raise, fluid challenge, or both may be used to complement exercise testing in characterization of pulmonary vascular function.
Clinical Outcomes Among Patients With Abnormal RV Exertional Function
Exercise capacity is a validated predictor of quality of life and survival among patients with PH.14,36,57 For example, among 43 patients with idiopathic PAH (mean ± SD age, 44 ± 2 years; 81% women; mean ± SD mPAP, 55 ± 2 mm Hg), improvement in VO2max after initiation of vasodilators (mean follow-up, 13 months [range, 3-25 months]) predicted survival (P < .01).14 Abnormalities in RV exertional hemodynamics likewise are associated with clinical outcomes among patients with suspected and established PH. Elevated exertional afterload among patients without resting PH correlates with reduced VO2max and predicts subsequent development of PH, cardiovascular hospitalization, and mortality.11,42,65 Among 714 individuals with chronic exertional dyspnea (mean ± SD age, 57 ± 16 years; 59% women), mPAP vs Qc slope of > 3 mm Hg/L/min was present in 41% of patients and was associated with reduced VO2max (mPAP vs Qc slope of >3 mm Hg/L/min vs < 3 mm Hg/L/min: 14.0 ± 4.1 mL/kg/min vs 18.8 ± 6.0 mL/kg/min; P < .05) and increased risk of cardiovascular hospitalization or all-cause mortality (HR, 2.03; 95% CI, 1.48-2.78; P < .01).65 Among 78 patients with connective tissue disease with resting mPAP of < 25 mm Hg (mean ± SD age, 58 ± 12 years; 91% women), mPAP vs Qc slope was greater among the 16 patients who later showed resting mPAP of ≥ 25 mm Hg vs those who did not (mPAP vs Qc slope, 6.1 ± 3.8 mm Hg/L/min vs 2.6 ± 4.3 mm Hg/L/min; P < .01).42 Similarly, among asymptomatic carriers of BMPR2 mutations, 16.7% of participants with exercise mPAP of > 30 mm Hg and total pulmonary resistance of > 3 Woods units demonstrated PAH during follow-up (6 years) vs 2.5% among individuals with normal exercise hemodynamics.66 Like patients at risk of PH, patients with established PH demonstrate increased mortality associated with abnormal mPAP vs Qc slope.10 For example, among 36 patients with PAH or inoperable CTEPH (mean ± SD age, 54 ± 15 years; 58% women; mean ± SD mPAP, 46 ± 11 mm Hg), mPAP vs Qc slope predicted mortality (χ2, 4.1; P < .05).10
Contractile reserve and ventricular-arterial coupling provide additional prognostication for development of PH, exercise capacity, clinical worsening, and mortality.12,17,36,54 Impaired global longitudinal systolic strain augmentation during exercise TTE is associated with the development of PH in patients with systemic sclerosis.67 Among patients with CTEPH, RV contractile reserve determined by exertional RV end-systolic pressure-volume relationship correlated with VO2max (r = 0.664; P < .01).54 Among 35 patients with known or suspected PH, exertional RVEF predicted time to clinical worsening (HR, 2.5 per 10% decline in exercise RVEF; 95% CI, 1.0-6.6; P = .05).12 Similarly, among 152 patients awaiting lung transplantation, RVEF during exercise (but not rest) independently predicted worsened survival (HR, 0.94; 95% CI, 0.88-0.99; P = .02).68 Finally, exercise Qc predicts survival.69 For example, among 55 patients with PAH (mean ± SD age, 54 ± 16 years; 55% women; mean ± SD mPAP, 52 ± 13 mm Hg), a 20% increase in cardiac index from rest to exercise showed 70% sensitivity and 80% specificity for determining survival at 18 months.69 Similarly, among 207 patients referred for exercise RHC for suspected PH (median age, 64 years [interquartile range, 54-72 years]; 69% women; median mPAP, 18 mm Hg [interquartile range, 15-21 mm Hg]), a peak exercise Qc of < 8.5 L/min corresponded to a mortality HR of 4.41 (95% CI, 2.01-9.68; P < .01).11
Contractile reserve may differ between disease phenotypes. Despite similar resting hemodynamics, patients with PAH associated with systemic sclerosis demonstrated markedly reduced contractile reserve (rest to exercise Ees, 0.45 ± 0.14 mm Hg/mL to 0.46 ± 0.16 mm Hg/mL) relative to those with idiopathic PAH (rest to exercise Ees, 0.99 ± 0.34 mm Hg/mL to 1.49 ± 0.94 mm Hg/mL; P < .001) resulting in ventricular-arterial uncoupling.15 Impaired contractile reserve may reflect disease-specific intrinsic RV systolic dysfunction and may contribute to the reduced response to pulmonary vasodilators observed among patients with PAH associated with systemic sclerosis compared with those with idiopathic PAH.15,53,70
Diagnostic Approach Using Exercise Testing
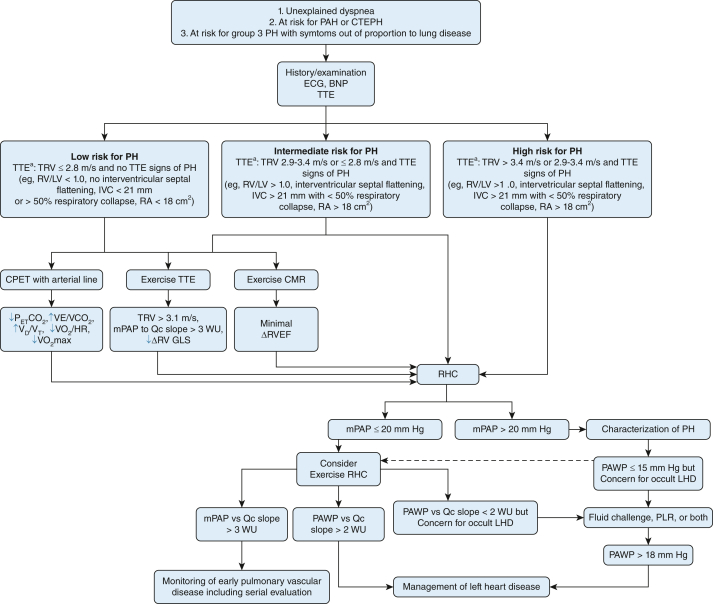
The 2022 joint European Society of Cardiology and European Respiratory Society guidelines outline two clinical scenarios in which exercise RHC is indicated: (1) patients with unexplained dyspnea and normal resting hemodynamics (to detect early pulmonary vascular disease or left heart dysfunction),2 and (2) patients at risk of PAH or CTEPH for prognostic and functional guidance.2 Exertional RV assessment also may be considered for patients with chronic lung diseases when mechanisms of impaired functional capacity are unclear. Detection of early pulmonary vascular disease may influence subsequent counseling and monitoring,11 whereas identification of left-sided heart dysfunction contributing to abnormal exertional pulmonary vascular response may influence risk factor modification and alternative therapies directed at heart failure with preserved ejection fraction.71 Although exertional hemodynamic abnormalities have been demonstrated to progress in some patients over time,42 whether exertional assessment should be performed serially among patients with suspected or established PH is relatively unexplored. Ultimately, the decision to repeat exercise assessment should be individualized based on a patient’s individual risk factors and may be considered particularly among patients with progressive symptoms refractory to treatment. Our approach to the use of exertional RV assessment in suspected pulmonary vascular disease is summarized in Figure 2.
Figure 2.
Flow diagram showing the approach to exercise testing in patients with suspected pulmonary vascular disease. aAdditional TTE risk stratification recommendations per 2022 European Society of Cardiology and European Respiratory Society guidelines for the diagnosis and treatment of pulmonary hypertension.2 BNP = brain natriuretic peptide; CMR = cardiac MRI; CPET = cardiopulmonary exercise testing; CTEPH = chronic thromboembolic pulmonary hypertension; Δ = change; GLS = global longitudinal strain; IVC = inferior vena cava diameter; LHD = left heart dysfunction; LV = left ventricle; mPAP = mean pulmonary artery pressure; mPAP vs Qc slope = mean pulmonary artery pressure vs cardiac output slope; PAH = pulmonary arterial hypertension; PAWP = pulmonary arterial wedge pressure; PAWP vs Qc slope = pulmonary artery wedge pressure vs cardiac output slope; PETCO2 = end-tidal partial pressure of CO2; PH = pulmonary hypertension; PLR = passive leg raise; RA = right atrial area; RHC = right heart catheterization; RV = right ventricle; RVEF = right ventricular ejection fraction; RV/LV = right ventricular to left ventricular diameter ratio; TTE = transthoracic echocardiography; TRV = tricuspid regurgitant jet velocity; VD/VT = dead space fraction (dead space volume to tidal volume ratio); VE/VCO2 = ventilatory equivalent for CO2; VO2/HR = oxygen pulse (oxygen consumption to heart rate ratio); VO2max = maximum oxygen uptake; Qc = cardiac output.
Limited data suggest benefits of PH-targeted therapy for abnormal exertional pulmonary vascular hemodynamics.72 For example, among 10 patients with exercise mPAP of ≥ 30 mm Hg and total pulmonary resistance or slope of mPAP vs Qc slope of ≥ 3 mmHg/L/min, treatment with pulmonary vasodilators for 283 ± 120 days resulted in reduced exercise total pulmonary resistance (4.4 ± 0.2 to 3.2 ± 0.3; P < .05) and increased cardiac index (5.0 ± 0.3 L/min/m2 to 6.1 ± 0.4 L/min/m2; P < .05).72 Among 12 patients with chronic thromboembolic disease, pulmonary endarterectomy reduced mPAP vs Qc slope (3.6 ± 1.0 mmHg/L/min to 2.3 ± 0.8 mmHg/L/min; P < .01), increased VO2max (1.2 ± 0.4 L/min to 1.5 ± 0.3 L/min; P < .05) and improved quality of life.73 Further investigation is needed in different populations of patients with abnormal exertional pulmonary vascular function to determine how exertional hemodynamics should be used to refine management recommendations.
Future Directions
Exercise stress testing will advance our understanding of RV dysfunction and its management. More precise phenotyping of RV response in heterogeneous disease states such as COPD and interstitial lung disease will help to clarify disease mechanisms. Exertional pulmonary vascular function should be assessed as an outcome in clinical trials, and therapies targeting exertional RV function should be explored. In particular, whether using exercise stress testing to diagnose early pulmonary vascular disease allows for modification of the natural history of disease is not known. However, exercise stress testing offers the potential to shift treatment paradigms among patients with PH by helping to identify interventions to treat early pulmonary vascular disease, monitoring response to therapy, and developing novel therapies for exertional RV function.
Summary
Exercise stress testing characterizes the RV response to increased afterload in PH including RV contractile reserve and ventricular-arterial coupling with important implications for exercise capacity, morbidity, and mortality. Exercise stress testing can be used in routine clinical practice to screen patients at risk of early pulmonary vascular disease. Among patients with established PH, exercise stress testing describes RV functional adaptations, identifies comorbid left-sided heart dysfunction, and predicts prognosis. Both noninvasive and invasive methods of exercise testing may be applied to the screening, diagnosis, and risk stratification of suspected and established PH. Ultimately, exercise stress testing may contribute to novel treatment strategies for patients with PH.
Funding/Support
L. M. F. is supported by the National Institutes of Health [Grants T32HL007085-48 and UL1 TR002535], the University of Colorado Division of Pulmonary Sciences and Critical Care Medicine ICON Award, the Colorado Pulmonary Vascular Disease Research Award, the International Society of Travel Medicine Research Award, and the Department of Veterans Affairs Airborne Hazards and Burn Pits Center of Excellence Pilot Project Program. T. L. is supported by the VA Merit Review Award [Grant 2 I01 BX002042-05], the National Institutes of Health [Grants 1R01HL144727-01A1 and 1P01HL158507-01], and the Borstein Family Foundation. B. J. M. is supported by the American Lung Association, the United States Department of Defense [Grant W81XWH-15-10705], the National Institutes of Health [Grants 5U01 HL089897-15 and 5R01 HL136682-05], and the University of Wisconsin. W. K. C. is supported by the University of Colorado Outstanding Early Career Scholarship Program. This work was supported through NIH/NHLBI T32HL007085-48 (L. M. F.).
Financial/Nonfinancial Disclosures
The authors have reported to CHEST the following: T. M. B. receives support from Bayer Corporation, Janssen Pharmaceuticals, Inc, and Liquidia Corporation. T. L. receives support from Altavant Sciences, Allinaire Therapeutics, Bayer Corporation, United Therapeutics Corporation, Guidepoint, and Gerson Lehrman Group, Inc. B. J. M. receives support from AstraZeneca, Boehringer Ingelheim Corp. USA, Glaxo Smith Kline, Integritas Communications, Novartis, Optimum Patient Care Global, PriMed Pharmaceuticals, Inc., Projects in Knowledge, Quintiles, Spiration, WebMD, and Wolters Kluwer Health. W. K. C. receives support from Merck & Co, Inc. None declared (L. M. F.).
Acknowledgments
Author contributions: L. M. F. and W. K. C. were involved in the conceptualization of this work. All authors were involved in the generation of this manuscript and its approval. L. M. F. and W. K. C. are guarantors of the article and take responsibility for the integrity of the work.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
References
- 1.Prins K.W., Rose L., Archer S.L., et al. Clinical determinants and prognostic implications of right ventricular dysfunction in pulmonary hypertension caused by chronic lung disease. J Am Heart Assoc. 2019;8(2):e01–e15. doi: 10.1161/JAHA.118.011464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humbert M., Kovacs G., Hoeper M.M., et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618–3731. doi: 10.1093/eurheartj/ehac237. [DOI] [PubMed] [Google Scholar]
- 3.Lahm T., Douglas I.S., Archer S.L., et al. Assessment of right ventricular function in the research setting: knowledge gaps and pathways forward. An official American Thoracic Society Research Statement. Am J Respir Crit Care Med. 2018;198(4):e15–e43. doi: 10.1164/rccm.201806-1160ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courand P.Y., Pina Jomir G., Khouatra C., et al. Prognostic value of right ventricular ejection fraction in pulmonary arterial hypertension. Eur Respir J. 2015;45(1):139–149. doi: 10.1183/09031936.00158014. [DOI] [PubMed] [Google Scholar]
- 5.Konstam M.A., Kiernan M.S., Bernstein D., et al. Evaluation and management of right-sided heart failure: a scientific statement from the American Heart Association. Circulation. 2018;137(20):e578–e622. doi: 10.1161/CIR.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 6.Cornwell W.K., Tran T., Cerbin L., et al. New insights into resting and exertional right ventricular performance in the healthy heart through real-time pressure-volume analysis. J Physiol. 2020;598(13):2575–2587. doi: 10.1113/JP279759. [DOI] [PubMed] [Google Scholar]
- 7.Edward J., Banchs J., Parker H., Cornwell W. Right ventricular function across the spectrum of health and disease. Heart. 2023;109(5):349–355. doi: 10.1136/heartjnl-2021-320526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliveira R.K.F., Waxman A.B., Hoover P.J., Dellaripa P.F., Systrom D.M. Pulmonary vascular and right ventricular burden during exercise in interstitial lung disease. Chest. 2020;158(1):350–358. doi: 10.1016/j.chest.2020.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degani-Costa L.H., Levarge B., Digumarthy S.R., Eisman A.S., Harris R.S., Lewis G.D. Pulmonary vascular response patterns during exercise in interstitial lung disease. Eur Respir J. 2015;46(3):738–749. doi: 10.1183/09031936.00191014. [DOI] [PubMed] [Google Scholar]
- 10.Blumberg F.C., Arzt M., Lange T., Schroll S., Pfeifer M., Wensel R. Impact of right ventricular reserve on exercise capacity and survival in patients with pulmonary hypertension. Eur J Heart Fail. 2013;15(7):771–775. doi: 10.1093/eurjhf/hft044. [DOI] [PubMed] [Google Scholar]
- 11.Douschan P., Avian A., Foris V., et al. Prognostic value of exercise as compared to resting pulmonary hypertension in patients with normal or mildly elevated pulmonary arterial pressure. Am J Respir Crit Care Med. 2022;206(11):1418–1423. doi: 10.1164/rccm.202112-2856LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ireland C.G., Damico R.L., Kolb T.M., et al. Exercise right ventricular ejection fraction predicts right ventricular contractile reserve. J Heart Lung Transplant. 2021;40(6):504–512. doi: 10.1016/j.healun.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brener M.I., Masoumi A., Ng V.G., et al. Invasive right ventricular pressure-volume analysis: basic principles, clinical applications, and practical recommendations. Circ Heart Fail. 2022;15(1) doi: 10.1161/CIRCHEARTFAILURE.121.009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groepenhoff H., Vonk-Noordegraaf A., van de Veerdonk M.C., Boonstra A., Westerhof N., Bogaard H.J. Prognostic relevance of changes in exercise test variables in pulmonary arterial hypertension. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0072013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu S., Houston B.A., Tampakakis E., et al. Right ventricular functional reserve in pulmonary arterial hypertension. Circulation. 2016;133(24):2413–2422. doi: 10.1161/CIRCULATIONAHA.116.022082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis G.D., Bossone E., Naeije R., et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation. 2013;128(13):1470–1479. doi: 10.1161/CIRCULATIONAHA.112.000667. [DOI] [PubMed] [Google Scholar]
- 17.Singh I., Oliveira R.K.F., Heerdt P., et al. Dynamic right ventricular function response to incremental exercise in pulmonary hypertension. Pulm Circ. 2020;10(3) doi: 10.1177/2045894020950187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilde J.M., Skjørten I., Hansteen V., et al. Haemodynamic responses to exercise in patients with COPD. Eur Respir J. 2013;41(5):1031–1041. doi: 10.1183/09031936.00085612. [DOI] [PubMed] [Google Scholar]
- 19.Portillo K., Torralba Y., Blanco I., et al. Pulmonary hemodynamic profile in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1313–1320. doi: 10.2147/COPD.S78180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Claessen G., La Gerche A., Dymarkowski S., Claus P., Delcroix M., Heidbuchel H. Pulmonary vascular and right ventricular reserve in patients with normalized resting hemodynamics after pulmonary endarterectomy. J Am Heart Assoc. 2015;4(3) doi: 10.1161/JAHA.114.001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tolle J.J., Waxman A.B., Van Horn T.L., Pappagianopoulos P.P., Systrom D.M. Exercise-induced pulmonary arterial hypertension. Circulation. 2008;118(21):2183–2189. doi: 10.1161/CIRCULATIONAHA.108.787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaouat A., Naeije R., Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J. 2008;32(5):1371–1385. doi: 10.1183/09031936.00015608. [DOI] [PubMed] [Google Scholar]
- 23.Stenmark K.R., Fagan K.A., Frid M.G. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99(7):675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 24.Panagiotou M., Church A.C., Johnson M.K., Peacock A.J. Pulmonary vascular and cardiac impairment in interstitial lung disease. Eur Respir Rev. 2017;26(143):160053. doi: 10.1183/16000617.0053-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kholdani C., Fares W.H., Mohsenin V. Pulmonary hypertension in obstructive sleep apnea: is it clinically significant? A critical analysis of the association and pathophysiology. Pulm Circ. 2015;5(2):220–227. doi: 10.1086/679995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Axell R.G., Messer S.J., White P.A., et al. Ventriculo-arterial coupling detects occult RV dysfunction in chronic thromboembolic pulmonary vascular disease. Physiol Rep. 2017;5(7):e13227. doi: 10.14814/phy2.13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tello K., Dalmer A., Axmann J., et al. Reserve of right ventricular-arterial coupling in the setting of chronic overload. Circ Heart Fail. 2019;12(1) doi: 10.1161/CIRCHEARTFAILURE.118.005512. [DOI] [PubMed] [Google Scholar]
- 28.Simonneau G., Montani D., Celermajer D.S., et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spruijt O.A., de Man F.S., Groepenhoff H., et al. The effects of exercise on right ventricular contractility and right ventricular-arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med. 2015;191(9):1050–1057. doi: 10.1164/rccm.201412-2271OC. [DOI] [PubMed] [Google Scholar]
- 30.Boerrigter B.G., Bogaard H.J., Trip P., et al. Ventilatory and cardiocirculatory exercise profiles in COPD: the role of pulmonary hypertension. Chest. 2012;142(5):1166–1174. doi: 10.1378/chest.11-2798. [DOI] [PubMed] [Google Scholar]
- 31.Maron B.A., Cockrill B.A., Waxman A.B., Systrom D.M. The invasive cardiopulmonary exercise test. Circulation. 2013;127(10):1157–1164. doi: 10.1161/CIRCULATIONAHA.112.104463. [DOI] [PubMed] [Google Scholar]
- 32.Sun X.G., Hansen J.E., Oudiz R.J., Wasserman K. Exercise pathophysiology in patients with primary pulmonary hypertension. Circulation. 2001;104(4):429–435. doi: 10.1161/hc2901.093198. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong H.F., Schulze P.C., Bacchetta M., Thirapatarapong W., Bartels M.N. Impact of pulmonary hypertension on exercise performance in patients with interstitial lung disease undergoing evaluation for lung transplantation. Respirology. 2014;19(5):675–682. doi: 10.1111/resp.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neder J.A., Phillips D.B., O’Donnell D.E., Dempsey J.A. Excess ventilation and exertional dyspnoea in heart failure and pulmonary hypertension. Eur Respir J. 2022;60(5):2200144. doi: 10.1183/13993003.00144-2022. [DOI] [PubMed] [Google Scholar]
- 35.McCabe C., Deboeck G., Harvey I., et al. Inefficient exercise gas exchange identifies pulmonary hypertension in chronic thromboembolic obstruction following pulmonary embolism. Thromb Res. 2013;132(6):659–665. doi: 10.1016/j.thromres.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 36.Grünig E., Tiede H., Enyimayew E.O., et al. Assessment and prognostic relevance of right ventricular contractile reserve in patients with severe pulmonary hypertension. Circulation. 2013;128(18):2005–2015. doi: 10.1161/CIRCULATIONAHA.113.001573. [DOI] [PubMed] [Google Scholar]
- 37.Kovacs G., Maier R., Aberer E., et al. Assessment of pulmonary arterial pressure during exercise in collagen vascular disease: echocardiography vs right-sided heart catheterization. Chest. 2010;138(2):270–278. doi: 10.1378/chest.09-2099. [DOI] [PubMed] [Google Scholar]
- 38.Arcasoy S.M., Christie J.D., Ferrari V.A., et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167(5):735–740. doi: 10.1164/rccm.200210-1130OC. [DOI] [PubMed] [Google Scholar]
- 39.Lancellotti P., Pellikka P.A., Budts W., et al. The clinical use of stress echocardiography in non-ischaemic heart disease: recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2016;17(11):1191–1229. doi: 10.1093/ehjci/jew190. [DOI] [PubMed] [Google Scholar]
- 40.Andersen M.J., Nishimura R.A., Borlaug B.A. The hemodynamic basis of exercise intolerance in tricuspid regurgitation. Circ Heart Fail. 2014;7(6):911–917. doi: 10.1161/CIRCHEARTFAILURE.114.001575. [DOI] [PubMed] [Google Scholar]
- 41.Argiento P., Chesler N., Mulè M., et al. Exercise stress echocardiography for the study of the pulmonary circulation. Eur Respir J. 2010;35(6):1273–1278. doi: 10.1183/09031936.00076009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kusunose K., Yamada H., Hotchi J., et al. Prediction of future overt pulmonary hypertension by 6-min walk stress echocardiography in patients with connective tissue disease. J Am Coll Cardiol. 2015;66(4):376–384. doi: 10.1016/j.jacc.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 43.Claessen G., La Gerche A., Voigt J.U., et al. Accuracy of echocardiography to evaluate pulmonary vascular and RV function during exercise. JACC Cardiovasc Imaging. 2016;9(5):532–543. doi: 10.1016/j.jcmg.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 44.D’Andrea A., Stanziola A.A., Saggar R., et al. Right ventricular functional reserve in early-stage idiopathic pulmonary fibrosis: an exercise two-dimensional speckle tracking Doppler echocardiography study. Chest. 2019;155(2):297–306. doi: 10.1016/j.chest.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee M., Mercurio V., Hsu S., et al. Assessment of right ventricular reserve utilizing exercise provocation in systemic sclerosis. Int J Cardiovasc Imaging. 2021;37(7):2137–2147. doi: 10.1007/s10554-021-02237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanz J., García-Alvarez A., Fernández-Friera L., et al. Right ventriculo-arterial coupling in pulmonary hypertension: a magnetic resonance study. Heart. 2012;98(3):238–243. doi: 10.1136/heartjnl-2011-300462. [DOI] [PubMed] [Google Scholar]
- 47.Jaijee S., Quinlan M., Tokarczuk P., et al. Exercise cardiac MRI unmasks right ventricular dysfunction in acute hypoxia and chronic pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2018;315(4):H950–H957. doi: 10.1152/ajpheart.00146.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.La Gerche A., Claessen G., Van de Bruaene A., et al. Cardiac MRI: a new gold standard for ventricular volume quantification during high-intensity exercise. Circ Cardiovasc Imaging. 2013;6(2):329–338. doi: 10.1161/CIRCIMAGING.112.980037. [DOI] [PubMed] [Google Scholar]
- 49.Boerrigter B.G., Waxman A.B., Westerhof N., Vonk-Noordegraaf A., Systrom D.M. Measuring central pulmonary pressures during exercise in COPD: how to cope with respiratory effects. Eur Respir J. 2014;43(5):1316–1325. doi: 10.1183/09031936.00016913. [DOI] [PubMed] [Google Scholar]
- 50.Oliveira R.K., Agarwal M., Tracy J.A., et al. Age-related upper limits of normal for maximum upright exercise pulmonary haemodynamics. Eur Respir J. 2016;47(4):1179–1188. doi: 10.1183/13993003.01307-2015. [DOI] [PubMed] [Google Scholar]
- 51.Nagel C., Marra A.M., Benjamin N., et al. Reduced right ventricular output reserve in patients with systemic sclerosis and mildly elevated pulmonary artery pressure. Arthritis Rheumatol. 2019;71(5):805–816. doi: 10.1002/art.40814. [DOI] [PubMed] [Google Scholar]
- 52.Singh I., Rahaghi F.N., Naeije R., et al. Dynamic right ventricular-pulmonary arterial uncoupling during maximum incremental exercise in exercise pulmonary hypertension and pulmonary arterial hypertension. Pulm Circ. 2019;9(3) doi: 10.1177/2045894019862435. 2045894019862435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tedford R.J., Mudd J.O., Girgis R.E., et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ Heart Fail. 2013;6(5):953–963. doi: 10.1161/CIRCHEARTFAILURE.112.000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Claeys M., Claessen G., La Gerche A., et al. Impaired cardiac reserve and abnormal vascular load limit exercise capacity in chronic thromboembolic disease. JACC Cardiovasc Imaging. 2019;12(8 Pt 1):1444–1456. doi: 10.1016/j.jcmg.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 55.Pellikka P.A., Arruda-Olson A., Chaudhry F.A., et al. Guidelines for performance, interpretation, and application of stress echocardiography in ischemic heart disease: from the American Society of Echocardiography. J Am Soc Echocardiogr. 2020;33(1):1–41.e48. doi: 10.1016/j.echo.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Hansen J.E., Wasserman K. Pathophysiology of activity limitation in patients with interstitial lung disease. Chest. 1996;109(6):1566–1576. doi: 10.1378/chest.109.6.1566. [DOI] [PubMed] [Google Scholar]
- 57.Farina S., Correale M., Bruno N., et al. The role of cardiopulmonary exercise tests in pulmonary arterial hypertension. Eur Respir Rev. 2018;27(148):170134. doi: 10.1183/16000617.0134-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.King C.S., Shlobin O.A. The trouble with group 3 pulmonary hypertension in interstitial lung disease: dilemmas in diagnosis and the conundrum of treatment. Chest. 2020;158(4):1651–1664. doi: 10.1016/j.chest.2020.04.046. [DOI] [PubMed] [Google Scholar]
- 59.Benza R.L., Miller D.P., Gomberg-Maitland M., et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122(2):164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 60.Fowler R.M., Maiorana A.J., Jenkins S.C., Gain K.R., O’Driscoll G., Gabbay E. A comparison of the acute haemodynamic response to aerobic and resistance exercise in subjects with exercise-induced pulmonary arterial hypertension. Eur J Prev Cardiol. 2013;20(4):605–612. doi: 10.1177/2047487312445424. [DOI] [PubMed] [Google Scholar]
- 61.Berlier C., Saxer S., Lichtblau M., et al. Influence of upright versus supine position on resting and exercise hemodynamics in patients assessed for pulmonary hypertension. J Am Heart Assoc. 2022;11(4):e023839. doi: 10.1161/JAHA.121.023839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vachiéry J.L., Tedford R.J., Rosenkranz S., et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53(1):1801897. doi: 10.1183/13993003.01897-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montané B., Tonelli A.R., Arunachalam A., et al. Hemodynamic responses to provocative maneuvers during right heart catheterization. Ann Am Thorac Soc. 2022;19(12):1977–1985. doi: 10.1513/AnnalsATS.202201-077OC. [DOI] [PubMed] [Google Scholar]
- 64.Kremer N., Rako Z., Douschan P., et al. Unmasking right ventricular-arterial uncoupling during fluid challenge in pulmonary hypertension. J Heart Lung Transplant. 2022;41(3):345–355. doi: 10.1016/j.healun.2021.11.019. [DOI] [PubMed] [Google Scholar]
- 65.Ho J.E., Zern E.K., Lau E.S., et al. Exercise pulmonary hypertension predicts clinical outcomes in patients with dyspnea on effort. J Am Coll Cardiol. 2020;75(1):17–26. doi: 10.1016/j.jacc.2019.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Montani D., Girerd B., Jaïs X., et al. Screening for pulmonary arterial hypertension in adults carrying a BMPR2 mutation. Eur Respir J. 2021;58(1):2004229. doi: 10.1183/13993003.04229-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu J., Jani V., Mercurio V., et al. Stress echocardiographic prediction of emerging pulmonary vascular disease in systemic sclerosis. J Am Soc Echocardiogr. 2023;36(2):259–261. doi: 10.1016/j.echo.2022.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Selimovic N., Andersson B., Bech-Hanssen O., Lomsky M., Riise G.C., Rundqvist B. Right ventricular ejection fraction during exercise as a predictor of mortality in patients awaiting lung transplantation: a cohort study. BMJ Open. 2013;3(4):e002108. doi: 10.1136/bmjopen-2012-002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chaouat A., Sitbon O., Mercy M., et al. Prognostic value of exercise pulmonary haemodynamics in pulmonary arterial hypertension. Eur Respir J. 2014;44(3):704–713. doi: 10.1183/09031936.00153613. [DOI] [PubMed] [Google Scholar]
- 70.Argula R.G., Karwa A., Lauer A., et al. Differences in right ventricular functional changes during treatment between systemic sclerosis-associated pulmonary arterial hypertension and idiopathic pulmonary arterial hypertension. Ann Am Thorac Soc. 2017;14(5):682–689. doi: 10.1513/AnnalsATS.201608-655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary. J Am Coll Cardiol. 2022;79(17):1757–1780. doi: 10.1016/j.jacc.2021.12.011. [DOI] [PubMed] [Google Scholar]
- 72.Wallace W.D., Nouraie M., Chan S.Y., Risbano M.G. Treatment of exercise pulmonary hypertension improves pulmonary vascular distensibility. Pulm Circ. 2018;8(3):e1–e12. doi: 10.1177/2045894018787381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guth S., Wiedenroth C.B., Rieth A., et al. Exercise right heart catheterisation before and after pulmonary endarterectomy in patients with chronic thromboembolic disease. Eur Respir J. 2018;52(3):1800458. doi: 10.1183/13993003.00458-2018. [DOI] [PubMed] [Google Scholar]