Abstract
Introduction
Refractory shock, which fails to respond to conventional vasopressor therapy, is a common complication of sepsis. Methylene blue has emerged as a potential adjunctive treatment option for reversing refractory shock in sepsis. The aim of this study was to evaluate the impact of intravenous methylene blue infusion on hemodynamic improvement and mortality in patients with refractory shock.
Methodology
This was an observational prospective study for the duration of six months conducted at intensive care a medical college and teaching hospital including 76 patients with a diagnosis of septic shock requiring vasopressor therapy. Intravenous (IV) methylene blue was infused as a bolus dose with 2 mg/kg dose in 20 minutes and its response to mean arterial blood pressure, decrease in vasopressor therapy, lactate level, and urine output was recorded in next 2 hours. Patients with improvement in mean arterial pressure (MAP) by 10% or decrease in vasopressor therapy in the next 2 hours were leveled as responder. The length of intensive care unit (ICU) stay, duration of mechanical ventilation, incidence of acute kidney injury (AKI), and mortality were compared between responder and non-responder.
Results
A total of 76 patients with refractory shock were included in the study. With the use of IV methylene blue, 41 (53.9%) patients showed significant improvement in MAP within 2 hours (70.17 ± 8.30 vs 64.28 ± 11.84, p = 0.005). Responders were 4.019 times more likely to have vasopressor-free time within 24 hours (18.4% vs 5.3%, p = 0.020, odds ratio 4.019, 95% confidence interval, 1.180–13.682). However, there was no significant difference in terms of mortality, length of ICU stay, ventilator free days, and incidence of AKI. In the responder group, there was a significant increase in the MAP and decrease in vasopressor requirement pre- and post-infusion of methylene blue (p < 0.05). Responder had shorter vasopressor-free days as compared with non-responder (5.34 vs 6.79, p = 0.008) while the mean survival time was longer with responders (21.97 vs 15.93 days, p = 0.024).
Conclusion
The use of IV methylene blue in refractory shock as an adjuvant therapy significantly improved the mean arterial blood pressure and decreased the requirement of vasopressor therapy as well as improvement in the survival time. However, there was no change in the mortality, length of ICU stay, ventilator-free days, or incidence of AKI in the patients.
How to cite this article
Rajbanshi LK, Bajracharya A, Arjyal B, Devkota D. Can Use of Intravenous Methylene Blue Improve the Hemodynamics and Outcome of the Patients with Refractory Septic Shock? An Observational Study. Indian J Crit Care Med 2023;27(9):669–674.
Keyword: Hemodynamics, Methylene blue, Refractory shock, Septic shock, Vasoplegia
Highlights
In this observational study, we examined the potential impact of methylene blue (MB) on the hemodynamics and outcomes of patients with refractory septic shock. By analyzing data from a group of patients, the study aimed to determine whether the use of MB could lead to improvements in hemodynamic variables and clinical outcomes. The findings of this study shed light on the potential benefits of MB in managing refractory septic shock.
Introduction
Sepsis is very often complicated by vasodilatory shock leading to circulatory failure and decreased perfusion to the various vital organs. The underlying inflammation in sepsis releases proinflammatory markers, complement factors, blood clotting factors, neuroendocrine mediators, and various other acute phase reactants leading to vascular endothelial dysfunction and vasodilation.1,2 The sepsis-induced vasodilation is mediated by dysregulated and sustained release of nitric oxide via inducible nitric oxide synthetase (iNOS) present in vascular endothelium.2 Nitric oxide diffuses across cell membranes, activating the second messenger guanylate cyclase, which converts guanosine triphosphate to cyclic guanosine monophosphate (cGMP), resulting in smooth muscle relaxation and severe vasoplegia.3 The nitric oxide-induced vasodilation initially increases the cardiac out-put but later on nitric oxide impairs the uses of ATP by the myocardial cells leading to decreased inotropy and a reduction in cardiac out-put.4 Extensive vasodilation coupled with reduced myocardial contractility gives rise to low blood pressure and, in some cases, can result in unresponsive hypotension that does not react to adrenergic medications.
The various vasoactive drugs like norepinephrine and epinephrine are the first choices of the drugs for the sepsis-induced hypotension. To optimize the hemodynamics in sepsis-induced hypotension may require large doses of the vasoactive drugs. At high dosages, it may be responsible for added risk for adverse events such as dysrhythmias, peripheral ischemia, and increased myocardial oxygen consumption.5
In specific situations, individuals may encounter low blood pressure that does not respond to usual vasoactive drugs, signifying a condition known as refractory shock. Refractory shock is characterized by the necessity for vasopressor dosages surpassing 0.2–0.5 mcg/kg/min of norepinephrine equivalents, or even higher. Additionally, there is a cardiac index of at least 2.2 L/min/m² and difficulties in sustaining mean arterial pressure (MAP) above 65 mm Hg.6,7
Nonadrenergic drugs such as low-dose corticosteroids, vasopressin, and angiotensin II can be considered as alternative management options for sepsis-induced refractory shock.8,9 These nonadrenergic drugs have a catecholamine-sparing effect, which means they can help reduce the required dose of catecholamines while still achieving the desired blood pressure target.8 Nevertheless, there exists an insufficiency of substantial evidence to firmly establish the effectiveness of these nonadrenergic medications in terms of enhancing morbidity and mortality results.
Methylene blue (MB) is one of the catecholamines sparing agent which is recently being used to treat the refractory shock in sepsis.10,11 Methylene blue is known to selectively inhibit iNOS and soluble guanylate cyclase, which helps to counteract the vasodilation caused by nitric oxide. Although norepinephrine is the first-line choice of vasopressor for septic shock in our intensive care unit (ICU) and is administered at a fixed dose protocol of 2.5–20 mcg/min in adult septic patients, MB is regularly administered in our ICU as an adjunctive treatment for refractory shock in septic patients. As outlined in our ICU protocol, refractory shock was defined as septic shock that required norepinephrine alone at a dose exceeding 15 mcg/min, or mandated the use of more than two vasopressor drugs to sustain a MAP more than 65 mm Hg. In such cases, adjuvant therapies were introduced. The specific adjuvant therapy was determined by the treating clinician. For patients receiving MB as a rescue therapy, a bolus dose of 2 mg/kg was administered over a 15-minute period, and the hemodynamic response was evaluated after two hours of infusion. The use of MB or other non-catecholamine vasopressor therapies, such as vasopressin and low-dose hydrocortisone, was primarily determined by the attending physician. Vasopressin was given within a dosage range of 0.01 units/min to 0.04 units/min, and a total of 200 mg of hydrocortisone was administered in four equally divided doses, as directed by the physician.
Similar to other nonadrenergic vasopressors, there are limited data available regarding the actual hemodynamic response of MB in patients with refractory shock. To bridge this knowledge gap, this study was undertaken to evaluate the influence of MB on the hemodynamic response among septic patients experiencing unresponsive shock, as well as its implications for morbidity and mortality outcomes.
The primary objective of the study was to observe the improvement in hemodynamics among patients with refractory septic shock following MB infusion. The secondary aims encompassed evaluating results in relation to 30-day mortality, duration of stay within the ICU, length of mechanical ventilation, and the incidence of acute kidney injury (AKI).
Methodology
Over a period of 6 months, a prospective observational study was carried out in the medical and surgical ICU of an educational medical college hospital located in eastern Nepal. Ethical approval for the study was granted by the Institutional Review Committee (IRC-PA-250/2078-79), and prior to the commencement of the study, informed written consent was obtained from all participants.
The study focused on patients with age more than 18 years with refractory septic shock, where MB was used as an adjunctive non-catecholamine vasopressor therapy. Patients with pre-existing conditions such as acute respiratory distress syndrome, acute or chronic kidney injury, pulmonary hypertension, pregnancy, known glucose-6-phosphate dehydrogenase deficiency, vasospastic diathesis (e.g., Raynaud's syndrome), coronary artery disease, or patients receiving monoamine oxidase inhibitors were excluded from the study.
In this study, a favorable hemodynamic reaction was defined as either a 10% rise in MAP or a reduction of 2 µg in noradrenaline dosage within a 2-hour timeframe subsequent to the administration of MB. For patients who showed initial improvement in hemodynamic variables (increased MAP or decreased norepinephrine dose), MB infusion was continued as a maintenance dose of 0.5 mg/kg/min. Data related to hemodynamics, including parameters like mean arterial blood pressure (MABP), heart rate, lactate levels, urine output, oxygen saturation, and outcome variables, were compiled from both the electronic medical record system and nursing observation sheets. The data retrieval process was conducted by a blinded individual, who was unaware of the study details, to ensure unbiased collection of the data.
Study participants were divided into two cohorts according to their reaction to MB: MB responders and nonresponders. Nonresponders continued to receive conventional treatment with noncatecholamine vasopressors other than MB. An analysis was conducted between the two groups utilizing diverse data elements, encompassing demographic particulars, the Sequential Organ Failure Assessment (SOFA) score assessed 24 hours post MB infusion, as well as hemodynamic metrics like MABP, heart rate, oxygen saturation (SpO2), arterial partial pressure of oxygen (PaO2), the PaO2/FIO2 ratio, mean airway pressure (p mean), vasopressor dosages (noradrenaline), and lactate levels.
In addition, the study analyzed outcome variables including 30-day mortality, duration of mechanical ventilation, length of ICU stay, and the incidence of AKI 24 hours after administering MB, with a focus on comparing the outcomes between the two groups of responders and nonresponders.
Results
The patient data were recorded in Microsoft Excel and subjected to statistical analysis using IBM SPSS version 21. The Kolmogorov-Smirnov test was used to evaluate the normal distribution of various parameters. Continuous variables were compared using either the Student t-test or the Mann–Whitney U test, depending on the characteristics of the data. Proportions were compared using either the Chi-square test or the Fisher exact test, with the choice based on their suitability for each specific analysis. Survival analysis and assessment of vasopressor-free days between responders and nonresponders were performed using the Kaplan–Meier method and Cox regression analysis. A significance level of p < 0.05 was considered statistically significant.
The study enrolled a total of 76 patients with refractory shock who received MB infusion as a non-catecholamine adjuvant therapy. Out of these, 41 (53.9%) patients exhibited improved hemodynamics following the infusion and were designated as the responder group. Conversely, 35 patients (46.1%) did not show any discernible effect from the MB infusion and were categorized as the non-responder group (Fig. 1).
Fig. 1.
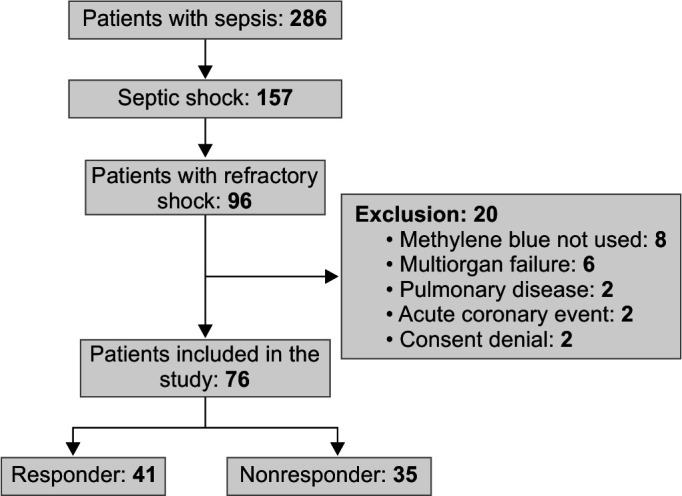
Flow diagram of patient participation in the study
Table 1 depicts a comparison among patients categorized as responders and nonresponders in terms of their demographic characteristics, clinical profiles, and hemodynamic parameters. There were no notable distinctions between the two groups in relation to age, gender, or disease severity evaluated through the SOFA score.
Table 1.
Comparison of the demographic, clinical profile as well as hemodynamic parameters
| Variables | Responder | Nonresponder | p-value |
|---|---|---|---|
| Age (years) | 41.5616.36 | 48.4820.36 | 0.105 |
| Sex | |||
| Male | 20 (26.3%) | 26 (34.2%) | 0.642 |
| Female | 15 (19.7%) | 15 (19.7%) | |
| SOFA | 9.024.65 | 9.975.58 | 0.423 |
| MABP pre-infusion (mm Hg) |
62.5110.54 | 62.9111.84 | 0.876 |
| MABP post-infusion (mm Hg) |
70.178.30 | 64.2811.84 | 0.005 |
| Lactate pre-infusion (mmol/L) |
4.291.83 | 4.602.10 | 0.499 |
| Lactate post-infusion (mmol/L) |
3.501.83 | 4.902.07 | 0.003 |
| UO pre-infusion (mL/2 h) |
70.39.30 | 69.7763.07 | 0.964 |
| UO post-infusion (mL/2 h) |
72.65 | 60.0947.30 | 0.259 |
| Vasopressor dose pre-infusion (µg/min) |
15.374.46 | 15.374.40 | 0.720 |
| Vasopressor post-infusion (µg/min) |
13.074.79 | 16.514.30 | 0.002 |
| FIO2 post-infusion (%) | 48.8018.46 | 54.9420.81 | 0.177 |
| P (cm H20) | 18.196.04 | 16.314.04 | 0.122 |
| PF ratio | 211.46111.25 | 173.8874.84 | 0.094 |
| HR (per minute) | 97.1727.40 | 95.0830.08 | 0.753 |
FIO2, fraction of inspired oxygen; HR, heart rate; MABP, mean arterial pressure; P, mean; PF, PaO2/FIO2; SOFA, sequential organ failure assessment; UO, urine output
Furthermore, there were no significant variations in the initial dose of MB infusion administered to either group. Both the responders and non-responder groups showed similar baseline values for MABP, lactate level, urine output, and vasopressor dose (noradrenaline) prior to MB infusion. However, at the 2-hour mark following MB administration, a statistically significant disparity was observed between the responder and non-responder groups in terms of MABP (p = 0.005), lactate levels (p = 0.003), and the quantity of vasopressor used (p = 0.002). No significant variations were noted between responders and nonresponders with respect to required FIO2, mean airway pressure, PaO2–FIO2 ratio, and heart rate.
Table 2 demonstrated a comparative analysis of hemodynamic variables, including MABP, vasopressor dose, urine output, and lactate levels, before and after the administration of MB in the responder group. The findings revealed a significant improvement in MABP (62.56 ± 10.44 vs. 67.82 ± 9.76, p = 0.021) and a noteworthy decrease in the requirement of noradrenaline (15.37 ± 4.46 vs. 11.07 ± 4.79, p = 0.027) following two hours after MB infusion. However, there was no statistically significant improvement observed in urine output or lactate levels subsequent to the administration of MB.
Table 2.
Subgroup analysis of hemodynamic variables (within responder group)
| Variables | Pre-infusion | Post-infusion | p-value |
|---|---|---|---|
| MABP (mm Hg) | 62.5610.44 | 67.829.76 | 0.021 |
| Vasopressor dose (µg/min) | 15.374.46 | 11.074.79 | 0.027 |
| Urine output (mL/2 h) | 70.3955.30 | 72.6546.38 | 0.841 |
| Lactate (mmol/L) | 4.291.83 | 3.501.83 | 0.058 |
MABP, mean arterial blood pressure
The study evaluated several outcome variables, including the number of ventilator days, length of ICU stay, vasopressor (noradrenaline)-free days within 24 hours and during the study period, incidence of AKI, and 30-day mortality (Table 3). In the 24-hour duration, the responders demonstrated an increased likelihood of being free from vasopressors, with an odds ratio (OR) of 4.019 [95% confidence interval (CI), 1.180–13.682]. Similarly, during the complete study period, a significantly higher number of patients in the responder group were free from vasopressors, with an OR of 2.625 (95% CI, 1.010–6.824, p = 0.045).
Table 3.
Outcome measurement
| Variables | Responder | Nonresponder | p-value | Odds ratio 95% CI |
|---|---|---|---|---|
| Ventilator days | 7.874.43 | 4.66 | 0.563 | 0.908 (0.672–1.225) |
| ICU stay (days) | 11.854.94 | 12.255.00 | 0.725 | 1.071 (0.817–1.406) |
| Vasopressor-free in 24 h (N) | 14 (18.4%) | 4 (5.3%) | 0.020 | 4.019 (1.180–13.682) |
| Total vasopressor free (N) | 21 (27.6) | 10 (13.2%) | 0.045 | 2.625 (1.010–6.824) |
| Acute kidney injury | 6 (7.9%) | 10 (13.2%) | 0.137 | 0.429 (0.138–1.333) |
| Mortality (30 days) | 12 (15.8%) | 16 (21.1%) | 0.138 | 0.491 (0.191–1.265) |
Regarding the number of ventilator days and overall ICU stay, no significant differences were observed among the responder group. However, the nonresponders were more likely to have longer ventilator days (OR 0.908, 95% CI, 0.672–1.225) and ICU stay (OR 1.071, 95% CI, 0.817–1.406), although these differences did not reach statistical significance. There was no significant difference in the incidence of AKI between the responder and non-responder groups.
Finally, there was no significant difference in 30-day mortality between the responder and non-responder groups.
The Kaplan–Meier test (Table 4) demonstrated a notable disparity in the number of vasopressor-free days between the responder and non-responder groups. The responder group achieved vasopressor-free status in an average of 5.34 days, whereas the non-responder group achieved this in 6.79 days (p = 0.008). The Cox regression for vasopressor-free days showed that the responders had a hazard ratio of 2.528 (95% CI, 1.184–5.399, p = 0.013), indicating a statistically significant association (Fig. 2). This suggests that the responders had a greater chance of remaining free from vasopressors compared with the nonresponders.
Table 4.
Log rank test for vasopressor-free days
| Group | Mean | Std. error | 95% CI | p-value |
|---|---|---|---|---|
| Responder | 5.34 | 0.497 | 4.370–6.319 | 0.008 |
| Nonresponder | 6.79 | 0.343 | 6.122–7.465 |
Fig. 2.
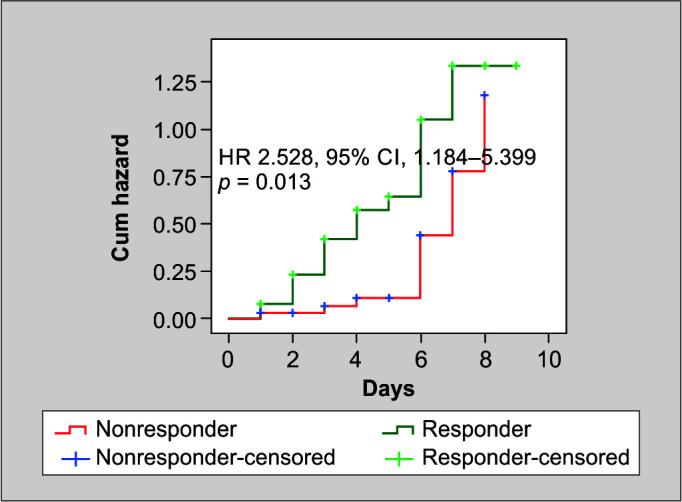
Cox regression analysis for vasopressor free days
The survival analysis was employed by using the Kaplan–Meier test specifically comparing the survival days between responders and nonresponders using the log-rank test (Table 5 and Fig. 3). The results indicated that responders had a significantly longer mean survival duration of 21.97 days, whereas nonresponders had a mean survival duration of 15.93 days (p = 0.024).
Table 5.
Log rank (Kaplan–Meier test) for survival analysis
| Group | Mean | Std. error | CI 95% | p-value |
|---|---|---|---|---|
| Responder | 21.97 | 1.88 | 18.271–25.672 | 0.024 |
| Nonresponder | 15.93 | 2.11 | 11.778–20.082 |
Fig. 3.
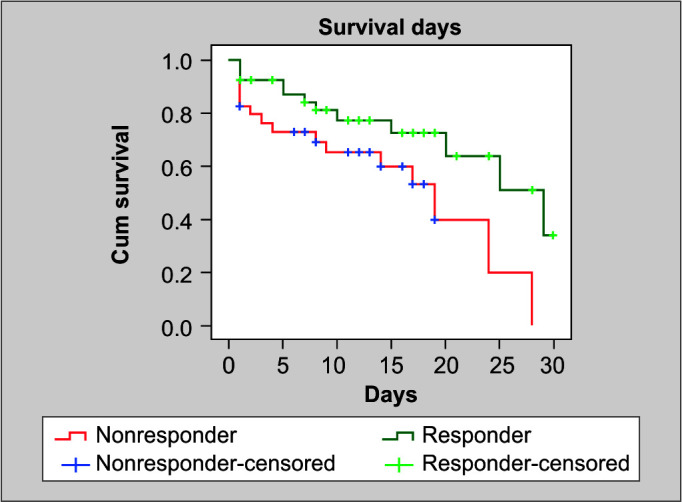
Kaplan–Meier test for survival analysis
Discussion
In this particular study, MB was administered as a rescue therapy for patients with refractory shock, aiming to enhance hemodynamics. The intervention involved administering MB at a dose of 1 mg/kg as a bolus infusion over a duration of half an hour. The results demonstrated a noteworthy improvement in MAP, lactate levels, and the required dosage of vasopressors (p < 0.05). Before the MB infusion, there were no significant differences observed between the groups in terms of hemodynamic variables such as MABP, lactate level, and urine output. Upon comparing the parameters before and after the infusion of MB, the responder group exhibited improvements in MABP and a reduction in the requirement for vasopressor medications. However, no significant changes were observed in terms of lactate levels and urine output in the same group. In this study, no significant difference was observed in the degree of tissue hypoxia, as indicated by lactate levels, among the responders prior to the administration of MB and 2 hours after its administration.
Research into the application of MB for septic shock has been explored in a restricted set of studies, yielding contradictory outcomes. Although a multitude of experimental studies have documented favorable hemodynamic effects, the clinical evidence supporting the use of MB is predominantly derived from case reports, small observational studies, and a limited number of controlled trials.12,13 Despite the observed improvements in MAP and systemic vascular resistance (SVR) following the administration of MB, the evidence supporting a statistically significant improvement in survival outcomes remains inconclusive.10,14–16
Methylene blue has been shown to enhance hemodynamics by improving peripheral vascular resistance and increasing cardiac output. These effects may be attributed to the inhibition of nitric oxide synthase and the restoration of endothelial function.12 Furthermore, MB's antioxidant properties may counteract oxidative stress, which plays a critical role in sepsis-induced organ dysfunction. The findings of our study align with several other studies, which have reported a significant increase in MABP, SVR, and a decrease in the requirement for vasopressor medications with the use of MB.15 Park et al. conducted a study where they observed similar findings, demonstrating that the administration of MB resulted in a transient elevation of MAP by increasing SVR.16 The infusion of MB in patients with refractory shock led to improvements in myocardial function and left ventricular stroke index. This beneficial effect was achieved by enhancing the sensitivity of the cardiovascular system to catecholamines through the inhibition of excessive production of cGMP.10
Our study findings revealed that a significantly higher proportion of patients who responded to MB infusion were able to discontinue the use of vasopressor (noradrenaline) within 24 hours and throughout the entire study period, in comparison to those who did not respond to MB. Nevertheless, there were no significant differences observed in terms of the number of ventilator days, length of stay in the ICU, and 30-day mortality between the two groups. Although there was no significant difference in terms of mortality, it is noteworthy that the mean survival of responders was significantly higher compared with nonresponders. Additionally, patients who responded to MB infusion had a significantly shorter duration of vasopressor-free days. Our study findings align with previous research, indicating that MB administration can lead to improvements in MABP and SVR. However, there is a lack of consistent evidence demonstrating a statistically significant improvement in survival outcomes associated with MB use.10,11,14 Several factors, including the dosing regimen of MB, potential delays in administering MB after the onset of refractory shock, and the initial severity of the patient's clinical condition at the time of intervention, could contribute to the lack of observed mortality benefit in response to MB treatment. A significant factor contributing to the improved survival of patients with refractory shock is the timing of MB infusion in relation to the onset of shock. Recent research supports this notion, as a study on vasoplegic cardiac surgical patients demonstrated that early perioperative administration of MB resulted in improved outcomes, including reduced mortality, compared with patients who received late postoperative intervention.17
Limitations
The study encountered several significant limitations. First, its observational design precluded the establishment of a causal relationship between the use of MB and patient outcomes. This design also made the study susceptible to the influence of confounding factors such as disease severity, timing of MB administration, and clinician preferences, which could have impacted the observed outcomes. Additionally, the absence of randomization introduced the potential for selection bias, limiting the generalizability of the findings. The study's small sample size further restricted its statistical power and precision, potentially affecting the reliability of the results. Furthermore, the limited follow-up period hindered a comprehensive evaluation of long-term outcomes, including survival rates and complications. Given that the study was conducted in a single center, the external validity of the findings to other healthcare settings and patient populations may be limited. Lastly, a major limitation was the lack of consideration for the timing of MB infusion in evaluating its potential mortality benefit. These limitations underscore the need for further research with more robust study designs and larger sample sizes to overcome these shortcomings and provide more definitive conclusions.
Conclusion
MB infusion led to improvement in MABP and a reduction in vasopressor therapy in a significant number of patients. However, these positive effects did not translate into decreased mortality rates, decreased incidence of AKI, reduced number of ventilator days, or length of ICU stay. These findings suggest that while MB may have beneficial hemodynamic effects, its overall impact on patient outcomes in refractory septic shock remains limited. Further research, including randomized controlled trials, is warranted to better understand the potential benefits and limitations of MB in this patient population.
Orcid
Lalit Kumar Rajbanshi https://orcid.org/0000-0001-7531-634X
Akriti Bajracharya https://orcid.org/0000-0001-5948-161X
Batsalya Arjyal https://orcid.org/0000-0001-6939-4544
Dikshya Devkota https://orcid.org/0000-0003-1192-1086
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 2.Jentzer JC, Vallabhajosyula S, Khanna AK, Chawla LS, Busse LW, Kashani KB. Management of refractory vasodilatory shock. Chest. 2018;154(2):416–426. doi: 10.1016/j.chest.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Murad F. The 1996 Albert Lasker medical research awards: Signal transduction using nitric oxide and cyclic guanosine monophosphate. JAMA. 1996;276(14):1189–1192. 8827977 [PubMed] [Google Scholar]
- 4.de Belder AJ, Radomski MW, Why HJ, Richardson PJ, Bucknall CA, Salas E, et al. Nitric oxide synthase activities in human myocardium. Lancet. 1993;341(8837):84–85. doi: 10.1016/0140-6736(93)92559-c. [DOI] [PubMed] [Google Scholar]
- 5.Dünser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: Adverse effects of adrenergic stress. J Intensive Care Med. 2009;24(5):293–316. doi: 10.1177/0885066609340519. [DOI] [PubMed] [Google Scholar]
- 6.Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H, et al. Angiotensin II for the treatment of vasodilatory shock. N Engl J Med. 2017;377(5):419–430. doi: 10.1056/NEJMoa1704154. [DOI] [PubMed] [Google Scholar]
- 7.Ortoleva JP, Cobey FC. A systematic approach to the treatment of vaso-plegia based on recent advances in pharmacotherapy. J Cardiothorac Vasc Anesth. 2019;33(5):1310–1314. doi: 10.1053/j.jvca.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 8.Puntillo F, Giglio M, Pasqualucci A, Brienza N, Paladini A, Varrassi G. Vasopressor-sparing action of methylene blue in severe sepsis and shock: A narrative review. Adv Ther. 2020;37(9):3692–3706. doi: 10.1007/s12325-020-01422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lahiry S, Thakur S, Chakraborty DS. Advances in vasodilatory shock: A concise review. Indian J Crit Care Med. 2019;23(10):475–480. doi: 10.5005/jp-journals-10071-23266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Memis D, Karamanlioglu B, Yuksel M, Gemlik I, Pamukcu Z. The influence of methylene blue infusion on cytokine levels during severe sepsis. Anaesth Intensive Care. 2002;30(6):755–762. doi: 10.1177/0310057X0203000606. [DOI] [PubMed] [Google Scholar]
- 11.Kirov MY, Evgenov OV, Evgenov NV, Egorina EM, Sovershaev MA, Sveinbjørnsson B, et al. Infusion of methylene blue in human septic shock: A pilot, randomized, controlled study. Crit Care Med. 2001;29(10):1860–1867. doi: 10.1097/00003246-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Jang DH, Nelson LS, Hoffman RS. Methylene blue for distributive shock: a potential new use of an old antidote. J Med Toxicol. 2013;9(3):242–249. doi: 10.1007/s13181-013-0298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaiswal A, Kumar M, Silver E. Extended continuous infusion of methylene blue for refractory septic shock. Indian J Crit Care Med. 2020;24(3):206–207. doi: 10.5005/jp-journals-10071-23376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donati A, Conti G, Loggi S, Münch C, Coltrinari R, Pelaia P, et al. Does methylene blue administration to septic shock patients affect vascular permeability and blood volume? Crit Care Med. 2002;30(10):2271–2277. doi: 10.1097/00003246-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Andresen M, Dougnac A, Díaz O, Hernández G, Castillo L, Bugedo G, et al. Use of methylene blue in patients with refractory septic shock: Impact on hemodynamics and gas exchange. J Crit Care. 1998;13(4):164–168. doi: 10.1016/s0883-9441(98)90001-6. [DOI] [PubMed] [Google Scholar]
- 16.Park BK, Shim TS, Lim CM, Lee SD, Kim WS, Kim DS, et al. The effects of methylene blue on hemodynamic parameters and cytokine levels in refractory septic shock. Korean J Intern Med. 2005;20(2):123–128. doi: 10.3904/kjim.2005.20.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehaffey JH, Johnston LE, Hawkins RB, Charles EJ, Yarboro L, Kern JA, et al. Methylene blue for vasoplegic syndrome after cardiac operation: Early administration improves survival. Ann Thorac Surg. 2017;104(1):36–41. doi: 10.1016/j.athoracsur.2017.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]


