Abstract
Estrogen plays a prominent role in regulating and coordinating energy homeostasis throughout the growth, development, reproduction, and aging of women. Estrogen receptors (ERs) are widely expressed in the brain and nearly all tissues of the body. Within the brain, central estrogen via ER regulates appetite and energy expenditure and maintains cell glucose metabolism, including glucose transport, aerobic glycolysis, and mitochondrial function. In the whole body, estrogen has shown beneficial effects on weight control, fat distribution, glucose and insulin resistance, and adipokine secretion. As demonstrated by multiple in vitro and in vivo studies, menopause-related decline of circulating estrogen may induce the disturbance of metabolic signals and a significant decrease in bioenergetics, which could trigger an increased incidence of late-onset Alzheimer’s disease, type 2 diabetes mellitus, hypertension, and cardiovascular diseases in postmenopausal women. In this article, we have systematically reviewed the role of estrogen and ERs in body composition and lipid/glucose profile variation occurring with menopause, which may provide a better insight into the efficacy of hormone therapy in maintaining energy metabolic homeostasis and hold a clue for development of novel therapeutic approaches for target tissue diseases.
Keywords: energy homeostasis, estrogen, glucose, lipid, menopause, obesity
Introduction
Abnormal energy metabolism may lead to a series of physical disorders and predispose postmenopausal women to obesity, type 2 diabetes mellitus, Alzheimer’s disease (AD), and cardiovascular disease. 1 The incidence of metabolic diseases shows a gender-specific character, with females being particularly at risk of age-correlated metabolic pathology.2,3 Premenopausal women exhibit a healthier metabolic pattern compared to age-matched men. However, in the postmenopausal state, with the absence of estrogen, women experience general variations in glucose and lipid profiles, as well as the redistribution of body fat, triggering a significant increase in metabolic-related diseases and all-cause mortality. 4 These findings verify that estrogen is a fundamental regulator in maintaining female energy homeostasis.
Estrogens, as a category of steroid hormones, include estradiol (E2), estriol (E3), and estrone (E1). In humans, estradiol is the primary circulating estrogen hormone that mediates signals through the intracellular nucleus, plasma, and membrane-associated estrogen receptors (ERs). 5 Estrogen is among the most essential hormones in women and gets involved in the initiation, development, and maintenance of reproductive and physiological functions across the body tissues. 6 With the improved life expectancy and the high prevalence of postmenopausal metabolic dysfunction, the beneficial effect of estrogen (mainly referred to estradiol) on central and peripheral energy balance has gained extensive attention. In this study, we review how estrogen contributes to bioenergetic systems in the brain and whole body through ERs signaling in women. Besides, the choice of a particular regimen, dosage, and timing of estrogen therapy have been discussed, with a special emphasis on its regulation of metabolism and clinical implications.
Estrogen and ERs
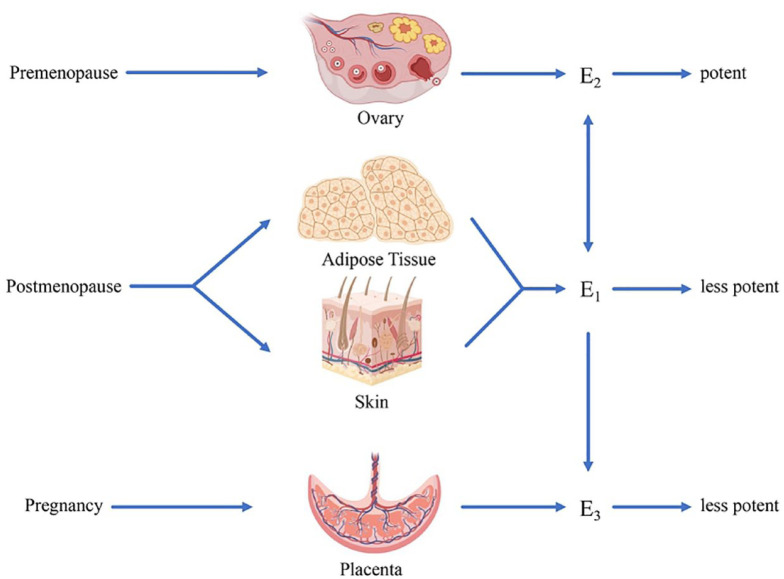
Estrogens refer to C18 steroids that are derived from cholesterol, catalyzed by the enzyme aromatase, and converted from the C19 precursor, androgen. The aromatase is detected in various human tissues, such as ovarian granulosa cells, adipose and skin fibroblasts, placental syncytiotrophoblast, brain, and bone. 7 With the highest affinity to ERs, E2 is primarily secreted by ovarian granulosa cells in premenopausal women. E1 and E3 are the metabolites of E2, both of which are much less potent than E2. 8 E1, as the main estrogen after menopause, is synthesized in peripheral adipose and skin tissue with precursor androstenedione produced by the adrenal cortex. E3 is mainly produced by the placenta in pregnant women (Figure 1). Orally ingested exogenous estrogen first passes from the intestine to the liver and is rapidly metabolized into less potent E1 before reaching the specific tissue. 9 The first pass decreases the estrogen level in circulation and affects other liver metabolic functions (i.e. coagulation and lipid metabolism). 10 Later, metabolized estrogen in the gut is reabsorbed into circulation through enterohepatic circulation with the help of enteral bacterial flora. Coadministration with drugs that disrupt the gut microbiota, such as antibiotics, may inhibit the effectiveness of estrogen replacement therapy.11,12 Therefore, it is essential to individually design the route of administration of estrogen replacement therapy and evaluate specific combined medication to avoid drug–drug interaction.
Figure 1.
Schematic representation of biosynthesis of estradiol (E2), estriol (E3), and estrone (E1). E2, with the highest affinity to ERs, is primarily secreted by ovarian granulosa cells in premenopausal women. E1 and E3 are the metabolites of E2, both of which are much less potent than E2. Besides, E1 is the main estrogen after menopause synthesized in peripheral adipose and skin tissue, while E3 is mainly produced by the placenta in pregnant women.
Estrogen signaling is mediated by ERs, which belong to the superfamily of nuclear receptors and act as ligand-activated transcription factors (TFs). 13 ERs have two subtypes: ERα and ERβ. The human ERα is located on chromosome 6 and composed of 595 amino acids, whereas ERβ is located on chromosome 14 with 530 amino acids. Both of them share similar structural and functional features, with six distinct domains. There are two highly conserved regions, in which one is the DNA-binding domain (region C) with 97% amino acid homology between the ERs, and the other is the ligand-binding domain (region E with 56% identity) harboring a hormone-binding site with a dimerization interface and ligand-dependent transactivation function (AF-2). 14 The distribution of ERs is tissue biased and cell specific. Although both ERs are widely expressed in the brain and nearly all tissues of the body, ERα is predominate in the uterus, hypothalamus/pituitary gland, breast, liver, skeletal muscle, bone, and adipose tissue (AT) where ERβ plays a subordinate role, whereas ERβ is considered to be critical in ovary, gastrointestinal, cardiovascular and central nervous systems (CNSs), etc. 15 Even in a single tissue (e.g. in the ovary), ERα is predominant in theca cells and ERβ is found in granulosa cells. 16 Thus, the differential expression of the ERs is also the foundation for exploring selective estrogen receptor modulators (SERMs), which bind to a certain subtype of ERs to induce either agonist or antagonist effects. 17 Monomeric ER is activated by E2 to form homo- or heterodimer regulating followed transcription. In heterodimer, ERα is reported as the dominant partner. 18
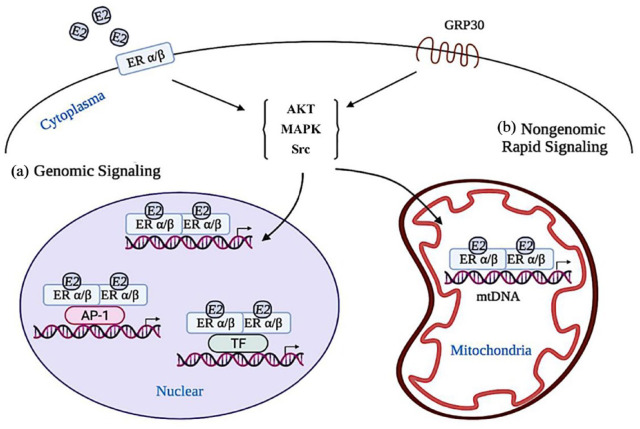
Estrogens are lipid-soluble and easily diffuse through the cell and nuclear membrane, attaching to dimerized ERs at the nuclear, mitochondria, and the peri-membrane (Figure 2). 19 ER-mediated nuclear signaling, as the classic cellular response to E2, includes two different models occurring within hours. Estrogen response element (ERE)-dependent signaling pathway is that E2-activated dimerized ERs directly bind to ERE on the promotor of target protein regulating transcriptional responses. The other ERE-independent signaling pathway is that E2-activated ERs modulate specific TFs (such as Fos/Jun) or activator protein 1 (AP-1) and bind to the TF/AP-1 response element of the target protein indirectly regulating transcriptional responses. 20 In comparison to ERα, ERβ has a lower affinity to ERE. 21 Except for the above genomic signaling, extranuclear events are mediated by nongenomic ERs rapid signaling that occurs within minutes or seconds across the plasma membrane. With the exposure of E2, member-embedded ERs or G protein-coupled ER (also called GPR30) are initiated and subsequently activate multiple signaling pathways, such as Src/ phosphatidylinositol-3-kinase (PI3K), Ras/Raf/MEKK/ extracellular signal-regulated kinase (ERK), mitogen-activated protein kinase (MAPK)/ERK, PI3K/protein kinase B (AKT), cAMP/ Protein kinase A (PKA), and JNK signaling cascades, to induce downstream ion fluxes and proteins kinases activation.22–24 Here, whether GPR 30 is a kind of member ER or a kind of G protein coupled with the original ER remains controversial.25,26 In recent years, other types of membrane estrogen receptors (mERs) have been reported. ER-X is reported as a new plasma membrane-associated estrogen-binding protein that participates in estrogen-activated MAPK cascades to alleviate brain injury. 27 Gαq-coupled mER (Gαq-mER) activated by E2 or selectively targeted by STX, a diphenylacrylamide compound that does not bind to either ERα or ERβ, modulates the gamma-aminobutyric acid type B signaling and induces both opposite effects on hypothalamic orexigenic and anorexigenic neurons.28,29 Recent evidence shows that mitochondria are also the target organelles of E2 where both ERα and ERβ are localized for maintaining cellular bioenergetics. 19 Meanwhile, mitochondria DNA (mtDNA) contains ERE-like sequences, indicating the regulation of E2 on mtDNA transcription via ERs signaling. Despite a large number of studies, the molecular mechanism of E2 signaling and the role of ERα or ERβ in regulating cellular events of phosphorylation, acetylation, glycosylation, and ubiquitination is still a developing area of investigation.
Figure 2.
Overview of intracellular 17β-estradiol (E2) signaling. E2 signaling is mediated by ERs, which include two typical isoforms, ERα and ERβ. (a) Genomic signaling involves two different models occurring within hours. E2-activated dimerized ERs directly bind to nuclear ERE on the promotor of target protein regulating transcriptional responses, or modulating specific transcription factors or activator protein 1 indirectly regulating transcriptional responses. (b) Nongenomic ERs rapid signaling occurs within minutes or seconds across the plasma membrane. Member-embedded ERs or G protein-coupled ER (GPR30) are initiated by E2 and subsequently activate multiple signaling pathways, such as Src, MAPK, and AKT signaling cascades, to induce downstream ion fluxes and protein kinases activation. Mitochondria is also the target organelle of E2, where both ERα and ERβ are localized for maintaining cellular bioenergetics. Mitochondrial DNA contains ERE-like sequences, which is activated by E2-ER to regulate mitochondrial function.
ER, estrogen receptor; ERE, estrogen response element.
Estrogen and central energy homeostasis
Many different cell types in the human brain, including astrocytes and neurons, may express aromatase. 30 Thus, E2, as an important modulator of central bioenergetics, may exert its effect by crossing the blood–brain barrier (BBB) into the brain from the peripheral circulation, or being made from cholesterol within the brain. 31 ERα and ERβ, which serve different roles in the brain, are widely expressed in the hypothalamus, hippocampus, neocortex, preoptic area, septum, amygdala, and the periaquaductal central gray. 32 The central bioenergetics system is modulated by E2 signaling mainly via (1) regulating glucose metabolism (the predominant preference in the brain) and providing adenosine triphosphate (ATP) and precursors for sustaining physiological brain function and (2) controlling the energy balance between energy expenditure and energy intake. 33
Estrogen regulation of central glucose metabolism
As revealed by recent clinical studies, heightened estrogen levels are correlated with increased cerebral glucose utilization, thereby affecting cognitive and behavioral functions.34–37 Estrogen induces a series of nongenomic signaling pathways including MAPK, PI3K/AKT, c-Fos, Protein kinase C (PKC), and Ca2+ influx in the brain to sustain mitochondria function and regulate the glucose transports (GLUTs) into the cell, aerobic glycolysis (glycolysis coupled to the citric acid cycle) derived oxidative phosphorylation and ATP generation.24,38,39
Glucose enters into the brain from the blood by utilizing its concentration gradient, which propels glucose-facilitated transport across the plasma membrane via GLUTs. GLUTs consist of three subfamilies with a total of 13 family members (GLUT1-12 and H+/myo-inositol transporter), among which GLUT1 and GLUT3 are found most popular in the brain, while other isoforms, such as GLUTs 4, 6, 8 have also been detected in brain with minor expression. 40 Neuronal energy deficit brought on by GLUTs may play an important role in AD development. 41 Meanwhile, scientific evidence from animal studies suggests a potential protective mechanism of estrogen associated with GLUTs. Ovariectomy (OVX) may directly decrease the GLUT1 and GLUT3 expression in the rat’s brain and cause impaired insulin sensitivity and oxidative stress.42,43 In vivo treatment with E2 demonstrates a notable augmentation in the uptake of 2-deoxy-[14C]glucose into the BBB endothelial cells of ovariectomized female rates, implying that the observed effect of E2 is attributable to its regulatory influence on GLUT-1 mRNA and protein expression. 44 Given that GLUT is insulin sensitive, so the neuroprotective estrogen upregulates insulin growth factor-1 (IGF-1) signaling via the synergistic effect of ERs and IGF-1 receptor, activates MARK and PI3K cascades, and increases the GLUT3,4 expression in cerebral cortical neurons of ovariectomized rhesus monkeys. 45
It has been reported in wild-type mice with an age-dependent decline of aerobic glycolysis enzymes and a concomitant decrease in lactate. Lactate is the metabolite of aerobic glycolysis and the substrate fuel for aerobic respiration that is associated with memory consolidation in the frontal cortex. 46 E2 promotes neuronal aerobic glycolysis by activating hexokinase (HK), phosphofructokinase, and pyruvate kinase within 4 h in rat brains. 47 While HK is considered to bind to voltage-dependent anion channel directly coupling intramitochondrial ATP synthesis, E2 has been announced to activate Akt, increase HK activity and herein, and trigger enhanced glucose metabolism in vitro study.2,48,49 As shown by additional evidence, E2 enhances glycolytic activity by increasing aconitase, decreasing malate dehydrogenase, and increasing glutamate dehydrogenase and glutamate oxaloacetate transaminase-2 in ovariectomized female rat. 50
Furthermore, the E2 signal has been identified as one of the major signals that converge upon mitochondria to exert its neuroprotective effect. Mitochondria malfunction may cause many neurocognitive and neurodegenerative disorders, such as AD, depression, and anxiety, which show a sex-specific prevalence.51–54 Proteomic analysis of brain mitochondria of female rats indicates that E2 regulates the expression of pyruvate dehydrogenase (PDH), a pivotal enzyme that transforms the pyruvate to acetyl CoA, provides substrate in the citric acid cycle, concomitantly increases oxidative phosphorylation and ATP synthase, and decreases β-oxidation. 50 According to another in vivo data, E2- and progesterone-treated rats brain mitochondria display enhanced respiratory function coupled with increased expression of the electron transport chain complex IV (cytochrome C oxidase). 55 Notably, the research by Irwin et al. 56 demonstrates that medroxyprogesterone acetate (MPA) inhibits the upregulation of brain mitochondrial activity by E2 and induces a decrease in the mitochondrial expression of PDH, cytochrome oxidase, ATP synthase, manganese-superoxide dismutase, and peroxiredoxin V in ovariectomized female rats, suggesting that different hormone regimens may exert opposing effects on mitochondria function. 56 Moreover, animal data show that E2 has a protective effect on the mitochondria against the accumulation of amyloid beta and hyperphosphorylation of tau, which is involved in increased mitochondrial calcium uptake and decreased ATP production. 57 Excessive intracellular calcium rise of hippocampal neurons in rat fetuses may promote glutamate excitotoxicity, which is attenuated by E2 treatment via improvement of the mitochondrial sequestration of cytosolic Ca (2+) coupled with an increased expression of anti-apoptotic protein Bcl-2 to sustain the Ca(2+) load tolerance. 58
Both ERα and ERβ were examined with SERMs in animal experiments, each of which displayed an independent capability in upregulating the mitochondria proteins. Targeting ERβ exhibits greater efficacy in mitochondrial respiration.59,60 Recent studies have also highlighted the role of ERβ in triggering more sensitive mitochondrial calcium permeability in females than in males. 61
Due to the pivotal function of mitochondria in central bioenergetics, many studies have been conducted to develop pharmaceuticals targeting the complex mechanism of mitochondria. 62 Currently, the treatment of neurodegenerative disorders by mitochondrial transplantation is also a hot topic. 63 However, it is noteworthy that many discoveries are based on animal research, raising questions about whether they can actually be applied to humans.
Estrogen regulation of central appetite and energy expenditure
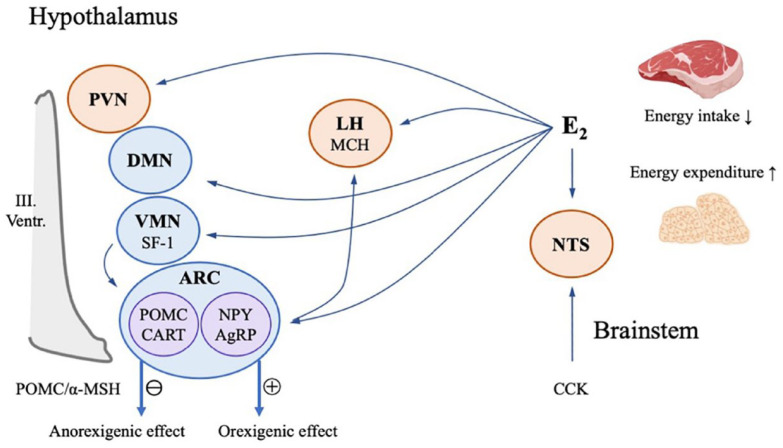
Energy homeostasis, a delicate balance between energy expenditure and energy intake, is regulated by two coordinated networks, namely homeostatic and hedonic neural circuits. 64 Homeostatic regulation of energy balance is mainly reliant on hypothalamic and brainstem-associated neuronal populations, including those in the arcuate nucleus (ARC), lateral hypothalamus (LH), ventromedial nucleus (VMN), dorsomedial nucleus, and paraventricular nucleus, which are modulated by E2 signaling, activate ER, and Gαq-mER to trigger later signaling cascades involving PI3K, PKC, PKA, and neuronal nitric oxide synthase, deliver orexigenic or anorexigenic signals to stimulate, or suppress the energy intake and the energy expenditure 65 (Figure 3). ERα is dominant in hypothalamic metabolic regulation. 66 The ERα mutant mice that only signal via nonclassical pathways are shown to be sufficient for restoring metabolic deficit to normal or near-normal values, indicating that the membrane-based signaling pathway also plays a pivotal role in maintaining energy homeostasis.28,67
Figure 3.
Model of 17β-estradiol (E2)’s homeostatic regulation of energy balance in the brain. Brain regulation of energy homeostasis is mainly reliant on hypothalamic and brainstem-associated neuronal populations, including those in the arcuate nucleus (ARC), lateral hypothalamus (LH), ventromedial nucleus (VMN), dorsomedial nucleus, and the paraventricular nucleus, which are modulated by E2 signaling, and deliver orexigenic or anorexigenic signals to stimulate or suppress the energy intake and the energy expenditure. Proopiomelanocortin (POMC)/cocaine- and amphetamine-regulated transcript neurons and neuropeptide Y (NPY)/agouti-related peptide (AgRP) neurons in ARC may regulate the body energy status through POMC/α-melanocortin stimulating hormone signaling. POMC neurons are impinged by steroidogenic factor-1 neurons in the VMN, while NPY/AgRP neurons project to melanin-concentrating hormone neurons in LH. Nucleus tractus solitarius in the brainstem, which receives abdominal vagal afferent projections activated by the peptide cholecystokinin, may modulate the feeding inhibition effect via E2 signaling.
Evidence shows a gender difference in food cravings, binge eating disorders, and obesity. 68 Women are 3% more likely to be overweight than men. In addition, female food consumption fluctuates across the menstrual cycle, as observed by preclinical and clinical studies. Women in the luteal phase present an increased food craving and eating behavior with a lower E2 level compared with the follicular phase. 69 As suggested by animal experiments, E2 might attenuate the cannabinoid-induced hypothermia, hyperphagia, and the decrease in glutamatergic neurotransmission at proopiomelanocortin (POMC) neurons in the ARC of ovariectomized female guinea pigs. 70 POMC neurons, impinged by steroidogenic factor-1 neurons in the VMN, are critical anorexigenic synapses. From the data on mice and rats, it could be observed that the activated POMC neurons release satiety mediator α-melanocortin stimulating hormone signaling (α-MSH), β-endorphin, as well as co-express cocaine- and amphetamine-regulated transcript, which are involved in the glucose metabolism.71,72 Conversely, together with agouti-related peptide (AgRP) co-expressing neurons in ARC, neuropeptide Y (NPY) promotes the central orexigenic or appetite-stimulating effect, ultimately inducing feeding and reducing energy expenditure through the inhibition of POMC/α-MSH signaling. 73 As elucidated by studies, NPY neurons can be modulated by E2. For example, increased NPY expression induced by OVX can be reversed by E2 administration in rats. 74 Due to its location, the ARC is in direct contact with circulating hormones, sustaining energy homeostasis through the communication of the energy status between the body and brain. 65
In addition to ARC neurons, other hypothalamic neurons also participate in E2’s regulation of homeostatic energy balance. Melanin-concentrating hormone (MCH) neurons are located in the LH area, receive inputs from NPY/AgRP neurons in ARC, and synergistically induce the orexigenic effect. MCH is upregulated when E2 is low. 75 As claimed by Musatov et al., 76 site-specific silencing of ERα in the ventromedial hypothalamus (VMH) decreases the E2-induced energy expenditure and leads to weight gain and visceral fat accumulation in female mice and rats. 76 Another animal study indicates that the feeding inhibition effect may also be mediated by ERα signaling in nucleus tractus solitarius, an area of the brain that receives abdominal vagal afferent projections, which may be activated by the peptide cholecystokinin, released by duodenal I cells. 77
Alongside the homeostatic neural circuits, energy homeostasis is also modulated by hedonic feeding behavior, namely reward-based food intake. Multiple evidence suggest that reward and drug addiction converge upon a common pathway to mediate motivated behavior within the limbic system.78,79 The hedonic regulation of feeding is reliant on the mesolimbic dopamine (A10) neurons that emanate from the ventral tegmental area (VTA) and project onto other regions, including the nucleus accumbens, prefrontal cortex, hippocampus, and amygdala. ERs are expressed in VTA. Activation of ERα enhances the ethanol-induced VTA sensitivity and causes binge-like alcohol drinking by female mice, not males. 80 Women present more rapid drug addiction and greater withdrawal response, due to increased dopamine release by E2 stimulation in the dorsolateral striatum, but not in nucleus accumbens. 81 Interestingly, intra-VTA microinjection of E2 conversely shows a significant decrease in food-motivated behavior of female rats. 82 Therefore, these variable results between the drug versus food rewards in women, as well as the sex differences in motivated behavior, promote us to further explore the role of estrogen in regulating hedonic feeding behavior.
Estrogen and peripheral energy homeostasis
In addition to the brain, estrogen is closely related to energy homeostasis in the whole body. During the reproductive age, the average level of circulating E2 is 100–250 pg/mg. However, along with the cessation of oocyte production, the E2 level declines up to 10 pg/ml. 83 Clinically, menopause is diagnosed with 12 months of amenorrhea following the final menstrual period. Epidemiological studies spanning 35 countries reported that the overall average age of menopause for women with natural menopause is 48.8 years, with regional differences. 84 The change in E2 with the menopausal transition may be related to a series of dysregulation of peripheral lipid and glucose metabolism, which affects the body weight, body fat redistribution, fatty acid metabolism, various lipid profiles, and adipokines circulating in serum, and leads to an increase in the development of type 2 diabetes mellitus, hypertension, and cardiovascular diseases in postmenopausal women. 85
Estrogen, obesity, and adiposity
Obesity, defined as the body mass index (BMI, weight in kg/height in m2) ⩾30, resulted from an imbalance between energy expenditure and energy intake. It was reported that women are twice as likely to suffer from obesity than men worldwide. 86 Earlier, it is commonly believed that weight gain in postmenopausal women is attributed to estrogen deficiency. 87 However, according to the 2016 Guideline of the International Menopause Society, multiple studies have obtained consistent results that the weight gain seen in midlife women is attributed to aging and environmental factors, such as inactivity, urbanization, higher parity, etc., not menopause. 88 Instead, the alternation of the E2 milieu at menopause is mainly associated with a significant increase in total fat mass and abdominal obesity, especially visceral adipose deposition.89,90 Abdominal obesity, defined as a raised waist circumference, is confirmed to be associated with higher cardiovascular mortality compared with high BMI-defined obesity.4,91,92
The distribution of regional AT is different between women versus men. The sexual dimorphism in total fat distribution is attributed to sex-linked genes. 93 Premenopausal women hold a relatively healthier glutei-femoral pattern of fat accumulation than age-matched men. After menopause, women experience a significant increase in total fat mass and redistribution of AT, resulting in a high risk of abdominal obesity. Although the underlying process is not yet fully understood, some studies have suggested that postmenopausal visceral and non-subcutaneous AT fat deposition is caused by tissue-specific control of estrogen through ERs signaling.94,95 Another study proposed that E2 exerts a lipolysis effect by upregulating α2A-adrenergic receptors in human subcutaneous AT, rather than visceral AT. 96
Both ER isoforms are present in human AT, but their distribution is not equal, with a large predominance of ERα expression. For instance, it was reported from a study of overweight-to-obese premenopausal women that ERα is dominant in abdominal subcutaneous AT, whereas ERβ is dominant in gluteal fat. 97 As suggested by animal research, the age-associated E2 deficiency alters the ERα/β ratio to greater ERβ in visceral AT of rats and causes increased adiposity. 98 Apart from that, the expression of ERa is identical between sexes, whereas ERβ level is higher in women than in men. In an In vitro study, E2 upregulates both ERa and ERβ expression in female subcutaneous adipocytes, but only ERα expression in both subcutaneous and visceral adipocytes in men. 99 In addition, ERα but not ERβ-deficient male mice develop obesity after sexual maturation. 100 This sex dimorphism in the distribution of ERs may explain the android and gynoid body shape between men and women. ERs mediate crucial estrogen signaling pathways in both women and men. Notably, the distinct estrogen levels, tissue-specific distributions of ERs, and intricate interactions with other hormones, such as testosterone, bestow distinct physiological and developmental effects in each sex.101,102
It was previously believed that ERa and ERβ perform opposing roles in the metabolism of glucose and lipids. 103 As demonstrated by Davis et al., 104 selective ERα silence in AT of adult mice increases adiposity and inflammation. Another piece of evidence indicates that ERα signaling has a protective effect on white AT of both sexes, and ERα-knockout (αERKO) mice show enhanced fat accumulation, namely adipocyte hypertrophy and hyperplasia. 105 The observations discussed above lead to certain speculation that with the absence of ERα, this unhealthy adipose phenotype may be regulated through ERβ signaling. 2 With a deepened understanding of ERβ, positive results have been reached in some investigations that specifically address the topic. 106 The activation of ERβ may lead to an anti-obesity development with increased mitochondrial function and energy expenditure in brown AT of mice, as mentioned by Ponnusamy et al. 107 According to the research of Yepuru et al., 108 ER-β-selective ligands reduce the fat mass in an animal model of OVX- and high-fat diet-induced obesity. 108 The conclusion of these studies supports that both ERα and ERβ participate in the anti-lipogenic action of estrogens and may have overlapping yet unique roles.109,110 To limit lipogenesis, E2 may also modulate the synthesis of adipose depot Lipoprotein lipase. 111 Moreover, vascular endothelial growth factors and peroxisome proliferator-activated receptor gamma (PPARγ) are involved in the regulation of E2 signaling in lipid deposition.111,112
Estrogen regulation of glucose profile
Insulin plays a pivotal role in maintaining central and peripheral glucose metabolism in women. The EPIC-InterAct study concluded that menopause occurring before 40 is associated with a higher risk of type 2 diabetes than that after the age of 50. 113 E2 mediates a protective effect against diabetes and AD by regulating insulin biosynthesis and release, insulin sensitivity, and pancreatic β-cell preservation.
In pancreatic islets, it was reported that postmenopausal women have a relatively lower insulin secretion, insulin elimination, and plasma C-peptide response compared with premenopausal women, whereas there is no significant variation in fast plasma glucose and insulin concentration between them. 114 Exposure to physiological level E2 may increase the β-cell insulin release, insulin content, and gene expression without the changes of pancreatic β-cell mass, according to the mice experiment by Alonso-Magdalena et al. 115 Consistent with these findings, in rodent models, E2 treatment also attenuates the type I and II diabetes-induced oxidative stress, lipotoxicity, and amyloid polypeptide toxicity in pancreatic β-cell. 20 The pancreatic β-cells of both humans and rodents express ERα, ERβ, and GPER. The protective effect of E2 on pancreatic islets is sustained mainly through a rapid extranuclear pathway involving Src, ERK, and NeuroD1.20,116,117
Insulin resistance is a status in which pancreatic islets pathologically hyper-secrete insulin to induce glucose uptake, and it is primarily associated with abdominal obesity. Therefore, E2-replete women with regular menstruation present higher insulin sensitivity normalized to lean mass than the age-matched men.109,118 The study by Kim et al. 119 also suggested that insulin resistance varies depending on the menopause status. E2 improves insulin resistance, resulting in decreased glucose uptake in insulin sensitivity organs, especially AT and skeletal muscle. 120 Skeletal muscle is known as the primary tissue responsible for oxidative metabolism and glucose disposal. Saengsirisuwan et al. 121 reported that OVX mice have lower insulin-activated GLUT in skeletal muscle and exhibit features of insulin resistance syndrome. According to a later study by Ribas et al., 122 muscle-specific αERKO mice are associated with impaired glucose homeostasis and increased adiposity with dysfunctional mitochondria in muscles. Both ERs are expressed in skeletal muscle, with ERβ predominating in mice. 123 ERα and ERβ exert varying effects on GLUT4 expression. Barros et al. 124 reported that ERβ has a suppressed role in GLUT4 expression in muscles, which could contribute to insulin resistance in male mice. Gorres et al. stated that ERα agonist PPT enhances the GLUT four expression, insulin action, and glucose uptake in skeletal muscles of OVX mice. 125 Hence, compared with ERβ, the positive role of ERα in glucose metabolism in skeletal muscle is relatively clear. In addition, another essential strategy to treat insulin resistance is to eliminate the excess free fatty acids (FFA) in the liver that are released from the abdominal visceral fat depot. αERKO mice show significant hepatic insulin resistance with upregulating in hepatic lipid biosynthesis and downregulating lipid transport. 126 Consistent with this observation, E2 treatment of the high-fat diet mice has decreased body weight, improved glucose tolerance and insulin sensitivity closely correlated to suppressed lipogenic genes in white AT and liver, and decreased hepatic expression of glucose-6-phosphatase. 127 The animal experiment by Gao et al. 128 indicated that E2 administration improves glucose tolerance and insulin sensitivity to glucose in ob/ob mice through activating hepatic ERα/Stat3 signaling. 128 Therefore, it is speculated that E2 deficit influences glucose homeostasis in the whole body, especially AT, skeletal muscle, and liver accompanied by impaired glucose utilization and ectopic lipid accumulation.
Notably, several in vitro and in vivo studies have emphasized that E2 needs to stay within a certain physiological concentration to maintain insulin sensitivity, otherwise, excessive E2 level may conversely reduce GLUT4 expression in muscle and overproduce insulin signaling, which subsequently provokes insulin resistance in the muscle and liver, as well as β-cell exhaustion.129,130 Postmenopausal high endogenous E2 level is associated with insulin resistance, glucose tolerance, and development of type 2 diabetes.131–134 Interestingly, hormone therapies on postmenopausal women or surgically ovariectomy primates show different consequences: conjugated equine estrogens (CEE) alone have a beneficial effect on adipocyte size with no other adverse effects, whereas additional adding dose-dependent MPA may harm insulin resistance.135–137 Therefore, the question of whether there is a maximum concentration at which estrogen may exert a positive effect is still open. The underlying mechanism of E2 in regulating glucose homeostasis is not fully understood, particularly given the disparate research samples, multiple types and routes of hormone therapy, and various testing standards for insulin resistance.
Estrogen regulation of lipid profile
Another noticeable adverse change arising with menopause is the dysregulation of lipid metabolism in the liver and plasma lipid profile, which accelerates the process of fatty liver and atherosclerotic plaque formation with an increased risk of later cardiovascular diseases.
The Study of Women’s Health Across the Nation (SWAN), which recruited 3302 women aged from 42 to 52 in 1996 with 17 visits through 2017, reported a sharp increase in apolipoprotein B, low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), triglycerides (TGs), lipoprotein(a) in late peri- and early postmenopausal stage, as well as high-density lipoprotein cholesterol (HDL-C). Subsequently, serum HDL-C levels gradually flatten till late post-menopause.138,139 Consistent with the SWAN study, elevated LDL-C, TGs, and TC levels were well recognized to be associated with menopause status in many other studies.140,141 However, the conclusions about changes in HDL-C during menopause are inconsistent, with some studies that report HDL-C to be maintained or slightly decreased.138,142,143 As argued by Anagnostis et al., 144 postmenopausal women have a more atherogenic lipid profile with lower HDL-C subfractions 2 compared with premenopausal women. According to Zago et al., 143 increased oxidized HDL in postmenopausal women leads to an impaired protective effect against LDL oxidation, which is independent of HDL-C plasma levels. Hence, measured serum HDL-C level may not fully reflect other metrics of HDL related to menopause alterations. 145
The circulating lipid profile is usually not directly absorbed from the gut but released into the blood after being processed by various tissues, especially the liver. The liver is the most important organ where TG, TC, and fatty acids are regulated to meet the physiological needs of the whole body. During the menopausal transition, increased FFA releases from AT, then transports to the liver to accelerate the synthesis of TGs rich very-low-density lipoprotein (VLDL) particles and subsequently, leads to enhanced export of VLDL-TGs in the liver, as well as the VLDL-TGs clearance rate.146,147 More than 1000 human liver genes have a sex-biased expression, especially the genes related to lipid metabolism and cardiovascular diseases, indicating that the liver is the primary target organ of E2.148,149 Meanwhile, ERs coupling lipid metabolism in the liver was reported to associate with the reproductive cycle in a mouse model. 150 As mentioned by several studies, oral administration of micronized estradiol increases VLDL production, whereas transdermal E2 has no influence on VLDL production, suggesting that hepatic processing is the primary cause of VLDL-TGs elevation due to estrogen action. 147 Both genomic and nongenomic signaling get involved in the E2’s regulation of the liver. Chen et al. 151 claimed that the knockdown of estrogen-related receptor γ in mice reduces the hepatic VLDL-TGs secretion and leads to lipid accumulation in the liver with non-alcoholic fatty liver disease, which is mediated via phospholipase A2G12B. E2 treatment with hepatic knockout ERα plus OVX mice has no protective effect against insulin sensitivity, lipogenesis, and liver TGs export. However, the mice do not develop adiposity, which may be reliant on the maintenance of E2 signaling in the CNS and other tissues. 152
E2 is also supposed to have a protective effect against fatty plaque formation by regulating cholesterol biosynthesis, uptake and reverse cholesterol transport (RCT) from peripheral tissues back and excreted by bile. The RCT is performed by HDL, which inhibits the accumulation of LDL-C. As suggested by Pedram et al., 153 E2 agonist PPT activates the membrane ERα signaling to induce adenosine monophosphate-activated protein kinase to phosphorylate sterol regulatory element-binding factor 1, and consequently prevents the cholesterol content synthesis in the mice liver. Furthermore, HDL mediates cholesterol efflux from foam cells. The cholesterol and cholesteryl esters are removed either directly through the hepatic scavenger receptor class B member I or transferred via cholesteryl ester transfer protein to LDL particles. Furthermore, free cholesteryl is released and excreted by bile. 154 According to the work by Wang et al., 155 E2 stimulates E2/ERα/SREBP-2 pathway in mice to increase the cholesterol secreted into bile.
Estrogen regulation of adipokine
Leptin
Leptin is a protein hormone produced mainly by white adipocytes. The secretion of leptin is proportional to the adiposity of the body. Crossing the BBB, leptin may regulate the central food intake and energy expenditure with receptors in the hypothalamus and brainstem.74,156,157 The leprb is the most important leptin receptor mainly located in VMH and ARC, which modulates various aspects of energy homeostasis, such as glucose balance, satiety, and hedonic eating. 158 Both E2 and leptin are critical hormones in maintaining the body’s energy homeostasis. However, many studies have different opinions over whether there is a cross-talk in CNS between E2 and leptin. As pointed out by Bennett et al., 159 E2 decreases the expression of leptin receptors in the hypothalamus and the changes in total leptin level in rats are inversely proportional to circulating E2 levels in the estrous cycle. Clegg et al. 156 reported that adding E2 directly in the female rats’ brain increases intra-third ventricular leptin sensitivity. Springer et al. analyzed 20 PubMed/Medline articles about the relationship between hormone therapy, leptin, and adiposity in healthy postmenopausal women. As shown by the results, there is no solid evidence verifying that E2 treatment changes circulating leptin levels or improves leptin action in postmenopausal women. 160 Kim et al. 161 also shared a similar result in mice experiment that E2 has a minimal direct effect on leprb in mediobasal hypothalamus. Independent of leprb/STAT3 signaling, E2 may still exert its anorexigenic effects in female mice. 161
Leptin regulates peripheral metabolic processes of glucose and lipids that are typically relevant to the pancreas, liver, skeletal muscle, ATs, immune cells, and cardiovascular system. 162 It was reported that obese postmenopausal women have worse insulin resistance and elevated leptin level. 163 Based on human data, Norata et al. 164 also suggested that the leptin/adiponectin ratio is a stronger predictor of cardiovascular diseases than single adipokine. The prospective, longitudinal study of Di Carlo et al. evaluated 44 healthy postmenopausal women who randomly received either transdermal E2 plus nomegestrol or no treatment. As demonstrated by the results, serum leptin as well as the total and percent fat mass is significantly increased 1 year after the study in untreated women, while transdermal E2 treatment shows no changes in leptin as well as body mass throughout the study. 165 Chu et al. compared oral and transdermal E2 treatment of obese postmenopausal women with metabolic syndrome (MBS). The transdermal E2 group shows a similar result as the aforementioned study, whereas the oral E2-treated women have an increased leptin level, resulting in an increased leptin/adiponectin ratio with impaired insulin resistance. 166 This once again indicates that different routes of E2 administration should be selected according to each woman’s individual condition of each woman.
Adiponectin
Adiponectin is an insulin-sensitive polypeptide hormone produced mainly by adipocytes.
Similar to leptin, the effect of adiponectin appears to be both centrally and peripherally mediated. 167 Multiple studies demonstrated that adiponectin has an anti-diabetic, anti-atherogenic, anti-inflammatory, and anti-oxidative effect mediated primarily by two receptors, adipoR1 and adipoR2, which activate the downstream signaling, including 5’-adenosine monophosphate (AMP)-activated protein kinase and PPARs.168–171 The action of adiponectin seems to be bidirectional modulated. Low adiponectin plasma levels are known to increase the risk of MBS, obesity, and cardiovascular diseases throughout the postmenopausal era.172,173 It was found that increased plasma adiponectin is an independent risk factor for the onset of AD and all-cause dementia in women in a large-scale study conducted in 2012. 174 De Franciscis et al. 175 also mentioned that serum adiponectin levels are associated with cognitive decline in postmenopausal women. 175
Both leptin and adiponectin levels are higher in women than in men.162,176 According to one study of Africa America women, visceral AT is inversely associated with adiponectin level, but no correlation is shown in men. 177 In an in vitro experiment by Foryst-Ludwig et al., 178 it was revealed that ERβ decreases the PPARγ transcription activity, which regulates adiponectin promoter in white AT. Whether the adiponectin level changes during the menopause transition, there is still no consistent conclusion. Some studies reported a negative association of E2 with adiponectin level,179,180 whereas others showed no significant difference in adiponectin levels between pre- and post-menopause women.181,182 Unlike leptin, Chalvatzas et al. 183 argued that oral estrogen administration does not affect adiponectin levels, whereas Chu et al. 166 suggested that adiponectin levels increase when transdermal E2 treatment is conducted on obese postmenopausal women with MBS.
Estrogen, timing, and hormone therapy
Menopause has long been considered a natural aging process in women along with menopausal disorders, such as hot flashes, night sweats, and insomnia, which affect not only the life quality but also long-term health with an improved incidence of obesity, osteoporosis, urogenital tract atrophy, and cardiovascular diseases. 184 From the first use of bovine ovarian for relieving menopausal symptoms to today’s standard menopausal hormone therapy (MHT), the concept that women’s aging needs medical attention is gradually gaining popularity.88,185 However, the history of understanding estrogen supplements and MHT is full of twists and turns. The first severe blow in the use of estrogen supplements occurred in the 1970s when it was reported that a sharply increased risk of endometrial cancer was associated with estrogen therapy. 186 Later, this risk was counteracted by extra progestin addition in women with a uterus, which initiated the revival of MHT usage. However, in the 1990s, the famous Women’s Health Initiative (WHI) trial enrolled over 16,000 postmenopausal women, used conjugated estrogen plus MPA for women with an intact uterus and reported that MHT worsens the risk of breast cancer, pulmonary embolism, stroke, and coronary heart disease. Throughout the decade from 2000 to 2009, the long-term impact of the WHI caused a continued decline in MHT prescription, and the treatment regimen shifted to favor low-dose oral or vagina preparations. 187 Furthermore, age-stratified analysis of the 13-year follow-up data in the WHI study yielded a more complete evaluation of MHT and proposed the ‘timing hypothesis’. 188 Women should initiate the MHT less than 10 years after menopause or within 60 years of age so that the benefits of symptom control and disease prevention outweigh the risks. Otherwise, MHT may stimulate different biological processes of vascular endothelium, smooth muscle cells, and inflammatory cells on established atherosclerosis inversely, leading to an increased risk of cardiovascular diseases.189,190
The concept of the ‘timing hypothesis’ also exists in E2’s protective effect against the development of AD.191,192 As pointed out by Brinton, 193 MHT should begin early in menopause when neurons are still in a healthy state, otherwise MHT may have no benefits but lead to a detrimental effect on the brain. 193 Henderson and Sherwin 194 suggested that although MHT should not be used to improve cognitive function, a short-term cognitive benefit is observed when initiating MHT at the time of surgical menopause. 194
As claimed by Li et al., 195 earlier puberty timing status is correlated with obesity. Later menarche was reported with a lower risk of type 2 diabetes and cardiovascular diseases (Zhang et al., 2020; Qiu et al., 2013). 196 , 197 Another study focused on the timing of pharmacologic sex hormone use during pregnancy. Indeed, oral contraceptive or diethylstilbestrol (DES) use during pregnancy is strongly associated with offspring overweight, especially when the oral contraceptive is used in the first 2 months of pregnancy or DES is used between months 3 and 5. 198 The above studies indicated that earlier elevated endogenous E2 levels or earlier exposure to exogenous estrogen may cause long-term negative effects on the energy metabolism of the body.
In addition to timing, the type, dosage, and route of the MHT regimen are equally important. 199 Godsland reviewed 248 studies about the effect of different MHT formulations on lipid profile and found that in all cases, estrogen alone decreases LDL and TC, and increases HDL-C; oral estrogen increases TG, while transdermal estrogen lowers TG. In addition, adding different types of progestogens may have the opposite effect on the estrogen-induced increase in HDL and TG. Tibolone decreases HDL-C and TG levels. 200 The above beneficial effects on LDL-C, TC, lipoprotein(a), insulin resistance, and harmful effects on TG are attributed to the hepatic first-pass effect. 201 Therefore, different routes of administration may influence hepatic lipid metabolism and energy homeostasis. 10 The ancillary Cognitive and Affective Study of the Kronos Early Estrogen Prevention Study also suggested that oral CEE has a beneficial mood effect, but transdermal CEE does not. 202 Beyond that, Villa et al. 203 stated that for healthy postmenopausal women, 1 mg oral micronized estradiol supplement has a favorable effect on insulin sensitivity, whereas it shows a neutral effect on lipid metabolism, but 2 mg preparation impairs insulin sensitivity and increases TGs, despite a favorable effect on LDL-C. The therapeutic consequences of hormones are not always dosage dependent. 204
In an in vitro study by Perkins et al., 205 the pharmacological features of synthetic ethinylestradiol (EE), pure E2, E3, and E1 standards, as well as bioidentical estrogen (bE2 and bE3) were compared through ERs. 205 As shown by results, E2 and E3 standards have similar binding affinities to the bE2 and bE3, while E1 has a lower affinity for ERβ, and EE a higher affinity for ERα. Bhavnani et al. 206 also claimed that E2, E1, and ring B unsaturated estrogen interact with human ERs with different affinities. The therapeutic effects of estrogens are mediated predominantly by ERα. 207 However, ERβ and GPR30 may also play an important role in specific organs.208,209 New pharmacological tools, such as membrane-selective SERM, have been developed to activate membrane, without activating nuclear ER, so as to enhance vascular protection without increasing the risk of breast and endometrial cancer.210,211 The above-mentioned studies can provide ideas for the design of high-affinity binding pharmacological tools that can selectively activate either genomic or nongenomic ER signaling in target organs.
However, there is still ongoing controversy about the MHT today. The healthcare provider prescribing these medications must comprehensively review the mechanism of compounds, adverse effects, contraindications, and the potential risks before initiating therapy, to avoid exacerbating cardiovascular risk and the development of AD. Moreover, the main objective of MHT is to alleviate menopausal symptoms. Although scientific evidence suggests that MHT may positively impact energy metabolism, it is important to note that it has not yet received approval for this specific indication. 212
Conclusion
Throughout this paper, we have systematically reviewed the critical role of estrogen via ERs signaling pathway in maintaining the overall energy homeostasis of the brain and the whole body. Cessation of the ovarian function with declined circulating E2 level leads to widespread changes in glucose and lipid metabolism. The adaption of women to menopause is individualized. Initiation of MHT at an early stage of menopause may effectively enhance the metabolic rate, and attenuate the development of obesity, insulin resistance, type 2 diabetes, fatty liver, and AD. However, the MHT remains a mixed picture of benefits and risks. Thus, more studies should be conducted to further explore the underlying molecular mechanism of E2 regulation, which may reveal an innovative pharmacological target for MHT beneficial action, and provide precise guidance on the type, dosage, and route of MHT usage.
Acknowledgments
None.
Footnotes
ORCID iD: Jing Zhu  https://orcid.org/0000-0002-3393-7225
https://orcid.org/0000-0002-3393-7225
Contributor Information
Jing Zhu, Center for Reproductive Medicine, Department of Reproductive Endocrinology, Zhejiang Provincial People’s Hospital, Hangzhou Medical College, Hangzhou, Zhejiang, China.
Yier Zhou, Center for Reproductive Medicine, Department of Reproductive Endocrinology, Zhejiang Provincial People’s Hospital, Hangzhou Medical College, Hangzhou, Zhejiang, China.
Bihui Jin, Center for Reproductive Medicine, Department of Reproductive Endocrinology, Zhejiang Provincial People’s Hospital, Hangzhou Medical College, Hangzhou, Zhejiang, China.
Jing Shu, Reproductive Medicine Center, the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang 310006, China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Jing Zhu: Writing – original draft; Writing – review & editing.
Yier Zhou: Visualization.
Bihui Jin: Funding acquisition; Software; Validation.
Jing Shu: Project administration; Supervision.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Medical Science and Technology Project of Zhejiang Province under Grant Nos. 2022RC012 and 2020KY414; and Natural Science Foundation of Zhejiang Province under Grant No. LQ19H04005.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Not applicable.
References
- 1. Lizcano F, Guzmán G. Estrogen deficiency and the origin of obesity during menopause. Biomed Res Int 2014; 2014: 757461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rettberg JR, Yao J, Brinton RD. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol 2014; 35: 8–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beigh SH, Jain S. Prevalence of metabolic syndrome and gender differences. Bioinformation 2012; 8: 613–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fang H, Berg E, Cheng X, et al. How to best assess abdominal obesity. Curr Opin Clin Nutr Metab Care 2018; 21: 360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klinge CM. Estrogenic control of mitochondrial function. Redox Biol 2020; 31: 101435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Santen RJ, Simpson E. History of estrogen: its purification, structure, synthesis, biologic actions, and clinical implications. Endocrinology 2019; 160: 605–625. [DOI] [PubMed] [Google Scholar]
- 7. Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol 2001; 45: S116–S124. [DOI] [PubMed] [Google Scholar]
- 8. Thomas MP, Potter BV. The structural biology of oestrogen metabolism. J Steroid Biochem Mol Biol 2013; 137: 27–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bagot CN, Marsh MS, Whitehead M, et al. The effect of estrone on thrombin generation may explain the different thrombotic risk between oral and transdermal hormone replacement therapy. J Thromb Haemost 2010; 8: 1736–1744. [DOI] [PubMed] [Google Scholar]
- 10. Stevenson JC. Type and route of estrogen administration. Climacteric 2009; 12 (Suppl. 1): 86–90. [DOI] [PubMed] [Google Scholar]
- 11. Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta 2009; 1791: 419–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas 2017; 103: 45–53. [DOI] [PubMed] [Google Scholar]
- 13. Dama A, Baggio C, Boscaro C, et al. Estrogen receptor functions and pathways at the vascular immune interface. Int J Mol Sci 2021; 22: 4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hewitt SC, Korach KS. Estrogen receptors: new directions in the new millennium. Endocr Rev 2018; 39: 664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris HA. Estrogen receptor-beta: recent lessons from in vivo studies. Mol Endocrinol 2007; 21: 1–13. [DOI] [PubMed] [Google Scholar]
- 16. Hewitt SC, Winuthayanon W, Korach KS. What’s new in estrogen receptor action in the female reproductive tract. J Mol Endocrinol 2016; 56: R55–R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science 2002; 295: 2465–2468. [DOI] [PubMed] [Google Scholar]
- 18. Li X, Huang J, Yi P, et al. Single-chain estrogen receptors (ERs) reveal that the ERalpha/beta heterodimer emulates functions of the ERalpha dimer in genomic estrogen signaling pathways. Mol Cell Biol 2004; 24: 7681–7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yaşar P, Ayaz G, User SD, et al. Molecular mechanism of estrogen-estrogen receptor signaling. Reprod Med Biol 2017; 16: 4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tiano JP, Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional β-cell mass in diabetes. Nat Rev Endocrinol 2012; 8: 342–351. [DOI] [PubMed] [Google Scholar]
- 21. Yi P, Driscoll MD, Huang J, et al. The effects of estrogen-responsive element- and ligand-induced structural changes on the recruitment of cofactors and transcriptional responses by ER alpha and ER beta. Mol Endocrinol 2002; 16: 674–693. [DOI] [PubMed] [Google Scholar]
- 22. Tang H, Zhang Q, Yang L, et al. GPR30 mediates estrogen rapid signaling and neuroprotection. Mol Cell Endocrinol 2014; 387: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu S, Mauvais-Jarvis F. Rapid, nongenomic estrogen actions protect pancreatic islet survival. Islets 2009; 1: 273–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arevalo MA, Ruiz-Palmero I, Scerbo MJ, et al. Molecular mechanisms involved in the regulation of neuritogenesis by estradiol: recent advances. J Steroid Biochem Mol Biol 2012; 131: 52–56. [DOI] [PubMed] [Google Scholar]
- 25. Kang L, Zhang X, Xie Y, et al. Involvement of estrogen receptor variant ER-α36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol 2010; 24: 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tutzauer J, Gonzalez de, Valdivia E, Swärd K, et al. Ligand-independent G protein-coupled estrogen Receptor/G protein-coupled receptor 30 activity: lack of receptor-dependent effects of G-1 and 17β-estradiol. Mol Pharmacol 2021; 100: 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Toran-Allerand CD. Estrogen and the brain: beyond ER-alpha and ER-beta. Exp Gerontol 2004; 39: 1579–1586. [DOI] [PubMed] [Google Scholar]
- 28. Smith AW, Rønnekleiv OK, Kelly MJ. Gq-mER signaling has opposite effects on hypothalamic orexigenic and anorexigenic neurons. Steroids 2014; 81: 31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qiu J, Bosch MA, Tobias SC, et al. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci 2003; 23: 9529–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garcia-Segura LM. Aromatase in the brain: not just for reproduction anymore. J Neuroendocrinol 2008; 20: 705–712. [DOI] [PubMed] [Google Scholar]
- 31. Nicholson K, MacLusky NJ, Leranth C. Synaptic effects of estrogen. Vitam Horm 2020; 114: 167–210. [DOI] [PubMed] [Google Scholar]
- 32. Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med 2013; 19: 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qi G, Mi Y, Yin F. Cellular specificity and inter-cellular coordination in the brain bioenergetic system: implications for aging and neurodegeneration. Front Physiol 2019; 10: 1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berman KF, Schmidt PJ, Rubinow DR, et al. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci USA 1997; 94: 8836–8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baxter LR, Jr, Mazziotta JC, Phelps ME, et al. Cerebral glucose metabolic rates in normal human females versus normal males. Psychiatry Res 1987; 21: 237–245. [DOI] [PubMed] [Google Scholar]
- 36. Wei SM, Baller EB, Kohn PD, et al. Brain-derived neurotrophic factor Val(66)Met genotype and ovarian steroids interactively modulate working memory-related hippocampal function in women: a multimodal neuroimaging study. Mol Psychiatry 2018; 23: 1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nguyen TV, Reuter JM, Gaikwad NW, et al. The steroid metabolome in women with premenstrual dysphoric disorder during GnRH agonist-induced ovarian suppression: effects of estradiol and progesterone addback. Transl Psychiatry 2017; 7: e1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brinton RD. Estrogen regulation of glucose metabolism and mitochondrial function: therapeutic implications for prevention of Alzheimer’s disease. Adv Drug Deliv Rev 2008; 60: 1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Del Río JP, Alliende MI, Molina N, et al. Steroid hormones and their action in women’s brains: the Importance of hormonal balance. Front Public Health 2018; 6: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review). Mol Membr Biol 2001; 18: 247–256. [DOI] [PubMed] [Google Scholar]
- 41. Koepsell H. Glucose transporters in brain in health and disease. Pflugers Arch 2020; 472: 1299–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. López-Grueso R, Gambini J, Abdelaziz KM, et al. Early, but not late onset estrogen replacement therapy prevents oxidative stress and metabolic alterations caused by ovariectomy. Antioxid Redox Signal 2014; 20: 236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morán J, Garrido P, Alonso A, et al. 17β-estradiol and genistein acute treatments improve some cerebral cortex homeostasis aspects deteriorated by aging in female rats. Exp Gerontol 2013; 48: 414–421. [DOI] [PubMed] [Google Scholar]
- 44. Shi J, Simpkins JW. 17β-estradiol modulation of glucose transporter 1 expression in blood-brain barrier. Am J Physiol 1997; 272: E1016–E1022. [DOI] [PubMed] [Google Scholar]
- 45. Cheng CM, Cohen M, Wang J, et al. Estrogen augments glucose transporter and IGF1 expression in primate cerebral cortex. FASEB J 2001; 15: 907–915. [DOI] [PubMed] [Google Scholar]
- 46. Harris RA, Tindale L, Lone A, et al. Aerobic glycolysis in the frontal cortex correlates with memory performance in wild-type mice but not the APP/PS1 mouse model of cerebral amyloidosis. J Neurosci 2016; 36: 1871–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kostanyan A, Nazaryan K. Rat brain glycolysis regulation by estradiol-17 beta. Biochim Biophys Acta 1992; 1133: 301–306. [DOI] [PubMed] [Google Scholar]
- 48. Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ 2008; 15: 521–529. [DOI] [PubMed] [Google Scholar]
- 49. Azoulay-Zohar H, Israelson A, Abu-Hamad S, et al. In self-defence: hexokinase promotes voltage-dependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. Biochem J 2004; 377: 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nilsen J, Irwin RW, Gallaher TK, et al. Estradiol in vivo regulation of brain mitochondrial proteome. J Neurosci 2007; 27: 14069–14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lindemer ER, Greve DN, Fischl B, et al. White matter abnormalities and cognition in patients with conflicting diagnoses and CSF profiles. Neurology 2018; 90: e1461–e1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol 2014; 35: 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mohajeri M, Martín-Jiménez C, Barreto GE, et al. Effects of estrogens and androgens on mitochondria under normal and pathological conditions. Prog Neurobiol 2019; 176: 54–72. [DOI] [PubMed] [Google Scholar]
- 54. Ferretti MT, Iulita MF, Cavedo E, et al. Sex differences in Alzheimer disease – the gateway to precision medicine. Nat Rev Neurol 2018; 14: 457–469. [DOI] [PubMed] [Google Scholar]
- 55. Irwin RW, Yao J, Hamilton RT, et al. Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinology 2008; 149: 3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Irwin RW, Yao J, Ahmed SS, et al. Medroxyprogesterone acetate antagonizes estrogen up-regulation of brain mitochondrial function. Endocrinology 2011; 152: 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shaw GA. Mitochondria as the target for disease related hormonal dysregulation. Brain Behav Immun Health 2021; 18: 100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nilsen J, Diaz Brinton R. Mechanism of estrogen-mediated neuroprotection: regulation of mitochondrial calcium and Bcl-2 expression. Proc Natl Acad Sci USA 2003; 100: 2842–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Irwin RW, Yao J, To J, et al. Selective oestrogen receptor modulators differentially potentiate brain mitochondrial function. J Neuroendocrinol 2012; 24: 236–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yao J, Zhao L, Mao Z, et al. Potentiation of brain mitochondrial function by S-equol and R/S-equol estrogen receptor β-selective phytoSERM treatments. Brain Res 2013; 1514: 128–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Burstein SR, Kim HJ, Fels JA, et al. Estrogen receptor beta modulates permeability transition in brain mitochondria. Biochim Biophys Acta Bioenerg 2018; 1859: 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yonutas HM, Hubbard WB, Pandya JD, et al. Bioenergetic restoration and neuroprotection after therapeutic targeting of mitoNEET: New mechanism of pioglitazone following traumatic brain injury. Exp Neurol 2020; 327: 113243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Espino De la Fuente-Muñoz C, Arias C. The therapeutic potential of mitochondrial transplantation for the treatment of neurodegenerative disorders. Rev Neurosci 2021; 32: 203–217. [DOI] [PubMed] [Google Scholar]
- 64. Berthoud HR. The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc 2012; 71: 478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gastelum C, Perez L, Hernandez J, et al. Adaptive changes in the central control of energy homeostasis occur in response to variations in energy status. Int J Mol Sci 2021; 22: 2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Geary N, Asarian L, Korach KS, et al. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology 2001; 142: 4751–4757. [DOI] [PubMed] [Google Scholar]
- 67. Park CJ, Zhao Z, Glidewell-Kenney C, et al. Genetic rescue of nonclassical ERα signaling normalizes energy balance in obese Erα-null mutant mice. J Clin Investig 2011; 121: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chao AM, Grilo CM, Sinha R. Food cravings, binge eating, and eating disorder psychopathology: exploring the moderating roles of gender and race. Eat Behav 2016; 21: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hallam J, Boswell RG, DeVito EE, et al. Gender-related differences in food craving and obesity. Yale J Biol Med 2016; 89: 161–173. [PMC free article] [PubMed] [Google Scholar]
- 70. Kellert BA, Nguyen MC, Nguyen C, et al. Estrogen rapidly attenuates cannabinoid-induced changes in energy homeostasis. Eur J Pharmacol 2009; 622: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hernandez J, Fabelo C, Perez L, et al. Nociceptin/orphanin FQ modulates energy homeostasis through inhibition of neurotransmission at VMN SF-1/ARC POMC synapses in a sex- and diet-dependent manner. Biol Sex Differ 2019; 10: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Horvath TL. The hardship of obesity: a soft-wired hypothalamus. Nat Neurosci 2005; 8: 561–565. [DOI] [PubMed] [Google Scholar]
- 73. Zhang L, Hernandez-Sanchez D, Herzog H. Regulation of feeding-related behaviors by arcuate neuropeptide Y neurons. Endocrinology 2019; 160: 1411–1420. [DOI] [PubMed] [Google Scholar]
- 74. Frank A, Brown LM, Clegg DJ. The role of hypothalamic estrogen receptors in metabolic regulation. Front Neuroendocrinol 2014; 35: 550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Messina MM, Boersma G, Overton JM, et al. Estradiol decreases the orexigenic effect of melanin-concentrating hormone in ovariectomized rats. Physiol Behav 2006; 88: 523–528. [DOI] [PubMed] [Google Scholar]
- 76. Musatov S, Chen W, Pfaff DW, et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA 2007; 104: 2501–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Thammacharoen S, Lutz TA, Geary N, et al. Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology 2008; 149: 1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci 2003; 73: 759–768. [DOI] [PubMed] [Google Scholar]
- 79. Georgescu D, Sears RM, Hommel JD, et al. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci 2005; 25: 2933–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vandegrift BJ, Hilderbrand ER, Satta R, et al. Estrogen receptor α regulates ethanol excitation of ventral tegmental area neurons and binge drinking in female mice. J Neurosci 2020; 40: 5196–5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Becker JB. Sex differences in addiction. Dialogues Clin Neurosci 2016; 18: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Richard JE, López-Ferreras L, Anderberg RH, et al. Estradiol is a critical regulator of food-reward behavior. Psychoneuroendocrinology 2017; 78: 193–202. [DOI] [PubMed] [Google Scholar]
- 83. Cervellati C, Bergamini CM. Oxidative damage and the pathogenesis of menopause related disturbances and diseases. Clin Chem Lab Med 2016; 54: 739–753. [DOI] [PubMed] [Google Scholar]
- 84. Davis SR, Lambrinoudaki I, Lumsden M, et al. Menopause. Nat Rev Dis Primers 2015; 1: 15004. [DOI] [PubMed] [Google Scholar]
- 85. Anagnostis P, Paschou SA, Katsiki N, et al. Menopausal hormone therapy and cardiovascular risk: where are we now? Curr Vasc Pharmacol 2019; 17: 564–572. [DOI] [PubMed] [Google Scholar]
- 86. Leeners B, Geary N, Tobler PN, et al. Ovarian hormones and obesity. Hum Reprod Update 2017; 23: 300–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Matthews KA, Abrams B, Crawford S, et al. Body mass index in mid-life women: relative influence of menopause, hormone use, and ethnicity. Int J Obes Relat Metab Disord 2001; 25: 863–873. [DOI] [PubMed] [Google Scholar]
- 88. Baber RJ, Panay N, Fenton A. 2016. IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric 2016; 19: 109–150. [DOI] [PubMed] [Google Scholar]
- 89. Ho SC, Wu S, Chan SG, et al. Menopausal transition and changes of body composition: a prospective study in Chinese perimenopausal women. Int J Obes 2010; 34: 1265–1274. [DOI] [PubMed] [Google Scholar]
- 90. Abdulnour J, Doucet Brochu M, et al. The effect of the menopausal transition on body composition and cardiometabolic risk factors: a Montreal-Ottawa New Emerging Team group study. Menopause 2012; 19: 760–767. [DOI] [PubMed] [Google Scholar]
- 91. Sahakyan KR, Somers VK, Rodriguez-Escudero JP, et al. Normal-weight central obesity: implications for total and cardiovascular mortality. Ann Intern Med 2015; 163: 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Alencar AKN, Wang H, Oliveira GMM, et al. Crossroads between estrogen loss, obesity, and heart failure with preserved ejection fraction. Arq Bras Cardiol 2021; 117: 1191–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chen X, McClusky R, Chen J, et al. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet 2012; 8: e1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jin B, Chen X, Xing L, et al. Tissue-specific effects of estrogen on glycerol channel aquaporin 7 expression in an ovariectomized mouse model of menopause. Climacteric 2017; 20: 385–390. [DOI] [PubMed] [Google Scholar]
- 95. Xing L, Jin B, Fu X, et al. Identification of functional estrogen response elements in glycerol channel Aquaporin-7 gene. Climacteric 2019; 22: 466–471. [DOI] [PubMed] [Google Scholar]
- 96. Pedersen SB, Kristensen K, Hermann PA, et al. Estrogen controls lipolysis by up-regulating alpha2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor alpha. Implications for the female fat distribution. J Clin Endocrinol Metab 2004; 89: 1869–1878. [DOI] [PubMed] [Google Scholar]
- 97. Gavin KM, Cooper EE, Hickner RC. Estrogen receptor protein content is different in abdominal than gluteal subcutaneous adipose tissue of overweight-to-obese premenopausal women. Metabolism 2013; 62: 1180–1188. [DOI] [PubMed] [Google Scholar]
- 98. Tomicek NJ, Lancaster TS, Korzick DH. Increased estrogen receptor β in adipose tissue is associated with increased intracellular and reduced circulating adiponectin protein levels in aged female rats. Gend Med 2011; 8: 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dieudonné MN, Leneveu MC, Giudicelli Y, et al. Evidence for functional estrogen receptors alpha and beta in human adipose cells: regional specificities and regulation by estrogens. Am J Physiol Cell Physiol 2004; 286: C655–C661. [DOI] [PubMed] [Google Scholar]
- 100. Ohlsson C, Hellberg N, Parini P, et al. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun 2000; 278: 640–645. [DOI] [PubMed] [Google Scholar]
- 101. Schulster M, Bernie AM, Ramasamy R. The role of estradiol in male reproductive function. Asian J Androl 2016; 18: 435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li XF, Wang SJ, Jiang LS, et al. Gender- and region-specific variations of estrogen receptor α and β expression in the growth plate of spine and limb during development and adulthood. Histochem Cell Biol 2012; 137: 79–95. [DOI] [PubMed] [Google Scholar]
- 103. Foryst-Ludwig A, Kintscher U. Metabolic impact of estrogen signalling through ERalpha and ERbeta. J Steroid Biochem Mol Biol 2010; 122: 74–81. [DOI] [PubMed] [Google Scholar]
- 104. Davis KE, D Neinast M, Sun K, et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab 2013; 2: 227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Heine PA, Taylor JA, Iwamoto GA, et al. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A 2000; 97: 12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Clart LM, Welly RJ, Queathem ED, et al. Role of ERβ in adipocyte metabolic response to wheel running following ovariectomy. J Endocrinol 2021; 249: 223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ponnusamy S, Tran QT, Harvey I, et al. Pharmacologic activation of estrogen receptor α increases mitochondrial function, energy expenditure, and brown adipose tissue. FASEB J 2017; 31: 266–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Yepuru M, Eswaraka J, Kearbey JD, et al. Estrogen receptor-{beta}-selective ligands alleviate high-fat diet- and ovariectomy-induced obesity in mice. J Biol Chem 2010; 285: 31292–31303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 2013; 34: 309–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhao C, Dahlman-Wright K, Gustafsson JA. Estrogen receptor beta: an overview and update. Nucl Recept Signal 2008; 6: e003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Katzer K, Hill JL, McIver KB, et al. Lipedema and the potential role of estrogen in excessive adipose tissue accumulation. Int J Mol Sci 2021; 22: 11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Applanat MP, Buteau-Lozano H, Herve MA, et al. Vascular endothelial growth factor is a target gene for estrogen receptor and contributes to breast cancer progression. Adv Exp Med Biol 2008; 617: 437–444. [DOI] [PubMed] [Google Scholar]
- 113. Brand JS, van der Schouw YT, Onland-Moret NC, et al. Age at menopause, reproductive life span, and type 2 diabetes risk: results from the EPIC-InterAct study. Diabetes Care 2013; 36: 1012–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Walton C, Godsland IF, Proudler AJ, et al. The effects of the menopause on insulin sensitivity, secretion and elimination in non-obese, healthy women. Eur J Clin Invest 1993; 23: 466–473. [DOI] [PubMed] [Google Scholar]
- 115. Alonso-Magdalena P, Ropero AB, Carrera MP, et al. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS One 2008; 3: e2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tiano JP, Delghingaro-Augusto V, Le May C, et al. Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents β cell failure in rodent models of type 2 diabetes. J Clin Investig 2011; 121: 3331–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wong WPS, Tiano JP, Liu S, et al. Extranuclear estrogen receptor-α stimulates NeuroD1 binding to the insulin promoter and favors insulin synthesis. Proc Natl Acad Sci U S A 2010; 107: 13057–13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Luchsinger JA, Small S, Biessels GJ. Should we target insulin resistance to prevent dementia due to Alzheimer disease? Arch Neurol 2011; 68: 17–18. [DOI] [PubMed] [Google Scholar]
- 119. Kim S, Lee JY, Im JA, et al. Association between serum osteocalcin and insulin resistance in postmenopausal, but not premenopausal, women in Korea. Menopause 2013; 20: 1061–1066. [DOI] [PubMed] [Google Scholar]
- 120. Bell GI, Polonsky KS. Diabetes mellitus and genetically programmed defects in beta-cell function. Nature 2001; 414: 788–791. [DOI] [PubMed] [Google Scholar]
- 121. Saengsirisuwan V, Pongseeda S, Prasannarong M, et al. Modulation of insulin resistance in ovariectomized rats by endurance exercise training and estrogen replacement. Metabolism 2009; 58: 38–47. [DOI] [PubMed] [Google Scholar]
- 122. Ribas V, Drew BG, Zhou Z, et al. Skeletal muscle action of estrogen receptor α is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci Transl Med 2016; 8: 334ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Jia M, Dahlman-Wright K, Gustafsson JÅ. Estrogen receptor alpha and beta in health and disease. Best Pract Res Clin Endocrinol Metab 2015; 29: 557–568. [DOI] [PubMed] [Google Scholar]
- 124. Barros RP, Machado UF, Warner M, et al. Muscle GLUT4 regulation by estrogen receptors ERbeta and ERalpha. Proc Natl Acad Sci USA 2006; 103: 1605–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Gorres BK, Bomhoff GL, Morris JK, et al. In vivo stimulation of oestrogen receptor α increases insulin-stimulated skeletal muscle glucose uptake. J Physiol 2011; 589: 2041–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bryzgalova G, Gao H, Ahren B, et al. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia 2006; 49: 588–597. [DOI] [PubMed] [Google Scholar]
- 127. Bryzgalova G, Lundholm L, Portwood N, et al. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am J Physiol Endocrinol Metab 2008; 295: E904–E912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gao H, Bryzgalova G, Hedman E, et al. Long-term administration of estradiol decreases expression of hepatic lipogenic genes and improves insulin sensitivity in ob/ob mice: a possible mechanism is through direct regulation of signal transducer and activator of transcription 3. Mol Endocrinol 2006; 20: 1287–1299. [DOI] [PubMed] [Google Scholar]
- 129. Barros RP, Morani A, Moriscot A, et al. Insulin resistance of pregnancy involves estrogen-induced repression of muscle GLUT4. Mol Cell Endocrinol 2008; 295: 24–31. [DOI] [PubMed] [Google Scholar]
- 130. Nadal A, Alonso-Magdalena P, Soriano S, et al. The pancreatic beta-cell as a target of estrogens and xenoestrogens: implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol 2009; 304: 63–68. [DOI] [PubMed] [Google Scholar]
- 131. Golden SH, Dobs AS, Vaidya D, et al. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab 2007; 92: 1289–1295. [DOI] [PubMed] [Google Scholar]
- 132. Ding EL, Song Y, Manson JE, et al. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia 2007; 50: 2076–2084. [DOI] [PubMed] [Google Scholar]
- 133. Kalyani RR, Franco M, Dobs AS, et al. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab 2009; 94: 4127–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Muka T, Nano J, Jaspers L, et al. Associations of steroid sex hormones and sex hormone-binding globulin with the risk of type 2 diabetes in women: a population-based cohort study and meta-analysis. Diabetes 2017; 66: 577–586. [DOI] [PubMed] [Google Scholar]
- 135. Odmark IS, Carlström K, Jonsson B, et al. Conjugated estrogen/progestagen versus tibolone hormone replacement therapy in postmenopausal women: effects on carbohydrate metabolism and serum sex hormone-binding globulin. Maturitas 2006; 53: 89–96. [DOI] [PubMed] [Google Scholar]
- 136. Shadoan MK, Anthony MS, Rankin SE, et al. Effects of tibolone and conjugated equine estrogens with or without medroxyprogesterone acetate on body composition and fasting carbohydrate measures in surgically postmenopausal monkeys. Metabolism 2003; 52: 1085–1091. [DOI] [PubMed] [Google Scholar]
- 137. Shadoan MK, Kavanagh K, Zhang L, et al. Addition of medroxyprogesterone acetate to conjugated equine estrogens results in insulin resistance in adipose tissue. Metabolism 2007; 56: 830–837. [DOI] [PubMed] [Google Scholar]
- 138. Derby CA, Crawford SL, Pasternak RC, et al. Lipid changes during the menopause transition in relation to age and weight: the Study of Women’s Health Across the Nation. Am J Epidemiol 2009; 169: 1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. El Khoudary SR, Greendale G, Crawford SL, et al. The menopause transition and women’s health at midlife: a progress report from the Study of Women’s Health Across the Nation (SWAN). Menopause 2019; 26: 1213–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Anagnostis P, Bitzer J, Cano A, et al. Menopause symptom management in women with dyslipidemias: an EMAS clinical guide. Maturitas 2020; 135: 82–88. [DOI] [PubMed] [Google Scholar]
- 141. Wang N, Qin MZ, Cui J. Lipid profile comparison between pre- and post-menopausal women. Zhonghua Xin Xue Guan Bing Za Zhi 2016; 44: 799–804. [DOI] [PubMed] [Google Scholar]
- 142. El Khoudary SR. HDL and the menopause. Curr Opin Lipidol 2017; 28: 328–336. [DOI] [PubMed] [Google Scholar]
- 143. Zago V, Sanguinetti S, Brites F, et al. Impaired high density lipoprotein antioxidant activity in healthy postmenopausal women. Atherosclerosis 2004; 177: 203–210. [DOI] [PubMed] [Google Scholar]
- 144. Anagnostis P, Stevenson JC, Crook D, et al. Effects of menopause, gender and age on lipids and high-density lipoprotein cholesterol subfractions. Maturitas 2015; 81: 62–68. [DOI] [PubMed] [Google Scholar]
- 145. Cho KH. The current status of research on high-density lipoproteins (HDL): a paradigm shift from HDL quantity to HDL quality and HDL functionality. Int J Mol Sci 2022; 23: 3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Matthan NR, Jalbert SM, Barrett PH, et al. Gender-specific differences in the kinetics of nonfasting TRL, IDL, and LDL apolipoprotein B-100 in men and premenopausal women. Arterioscler Thromb Vasc Biol 2008; 28: 1838–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Palmisano BT, Zhu L, Stafford JM. Role of estrogens in the regulation of liver lipid metabolism. Adv Exp Med Biol 2017; 1043: 227–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Della Torre S. Beyond the X Factor: relevance of sex hormones in NAFLD pathophysiology. Cells 2021; 10: 2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zhang Y, Klein K, Sugathan A, et al. Transcriptional profiling of human liver identifies sex-biased genes associated with polygenic dyslipidemia and coronary artery disease. PLoS One 2011; 6: e23506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Della Torre S, Mitro N, Fontana R, et al. An essential role for liver ERα in coupling hepatic metabolism to the reproductive cycle. Cell Rep 2016; 15: 360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Chen L, Wu M, Zhang S, et al. Estrogen-related receptor γ regulates hepatic triglyceride metabolism through phospholipase A2 G12B. FASEB J 2019; 33: 7942–7952. [DOI] [PubMed] [Google Scholar]
- 152. Zhu L, Brown WC, Cai Q, et al. Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes 2013; 62: 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Pedram A, Razandi M, O’Mahony F, et al. Estrogen reduces lipid content in the liver exclusively from membrane receptor signaling. Sci Signal 2013; 6: ra36. [DOI] [PubMed] [Google Scholar]
- 154. Ko SH, Kim HS. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients 2020; 12: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Wang HH, Afdhal NH, Wang DQ. Overexpression of estrogen receptor alpha increases hepatic cholesterogenesis, leading to biliary hypersecretion in mice. J Lipid Res 2006; 47: 778–786. [DOI] [PubMed] [Google Scholar]
- 156. Clegg DJ, Brown LM, Woods SC, et al. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 2006; 55: 978–987. [DOI] [PubMed] [Google Scholar]
- 157. Elmquist JK, Ahima RS, Maratos-Flier E, et al. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology 1997; 138: 839–842. [DOI] [PubMed] [Google Scholar]
- 158. Leinninger GM. Location, location, location: the CNS sites of leptin action dictate its regulation of homeostatic and hedonic pathways. Int J Obes 2009; 33 (Suppl. 2): S14–S17. [DOI] [PubMed] [Google Scholar]
- 159. Bennett PA, Lindell K, Wilson C, et al. Cyclical variations in the abundance of leptin receptors, but not in circulating leptin, correlate with NPY expression during the oestrous cycle. Neuroendocrinology 1999; 69: 417–423. [DOI] [PubMed] [Google Scholar]
- 160. Springer AM, Foster-Schubert K, Morton GJ, et al. Is there evidence that estrogen therapy promotes weight maintenance via effects on leptin? Menopause 2014; 21: 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Kim JS, Rizwan MZ, Clegg DJ, et al. Leptin signaling is not required for anorexigenic estradiol effects in female mice. Endocrinology 2016; 157: 1991–2001. [DOI] [PubMed] [Google Scholar]
- 162. Pereira S, Cline DL, Glavas MM, et al. Tissue-specific effects of leptin on glucose and lipid metabolism. Endocr Rev 2021; 42: 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Chu MC, Cosper P, Orio F, et al. Insulin resistance in postmenopausal women with metabolic syndrome and the measurements of adiponectin, leptin, resistin, and ghrelin. Am J Obstet Gynecol 2006; 194: 100–104. [DOI] [PubMed] [Google Scholar]
- 164. Norata GD, Raselli S, Grigore L, et al. Leptin:adiponectin ratio is an independent predictor of intima media thickness of the common carotid artery. Stroke 2007; 38: 2844–2846. [DOI] [PubMed] [Google Scholar]
- 165. Di Carlo C, Tommaselli GA, Sammartino A, et al. Serum leptin levels and body composition in postmenopausal women: effects of hormone therapy. Menopause 2004; 11: 466–473. [DOI] [PubMed] [Google Scholar]
- 166. Chu MC, Cosper P, Nakhuda GS, et al. A comparison of oral and transdermal short-term estrogen therapy in postmenopausal women with metabolic syndrome. Fertil Steril 2006; 86: 1669–1675. [DOI] [PubMed] [Google Scholar]
- 167. Bloemer J, Pinky PD, Govindarajulu M, et al. Role of adiponectin in central nervous system disorders. Neural Plast 2018; 2018: 4593530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hatzis G, Deftereos S, Tousoulis D, et al. Adiponectin: merely a bystander or the missing link to cardiovascular disease? Curr Top Med Chem 2013; 13: 139–163. [DOI] [PubMed] [Google Scholar]
- 169. Chan KH, Lam KS, Cheng OY, et al. Adiponectin is protective against oxidative stress induced cytotoxicity in amyloid-beta neurotoxicity. PLoS One 2012; 7: e52354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Gagliardi D, Meneri M, Saccomanno D, et al. Diagnostic and prognostic role of blood and cerebrospinal fluid and blood neurofilaments in amyotrophic lateral sclerosis: a review of the literature. Int J Mol Sci 2019; 20: 4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. de Oliveira C, de Mattos AB, Silva CB, et al. Nutritional and hormonal modulation of adiponectin and its receptors adipoR1 and adipoR2. Vitam Horm 2012; 90: 57–94. [DOI] [PubMed] [Google Scholar]
- 172. Soni AC, Conroy MB, Mackey RH, et al. Ghrelin, leptin, adiponectin, and insulin levels and concurrent and future weight change in overweight, postmenopausal women. Menopause 2011; 18: 296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Everson-Rose SA, Barinas-Mitchell EJM, El Khoudary SR, et al. Adipokines and subclinical cardiovascular disease in post-menopausal women: Study of Women’s Health Across the Nation. J Am Heart Assoc 2021; 10: e019173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. van Himbergen TM, Beiser AS, Ai M, et al. Biomarkers for insulin resistance and inflammation and the risk for all-cause dementia and Alzheimer disease: results from the Framingham Heart Study. Arch Neurol 2012; 69: 594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. De Franciscis P, Barbieri M, Leo S, et al. Serum adiponectin levels are associated with worse cognitive function in postmenopausal women. PLoS One 2017; 12: e0186205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Andreasson AN, Undén AL, Elofsson S, et al. Leptin and adiponectin: distribution and associations with cardiovascular risk factors in men and women of the general population. Am J Hum Biol 2012; 24: 595–601. [DOI] [PubMed] [Google Scholar]
- 177. Bidulescu A, Liu J, Hickson DA, et al. Gender differences in the association of visceral and subcutaneous adiposity with adiponectin in African Americans: the Jackson Heart Study. BMC Cardiovasc Disord 2013; 13: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Foryst-Ludwig A, Clemenz M, Hohmann S, et al. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet 2008; 4: e1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Gavrila A, Chan JL, Yiannakouris N, et al. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab 2003; 88: 4823–4831. [DOI] [PubMed] [Google Scholar]
- 180. Tamakoshi K, Yatsuya H, Wada K, et al. The transition to menopause reinforces adiponectin production and its contribution to improvement of insulin-resistant state. Clin Endocrinol 2007; 66: 65–71. [DOI] [PubMed] [Google Scholar]
- 181. Hong SC, Yoo SW, Cho GJ, et al. Correlation between estrogens and serum adipocytokines in premenopausal and postmenopausal women. Menopause 2007; 14: 835–840. [DOI] [PubMed] [Google Scholar]
- 182. Ryan AS, Berman DM, Nicklas BJ, et al. Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide ranges of age and obesity. Diabetes Care 2003; 26: 2383–2388. [DOI] [PubMed] [Google Scholar]
- 183. Chalvatzas N, Dafopoulos K, Kosmas G, et al. Effect of ovarian hormones on serum adiponectin and resistin concentrations. Fertil Steril 2009; 91: 1189–1194. [DOI] [PubMed] [Google Scholar]
- 184. Minkin MJ. Menopause: hormones, lifestyle, and optimizing aging. Obstet Gynecol Clin North Am 2019; 46: 501–514. [DOI] [PubMed] [Google Scholar]
- 185. Kopera H. The dawn of hormone replacement therapy. Maturitas 1991; 13: 187–188. [DOI] [PubMed] [Google Scholar]
- 186. Kohn GE, Rodriguez KM, Hotaling J, et al. The history of estrogen therapy. Sex Med Rev 2019; 7: 416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Steinkellner AR, Denison SE, Eldridge SL, et al. A decade of postmenopausal hormone therapy prescribing in the United States: long-term effects of the Women’s Health Initiative. Menopause 2012; 19: 616–621. [DOI] [PubMed] [Google Scholar]
- 188. Gurney EP, Nachtigall MJ, Nachtigall LE, et al. The Women’s Health Initiative trial and related studies: 10 years later: a clinician’s view. J Steroid Biochem Mol Biol 2014; 142: 4–11. [DOI] [PubMed] [Google Scholar]
- 189. Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science 2005; 308: 1583–1587. [DOI] [PubMed] [Google Scholar]
- 190. Prabakaran S, Schwartz A, Lundberg G. Cardiovascular risk in menopausal women and our evolving understanding of menopausal hormone therapy: risks, benefits, and current guidelines for use. Ther Adv Endocrinol Metab 2021; 12: 20420188211013917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Henderson VW. Estrogen-containing hormone therapy and Alzheimer’s disease risk: understanding discrepant inferences from observational and experimental research. Neuroscience 2006; 138: 1031–1039. [DOI] [PubMed] [Google Scholar]
- 192. Stute P, Wienges J, Koller AS, et al. Cognitive health after menopause: does menopausal hormone therapy affect it? Best Pract Res Clin Endocrinol Metab 2021; 35: 101565. [DOI] [PubMed] [Google Scholar]
- 193. Brinton RD. Impact of estrogen therapy on Alzheimer’s disease: a fork in the road? CNS Drugs 2004; 18: 405–422. [DOI] [PubMed] [Google Scholar]
- 194. Henderson VW, Sherwin BB. Surgical versus natural menopause: cognitive issues. Menopause 2007; 14: 572–579. [DOI] [PubMed] [Google Scholar]
- 195. Li W, Liu Q, Deng X, et al. Association between obesity and puberty timing: a systematic review and meta analysis. Int J Environ Res Public Health 2017; 14: 1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Zhang L, Li Y, Dong X, et al. Effect of the age at menarche and menopause status interaction on type 2 diabetes: the henan rural cohort study. J Clin Endocrinol Metab 2020; 105: e139–e147. [DOI] [PubMed] [Google Scholar]
- 197. Qiu C, Chen H, Wen J, et al. Associations between age at menarche and menopause with cardiovascular disease, diabetes, and osteoporosis in Chinese women. J Clin Endocrinol Metab 2013; 98: 1612–1621. [DOI] [PubMed] [Google Scholar]
- 198. Jensen ET, Longnecker MP. Pharmacologic sex hormones in pregnancy in relation to offspring obesity. Obesity (Silver Spring, MD) 2014; 22: 2406–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Files J, Kling JM. Transdermal delivery of bioidentical estrogen in menopausal hormone therapy: a clinical review. Expert Opin Drug Deliv 2020; 17: 543–549. [DOI] [PubMed] [Google Scholar]
- 200. Godsland IF. Effects of postmenopausal hormone replacement therapy on lipid, lipoprotein, and apolipoprotein (a) concentrations: analysis of studies published from 1974–2000. Fertil Steril 2001; 75: 898–915. [DOI] [PubMed] [Google Scholar]
- 201. Mehta J, Kling JM, Manson JE. Risks, benefits, and treatment modalities of menopausal hormone therapy: current concepts. Front Endocrinol 2021; 12: 564781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS–Cognitive and Affective Study. PLoS Med 2015; 12: e1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Villa P, Sagnella F, Perri C, et al. Low- and standard-estrogen dosage in oral therapy: dose-dependent effects on insulin and lipid metabolism in healthy postmenopausal women. Climacteric 2008; 11: 498–508. [DOI] [PubMed] [Google Scholar]
- 204. Shufelt CL, Manson JE. Menopausal hormone therapy and cardiovascular disease: the role of formulation, dose, and route of delivery. J Clin Endocrinol Metab 2021; 106: 1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Perkins MS, Louw-du Toit R, Africander D. A comparative characterization of estrogens used in hormone therapy via estrogen receptor (ER)-α and -β. J Steroid Biochem Mol Biol 2017; 174: 27–39. [DOI] [PubMed] [Google Scholar]
- 206. Bhavnani BR, Tam SP, Lu X. Structure activity relationships and differential interactions and functional activity of various equine estrogens mediated via estrogen receptors (ERs) ERalpha and ERbeta. Endocrinology 2008; 149: 4857–4870. [DOI] [PubMed] [Google Scholar]
- 207. Arnal JF, Lenfant F, Metivier R, et al. Membrane and nuclear estrogen receptor alpha actions: from tissue specificity to medical implications. Physiol Rev 2017; 97: 1045–1087. [DOI] [PubMed] [Google Scholar]
- 208. Feldman RD, Limbird LE. GPER (GPR30): a nongenomic receptor (GPCR) for steroid hormones with implications for cardiovascular disease and cancer. Annu Rev Pharmacol Toxicol 2017; 57: 567–584. [DOI] [PubMed] [Google Scholar]
- 209. Muka T, Vargas KG, Jaspers L, et al. Estrogen receptor β actions in the female cardiovascular system: a systematic review of animal and human studies. Maturitas 2016; 86: 28–43. [DOI] [PubMed] [Google Scholar]
- 210. Chambliss KL, Wu Q, Oltmann S, et al. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Investig 2010; 120: 2319–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Valéra MC, Fontaine C, Dupuis M, et al. Towards optimization of estrogen receptor modulation in medicine. Pharmacol Ther 2018; 189: 123–129. [DOI] [PubMed] [Google Scholar]
- 212. “The 2022 Hormone Therapy Position Statement of The North American Menopause Society” Advisory Panel. The 2022 hormone therapy position statement of the North American Menopause Society. Menopause 2022; 29: 767–794. [DOI] [PubMed] [Google Scholar]