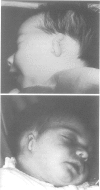
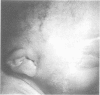
Abstract
The epidemiology and genetics of microtia-anotia (M-A) were studied using data collected from the Italian Multicentre Birth Defects Registry (IPIMC) from 1983 to 1992. Among 1,173, 794 births, we identified 172 with M-A, a rate of 1.46/10,000; 38 infants (22.1%) had anotia. Of the 172 infants, 114 (66.2%) had an isolated defect, 48 (27.9%) were multiformed infants (MMI) with M-A, and 10 (5.8%) had a well defined syndrome. The frequency of bilateral defects among non-syndromic cases was 12% compared to 50% of syndromic cases (p = 0.007). Among the MMI only holoprosencephaly was preferentially associated with M-A (four cases observed upsilon 0.7 expected, p = 0.005). No significant variations were identified in the prevalence of non-syndromic cases by geographical area (range 0.62-2.37/10,000 births) or by five month time periods (range 0.21-2.58/10,000 births), nor was there evidence of time trends. When M-A cases were compared to controls, we found that mothers with parity 1 had a higher risk of giving birth to an MMI with M-A, and that mothers with chronic maternal insulin dependent diabetes were at significantly higher risk for having a child with M-A. MMI with M-A had higher rates of prematurity, low birth weight, reduced intrauterine growth, and neonatal mortality than infants with isolated M-A and controls. Babies with isolated M-A had, on average, a lower birth weight than controls; the difference was higher for females. The analysis of pedigrees and familial cases suggests an autosomal dominant trait with variable expression and incomplete penetrance in a proportion of cases, or a multifactorial aetiology. Three cases had consanguineous parents, but the absence of M-A among previous sibs does not support autosomal recessive inheritance.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aase J. M., Tegtmeier R. E. Microtia in New Mexico: evidence for multifactorial causation. Birth Defects Orig Artic Ser. 1977;13(3A):113–116. [PubMed] [Google Scholar]
- Castilla E. E., Orioli I. M. Prevalence rates of microtia in South America. Int J Epidemiol. 1986 Sep;15(3):364–368. doi: 10.1093/ije/15.3.364. [DOI] [PubMed] [Google Scholar]
- Hollister D. W., Klein S. H., De Jager H. J., Lachman R. S., Rimoin D. L. The lacrimo-auriculo-dento-digital syndrome. J Pediatr. 1973 Sep;83(3):438–444. doi: 10.1016/s0022-3476(73)80268-9. [DOI] [PubMed] [Google Scholar]
- Khoury M. J., James L. M., Erickson J. D. On the measurement and interpretation of birth defect associations in epidemiologic studies. Am J Med Genet. 1990 Oct;37(2):229–236. doi: 10.1002/ajmg.1320370213. [DOI] [PubMed] [Google Scholar]
- Liberatos P., Link B. G., Kelsey J. L. The measurement of social class in epidemiology. Epidemiol Rev. 1988;10:87–121. doi: 10.1093/oxfordjournals.epirev.a036030. [DOI] [PubMed] [Google Scholar]
- Lynberg M. C., Khoury M. J., Lammer E. J., Waller K. O., Cordero J. F., Erickson J. D. Sensitivity, specificity, and positive predictive value of multiple malformations in isotretinoin embryopathy surveillance. Teratology. 1990 Nov;42(5):513–519. doi: 10.1002/tera.1420420508. [DOI] [PubMed] [Google Scholar]
- Smahel Z., Horák I. Craniofacial changes in unilateral microtia: I. An anthropometric study. J Craniofac Genet Dev Biol. 1984;4(1):7–16. [PubMed] [Google Scholar]