Abstract
Neuraxial analgesia and anaesthesia are widely accepted and well-tolerated modes of delivery analgesia, being employed in up to 76% of vaginal deliveries and 94% of caesarean deliveries in the United States. 1 A cause of considerable concern for postpartum women, their family and caring health professionals is the occurrence of unexplained postpartum complications, not only for management in the index pregnancy, but the uncertain risk of recurrence in future pregnancies. Complications of neuraxial blocks may impact significantly on the ability of mothers to care for and bond with their newborn. The reported incidence of temporary neurological deficit following obstetric neuraxial blocks is 1 in 3900 procedures, and the risk of permanent neurological harm estimated to be between 1 in 80,000 and 1 in 320,425 procedures. 2 Obstetric physicians may be asked to review women with postpartum complications following neuraxial blocks. This article reviews complications that may be seen following neuraxial blocks for delivery.
Keywords: Pregnancy, spinal anaesthesia, epidural anaesthesia, complications
Introduction
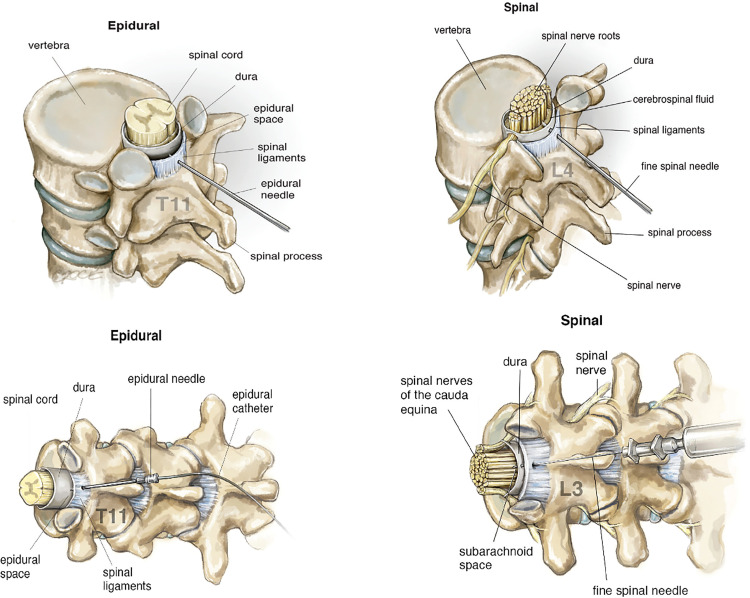
Analgesia is a pain-free state, whereas anaesthesia is a state achieved when there is a loss of touch, pain, and temperature sensations with or without loss of consciousness. Epidural blockade involves insertion of a needle into the epidural space, located outside the spinal cord and spinal fluid. (Figure 1) Local anaesthetic, often combined with other medications, may be injected directly via the needle or as boluses or a continuous infusion via a catheter inserted through the needle. Epidural needles are typically 17–18 gauge. Spinal blocks almost always involve a single injection through a fine needle into the cerebrospinal fluid. (Figure 2) Spinal needles are typically 25–27 gauge with a pencil-point tip, much finer than epidural needles. Spinal blocks may be combined with an epidural by inserting a long fine needle through the epidural needle into the cerebrospinal fluid before insertion of the epidural catheter.
Figure 1.
Anatomy of epidural and spinal blocks.
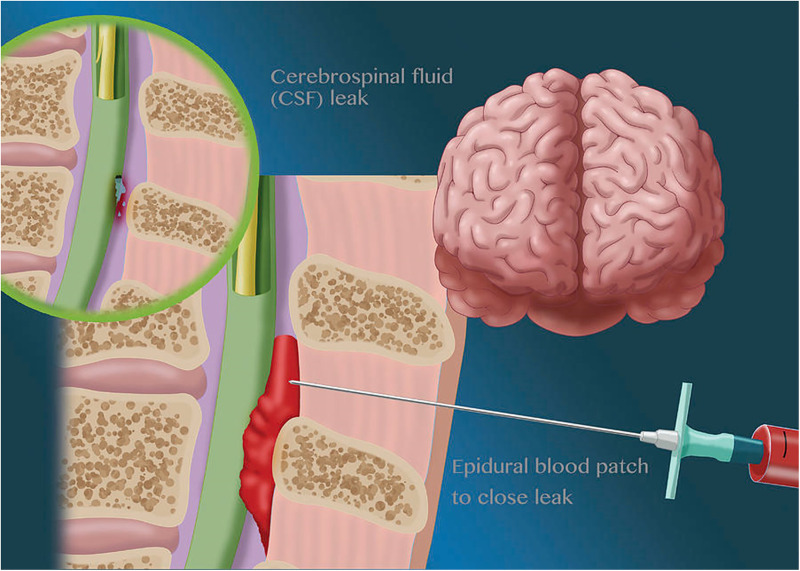
Figure 2.
Epidural blood patch.
Table 1.
Complications following neuraxial analgesia and anaesthesia.
| Cranial |
| Post-dural puncture headache |
| Haemorrhage |
| Intracranial subdural haemorrhage |
| Subarachnoid haemorrhage |
| Pituitary haemorrhage |
| Cerebral venous sinus thrombosis |
| Posterior reversible encephalopathy syndrome |
| Horner's syndrome |
| Cranial nerve palsies |
| Auditory loss |
| Meningitis |
| Aseptic meningitis |
| Tonic-clonic seizures |
| Pneumocephalus |
| Spinal |
| Spinal or epidural haematoma |
| Spinal epidural abscess |
| Anterior spinal artery syndrome |
| Epidural blood patch |
| Spinal myoclonus |
| Other |
| Fever |
| Arrhythmias and cardiac arrest |
| Transient neurologic symptoms |
| Nerve irritation – direct needle or catheter trauma |
| Necrotising fasciitis |
CSF pressure is unchanged during healthy pregnancy, with preeclampsia, chronic hypertension or with idiopathic seizures in pregnancy. 3 Uterine contractions alone do not cause increased CSF pressure on continuous monitoring during labour, however, Valsalva manoeuvres or pushing with contractions cause CSF pressure to increase to an average of 71cm H2O.4,5 Transient increases in intracranial pressure (ICP) with epidural injection depend on the basal ICP and the rate and volume injected. 6
Leak of cerebrospinal fluid (CSF) following dural puncture leading to intracranial hypotension is the predominant mechanism by which postpartum complications may occur following neuraxial blocks. Inadvertent dural puncture occurs in 0%–2.6% of epidural blocks, with this being unrecognised at the time of the procedure in 16%–38% of cases. 7 Post-dural puncture headache (PDPH) complicates 51%–85% of episodes of dural puncture with epidural blocks. The risk of PDPH following spinal anaesthesia is lower at 1%–10% due to the much finer needle.8–11 PDPH is associated with a statistically increased risk of subdural haematoma, cranial nerve palsy, meningitis, cerebral vein sinus thrombosis, depression, headache and persistent low back pain compared with women without PDPH. 10 A challenge for the obstetric physician is in deciding when to investigate women with complications following neuraxial anaesthesia. Numerous rarer complications have also been reported following neuraxial blocks which physicians should be aware of, despite their reassuringly low incidence (Table 1).
Cranial
Post-dural puncture headache (PDPH)
The International Headache Society defines PDPH as headache onset within 5 days of dural puncture, usually associated with neck stiffness and subjective hearing symptoms. 12 PDPH is classically positional, appearing or becoming aggravated within 15 min after sitting or standing, and improving within 15 min of lying down, however, 5.6% of presentations with PDPH may be non-positional. 13 Hearing loss, photophobia and vertigo may occur. 8 The mechanism of PDPH is thought to be reduced ICP, compensatory meningeal venous dilation, downward displacement of the brain and alterations in craniospinal compliance. 14 Accordingly, radiological features of intracranial hypotension following dural puncture include venous sinus distension, diffuse pachymeningeal enhancement, pituitary enlargement, transtentorial brain sagging and subdural fluid collection. 15 Cranial nerve abnormalities may occur due to mechanical traction on the cranial nerves when in an upright position. 15
PDPH is uncommon, complicating 0.4%–1.6% of epidural and combined spinal-epidural blocks, and less than 0.5% of cases of spinal anaesthesia.1,10,16
Development of headache within 24 h of delivery and lack of side predominance differentiates PDPH from migraine. 17 Characteristics suggesting the need for investigation of secondary causes of headache include associated neurological symptoms or signs, headache peaking in severity in less than 5 min, fever, precipitation of headache by physical activity or Valsalva, headache waking the patient from sleep, change in the type or pattern of previous headache (e.g., postural to non-postural), and failure of headache to resolve within 2 weeks.18,19 In a study of 95 women with postpartum headache, cerebral imaging in 22 women whose headache did not resolve or who had additional neurological symptoms or signs revealed abnormal findings in 15 cases (68%). 18
Conservative treatment may be considered where the headache is not severe, with the natural course being resolution in 2 weeks. Regular simple oral analgesia should be offered, with the short-term use of opioid analgesia considered if simple oral analgesia is ineffective. Prolonged bed rest is not recommended because of the risk of thromboembolism. There is no evidence for excessive fluid administration and abdominal binders, and insufficient evidence to recommend the use of theophylline, ACTH, glucocorticoids, triptans, gabapentinoids, acupuncture, greater occipital nerve, or sphenopalatine ganglion blocks. There is limited evidence to support the use of caffeine in conservative management, and this should be limited to 24 h, with a maximal dose of 900 mg during this time. The mechanism of action is thought to be cerebral vasoconstriction and increased cerebrospinal fluid production. 20 Epidural blood patch is the “gold standard” treatment, with greater resolution when this is delayed for 24–48 h after dural puncture. 21
Intracranial subdural haemorrhage
Intracranial subdural haematoma (ISDH) following neuraxial anaesthesia is thought to occur due to intracranial hypotension following dural puncture as previously described, with subsequent tearing of bridging veins. Accordingly, the incidence of ISDH is higher with recognised accidental dural puncture (110 compared with 26 per 100,000 epidural blocks), especially if PDPH occurs (83–147 per 100,000).22,23 The adjusted odds ratio of subdural haemorrhage following neuraxial block with PDPH is 76.7 compared with all neuraxial blocks. 10 The incidence of ISDH with spinal anaesthesia has been estimated to be 1 in 500,000 procedures. 24 However, incidence of ISDH is likely underestimated, with many patients treated as for PDPH alone without imaging and eventual resolution of symptoms. 25 Complication of PDPH by ISDH can manifest as persistent headache, failure to respond to conservative therapies and change from postural to non-postural nature, with non-postural headache present in 74%–91%.19,22,26 Other symptoms of ISDH include nausea/vomiting (31%–41%), altered mental status (31%–40%), focal motor deficits (23%–28%), visual changes (14%–20%) and aphasia/dysarthria (11%–13%). 22
The duration from dural puncture to the time of diagnosis of ISDH ranges widely from 4 h to 29 weeks, with most readmissions for ISDH occurring within 5 days of discharge. 27 Post-dural puncture ISDH is associated with mortality of 7%–10% and a 10% risk of permanent neurological injury.25,26 Diagnosis is with computerised axial tomography (CT) or magnetic resonance imaging (MRI). Management generally involves surgical drainage, which occurred in 16 of 24 ISDH following neuraxial block for delivery reported by Zeidan et al., 19 or conservative management if the haematoma is small and without midline shift or neurological deficit. If conservative management has undertaken the risk of haematoma enlargement and neurologic deterioration is highest in the first 36 h after ISDH onset. Repeat CT/MRI should be performed within 6–8 h of the initial scan in patient managed non-operatively, and neurological examinations performed every 1–2 h for the first 24 h after presentation.
Other intracerebral haemorrhage
Four cases of subarachnoid haemorrhage (SAH) and two cases of intracerebral haemorrhage have been reported following neuraxial anaesthesia in the absence of an alternative precipitating cause.28–33 The risk of SAH in the postpartum period has been estimated to be 5.8 cases per 100,000 deliveries. 34
Three cases of pituitary haemorrhage have been described following spinal anaesthesia in the absence of an alternative precipitating cause, presenting between five minutes and one week following delivery.35–37 The mechanism and significance of contribution by neuraxial anaesthesia are unclear, however, pituitary apoplexy after spinal anaesthesia has been described outside pregnancy, possibly precipitated by intraoperative hypotension causing ischaemic necrosis in a region susceptible to changes in mean arterial pressure, especially where an a pituitary tumour is present. 38
Cerebral vein sinus thrombosis
The estimated peripartum and postpartum incidence of cerebral vein sinus thrombosis (CVT) is 9–12 per 100,000 births. 10 With neuraxial anaesthesia the incidence is 15.2 per 100,000 births, and 166 per 100,000 births in the setting of PDPH (aOR 11.48). 10 In a review of 58 case reports of CVT following neuraxial anaesthesia, 46 women (79%) experienced a PDPH. 39 Twelve deaths from CVT were reported in the United Kingdom over the 15 years between 2006 and 2021. 39 Several mechanisms have been postulated for CVT following dural puncture. The reduction in pressure and volume of CSF result in compensatory cerebral venous and arterial dilation, reducing the velocity of blood flow in the straight sinus by 50%, increasing blood viscosity. 40 Traction on cerebral vessels by a negative spinal-cranial gradient increases the predisposition of the venous epithelium to damage. Combined with the physiological hypercoagulability of pregnancy these factors predispose to thrombosis.
The median time from delivery to the diagnosis of CVT is 6.5 days, with 72% of women presenting within 2 weeks of delivery. 39 Headache occurs in 98% of women with CVT. 39 CVT-related headache is variable with no specific characteristics, and is difficult to differentiate from PDPH. Onset may be subacute or thunderclap, throbbing, migraine-like or constant. It may be localised or generalised. Headache with CVT has been described to lack a postural element; however, a review of cases reported a postural element in 72% of cases following neuraxial anaesthesia. 41 A change in the character of headache accompanied by the loss of a postural element, headache becoming constant, and failure of epidural blood patch to provide relief should prompt consideration of CVT. Other features of CVT may include seizures (45%) visual disturbance, papilloedema, fever, motor deficit, speech disturbance, cranial nerve signs, and coma. 39 Additional risk factors include thrombophilia (24%) and preeclampsia (17%). 39 Complications reported with CVT included intracranial venous infarction (26%), subarachnoid haemorrhage (10%), subdural haematoma (5%) and other intracranial haemorrhages (5%). 39 Diagnosis is by MRI venography, or CT venography if MRI is not available. Treatment is with low molecular weight heparin (LMWH), or intravenous unfractionated heparin if invasive procedures are planned or LMWH is contraindicated.
Posterior reversible encephalopathy and reversible cerebral vasoconstriction syndromes
Posterior reversible encephalopathy syndrome (PRES) is a clinical disorder which may present with headache, tonic-clonic seizures, visual disturbance (hemianopia, cortical blindness, visual neglect), confusion or decreased level of consciousness, and motor and/or sensory loss, accompanied by characteristic neuroimaging findings of subcortical white matter oedema typically affecting the parieto-occipital lobes. Eighteen cases have been described postpartum in the setting of PDPH, ten following epidural blocks, six following spinal anaesthesia, and two following CSE. 42 Initial presentation was with typical PDPH, with the subsequent development of seizures (83%), visual changes (50%), altered conscious state (28%) and focal weakness/sensory loss (17%) 2–7 days later. Diagnosis is by MRI. Management consists of control of hypertension, and magnesium sulphate if seizures occur. Three of the 14 cases of PRES also demonstrated features of diffuse arterial vasospasm on imaging suggestive of reversible cerebral vasoconstriction syndrome. All of the women made a full recovery with no residual neurological deficit.
Horner's syndrome
Eighty-three cases of Horner's syndrome have been reported following obstetric neuraxial blockade. 43 The mode of anaesthesia employed was epidural blockade in 68 cases (82%), caudal labour analgesia in 11 cases (13%), spinal anaesthesia in 3 cases (3.6%) and 1 case (1.2%) following combined spinal-epidural. 43 In the vast majority of cases Horner's syndrome was unilateral. Associated trigeminal nerve palsy occurred in 9 cases and hypoglossal nerve palsy in 1 case. Other associated features included hypotension, blurred vision, neck pain and sensorimotor upper limb involvement. Ptosis and miosis were noted in 94% of women and anhidrosis in 31%. Horner syndrome occurred within 1 h of the local anaesthetic bolus in 74%, and the median time for resolution of Horner's syndrome was 2 h. One case of permanent Horner's syndrome was reported thought secondary to neuraxial anaesthesia. 44 The mechanism is thought to be blockade of the stellate ganglion sympathetic fibres due to unexpected migration of local anaesthetic. An important aetiology to exclude in women with postpartum Horner's syndrome is spontaneous carotid artery dissection (SCAD). Horner's syndrome occurs in 25% of individuals with SCAD. Features suggestive of SCAD include severe headache (78%) ipsilateral to the Horner's syndrome, pulsatile tinnitus, symptoms or signs of anterior circulation stroke, and delayed presentation with onset a mean time of onset 11days (range 0–53 days) following delivery. 45 SCAD may be diagnosed using MR or CT angiography.
Cranial nerve palsies
A review of 43 cases of cranial nerve palsies (CNP) following obstetric neuraxial block showed that the most common nerves involved were abducens (17), followed by facial (12), vestibulocochlear (10) and trigeminal (7). 46 Twenty-seven cases (63%) were preceded by PDPH, whereas subdural haematoma and transverse sinus thrombosis were more rarely associated, with 6 and 1 cases respectively. Complete resolution of CNP occurred in 35 patients (81%), ranging from weeks to months after presentation. A case report described resolution of trigeminal nerve palsy associated with PDPH following epidural blood patch (EBP). 47 Not all PDPH CNP cases improve following EBP; some persist despite EBP or occur following EBP. 46
Auditory loss
Although transient clinically significant hearing loss has been reported by 0.2%–0.8% of non-pregnant individuals following spinal anaesthesia, and audiometrically-measurable hearing loss has been found in 10%–50% of individuals after dural puncture, these events appear to be rare in obstetric populations. 48 In two studies totalling 81 women who received neuraxial anaesthesia for elective caesarean, none developed hearing loss on audiometry postpartum.49,50 Sudden sensorineural hearing loss post-neuraxial anaesthesia is thought to occur during due to the transmission of decreased CSF pressure to the cochlear perilymph. A case report described complete resolution of spinal anaesthesia-associated hearing loss following EBP, congruent with the common mechanism of decreased CSF pressure. 51
Meningitis
The incidence of meningitis following neuraxial anaesthesia in pregnancy has been reported to be 2.5 per 100,000 procedures. 10 Where PDPH occurs the rate of meningitis is 83 per 100,000 (aOR 39.7). 10 A retrospective study found the risk was lower after epidural blocks than after spinal anaesthesia. 52 Streptococcus viridans is the most common pathogen in bacterial meningitis post-dural puncture, accounting for 43%–60% of cases. 53 Other organisms include gram negatives (Pseudomonas, Escherichia coli, Neisseria meningitidis) and Staphylococci. In pregnant women with meningitis unrelated to neuraxial anaesthesia the most common organisms are Streptococcus pneumoniae and Listeria monocytogenes. 54 Droplet contamination or needle contamination from incompletely sterilised skin are the major routes of infection. Headache is universally present, fever occurs in approximately 70% of cases, with other symptoms including vomiting, confusion and urinary retention. 55 The triad of severe headache, high fever and nuchal rigidity occurred in only 48% of cases. 55 Latency from neuraxial procedure to onset of symptoms ranged from 8 h to 8 days, with a median of 24 h. The exception is Aspergillus meningitis which may take weeks for symptoms to occur. Diagnosis is by assessment of CSF obtained by lumbar puncture. A CT should be performed prior to lumbar puncture in the setting of new-onset seizure, papilloedema, altered level of consciousness or focal neurological deficit. Antimicrobial therapy, with dexamethasone if indicated, should not be delayed if imaging is performed prior to lumbar puncture. Empiric antimicrobial therapy should consist of vancomycin combined with ceftazidime, cefipime or meropenem.
Aseptic meningitis
Aseptic meningitis is uncommon with neuraxial anaesthesia. Neurotoxicity from unintentional injection of an irritant or inflammatory response to medication in the intrathecal space may result in fever, headache, neck stiffness, photophobia and low back pain, typically onset within 24 h of spinal anaesthesia, with apyrexia, high CSF protein, normal CSF glucose, elevated CSF polymorphs, absent of organisms on CSF gram stain and full recovery within 48 h.56,57
Tonic-clonic seizures
Fourteen case reports have described otherwise unexplained tonic-clonic seizures in women with PDPH.58,59 Shearer et al. described eight cases where the seizure was preceded by visual disturbance including cortical blindness in three women. 58 Seizures occurred between 2 and 7 days following dural puncture. Imaging revealed changes of intracranial hypotension with diffuse dural thickening and enhancement, venous sinus dilatation, brainstem slumping, reversible pituitary enlargement, and changes in cerebral vasospasm.
Pneumocephalus
Pneumocephalus may occur as the result of unintentional injection of air into the subarachnoid or subdural space. Symptoms include headache, seizures, focal neurologic deficits and neck stiffness. 60
Spinal
Spinal or epidural haematoma
The estimated incidence of spinal epidural haematoma (SEH) is 0.55–5.5 per 100,000 epidural catheterisations, and 1 per 220,000 spinal anaesthetics.14,61,62 Puncture of vessels in the venous plexus and resulting accumulation of blood may result in ischaemia/infarction of the spinal cord or cauda equina. 14 The epidural venous plexus lacks valves and is susceptible to changes in intra-abdominal and intrathoracic pressure. Due to the anatomical location of neuraxial block, 95% of haematomas involve the dorsal spinal canal.63,64 Haematoma may be asymptomatic for some time. Symptoms suggestive of SEH include bladder or bowel dysfunction, progressive motor and/or sensory block and back pain, though the last may occur in only 25% of cases. Initial symptoms occur during puncture in 7%, during anaesthesia with an indwelling epidural catheter in 36%, and after catheter removal in 56%. 65 Symptom onset may post-date catheter removal by several days.66,67 Early recognition of haematoma formation and neurological sequelae is critical as immediate surgical decompression is required, optimal neurologic outcomes occurring with surgery within 12 h of initial symptoms. 68 Emergency MRI (or CT if MRI is not available/contraindicated) should be performed if SEH is considered. Nonoperative management can be successful in the absence of significant neurology. 69 Additional risk factors for spinal haematoma include coagulopathy, thrombocytopenia and vascular and spinal malformations. 70 Interestingly, spontaneous spinal epidural haematoma unrelated to or distant from the site of neuraxial anaesthesia have been reported in second and third trimester, usually involving the cervicothoracic spine, highlighting that increased pressure in the venous plexus can cause spontaneous vascular rupture.67,71
Spinal epidural abscess
The incidence of spinal epidural abscess (SEA) has been reported to be 0–1.6 per 100,000 epidural catheterizations.52,61,62 Women with SEA typically present with fever (74%), severe localised back pain (70–100%), with or without symptoms and signs of cord and nerve root involvement (50%). Spinal tenderness is present in 17%–98% of cases. The median time from procedure to onset of symptoms of epidural abscess is 12 days in the non-obstetric population. 72 The risk is higher with epidural catheter placement. C-reactive protein is typically elevated. MRI with gadolinium is the imaging modality of choice. Staphylococci are the most common causative organism, followed by gram-negative bacilli, streptococci, and more rarely, anaerobes and mycobacteria. 73 The causative organism may be obtained from the abscess or culture of blood and CSF. Management usually involves a combination of surgical drainage and systemic antibiotic therapy. Antibiotics should be commenced as soon as the diagnosis of SEA is suspected and blood cultures have been collected. Empiric therapy should consist of vancomycin with a third-generation cephalosporin or meropenem.
Anterior spinal artery syndrome
Five cases of paraplegia due to anterior spinal artery syndrome (ASAS) have been described following neuraxial anaesthesia. 74 ASAS following neuraxial block presents as failure to regain motor function and pain and temperature sensation below the level of the lesion, with sparing of proprioception and vibration. Autonomic neuropathy with sphincter dysfunction may be present. Possible factors related to delivery include peripartum hypotension, sympathectomy and vasospasm due to anaesthetic agents, the prothrombotic state of pregnancy, spinal vascular malformations, thoracoabdominal aortic disease, uterine-mediated compression of the spinal vasculature, increased CSF pressure and venous obstruction. 74 Thoracolumbar magnetic resonance imaging may show hyperintense lesions due to anterior spinal ischaemia, however, may not identify pathology. 74
Epidural blood patch (EBP) complications
EBP remains the gold standard therapy for PDPH, with complete and permanent resolution of headache in up to third of cases of PDPH in with a single epidural blood patch, and partial relief in 50%–80%. 75 Smaller Australian prospective studies report similar success, with complete relief in 50%, partial in 38% and no relief in only 12%. 76 EBP can also prevent development of PDPH after an accidental dural puncture, although when used to treat PDPH it is best performed 48 h after headache onset.21,77 There is insufficient evidence as to whether EBP reduces the risk of subdural haematoma or cerebral vein sinus thrombosis, or improves CNP in women with obstetric PDPH. EBP should not be performed if the woman is febrile or there are any signs suggestive of systemic infection. EBP requires two anaesthetists both employing a fully aseptic technique. Twenty millilitres of blood should be drawn from an arm vein by the second anaesthetist and injected slowly into the epidural space at the site of the previous epidural or at one space higher or lower by the first anaesthetist Complications of EBP include further dural puncture (∼1:100), failure, back pain, nerve injury, meningitis, abscess, seizures, ISDH, CNP, tinnitus and arachnoiditis. 78
Spinal myoclonus
Six cases of spinal myoclonus have been reported following neuraxial anaesthesia in pregnancy.79–82 The disorder is characterised by the onset of involuntary myoclonic movements of the upper or lower limbs or trunk within 4 h of the administration of anaesthetic. Symptoms typically resolved within 2 days, though one case persisted until 6 months postpartum. 79
Other
Fever
A meta-analysis of 39 studies found that pregnant women managed with epidural blocks had an odds ratio of 5.26 of developing intrapartum hyperthermia. 83 Subgroup analysis of the 13 randomised control trials found this association persisted with an odds ratio of 4.21. 83 Similarly, an earlier meta-analysis of 12 RCTs reported a risk ratio for fever after epidural anaesthesia of 3.54. 84 The observed maternal temperature increase during labour averages 0.1°C per hour of epidural analgesia, following a lag of 4 to 5 h. 85 Approximately 20% of women labouring with neuraxial anaesthesia will experience temperature above 38°C. A previous systematic review concluded it was unlikely that epidural anaesthesia increased the risk of intrapartum infection. 84 The most likely explanation for epidural hyperthermia is that it represents a distinct condition from immunomodulation and/or cholinergic sympathetic blockade. 83 A study of the Swedish Birth Registry suggested that fever associated with epidural analgesia was a benign rise in temperature not associated with adverse neonatal neurologic outcomes. 86 Assessment of peripartum fever is complicated by the physiological rise in white blood cell count and erythrocyte sedimentation rate during pregnancy. C-reactive protein levels are unchanged during pregnancy though may rise up to fourfold in the first 48 h postpartum. 87 It is, however, critical, that a fever in labour is not attributed to neuraxial anaesthesia without thorough history, examination and investigations to exclude an infective or other pathological cause.
Arrhythmias and cardiac arrest
Spinal anaesthesia may be associated with atrioventricular (AV) block in pregnant women during CS, as well as in nonpregnant individuals. A prospective study of 254 healthy women with neuraxial anaesthesia for CS found first-degree atrioventricular (AV) block in 3.5%, second-degree AV block in 3.5%, and severe bradycardia (less than 50 bpm) in 6.7% of women, with no reported adverse maternal outcome. 88 Two cases of third-degree AV block have been described soon after induction of spinal anaesthesia, both responding to atropine.89,90 Ventricular arrhythmias are also rarely observed, with one case of ventricular fibrillation and cardiac arrest described post-CS confounded by hypomagnesaemia. 91 Severe sinus bradycardia followed by asystole has also been described during spinal anaesthesia for CS.92,93 This may be related to overlapping phenomena of a vasovagal response or the Bezold–Jarisch reflex, a cardioinhibitory reflex whereby poor ventricular filling causes reflex bradycardia, vasodilation and hypotension. 93
Transient neurologic symptoms
Transient neurologic symptoms present as pain and/or dysesthesia which radiates from the gluteal region to the posterior thigh within 2–24 h of spinal anaesthesia. 94 Ambulation and the administration of nonsteroidal anti-inflammatory medications may provide relief. Symptoms usually resolve spontaneously within 2–3 days. The pathogenesis is not understood. The presence of sensory or motor signs on physical examination may be useful to guide whether electromyographic (EMG) testing and MRI should be performed.
Direct nerve injury
The reported incidence of postpartum lumbosacral plexus and peripheral nerve injury following obstetric neuraxial blocks ranges between 0.1% and 9.2% depending on studies and methodology. 95 One study of nerve injury following obstetric neuraxial blocks attributed 80% of nerve injuries to intrinsic obstetric palsy and 20% to anaesthetic causation. 95 Eighty-four percent of postpartum neuropathy occurs in the femoral nerve distribution. 96 Nerve injury to the spinal cord, conus medullaris and spinal nerves may occur due to direct needle or catheter trauma, and is typically associated with acute pain at the time of needle insertion. Pain and dysesthesia due to spinal nerve injury usually resolve spontaneously within 72 h, and persistent injury is rare. 97 Direct trauma to the spinal cord may present as cauda equina syndrome, radiculopathy or focal neurologic deficit including foot drop. Neurological examination should guide further testing with EMG and MRI. EMG may not disclose an abnormality in the first 72 h after injury, and may need to be repeated if symptoms or signs persist. 98
Necrotising fasciitis
Necrotising fasciitis at the site of procedure has been described in five postpartum women following spinal anaesthesia in India, with two fatalities.99–103 Organisms isolated included streptococci, gram negatives, anaerobes and fungi.
Conclusion
Postpartum complications of uncertain aetiology raise considerable concern for the patient, relatives and caring healthy professionals, both acutely and with regard to risk of recurrence with subsequent pregnancy. Postpartum complications should not be attributed to neuraxial anaesthesia until other causes have been excluded. Obstetric physicians’ awareness of the array of complications potentially related to neuraxial anaesthesia is crucial for management of the postpartum woman.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Not applicable.
Informed consent: Not applicable.
ORCID iD: Adam Morton https://orcid.org/0000-0001-9887-714X
References
- 1.D'Angelo R, Smiley RM, Riley ET, et al. Serious complications related to obstetric anesthesia: the serious complication repository project of the Society for Obstetric Anesthesia and Perinatology. Anesthesiology 2014; 120: 1505–1512. [DOI] [PubMed] [Google Scholar]
- 2.Royal College of Anaesthetists. Major complications of central neuraxial in the UK: Report on the Third National Audit Project, (2009, accessed 27 August 2020).
- 3.Fish SA, Morrison JC, Bucovaz ET, et al. Cerebral spinal fluid studies in eclampsia. Am J Obstet Gynecol 1972; 112: 502–512. [DOI] [PubMed] [Google Scholar]
- 4.Marx GF, Zemaitis MT, Orkin LR. Cerebrospinal fluid pressures during labor and obstetrical anesthesia. Anesthesiology 1961; 22: 348–354. [DOI] [PubMed] [Google Scholar]
- 5.Vasicka A, Kretchmer H, Lawas F. Cerebrospinal fluid pressures during labor. Am J Obstet Gynecol 1962; 84: 206–212. [DOI] [PubMed] [Google Scholar]
- 6.Leffert LR, Schwamm LH. Neuraxial anesthesia in parturients with intracranial pathology: a comprehensive review and reassessment of risk. Anesthesiology 2013; 119: 703–718. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds F. Dural puncture and headache. Br Med J 1993; 306: 874–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turnbull DK, Shepherd DB. Post-dural puncture headache: pathogenesis, prevention and treatment. Br J Anaesth 2003; 91: 718–729. [DOI] [PubMed] [Google Scholar]
- 9.Choi PT, Galinski SE, Takeuchi L, et al. PDPH Is a common complication of neuraxial blockade in parturients: a meta-analysis of obstetrical studies. Can J Anaesth 2003; 50: 460–469. [DOI] [PubMed] [Google Scholar]
- 10.Guglielminotti J, Landau R, Li G. Major neurologic complications associated with postdural puncture headache in obstetrics: a retrospective cohort study. Anesth Analg 2019; 129: 1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peralta F, Higgins N, Lange E, et al. The relationship of body mass index with the incidence of postdural puncture headache in parturients. Anesth Analg 2015; 121: 451–456. [DOI] [PubMed] [Google Scholar]
- 12.International Headache Society. IHS Classification ICHD-3 Beta, https://ichd-3.org/ (accessed 13 February 2022).
- 13.Loures V, Savoldelli G, Kern K, et al. Atypical headache following dural puncture in obstetrics. Int J Obstet Anesth 2014; 23: 246–252. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Brown M, Sun L, et al. Complications and liability related to regional and neuraxial anesthesia. Best Pract Res Clin Anaesthesiol 2019; 33: 487–497. [DOI] [PubMed] [Google Scholar]
- 15.Lee G-H, Kim J, Kim H-W, et al. Comparisons of clinical characteristics, brain MRI findings, and responses to epidural blood patch between spontaneous intracranial hypotension and post-dural puncture headache: retrospective study. BMC Neurol 2021; 21: 1–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abela GP, Tan T. Accidental dural puncture and post-dural puncture headache: a retrospective review in an Irish maternity hospital. Ir J Med Sci 2019; 189: 657–660. [DOI] [PubMed] [Google Scholar]
- 17.Vgontzas A, Robbins MS. A hospital based retrospective study of acute postpartum headache. Headache 2018; 58: 845–851. [DOI] [PubMed] [Google Scholar]
- 18.Stella CL, Jodicke CD, How HY, et al. Postpartum headache: is your work-up complete? Am J Obstet Gynecol 2007; 196: e311–e317. [DOI] [PubMed] [Google Scholar]
- 19.Zeidan A, Farhat O, Maaliki H, et al. Does postdural puncture headache left untreated lead to subdural hematoma? Case report and review of the literature. Int J Obstet Anesth 2006; 15: 50–58. [DOI] [PubMed] [Google Scholar]
- 20.Association of Obstetric Anaesthetists. Treatment of obstetric post-dural puncture headache, https://www.oaa-anaes.ac.uk/Postdural_Puncture_Headache (2018, accessed 8 August 2022).
- 21.Kokki M, Sjovall S, Keinanen M, et al. The influence of timing on the effectiveness of epidural blood patches in parturients. Int J Obstet Anesth 2013; 22: 303–309. [DOI] [PubMed] [Google Scholar]
- 22.Han J, Lim DJ, Kim SD, et al. Subdural hematoma without subarachnoid hemorrhage caused by the rupture of middle cerebral artery aneurysm. J Cerebrovasc Endovasc Neurosurg 2016; 18: 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore AR, Wieczorek PM, Carvalho JCA. Association between post-dural puncture headache after neuraxial anesthesia in childbirth and intracranial subdural hematoma. JAMA Neurol 2020; 77: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott DB, Hibbard BM. Serious non-fatal complications associated with extradural block in obstetric practice. Br J Anaesth 1990; 64: 537–541. [DOI] [PubMed] [Google Scholar]
- 25.Szeto V, Kosirog J, Eilbert W. Intracranial subdural hematoma after epidural anesthesia: a case report and review of the literature. Int J Emerg Med 2018; 11: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuypers V, Van de Velde M, Devroe S. Intracranial subdural haematoma following neuraxial anaesthesia in the obstetric population: a literature review with analysis of 56 reported cases. Int J Obstet Anesth 2016; 25: 58–65. [DOI] [PubMed] [Google Scholar]
- 27.Amorim JA, Remigio DS, Damazio Filho O, et al. Intracranial subdural hematoma post-spinal anesthesia: report of two cases and review of 33 cases in the literature. Rev Bras Anestesiol 2010; 60: 620–629, 344–629. [DOI] [PubMed] [Google Scholar]
- 28.Eggert SM, Eggers KA. Subarachnoid haemorrhage following spinal anaesthesia in an obstetric patient. Br J Anaesth 2001; 86: 442–444. [DOI] [PubMed] [Google Scholar]
- 29.Mishra V LS, Mistry K, Verneker R. Subarachnoid haemorrhage following spinal anaesthesia in pregnant women: a rare occurrence. J Clin Diagn Res 2018; 12: QD01–QD02. [Google Scholar]
- 30.Shay A LL, Chu A, Theil Fet al. et al. Subarchnoid haemorrhage following spinal anesthesia for cesarean sections. MOJ Women's Health 2016; 2: 89–91. [Google Scholar]
- 31.Singh N ST, Singh M, Laithangbam P. Subarachnoid hemorrhage after central neuroaxial blockade: an accidental finding. Journal of Medical Society 2013; 27: 154–155. [Google Scholar]
- 32.Sharma K. Intracerebral hemorrhage after spinal anesthesia. J Neurosurg Anesthesiol 2002; 14: 234–237. [DOI] [PubMed] [Google Scholar]
- 33.Swei SC, Chiu WH, Liang HC, et al. Cerebral hemorrhage following spinal anesthesia for cesarean section: a case report. Acta Anaesthesiol Sin 1994; 32: 279–281. [PubMed] [Google Scholar]
- 34.Bateman BT, Olbrecht VA, Berman MF, et al. Peripartum subarachnoid hemorrhage: nationwide data and institutional experience. Anesthesiology 2012; 116: 324–333. [DOI] [PubMed] [Google Scholar]
- 35.Schrupp Berg HL, Edlow JA. Post-partum pituitary apoplexy: a case report. Intern Emerg Med 2007; 2: 311–314. [DOI] [PubMed] [Google Scholar]
- 36.Grand'Maison S, Weber F, Bedard MJ, et al. Pituitary apoplexy in pregnancy: a case series and literature review. Obstet Med 2015; 8: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathur D, Lim LFM, Mathur M, et al. Pituitary apoplexy with reversible cerebral vasoconstrictive syndrome after spinal anaesthesia for emergency caesarean section: an uncommon cause for postpartum headache. Anaesth Intensive Care 2014; 42: 99–105. [DOI] [PubMed] [Google Scholar]
- 38.Lennon M, Seigne P, Cunningham AJ. Pituitary apoplexy after spinal anaesthesia. Br J Anaesth 1998; 81: 616–618. [DOI] [PubMed] [Google Scholar]
- 39.Chambers DJ, Bhatia K, Columb M. Postpartum cerebral venous sinus thrombosis following obstetric neuraxial blockade: a literature review with analysis of 58 case reports. Int J Obstet Anesth 2022; 49: 103218. [DOI] [PubMed] [Google Scholar]
- 40.Canhão P, Batista P, Falcão F. Lumbar puncture and dural Sinus thrombosis – a causal or casual association? Cerebrovasc Dis 2005; 19: 53–56. [DOI] [PubMed] [Google Scholar]
- 41.Kate MP, Thomas B, Sylaja PN. Cerebral venous thrombosis in post-lumbar puncture intracranial hypotension: case report and review of literature. F1000Res 2014; 3: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng YY, Weng XP, Fu FW, et al. Cerebrospinal fluid hypovolemia and posterior reversible encephalopathy syndrome. Front Neurol 2020; 11: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chambers DJ, Bhatia K. Horner’s syndrome following obstetric neuraxial blockade – a systematic review of the literature. Int J Obstet Anesth 2018; 35: 75–87. [DOI] [PubMed] [Google Scholar]
- 44.Goel S, Burkat CN. Unusual case of persistent Horner's syndrome following epidural anaesthesia and caesarean section. Indian J Ophthalmol 2011; 59: 389–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruan CY, Gao BL, Pang HL, et al. Postpartum cerebral arterial dissections: clinical features and treatment. Medicine (Baltimore) 2021; 100: e27798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chambers DJ, Bhatia K. Cranial nerve palsy following central neuraxial block in obstetrics – a review of the literature and analysis of 43 case reports. Int J Obstet Anesth 2017; 31: 13–26. [DOI] [PubMed] [Google Scholar]
- 47.Zajac AE, Richardson MG. Postdural puncture headache treated with epidural blood patch and subsequent resolution of atypical odontalgia: a case report. A A Pract 2018; 11: 73–75. [DOI] [PubMed] [Google Scholar]
- 48.Sprung J, Bourke DL, Contreras MG, et al. Perioperative hearing impairment. Anesthesiology 2003; 98: 241–257. [PubMed] [Google Scholar]
- 49.Finegold H, Mandell G, Vallejo M, et al. Does spinal anesthesia cause hearing loss in the obstetric population? Anesth Analg 2002; 95: 198–203. [DOI] [PubMed] [Google Scholar]
- 50.Karabayirli S, Ugur KS, Ayrim A, et al. Hearing loss after spinal anesthesia : a comparative prospective randomized cohort study. Acta Anaesthesiol Belg 2016; 67: 87–95. [PubMed] [Google Scholar]
- 51.Lee CM, Peachman FA. Unilateral hearing loss after spinal anesthesia treated with epidural blood patch. Anesth Analg 1986; 65: 12. [DOI] [PubMed] [Google Scholar]
- 52.Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990-1999. Anesthesiology 2004; 101: 950–959. [DOI] [PubMed] [Google Scholar]
- 53.O'Neal MA. Obstetric anaesthesia: what a neurologist needs to know. Pract Neurol 2019; 19: 238–245. [DOI] [PubMed] [Google Scholar]
- 54.Adriani KS, Brouwer MC, van der Ende A, et al. Bacterial meningitis in pregnancy: report of six cases and review of the literature. Clin Microbiol Infect 2012; 18: 345–351. [DOI] [PubMed] [Google Scholar]
- 55.Sandkovsky U, Mihu MR, Adeyeye A, et al. Iatrogenic meningitis in an obstetric patient after combined spinal-epidural analgesia: case report and review of the literature. South Med J 2009; 102: 287–290. [DOI] [PubMed] [Google Scholar]
- 56.Ducornet A, Brousous F, Jacob C, et al. [Meningitis after spinal anesthesia: think about bupivacaine!]. Ann Fr Anesth Reanim 2014; 33: 288–290. [DOI] [PubMed] [Google Scholar]
- 57.Doghmi N, Meskine A, Benakroute A, et al. Aseptic meningitis following a bupivacaine spinal anesthesia. Pan Afr Med J 2017; 27: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shearer VE, Jhaveri HS, Cunningham FG. Puerperal seizures after post-dural puncture headache. Obstet Gynecol 1995; 85: 255–260. [DOI] [PubMed] [Google Scholar]
- 59.Taylor CR, Mehdiratta JE, Yurashevich M, et al. Tonic-clonic seizure after unrecognized unintentional dural puncture. Int J Obstet Anesth 2020; 44: 77–80. [DOI] [PubMed] [Google Scholar]
- 60.Avila JD. Thunderclap headache secondary to pneumocephalus following epidural anesthesia. Neurohospitalist 2016; 6: 132–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosero EB, Joshi GP. Nationwide incidence of serious complications of epidural analgesia in the United States. Acta Anaesthesiol Scand 2016; 60: 810–820. [DOI] [PubMed] [Google Scholar]
- 62.Ruppen W, Derry S, McQuay H, et al. Incidence of epidural hematoma, infection, and neurologic injury in obstetric patients with epidural analgesia/anesthesia. Anesthesiology 2006; 105: 394–399. [DOI] [PubMed] [Google Scholar]
- 63.Kreppel D, Antoniadis G, Seeling W. Spinal hematoma: a literature survey with meta-analysis of 613 patients. Neurosurg Rev 2003; 26: 1–49. [DOI] [PubMed] [Google Scholar]
- 64.Gruzman I, Shelef I, Weintraub AY, et al. Puerperal ventral epidural hematoma after epidural labor analgesia. Int J Obstet Anesth 2017; 31: 100–104. [DOI] [PubMed] [Google Scholar]
- 65.Bos EME, Haumann J, de Quelerij M, et al. Haematoma and abscess after neuraxial anaesthesia: a review of 647 cases. Br J Anaesth 2018; 120: 693–704. [DOI] [PubMed] [Google Scholar]
- 66.Svelato A, Rutili A, Bertelloni C, et al. Case report: difficulty in diagnosis of delayed spinal epidural hematoma in puerperal women after combined spinal epidural anaesthesia. BMC Anesthesiol 2019; 19: 54–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng H-X, Eric Nyam T-T, Liu C-A, et al. Spontaneous spinal epidural hematoma after normal spontaneous delivery with epidural analgesia: case report and literature review. World Neurosurg 2020; 137: 214–217. [DOI] [PubMed] [Google Scholar]
- 68.Lagerkranser M, Lindquist C. Neuraxial blocks and spinal haematoma: review of 166 cases published 1994–2015. Part 2: diagnosis, treatment, and outcome. Scand J Pain 2017; 15: 130–136. [DOI] [PubMed] [Google Scholar]
- 69.Siasios I, Vakharia K, Gibbons K, et al. Large, spontaneous spinal subdural-epidural hematoma after epidural anesthesia for caesarean section: conservative management with excellent outcome. Surg Neurol Int 2016; 7: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lagerkranser M. Neuraxial blocks and spinal haematoma: review of 166 case reports published 1994–2015. Part 1: demographics and risk-factors. Scand J Pain 2017; 15: 118–129. [DOI] [PubMed] [Google Scholar]
- 71.Soltani S, Nogaro M-C, Jacqueline Kieser SC, et al. Spontaneous spinal epidural hematomas in pregnancy: a systematic review. World Neurosurg 2019; 128: 254–258. [DOI] [PubMed] [Google Scholar]
- 72.Christie IW, McCabe S. Major complications of epidural analgesia after surgery: results of a six-year survey. Anaesthesia 2007; 62: 335–341. [DOI] [PubMed] [Google Scholar]
- 73.Darouiche RO. Spinal epidural abscess. N Engl J Med 2006; 355: 2012–2020. [DOI] [PubMed] [Google Scholar]
- 74.Gong J, Gao H, Gao Y, et al. Anterior spinal artery syndrome after spinal anaesthesia for caesarean delivery with normal lumbar and thoracic magnetic resonance imaging. J Obstet Gynaecol 2016; 36: 855–856. [DOI] [PubMed] [Google Scholar]
- 75.Russell R, Laxton C, Lucas DN, et al. Treatment of obstetric post-dural puncture headache. Part 2: epidural blood patch. Int J Obstet Anesth 2019; 38: 104–118. [DOI] [PubMed] [Google Scholar]
- 76.Banks S, Paech M, Gurrin L. An audit of epidural blood patch after accidental dural puncture with a Tuohy needle in obstetric patients. Int J Obstet Anesth 2001; 10: 172–176. [DOI] [PubMed] [Google Scholar]
- 77.Bradbury CL, Singh SI, Badder SR, et al. Prevention of post-dural puncture headache in parturients: a systematic review and meta-analysis. Acta Anaesthesiol Scand 2013; 57: 417–430. [DOI] [PubMed] [Google Scholar]
- 78.Iga K, Murakoshi T, Kato A, et al. Repeat epidural blood patch at the level of unintentional dural puncture and its neurologic complications: a case report. JA Clin Rep 2019; 5: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Menezes FV, Venkat N. Spinal myoclonus following combined spinal-epidural anaesthesia for Caesarean section. Anaesthesia 2006; 61: 597–600. [DOI] [PubMed] [Google Scholar]
- 80.Lev A, Korn-Lubezki I, Steiner-Birmanns B, et al. Prolonged propriospinal myoclonus following spinal anesthesia for cesarean section: case report and literature review. Arch Gynecol Obstet 2012; 286: 271–272. [DOI] [PubMed] [Google Scholar]
- 81.Ji TT, Shih CK, Hsieh YJ, et al. Desultory propriospinal myoclonus after epidural analgesia in a healthy parturient. Int J Obstet Anesth 2015; 24: 285–286. [DOI] [PubMed] [Google Scholar]
- 82.Kang HY, Lee SW, Hong EP, et al. Myoclonus-like involuntary movements following cesarean delivery epidural anesthesia. J Clin Anesth 2016; 34: 392–394. [DOI] [PubMed] [Google Scholar]
- 83.Morton S, Kua J, Mullington CJ. Epidural analgesia, intrapartum hyperthermia, and neonatal brain injury: a systematic review and meta-analysis. Br J Anaesth 2021; 126: 500–515. [DOI] [PubMed] [Google Scholar]
- 84.Jansen S, Lopriore E, Naaktgeboren C, et al. Epidural-related fever and maternal and neonatal morbidity: a systematic review and meta-analysis. Neonatology 2020; 117: 259–270. [DOI] [PubMed] [Google Scholar]
- 85.Kuczkowski KM. The epidural "fever": what does an obstetrician need to know? Arch Gynecol Obstet 2007; 276: 71–72. [DOI] [PubMed] [Google Scholar]
- 86.Törnell S, Ekéus C, Hultin M, et al. Low Apgar score, neonatal encephalopathy and epidural analgesia during labour: a Swedish registry-based study. Acta Anaesthesiol Scand 2015; 59: 486–495. [DOI] [PubMed] [Google Scholar]
- 87.Nielsen FR, Bek KM, Rasmussen PE, et al. C-reactive protein during normal pregnancy. Eur J Obstet Gynecol Reprod Biol 1990; 35: 23–27. [DOI] [PubMed] [Google Scholar]
- 88.Shen CL, Ho YY, Hung YC, et al. Arrhythmias during spinal anesthesia for Cesarean section. Can J Anaesth 2000; 47: 393–397. [DOI] [PubMed] [Google Scholar]
- 89.Matta BF, Magee P. Wenckebach type heart block following spinal anaesthesia for caesarean section. Can J Anaesth 1992; 39: 1067–1068. [DOI] [PubMed] [Google Scholar]
- 90.Joseph SE, Minehart RD. Third-degree heart block during spinal anesthesia for cesarean delivery. A A Case Rep 2014; 3: –5. [DOI] [PubMed] [Google Scholar]
- 91.Scull TJ, Carli F. Cardiac arrest after Caesarean section under subarachnoid block. Br J Anaesth 1996; 77: 274–276. [DOI] [PubMed] [Google Scholar]
- 92.Jang YE, Do SH, Song IA. Vasovagal cardiac arrest during spinal anesthesia for Cesarean section -a case report. Korean J Anesthesiol 2013; 64: 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Comito C, Bechi L, Serena C, et al. Cardiac arrest in the delivery room after spinal anesthesia for cesarean section: a case report and review of literature. J Matern Fetal Neonatal Med 2020; 33: 1456–1458. [DOI] [PubMed] [Google Scholar]
- 94.Liguori GA, Zayas VM, Chisholm MF. Transient neurologic symptoms after spinal anesthesia with mepivacaine and lidocaine. Anesthesiology 1998; 88: 619–623. [DOI] [PubMed] [Google Scholar]
- 95.Haller G, Pichon I, Gay FO, et al. Risk factors for peripheral nerve injuries following neuraxial labour analgesia: a nested case-control study. Acta Anaesthesiol Scand 2017; 61: 1203–1214. [DOI] [PubMed] [Google Scholar]
- 96.Tournier A, Doremieux AC, Drumez E, et al. Lower-limb neurologic deficit after vaginal delivery: a prospective observational study. Int J Obstet Anesth 2020; 41: 35–38. [DOI] [PubMed] [Google Scholar]
- 97.Dripps RD, Vandam LD. Long-term follow-up of patients who received 10,098 spinal anesthetics: failure to discover major neurological sequelae. J Am Med Assoc 1954; 156: 1486–1491. [DOI] [PubMed] [Google Scholar]
- 98.McCombe K, Bogod DG. Learning from the law. A review of 21 years of litigation for nerve injury following central neuraxial blockade in obstetrics. Anaesthesia 2020; 75: 541–548. [DOI] [PubMed] [Google Scholar]
- 99.Yagnik VD. Necrotizing fasciitis following spinal anesthesia: a rare and devastating complication. Saudi J Anaesth 2018; 12: 140–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kundra S, Singh RM, Grewal A, et al. Necrotizing fasciitis after spinal anesthesia. Acta Anaesthesiol Scand 2013; 57: 257–261. [DOI] [PubMed] [Google Scholar]
- 101.Chakaravarthi K, Gupta A, Kaman L. Neglected necrotizing fasciitis – a rare complication of spinal anaesthesia. J Clin Diagn Res 2017; 11: Pd03–pd04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Singh RK, Dutta G. Fatal necrotising fasciitis after spinal anaesthesia. J Cutan Aesthet Surg 2013; 6: 165–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Agarwal A, Babu MS, Verma M, et al. A rare case of necrotising fasciitis after spinal anaesthesia. Ind J Anesth 2013; 57: 316–318. [DOI] [PMC free article] [PubMed] [Google Scholar]