Abstract
Restriction digest profiling of pneumococcal pbp2b-specific amplicons was effective for screening penicillin resistance. The pbp2b amplicon of all pneumococcal isolates for which the MICs of penicillin were ≤0.03 μg/ml had one of two different susceptible restriction profiles, and all 33 isolates for which MICs were 0.5 μg/ml or greater had one of seven distinct resistant profiles. Low-concentration penicillin resistance (MICs = 0.06 μg/ml to 0.25 μg/ml) was associated with sensitive HaeIII profiles in some isolates; however, RsaI profiling and pbp2b sequence analysis of such isolates revealed that some isolates contained low-level resistant pbp2b alleles, while others had susceptible pbp2b alleles. This data indicates that low-level penicillin resistance is sometimes conferred by determinants other than pbp2b.
Penicillin resistance in Streptococcus pneumoniae is believed to be due to interspecies recombination events that have occurred between penicillin-binding protein (PBP) genes, resulting in pneumococcal PBPs with decreased affinity for penicillin (1, 3–12). Penicillin-resistant pneumococci are characterized by mosaic PBP genes that exhibit a remarkable degree of variation; PBP genes of penicillin-sensitive pneumococci typically show little variation (4, 6, 8, 11).
Previous studies have indicated that alterations in pbp2b are usually associated with penicillin MICs of ≥0.1 μg/ml (9, 11), suggesting that the pbp2b gene might be the single best target for deducing penicillin nonsusceptibility. Using frequently cutting restriction enzymes for fingerprinting PBP genes (1, 7), we noted that the enzyme cleavage patterns of the resistant pbp2b alleles in the GenBank database differed from those of the sensitive pbp2b alleles. In the present study we tested this approach with a diverse group of pneumococcal clinical isolates.
Bacterial strains.
All pneumococci were isolated from blood obtained from hospital patients from metropolitan Atlanta, Ga., with invasive pneumococcal disease during January and February of 1998. Isolates representative of many different serotypes and for which penicillin MICs varied widely were chosen. Isolate serotypes were determined by latex agglutination and confirmed by positive Quellung reactions. Penicillin MICs were determined by the Pasco MIC/ID system (Difco Laboratories, Detroit, Mich.). Centers for Disease Control and Prevention stock cultures of Streptococcus sanguis (SS910), Streptococcus gordonii (SS983), Streptococcus mitior (SS1165), Streptococcus oralis (SS1236 and SS1519), Streptococcus mitis (SS1246), Enterococcus faecium (SS935), and Streptococcus pyogenes (SS1457) were used to test the specificity of the primer pair used.
PCR and restriction analysis.
Strains were grown overnight on Trypticase soy agar containing 5% sheep blood. For the template, crude pneumococcal lysates were prepared by resuspending a loopful of overnight growth in 300 μl of 0.85% saline, followed by 5 min of incubation at 75°C. The cells were centrifuged, resuspended in 50 μl of TE buffer (10 mM Tris [pH 8], 1 mM EDTA) containing 9 U of mutanolysin (Sigma), and incubated at 37°C for 10 min. These samples were heated at 100°C for 3 min and centrifuged 30 s before 1 μl was removed from the surface to be used in PCRs. PCR primers pF (GATCCTCTAAATGATTCTCAGGTGGCTGT) and pR (GTCAATTAGCTTAGCAATAGGTGTTGGAT) flank the 3′ 486 codons of pbp2B (4). The Expand High Fidelity PCR system (Boehringer Mannheim) was used for 20-μl reaction mixtures containing 15 mM MgCl2, 1.4 μM (each) primer, 200 μM deoxyribonucleotides, and 0.5 U of the thermostable DNA polymerase mixture. The samples were subjected to an initial denaturation cycle at 95°C for 1 min, followed by 10 cycles at 94°C for 15 s, annealing at 58°C for 30 s, and elongation at 72°C for 1 min. This procedure was followed by 20 cycles of the same parameters but with sequential 10-s increments of the elongation cycle. A 7-min extension followed the final cycle.
Three units of HaeIII or RsaI (Gibco-BRL) was added to 5 μl of unpurified PCR product. Following a 20-min incubation at 37°C, the restriction digests were electrophoresed for 20 min at 150 mA through 2% agarose gels containing ethidium bromide and photographed with a UV transilluminator.
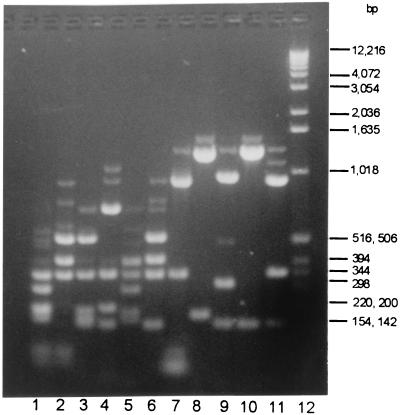
The HaeIII reaction conditions used were close to optimal (data not shown). Even so, all HaeIII profiles generated in this study are comprised of bright bands, which depict the true restriction pattern, and a minor background pattern of incomplete pbp2b amplicon digest fragments (Fig. 1). Both the major and the minor components of these profiles were identical for all members exhibiting a given profile.
FIG. 1.
HaeIII profiles of pbp2b amplicons from penicillin-susceptible (MICs, ≤0.03 μg/ml) (s1 and s2) and penicillin-resistant (MICs, 0.06 to 4.0 μg/ml) (r1 to r9) clinical blood isolates of pneumococci. Lane 1, r2; lane 2, r8; lane 3, r5; lane 4, r4; lane 5, r3; lane 6, r1; lane 7, r9; lane 8, r7; lane 9, r6; lane 10, s2; lane 11, s1, lane 12, Gibco-BRL kilobase ladder.
DNA sequence analysis.
Dye-deoxy terminator kits were used as described by the manufacturer (Applied Biosystems, Inc.) with oligonucleotides pF, pR, p5 (5a), and p6 (5a). Reaction mixtures were loaded onto 4.25% polyacrylamide gels and electrophoresed on an Applied Biosystems, Inc., model 377 sequencer.
Results and discussion.
PCR with primers pF and pR with crude pneumococcal template yielded a single abundant product of approximately 1.5 kb. None of the other species tested yielded a product. As shown in Table 1, all 63 isolates for which penicillin MICs were ≤0.03 μg/ml had HaeIII profile s1 or s2 (Fig. 1, lanes 11 and 12) and represented 20 capsular serotypes. Additionally, five of six isolates for which MICs were 0.06 μg/ml, one of seven isolates for which MICs were 0.12 μg/ml, and four of nine isolates for which MICs were 0.25 μg/ml exhibited one of these two profiles.
TABLE 1.
HaeIII profile distribution among systemic pneumococcal isolates of various serotypes for which penicillin MICs vary
| MIC (μg/ml) | HaeIII pattern (no. of isolates) | Serotype(s) (no. of isolates) |
|---|---|---|
| <0.03 | s1 (36) | 14 (7), 7F (4), 9V (6), 18C (3), 22F (6), 23F (2), 1 (1), 3 (1), 4 (1), 7B (1), 9A (1), 23B (1), 35B (1), 35F (1) |
| s2 (27) | 4 (10), 14 (3), 12F (3), 18C (2), 11A (2), 33F (1), 22A (1), 19F (2), 9V (1), 4 (1), 6A (1) | |
| 0.06 | s1 (3) | 9V (2), 3 (1) |
| s2 (2) | 6A (1), 14 (1) | |
| r4 (1) | 19A (1) | |
| 0.12 | r4 (4) | 19A (3), 19F (1) |
| r3 (1) | 14 (1) | |
| r5 (1) | 9V (1) | |
| s1 (1) | 19F (1) | |
| 0.25 | r3 (3) | 15C (1), 9A (1), 9V (1) |
| r6 (2) | 15C (2) | |
| s1 (3) | 14 (2), 19F (1) | |
| s2 (1) | 23F (1) | |
| 0.5 | r1 (4) | 6A (1), 9V (1), 14 (1), 23F (1) |
| r3 (2) | 9V (2) | |
| r2 (1) | 6B (1) | |
| r5 (1) | 35B (1) | |
| 1.0 | r1 (5) | 23F (1), 9V (3), 14 (1) |
| r5 (2) | 35B (1), 6A (1) | |
| r7 (2) | 6B (2) | |
| r8 (1) | 6B (1) | |
| r9 (1) | 23F (1) | |
| 2.0 | r1 (3) | 9V (1), 23F (2) |
| r2 (1) | 19A (1) | |
| r9 (1) | 12F (1) | |
| 4.0 | r2 (5) | 23F (5) |
| r1 (3) | 23F (3) | |
| r8 (1) | 14 (1) |
All 33 isolates for which MICs were ≥0.5 μg/ml exhibited one of seven HaeIII profiles (Fig. 1) that differed from the two susceptible profiles and represented nine capsular serotypes (Table 1). Pattern r1, the most-frequent resistant profile, was possessed by 15 isolates with four different capsular serotypes. Each of the other resistant profiles was exhibited by at least two independent isolates.
Two pbp2b profiles were found only with isolates for which MICs were 0.06 to 0.25 μg/ml. Pattern r4 appeared only in isolates for which penicillin MICs were 0.06 to 0.12 μg/ml, suggesting that this profile might represent a pbp2b allele that confers no more than low-level penicillin resistance. Similarly, pattern r6 was found only with isolates for which MICs were 0.25 μg/ml.
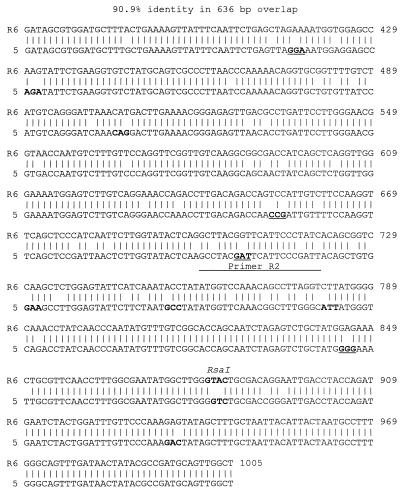
Previous studies have demonstrated that resistant isolates for which MICs were ≥0.125 μg/ml had alterations within the 300-bp transpeptidase-encoding region encompassed between annealing sites for P5 and P6 (4, 5a, 11). However, the presence of the s1 and s2 profiles in 10 of 22 isolates for which penicillin MICs ranged from 0.06 to 0.25 μg/ml indicated that in these strains one or more other PBP genes might confer this low level of penicillin resistance. To test this hypothesis and to analyze the rest of the pbp2b sequence, we sequenced the entire pbp2b 1,500-bp amplicon from two isolates with susceptible pbp2b HaeIII profiles for which MICs were 0.12 to 0.25 μg/ml. One of these isolates, designated isolate 11, was serotype 23F with pbp2b profile s2. The 1.5-kb pbp2b amplicon from isolate 11 contained three base changes compared with the susceptible strain R6 pbp2b allele (4), but these differences did not result in an amino acid change. The other isolate, designated isolate 5, was serotype 14 with pbp2b profile s1. Sequence analysis of pbp2b from isolate 5 revealed numerous differences throughout the 1.5-kb fragment compared to pbp2b in the susceptible strain R6. Extensive differences were observed within the 636 bp between primer P5 and P6 annealing sites (Fig. 2). Within this region, the two alleles had about 91% sequence identity, resulting in four nonconservative and seven nonconservative amino acid substitutions (Fig. 2). The sequence encoding one of the nonconservative substitutions is overlapped by a perfect match to primer R2 devised by previous investigators to detect pneumococcal penicillin resistance (5a). This PCR approach (5a) with isolates 5 and 11 would have detected resistance only in the former strain.
FIG. 2.
Comparison of the pbp2b sequence of low-level penicillin-resistant (MIC, 0.25) pneumococcal isolate 5 with that of penicillin-susceptible strain R6. Nucleotide numbering was taken from reference 4. Codons encoding amino acid substitutions and the indicated RsaI site are in bold-faced type. Nonconservative codon substitutions and a perfect match to the R2 primer sequence used by du Plessis et al. in detecting penicillin resistance (5a) are underlined.
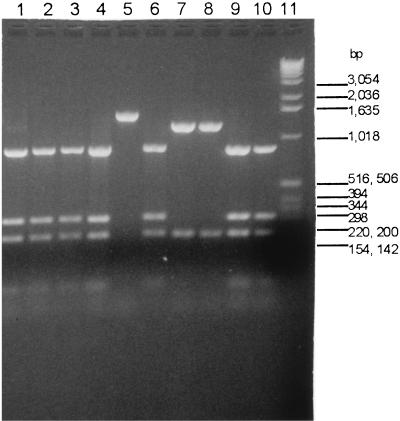
Sequence analysis revealed that the isolate 5 pbp2b amplicon could be differentiated from susceptible pbp2b allele amplicons by RsaI profiles. In Fig. 2, the RsaI site shown in the pbp2B gene of strain R6 is absent from the corresponding pbp2b sequence in isolate 5. RsaI profiles of pbp2b amplicons from all 63 sensitive isolates for which MICs were ≤0.03 μg/ml revealed that one sensitive RsaI profile differed from the RsaI profile for isolate 5 (Fig. 3, compare lanes 2 to 4, 9, and 10 with lane 8). The MICs for three other isolates, besides isolates 5 and 29, were 0.25 to 0.25 μg/ml and were associated with sensitive HaeIII profiles (Table 1). Two of these isolates had a pbp2b amplicon RsaI profile that differed from that for the susceptible profile (Fig. 3, compare lanes 5 and 7 with lanes 2 to 4, 9, and 10), and limited sequence analysis of the isolates represented in lanes 5 and 7 revealed pbp2b sequences that diverged extensively from that of the sensitive R6 allele (data not shown). The third isolate was like isolate 11 in that it had the susceptible RsaI profile (Fig. 3, lane 6) and HaeIII profile, with a pbp2b sequence nearly identical to that of the R6 pbp2b allele. In summary, it appears that pbp2b alleles conferring low to intermediate penicillin resistance can be detected more efficiently with RsaI or RsaI plus HaeIII double digests than with HaeIII alone. This method cannot detect all isolates for which MICs are lower because, as seen here, isolates sometimes have wild-type susceptible pbp2b alleles (e.g., isolate 11 and the strain represented in Fig. 3, lane 6).
FIG. 3.
pbp2b amplicon RsaI profiles of pneumococci susceptible to penicillin and of pneumococci with low-level resistance. Lanes 1, 5, and 8 are profiles of isolates for which MICs were 0.25 μg/ml; lanes 1 and 8 are from isolates 11 and 5, respectively. Lanes 6 and 7 are profiles of isolates for which MICs were 0.12 μg/ml. Lanes 2 to 4 and 9 to 10 are profiles of penicillin-susceptible isolates (MICs, ≤0.03 μg/ml). Lane 11, Gibco-BRL 1-kb ladder.
The greatest range in MICs (0.5 to 4.0 μg/ml) was for isolates with the r1 and r2 HaeIII profiles, indicating that with these alleles, variation in another PBP gene(s) probably causes incremental resistance. Studies have shown that MICs of ≤0.1 μg/ml result from alterations in pbp1a and pbp2x, while higher resistances (MICs, ≥0.25 μg/ml) are associated with alterations in pbp2b (2, 5, 10). The results presented here for isolates with the wild-type sensitive pbp2b gene sequence for which MICs are 0.12 to 0.25 μg/ml suggest that intermediate MICs of 0.06 to 0.25 μg/ml sometimes arise due to alterations in PBP genes other than pbp2b.
Pneumococcal pneumonia or bacteremia with isolates for which MICs are within the range of ≤0.03 to 0.25 μg/ml is generally treated with penicillin. All of the MICs for isolates with susceptible HaeIII patterns were between ≤0.03 and 0.25 μg/ml, and susceptible HaeIII patterns were exhibited by 73 of the 85 isolates (86%) for which MICs fell in this range. Therefore, if the results for these isolates are generally applicable, this system could allow the rapid identification of a majority of pneumococcal pneumonia and bacteremia cases treatable with penicillin. Cases of otitis media and meningitis would require antibiotic therapies other than penicillin; however, resistant pbp2b profiles could influence the choice of therapies for these infections.
An approach based on the simultaneous examination of the PBP gene amplicon profiles representing pbp2b, pbp1a, pbp2a, and pbp2x will possibly allow the detection of all levels of penicillin resistance in pneumococci. In addition, this approach may allow a more quantitative assessment of penicillin resistance by correlating different combinations of pbp alleles with different levels of resistance. Additionally, as noted previously (1, 2, 7), this approach allows the differentiation of different pbp alleles for epidemiologic purposes.
The speed with which antibiotic susceptibility is determined is critical in treating pneumococcal bacteremia. Deducing pneumococcal penicillin sensitivity by HaeIII and RsaI profiling requires only about 3 h, whereas susceptibility testing requires approximately 24 h.
Culture methods currently used to identify pneumococci require about 24 h. Another potential use of this approach is the detection of penicillin-sensitive and -resistant pneumococcal isolates from clinical specimens, since the primer pair used for amplification appears to be specific for the pneumococcal pbp2b gene.
Acknowledgments
We thank Alma Ruth Franklin and Lesyle LaClaire for assistance with serotyping and susceptibility testing and the Georgia Emerging Infection Program for collection of isolates used in this study. We are grateful to Benjamin Schwartz for information concerning treatment of pneumococcal infections.
REFERENCES
- 1.Coffey T J, Dowson C G, Daniels M, Zhou J, Martin C, Spratt B G, Musser J M. Horizontal transfer of multiple penicillin-binding protein genes, and capsular biosynthetic genes, in natural populations of Streptococcus pneumoniae. Mol Microbiol. 1991;5:2255–2260. doi: 10.1111/j.1365-2958.1991.tb02155.x. [DOI] [PubMed] [Google Scholar]
- 2.Coffey T J, Dowson C G, Daniels M, Spratt B G. Genetics and molecular biology of β-lactam-resistant pneumococci. Microb Drug Resist. 1995;1:29–34. doi: 10.1089/mdr.1995.1.29. [DOI] [PubMed] [Google Scholar]
- 3.Dowson C G, Hutchison A, Brannigan J A, George R C, Hansman D, Liñares J, Tomasz A, Maynard Smith J, Spratt B G. Horizontal transfer of penicillin-binding protein genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Proc Natl Acad Sci USA. 1989;87:5858–5862. doi: 10.1073/pnas.86.22.8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dowson C G, Hutchison A, Spratt G G. Extensive remodeling of the transpeptidase domain of penicillin-binding protein 2B of a penicillin-resistant South African isolate of Streptococcus pneumoniae. Mol Microbiol. 1989;3:95–102. doi: 10.1111/j.1365-2958.1989.tb00108.x. [DOI] [PubMed] [Google Scholar]
- 5.Dowson C G, Johnson A P, Cercenado E, George R C. Genetics of oxacillin resistance in clinical isolates of Streptococcus pneumoniae that are oxacillin resistant and penicillin susceptible. Antimicrob Agents Chemother. 1994;38:49–53. doi: 10.1128/aac.38.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.du Plessis M, Smith A M, Klugman K P. Rapid detection of penicillin-resistant Streptococcus pneumoniae in cerebrospinal fluid by a seminested-PCR strategy. J Clin Microbiol. 1998;36:453–457. doi: 10.1128/jcm.36.2.453-457.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hakenbeck R, Tarpay M, Tomasz A. Multiple changes of penicillin binding proteins in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980;17:364–371. doi: 10.1128/aac.17.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kell C M, Jordens J Z, Daniels M, Coffey T J, Bates J, Paul J, Gilks C, Spratt B G. Molecular epidemiology of penicillin-resistant pneumococci isolated in Nairobi, Kenya. Infect Immun. 1993;61:4382–4391. doi: 10.1128/iai.61.10.4382-4391.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laible G, Spratt B G, Hakenbeck R. Interspecies recombinational events during the evolution of altered PBP 2x genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Mol Microbiol. 1991;5:1993–2002. doi: 10.1111/j.1365-2958.1991.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 9.Martin C, Sibold C, Hakenbeck R. Relatedness of penicillin-binding protein 1a genes from different clones of penicillin-resistant Streptococcus pneumoniae isolated in South Africa and Spain. EMBO J. 1992;11:3831–3836. doi: 10.1002/j.1460-2075.1992.tb05475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muñoz R, Dowson C G, Daniels M, Coffey T J, Martin C, Hakenbeck R, Spratt B G. Genetics of resistance to third-generation cephalosporins in clinical isolates of Streptococcus pneumoniae. Mol Microbiol. 1992;6:2461–2465. doi: 10.1111/j.1365-2958.1992.tb01422.x. [DOI] [PubMed] [Google Scholar]
- 11.Smith A M, Klugman K P. Alterations in penicillin-binding protein 2B from penicillin-resistant wild-type strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1995;39:859–867. doi: 10.1128/aac.39.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith A M, Klugman K P, Coffey T J, Spratt B G. Genetic diversity of penicillin-binding protein 2B and 2X genes from Streptococcus pneumoniae in South Africa. Antimicrob Agents Chemother. 1993;37:1938–1944. doi: 10.1128/aac.37.9.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]