Abstract
Purpose of Review
Chimeric antigen receptor (CAR) T cell therapy is an immunotherapy that has resulted in tremendous progress in the treatment of patients with B cell malignancies. However, the remarkable efficacy of therapy is not without significant safety concerns. Herein, we will review the unique and potentially life-threatening toxicities associated with CAR-T cell therapy and their association with treatment efficacy.
Recent Findings
Currently, CAR-T cell therapy is approved for the treatment of B cell relapsed or refractory leukemia and lymphoma, and most recently, multiple myeloma (MM). In these different diseases, it has led to excellent complete and overall response rates depending on the patient population and therapy. Despite promising efficacy, CAR-T cell therapy is associated with significant side effects; the two most notable toxicities are cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). The treatment of CAR-T-induced toxicity is supportive; however, as higher-grade adverse events occur, toxicity-directed therapy with tocilizumab, an IL-6 receptor antibody, and steroids is standard practice. Overall, a careful risk–benefit balance exists between the efficacy and toxicities of therapies. The challenge lies in the underlying pathophysiology of CAR-T-related toxicity which relies upon the activation of CAR-T cells.
Summary
Some degree of toxicity is expected to achieve an effective response to therapy, and certain aspects of treatment are also associated with toxicity. As progress is made in the investigation and approval of new CARs, novel toxicity-directed therapies and toxicity-limited constructs will be the focus of attention.
Keywords: CAR-T, Immunotherapy, CAR-T toxicity, CAR-T efficacy, CRS, ICANS, CAR-T neurotoxicity
Introduction
In the past two decades, the development of chimeric antigen receptor (CAR) T cell therapy has resulted in tremendous progress in the treatment of hematological malignancies [1, 2]. CAR-T cell therapy uses engineered T cells to target overexpressed tumor cell surface antigens [3]. CAR-T cells are typically autologous but can also be derived from an allogeneic donor [4, 5]. The T cells are engineered to express a CAR, a synthetic receptor protein, that allows T cells to target a specific antigen [6–8]. Improving upon the first generation of CARs, higher-generation CAR constructs have incorporated co-stimulatory domains which increase T cell persistence and anti-tumor activity [7, 9–11]. Currently, there are six FDA-approved CAR-T cell therapies for use in the USA, and there are numerous ongoing clinical trials assessing the application of CAR-T cell therapy across a myriad of diseases [12••, 13–15, 16••, 17–21].
The currently approved therapies have resulted in excellent response rates for patients across disease groups and have led to durable disease remission. However, there are unique and serious side effects to treatment, most notably, cytokine release syndrome (CRS), and immune effector cell-associated neurotoxicity syndrome (ICANS) (Fig. 1) [22, 23••, 24]. In evaluating the potential benefit for patients, a risk–benefit balance exists between the efficacy and the associated toxicities of therapy. This risk–benefit is complicated by the fact that many treatment-associated toxicities are related to immune and CAR-T cell activation. This review focuses on the efficacy and toxicities associated with current CAR-T cell therapies and their current limitations. We will additionally review treatment barriers and toxicity-directed therapies.
Fig. 1.
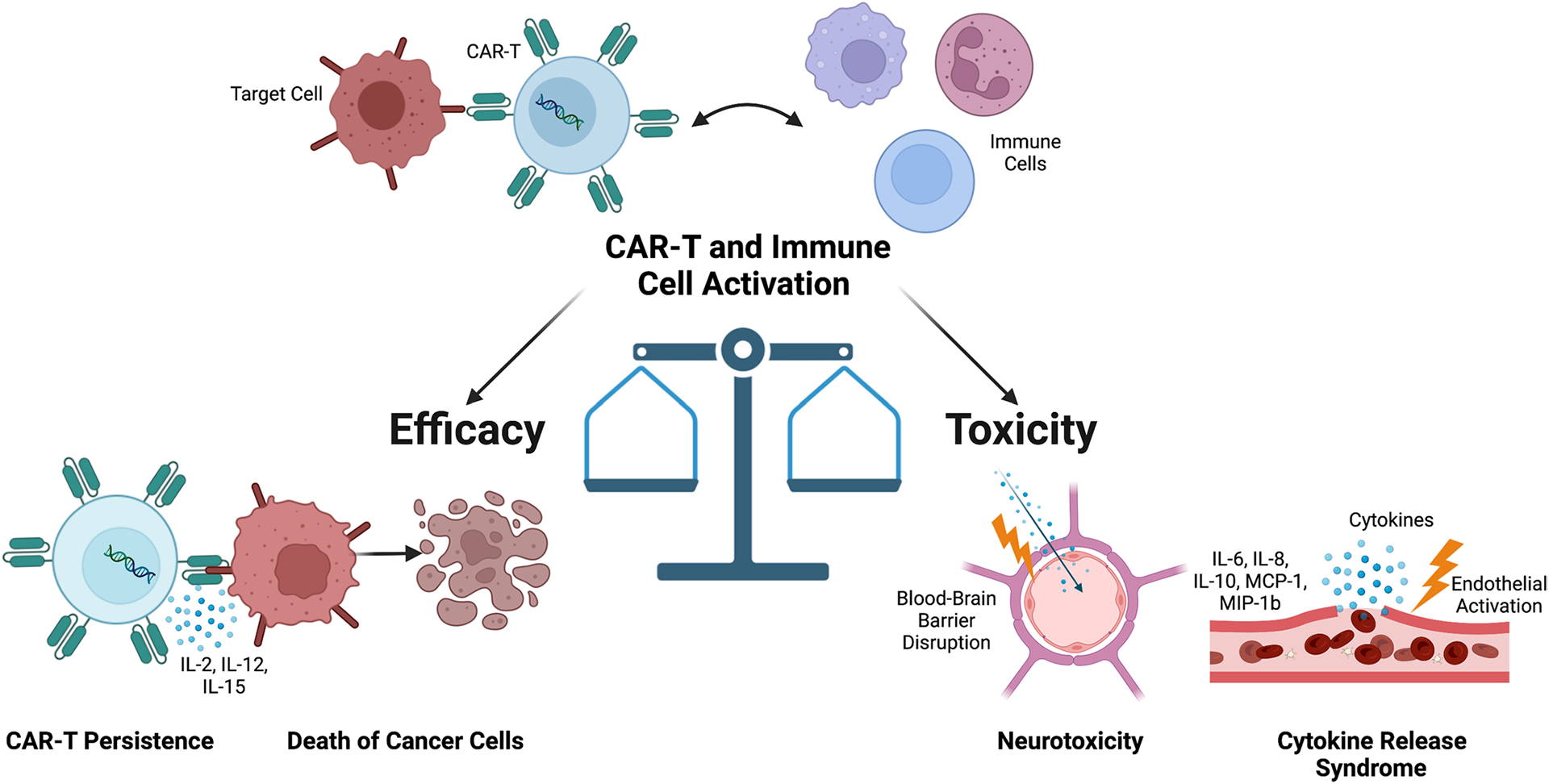
Toxicity and efficacy balance in CAR-T cell therapy. Illustration representing immune and CAR-T cell activation leading to tumor death, cytokine release syndrome (CRS), and neurotoxicity with overview of associated pathophysiology. IL, interleukin; MCP-1, monocyte chemoattractant protein-1; MIP-1b, macrophage inflammatory protein-1b. Figure created with BioRender.com
Treatment Efficacy and Barriers
CAR-T cell therapy is currently FDA-approved for the treatment of acute lymphoblastic leukemia (ALL), large B cell lymphoma (LBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), and multiple myeloma (MM) [12••, 13–15, 16••, 17–21]. Later in the text, we will describe specific CARs, as well as their associated efficacies and toxicities. Currently, there are several limitations to the efficacy of CAR-T cell therapy, which are a focus of recent research. These limitations include on-target/off-tumor targeting, antigen escape, and CAR-T cell dysfunction [2, 25, 26]. As CARs are developed to recognize a specific antigen, depending on the degree of target antigen expression on normal cells, this can lead to off-tumor targeting of the correct antigen on healthy tissue. There are ongoing investigations into modulating CAR affinity to detect tumor cells with high antigen density but not normal cells with lower antigen density [26, 27]. Another limitation that impairs treatment efficacy is antigen escape, where antigen expression on cancer cells is either reduced or nullified through downregulation or mutation [28]. Antigen escape can lead to disease resistance and relapse after CAR-T cell therapy. Lastly, inhibitory immune cells and cytokines in the tumor microenvironment can result in T cell exhaustion and death, especially in solid tumor malignancies [29–31]. Checkpoint blockade has emerged as a promising complement strategy that can help potentially reverse CAR-T cell inhibition and improve persistence [32–34].
Toxicities of Therapy
Cytokine Release Syndrome
CRS is one of the most common adverse events (AEs) associated with CAR-T cell therapy and is associated with symptoms that can range from mild to life-threatening [35]. CRS can progress from mild infusion reactions and fever to constitutional symptoms including hypotension, capillary leak, and end-organ dysfunction. There have been several toxicity scoring systems utilized in trials, complicating toxicity comparisons between studies; however, recent guidelines by the American Society for Transplantation and Cellular Therapy (ASTCT) have helped to establish a consensus grading system (Grades 1–4) for both CRS and ICANS [23••, 36–39].
The pathophysiology underlying the development of CRS stems from the activation of T cells upon antigen engagement with the CAR. This activation results in the proliferation and release of several cytokines and chemokines produced by CAR-T cells themselves and other immune cells. The most commonly reported elevated cytokines are interleukin (IL)-6, IL-8, IL-10, interferon (INF)-γ, granulocyte–macrophage colony-stimulating factor (GM-CSF), macrophage inflammatory protein-1β, and monocyte chemoattractant protein-1 [40–42]. IL-6 is a key mediator of CRS and forms the basis of CRS-directed management with tocilizumab, a monoclonal antibody that blocks signaling through the IL-6 receptor [43–45]. Factors that have been found to be predictors of CRS include higher tumor burden, thrombocytopenia prior to lymphodepletion, failure to select for CD8 + memory cells during CAR-T manufacturing, and lymphodepletion using cyclophosphamide and fludarabine [35, 38, 46, 47]. The diagnosis of CRS is clinical and can be graded based on the presence of fever, hypotension, and hypoxia. In monitoring CRS, C-reactive protein, ferritin, D-dimer, fibrinogen levels, and triglycerides are often regularly obtained [35, 48, 49].
ICANS
The spectrum of neurotoxicity following CAR-T cell therapy can range from encephalopathy to seizures, obtundation, and possible death [50]. Atypical findings can include transient aphasia, facial paresis, myoclonus, and hemifacial spasms. The pathophysiology of ICANS is poorly understood, and neurological events may occur independently of CRS-related toxicities, highlighting at least some underlying pathophysiologic differences between the conditions [51]. Generally, however, neurotoxicity is observed with CRS symptoms. Endothelial activation is felt to play a considerable role in neurotoxicity, where increased blood–brain barrier (BBB) permeability allows entry of pro-inflammatory cytokines, including IFNγ, as well as CAR-T cells into the central nervous system (CNS) [52, 53].
Previously, factors including older age, bone marrow disease burden, lymphodepletion with fludarabine and cyclophosphamide, pre-existing neurological conditions, and, importantly, the presence and severity of CRS have been found to be risk factors for the development of ICANS [53, 54]. Physical examination is integral to early ICANS detection, where careful assessment for inattention and language deficits is made.
Toxicity-Directed Therapies
For most patients, management of toxicities relies primarily upon supportive care, tocilizumab, and steroids. Tocilizumab is an IL-6 receptor antagonist which is used in rheumatologic disorders and is widely applied for CRS following CAR-T infusion [44]. The level of toxicity required for tocilizumab use varies by center, but generally grade 3 or higher symptoms are a common threshold [55]. For patients with CRS refractory to tocilizumab, systemic corticosteroids are generally administered; however, caution is taken due to the potential of reducing treatment efficacy [56]. For the treatment of neurotoxicity, tocilizumab is generally not effective, which is likely secondary to the antibody’s inability to cross the BBB [57]. Generally, neurotoxicity is treated with systemic corticosteroids and anti-epileptics as needed. Dexamethasone is often the agent of choice given its high CNS penetration [58, 59]. The use of pre-emptive corticosteroids and tocilizumab has also been investigated, but this is not standard of practice [60•]. Other agents which have been investigated and are generally reserved for refractory CRS include siltuximab (anti-IL-6), etanercept (anti-TNF), infliximab (anti-TNF), and anakinra (anti-IL-1) [61–64]. Currently, there is an investigation underway into targeting granulocyte–macrophage colony-stimulating factor (GM-CSF) which is produced by activated CAR-T and myeloid cells and can lead to myeloid activation, expansion, and cytokine production [41, 65, 66]. Lenzilumab is a humanized monoclonal antibody capable of neutralizing GM-CSF, and preclinical studies have shown that it can prevent CRS and neurotoxicity while enhancing CAR-T antitumor efficacy [41]. The ZUMA-19 study (NCT04314843) is underway to assess the potential efficacy of lenzilumab in improving the safety of CAR-T cell therapy [67]. Lastly, one of the most important but often overlooked aspects of toxicity prevention is careful patient selection. In pivotal clinical trials for CAR-T cell therapy, rigorous safety-related eligibility criteria were implemented, which should be considered when selecting patients for therapy in a real-world setting.
Efficacy and Toxicities of Current Approved Therapies
Herein, we will review the efficacy and toxicities associated with currently approved CAR-T cell therapies, which are summarized in Table 1.
Table 1.
FDA-approved products and the associated efficacy and toxicity outcomes from key clinical trials
| Disease | Product | Trial | N | ORR (%) | CR (%) | CRS Any/Grade ≥ 3 (%) | NT Any/Grade ≥ 3 (%) | Ref |
|---|---|---|---|---|---|---|---|---|
| ALL | Tisagenlecleucel | ELIANA | 75 | 81 | 60 | 77/46 | 40/13 | 19 |
| KTE-X19 | ZUMA-3 | 71 | 71 | 56 | 89/24 | 60/25 | 20 | |
| LBCL | Axi-cel | ZUMA-1 | 111 | 82 | 54 | 93/13 | 64/28 | 14 |
| ZUMA-7 | 180 | 83 | 65 | 92/6 | 60/21 | 76 | ||
| Tisagenlecleucel | JULIET | 93 | 52 | 40 | 58/22 | 21/12 | 13 | |
| BELINDA | 162 | 46 | 28 | 61/5 | 10/2 | 77 | ||
| Liso-cel | TRANSCEND NHL 001 | 256 | 73 | 53 | 42/2 | 30/10 | 18 | |
| TRANSFORM | 92 | 86 | 66 | 49/1 | 12/4 | 78 | ||
| MCL | KTE-X19 | ZUMA-2 | 60 | 93 | 67 | 91/15 | 63/31 | 15 |
| FL/MZL | Axi-cel | ZUMA-5 | 104 | 92 | 74 | 82/7 | 59/19 | 16 |
| FL | Tisagenlecleucel | ELARA | 97 | 86 | 69 | 49/0 | 37/3 | 12 |
| MM | Ide-cel | KarMMa | 128 | 73 | 33 | 84/5 | 18/3 | 21 |
| Cilta-cel | CARTITUDE-1 | 113 | 97 | 67 | 95/5 | 21/10 | 17 |
ORR listed for ZUMA 3 trial included patients with CR and CR with incomplete hematological recovery
ALL acute lymphoblastic leukemia, LBCL large B cell lymphoma, FL follicular lymphoma, MCL mantle cell lymphoma, MZL marginal zone lymphoma, MM multiple myeloma, N number of patients, ORR overall response rate, CR complete remission, NT neurotoxicity, CRS cytokine release syndrome
CART-19 in ALL
CD19 is a cell surface protein that is generally restricted to B cells and their precursors and is expressed by most B cell malignancies [68, 69]. Tisagenlecleucel and brexucabtagene autoleucel are anti-CD19 CAR-T (CART-19) cell therapies approved for the treatment of relapsed/refractory (rel/ref) ALL [19, 20].
The initial single-center phase 1–2a study utilizing tisagenlecleucel in patients with rel/ref B cell ALL demonstrated promising results with a 93% CR rate and long-term disease control, which prompted a phase 2 multisite trial (ELIANA) [70]. Among 75 patients who received an infusion of tisagenlecleucel, the ORR and CR rates were found to be 81% and 60% [19]. The event-free survival rate at 6 months and 12 months was 73% and 50%, respectively. In terms of toxicity, CRS occurred in 77%, and neurological events in 40%. Grade 3 or greater CRS and neurotoxicity occurred in 46% and 13% of patients, respectively. Among those with CRS, tocilizumab was given in 48%, and neurological events were managed with supportive care [19]. Brexucabtagene autoleucel (KTE-X19) is another CART-19 cell therapy that is produced by a manufacturing process that removes circulating CD19-expressing malignant cells which reduces the potential for ex vivo CAR-T activation and exhaustion [20]. The phase 2 ZUMA-3 trial evaluated KTE-X19 in rel/ref adult patients with ALL where 71 patients underwent leukapheresis [20]. The rate of CR or CR with incomplete hematological recovery was found to be 71%. With a median follow-up time of 16.4 months, the relapse-free survival was 11.6 months for all treated patients. In terms of toxicity, a higher proportion of patients developed CRS (89%) compared to the ELIANA trial, with grade 3 or higher findings in 24%. Furthermore, neurological events occurred in 60%, with 25% having grade 3 or greater events. The authors of the study additionally noted that they found higher CAR-T levels associated with neurotoxicity grade ≥ 3 and CRS grade ≥ 2. In total, 80% of patients required tocilizumab, 75% required steroids, and vasopressors were administered to 40% of patients. Overall, despite the cohort age differences, both agents demonstrated similar rates of durable efficacy, with similar toxicity profiles, but a higher proportion of neurological events occurring in patients receiving KTE-X19.
In a systematic review and meta-analysis assessing CART-19 cell therapy for rel/ref patients with ALL by Anagnostou et al., 35 studies including 953 patients were assessed for efficacy and toxicity-related outcomes [71]. The analysis included 18 studies that used 4–1BB co-stimulated, 12 that used CD28 co-stimulated, four that used fourth-generation, and one that used third-generation CART-19 cells. After the application of inclusion and exclusion criteria, 80% of patients with available data achieved a CR to CART-19 cell infusion. At 1-year post-infusion, 37% were progression-free, and the pooled 1-year progression-free survival (PFS) did not differ between adults and children. Assessing toxicities, 82% developed CRS of any grade, and 26% developed grade 3 or greater CRS. In relation to ICANS, 29% developed neurotoxicity of any grade, with 12% developing grade 3 or greater. The authors additionally noted that a higher proportion of children were found to have neurotoxicity as compared to adult patients, and autologous CART-19 had a higher proportion of neurotoxicity (33% vs 3%, p = 0.01) as compared to allogeneic CART-19 cell therapy [71].
CART-19 in DLBCL
Axicabtagene ciloleucel (axi-cel) is an autologous CART-19 cell therapy that is approved for the treatment of patients with rel/ref LBCL [14]. After phase 1 studies showed good clinical efficacy, a multicenter phase 2 study, ZUMA-1, was conducted in 111 patients with refractory DLBCL, primary mediastinal B cell lymphoma, and transformed FL [14, 72]. The authors found axi-cel resulted in an 82% ORR with a 54% CR rate among the 101 patients receiving therapy. With a median follow-up of 15.4 months, 40% continued to have a CR, and the OS at 18 months was 52%. In terms of toxicity, 93% of patients were found to have CRS, with 13% having grade 3 or higher findings. Neurotoxicity occurred in 64% of patients, where 28% were found to have grade 3 or higher events. Forty-three percent of patients received tocilizumab, and 27% received glucocorticoids for management of toxicities. The authors further highlighted that they did not observe an association between the use of tocilizumab and glucocorticoids with the overall response. Locke et al. presented 2-year follow-up data to the ZUMA-1 study which exhibited a median duration of response of 11.1 months, and no additional treatment-related serious AEs [72]. In comparison to real-world studies, similar efficacy and toxicity rates were found. Nastoupil et al. published results from the US Lymphoma CAR-T Consortium, in a multicenter retrospective study of 275 patients who received axi-cel therapy, including 43% who would not have met ZUMA-1 eligibility [73]. The study found similar efficacy with an ORR of 82%, CR rate of 64%, and a median PFS of 8.3 months at 12.9 months follow-up. Additionally, a similar safety profile was observed, where grades 3 and greater CRS and neurotoxicity were observed in 7% and 31% of patients, respectively. Tocilizumab was used in 62% of patients, and 55% required corticosteroids [73]. In another multicenter real-world study including 122 patients, with 62% ineligible for ZUMA-1, a similar rate of overall response (70%), and CR (50%), was observed compared to the trial [74]. The authors however did report that ZUMA-1-eligible patients had improved efficacy (CR, duration of response, OS, and PFS), compared to ZUMA-1-ineligible patients. Safety results showed a rate of grade 3 or greater CRS and neurotoxicity comparable to ZUMA-1 at 16% and 35%, respectively. Toxicity-directed therapies involved the use of tocilizumab in 66% of patients and steroids in 53% [74]. A post-market study that assessed post-approval safety and efficacy of axi-cel was conducted by the Center for International Blood and Marrow Transplant and presented at the American Society of Hematology 2019 meeting [75]. Among 453 recipients with a median follow-up of 6 months, ORR was 70%, with a CR rate of 52%. CRS of any grade was observed in 83%, with 14% having grade 3 or higher findings. Tocilizumab was used in 70%, corticosteroids in 26%, and siltuximab in 1%. Sixty-one percent of patients had neurological events, with one patient dying from cerebral edema. Overall, axi-cel demonstrated excellent efficacy outcomes for patients with LBCL, with real-world studies demonstrating similar safety and efficacy compared to the pivotal ZUMA-1 trial.
The JULIET trial assessed tisagenlecleucel in 93 adult patients with rel/ref DLBCL, within an international phase 2 study [13]. Efficacy outcomes were favorable, with an ORR and CR rate of 52% and 40%, respectively. Relapse-free survival at 12 months was found to be 65% and 79% among those who achieved a CR. The rate of CRS was found to be 58%, with grade 3 or higher findings in 22% of patients. Neurological events of any grade occurred in 21% and 12% had grade 3 or higher complications. Fourteen percent of patients required tocilizumab, and 10% received both tocilizumab and glucocorticoids. Recently approved, lisocabtagene maraleucel (liso-cel) is an autologous CART-19 cell therapy that was assessed in a multicenter, seamless design study (TRANSCEND NHL 001) for patients with rel/ref adult LBCL [18]. Among 256 patients evaluated in the efficacy analysis, ORR was observed in 73%, with CR in 53%. CRS occurred in 42% of patients, with grade 3 or greater CRS in 2%. Neurological events occurred in 30% of patients, with grade 3 or higher symptoms in 10%. Overall, both trials assessing tisagenlecleucel and liso-cel revealed robust efficacy, with liso-cel revealing a favorable safety profile with low rates of grade 3 or greater CRS and neurotoxicity.
Recently, there has also been investigation into axi-cel (ZUMA-7), tisagenlecleucel (BELINDA), and liso-cel (TRANSFORM) being used in the second-line setting for LBCL. All three-trial assessed event-free survival as their primary endpoint. The phase 3 Zuma-7 trial randomized patients to receive axi-cel (n = 180) to standard of care second-line therapy (n = 179) [76]. Axi-cel resulted in superior 24-month event-free survival (41% vs 16%), with similar grade 3 or higher adverse events (91% vs 83%) compared to standard of care. Grade 3 or greater CRS occurred in 6%, and neurological events occurred in 21% of patients in the axi-cel group [76]. The phase 3 BELINDA trial assessed tisagenlecleucel (n = 162) compared to salvage chemotherapy and autologous hematopoietic stem cell transplant (n = 160) (standard-care) in patients with aggressive lymphoma refractory to or progressing within 12 months of frontline therapy [77]. The authors found tisagenlecleucel was not superior to standard salvage therapy, with a median event-free survival in both groups being 3.0 months. Grade 3 or higher adverse events were similar in the tisagenlecleucel (84%) and standard therapy groups (90%). In the tisagenlecleucel group, rates of grade 3 or higher CRS or neurotoxicity were low, occurring in 5% and 2% of patients, respectively [77]. In the phase 3 TRANSFORM trial, patients were randomized to liso-cel (n = 92) or standard-of-care therapy (n = 92) which included salvage immunochemotherapy followed by high-dose chemotherapy and autologous stem cell transplant in responders [78]. After a median follow-up of 6.2 months, the median event-free survival was higher in the liso-cel group (10.1 vs 2.3 months) compared to standard-of-care. Adverse events of grade 3 or higher were similar in both liso-cel (92%) and standard-of-care (87%) groups. Grade 3 or higher CRS and neurological events occurred in 1% and 4% of patients in the liso-cel group, respectively [78]. Overall, these trials raise the question of introducing CAR-T therapy earlier into the treatment algorithms; however, further long-term data on the safety and cost-effectiveness are needed. The difference in the efficacy of CART-19 as a second line therapy in the ZUMA-7 and TRANSFORM trials compared to the BELINDA trial is intriguing and can be related to several factors including patient selection and time to manufacture [79].
CART-19 in MCL
The single-group, multicenter, open-label, phase 2 ZUMA-2 trial evaluated KTE-X19 in patients with rel/ref MCL, among a cohort that had previously failed Bruton’s tyrosine kinase (BTK) inhibitor therapy [15]. Assessing efficacy of KTE-X19 in 60 patients, the ORR was 93%, with 67% having CR. At 12 months, the OS and PFS were found to be 61% and 83%, respectively. All treated patients had at least one AE, with CRS observed in 91% of patients, and neurological events in 63%. Of patients with CRS, grade 3 or higher findings were observed in 15%, 59% were managed with tocilizumab, 22% received glucocorticoids, and 16% received vasopressors. Neurotoxicity of grade 3 and higher were reported in 31%, with 26% receiving tocilizumab, 38% receiving glucocorticoids, and one patient requiring multi-modal therapy including ventriculostomy. Recently, a real-world study of 39 patients assessed KTE-X19 in rel/ref MCL patients [80•]. Among 33 infused patients, an ORR and CR of 91% and 79% were observed. Furthermore, at 12 months post-infusion, OS and PFS were 61% and 51%, respectively. Concerning toxicity, 91% developed CRS, and 3% had grade 3 or greater findings. Neurotoxicity developed in 64%, and 36% developed grade 3 or greater symptoms. Overall, this real-world investigation showed a similar treatment response and rate of neurotoxicity compared to the ZUMA-2 trial; however, CRS of grade 3 or higher was observed at a lower rate. Further studies are needed to establish efficacy and safety parameters, especially with longer follow-ups.
CART-19 in Indolent Lymphomas
In the ZUMA-5 trial, axi-cel was tested in patients with rel/ref FL and MZL [16••]. Among 104 patients eligible for primary analysis (84 FL and 20 MZL), the ORR was 92%, and 74% of patients achieved a CR. At 18 months of follow-up, the estimated PFS and OS were 66% and 87%, respectively. In terms of toxicity, CRS was reported in 82%, and neurological events in 59%. Grade 3 or higher CRS and neurological events were reported in 7% and 19%, respectively. Tocilizumab was utilized in 50% of patients with CRS, corticosteroids in 18%, and vasopressors in 5%. Amongst patients with indolent non-Hodgkin lymphoma, the ZUMA-5 trial demonstrated similar efficacy, with an overall lower rate of high-grade CRS and neurotoxicity compared to other trials [16••]. Overall, there was a highly durable response seen, with a reasonable safety profile, highlighting the treatment’s potential for patients with rel/ref FL and MZL. Assessing tisagenlecleucel in patients with rel/ref FL, the phase 2 ELARA trial found an ORR and CR of 86% and 69%, respectively, in 94 efficacy-evaluable patients [12••]. The safety profile was favorable with CRS occurring in 49% of patients, but no patients having grade 3 or higher findings. Additionally, neurotoxicity was observed in 37%, with only 3% having grade 3 or higher events. The promising efficacy and toxicity profile demonstrated in the ELARA trial led to the approval of tisagenlecleucel for patients with rel/ref FL [12••].
BCMA-CART in MM
B cell maturation antigen (BCMA) is a cell surface protein that plays an important role in B cell proliferation and is highly expressed on abnormal plasma cells in MM [81, 82]. Idecabtagene vicleucel (ide-cel) is a BCMA-directed CAR-T (BCMA-CAR-T) cell that has received recent approval for the treatment of rel/ref MM [21]. Among the 128 patients who received ide-cel in the KarMMA trial, the ORR was 73%, with a 33% rate of CR. With a median follow-up time of 13.3 months, the median PFS was 8.8 months. CRS was reported in 84% of patients, and grade 3 or higher CRS was noted in 5%. Neurotoxicity was observed in 18%, and 3% had grade 3 or higher findings. Most recently, the FDA approved ciltacabtagene autoleucel (cilta-cel) for the treatment of rel/ref MM [17]. Cilta-cel expresses two BCMA-targeting single-domain antibodies. The study was a single-arm, open-label, phase 1b/2 study, with 113 patients enrolled. In terms of efficacy, an excellent ORR of 97% was observed, with 67% of patients achieving CR. The 12-month PFS and OS rates were 77% and 89%, respectively. CRS occurred in almost all patients (95%), but only 5% had grade 3 or higher symptoms. Neurotoxicity occurred in 21% of patients, and 10% had grade 3 or greater toxicity. Overall, both BCMA-CAR-T cell therapies revealed a robust response, with cilta-cel having a more promising rate of CR. Both therapies had an excellent safety profile, with very few patients having severe CRS and neurotoxicity symptoms.
Balancing Efficacy and Toxicity
A difficult aspect of evaluating the potential of new CAR-T cell therapies is balancing both the efficacy and toxicity of treatment. The challenge lies in the underlying pathophysiology of CAR-T-related toxicity, which relies upon the activation of CAR-T cells. Some degree of toxicity is expected to achieve an effective response to therapy, and toxicity is also correlated with high CAR-T expansion [15]. Certain aspects of treatment are also associated with toxicity, such as the use of lymphodepletion regimens. Lymphodepletion is important in creating a favorable immune environment for CAR-T cell therapy; however, it can result in a greater degree of CRS. Recent retrospective data found that lymphodepletion using bendamustine before tisagenlecleucel resulted in similar efficacy and reduced rates of CRS, neurotoxicity, hematological toxicity, and infection as compared to fludarabine/cyclophosphamide [83]. These data suggest that certain lymphodepletion regimens may have preferable safety profiles compared to others. Another recent aspect of concern is the addition of checkpoint blockade, which can make the tumor microenvironment more favorable to the CAR-T cells and improve efficacy but has the potential to result in greater therapy-associated toxicities [84, 85].
There often appears to be a trade-off between efficacy and toxicity in CAR-T cell therapy. In numerous preclinical studies seeking to enhance CAR-T efficacy, additional components have been built into the CAR construct, including dual antigen-targeting CARs, membrane-bound cytokines, costimulatory molecules, and immune checkpoint inhibitors. While these strategies have improved CAR-T proliferation, persistence, and cytotoxicity, safety issues of hyperactive CAR-T cells remain concerning [86–89]. Many strategies have been employed to generate safer CAR-T cells through remote control of CAR-T cell apoptosis. Suicide genes trigger pharmacologically activated apoptosis in CAR-T cells expressing the transgene [90, 91]. Selection markers expressed on CAR-T cells allow specific CAR-T ablation with a neutralizing antibody [92, 93]. However, these are largely irreversible approaches that also terminate CAR-T therapeutic effects [94].
The lack of durable responses to CAR-T cell therapy and the progression of therapy-associated toxicities have been linked to activated monocytes, M2-polarized macrophages, and myeloid-derived suppressor cells [41, 95–98]. Therefore, strategies that target myeloid cells and myeloid-associated cytokines may be particularly useful to ameliorate both CAR-T cell dysfunction and toxicity concurrently. GM-CSF is crucial for myeloid cell activation. Preclinical studies of GM-CSF blockade resulted in the prevention of CRS and reduction of neuroinflammation and also enhanced CAR-T anti-tumor activity in patient-derived xenograft models [41]. The BTK inhibitor ibrutinib has also displayed reduced CRS incidence and improved clinical outcomes in patients when administered concomitantly with CAR-T cell therapy, possibly due to impacts on myeloid cells as well as direct modulation of T cells [99]. More studies are needed into the mechanisms of myeloid cells on both CAR-T efficacy and toxicity to develop novel strategies to overcome these two major hurdles to successful CAR-T cell therapy.
Conclusion
Overall, the approval of CAR-T cell therapy for patients with rel/ref hematological malignancies has led to tremendous benefits for patients with previously incurable diseases. However, this benefit has not been without a careful balance between the efficacy and unique toxicities associated with treatment. Novel toxicity-directed therapies and low-toxicity constructs which do not compromise treatment efficacy are desperately needed. As progress is made in the development of new CAR-T cell therapies, clinical trials should continue to assess both efficacy and toxicity simultaneously when evaluating the potential clinical applicability for patients.
Conflict of Interest
SSK is inventor on patents in the CART cell field that are licensed to Novartis (through an agreement between Mayo Clinic, University of Pennsylvania, and Novartis), Humanigen (through Mayo Clinic), Mettaforge (through Mayo Clinic), MustangBio (through Mayo Clinic), and Sendero (through Mayo Clinic). SSK has received funding from Novartis, Kite/Gilead, Juno/BMS, Lentigen, Humanigen, Tolero, Morphosys, Sunesis, and Viracta, and LeahLabs. SSK has participated in scientific advisory board meetings with Kite/Gilead, Novartis, BMS, CapstanBio, Luminary therapeutics, and Humanigen. SSK has participated in DSMB meetings with Humanigen. SSK has participated in consulting activities with Novartis, Torque, CapstanBio, Luminary therapeutics, and Calibr. SSK hold equity in Life Engine, Inc. and Leahlabs. KLC and ELS have no conflicts to report.
Footnotes
Human and Animal Rights and Informed Consent. This article does not contain any studies with human or animal subjects performed by any of the authors.
Data Availability
This review article does not present any original unpublished data. All data presented is associated with the corresponding reference.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Almasbak H, Aarvak T, Vemuri MC. CAR T cell therapy: a game changer in cancer treatment. J Immunol Res. 2016;2016:5474602. 10.1155/2016/5474602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69. 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guedan S, Calderon H, Posey AD Jr, Maus MV. Engineering and design of chimeric antigen receptors. Mol Ther Methods Clin Dev. 2019;12:145–56. 10.1016/j.omtm.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao J, Lin Q, Song Y, Liu D. Universal CARs, universal T cells, and universal CAR T cells. J Hematol Oncol. 2018;11:132. 10.1186/s13045-018-0677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19:185–99. 10.1038/s41573-019-0051-2. [DOI] [PubMed] [Google Scholar]
- 6.Brudno JN, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34:1112–21. 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinkove R, George P, Dasyam N, McLellan AD. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin Transl Immunology 8, e1049. 10.1002/cti2.1049 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–5. 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 9.Finney HM, Lawson AD, Bebbington CR, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998;161:2791–7. [PubMed] [Google Scholar]
- 10.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20:70–5. 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y, et al. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol. 2009;183:5563–74. 10.4049/jimmunol.0900447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.••.Fowler NH et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med 28, 325–332. 10.1038/s41591-021-01622-0. [DOI] [PubMed] [Google Scholar]; Recent trial leading to the approval of Tisagenlecleucel for FL.
- 13.Schuster SJ, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 14.Neelapu SS, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44. 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331–42. 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.••.Jacobson CA et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol 23, 91–103. [DOI] [PubMed] [Google Scholar]; Recent clinical trial forming the basis of approval of axi-cel for the treatment of indolent lymphomas.
- 17.Berdeja JG, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTI-TUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398:314–24. 10.1016/S0140-6736(21)00933-8. [DOI] [PubMed] [Google Scholar]
- 18.Abramson JS, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–52. 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 19.Maude SL, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48. 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah BD, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398:491–502. 10.1016/S0140-6736(21)01222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munshi NC, et al. Idecabtagene Vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384:705–16. 10.1056/NEJMoa2024850. [DOI] [PubMed] [Google Scholar]
- 22.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.••.Lee DW et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 25, 625–638. 10.1016/j.bbmt.2018.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]; Guidelines by the American Society for Transplantation and Cellular Therapy (ASTCT) which have established a consensus grading system (Grades 1–4) for both CRS and ICANS.
- 24.Yakoub-Agha I, et al. Management of adults and children undergoing chimeric antigen receptor T-cell therapy: best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Haematologica. 2020;105:297–316. 10.3324/haematol.2019.229781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sotillo E, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5:1282–95. 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castellarin M et al. A rational mouse model to detect on-target, off-tumor CAR T cell toxicity. JCI Insight 5. 10.1172/jci.insight.136012 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghorashian S, et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat Med. 2019;25:1408–14. 10.1038/s41591-019-0549-5. [DOI] [PubMed] [Google Scholar]
- 28.Majzner RG, Mackall CL. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8:1219–26. 10.1158/2159-8290.CD-18-0442. [DOI] [PubMed] [Google Scholar]
- 29.Ahmadzadeh M, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–44. 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baitsch L, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121:2350–60. 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long AH, et al. 4–1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21:581–90. 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gargett T, et al. GD2-specific CAR T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade. Mol Ther. 2016;24:1135–49. 10.1038/mt.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heczey A, et al. CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol Ther. 2017;25:2214–24. 10.1016/j.ymthe.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 35.Hay KA, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–306. 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pennisi M, et al. Comparing CAR T-cell toxicity grading systems: application of the ASTCT grading system and implications for management. Blood Adv. 2020;4:676–86. 10.1182/bloodadvances.2019000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neelapu SS, et al. Chimeric antigen receptor T-cell therapy — assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park JH, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–59. 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuster SJ, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–54. 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murthy H, Iqbal M, Chavez JC, Kharfan-Dabaja MA. Cytokine release syndrome: current perspectives. Immunotargets Ther. 2019;8:43–52. 10.2147/ITT.S202015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sterner RM, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2019;133:697–709. 10.1182/blood-2018-10-881722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20:119–22. 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gabay C, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381:1541–50. 10.1016/S0140-6736(13)60250-0. [DOI] [PubMed] [Google Scholar]
- 44.Bijlsma JWJ, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet. 2016;388:343–55. 10.1016/S0140-6736(16)30363-4. [DOI] [PubMed] [Google Scholar]
- 45.Le RQ, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23:943–7. 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li M, et al. The differential effects of tumor burdens on predicting the net benefits of ssCART-19 cell treatment on r/r B-ALL patients. Sci Rep. 2022;12:378. 10.1038/s41598-021-04296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17. 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teachey DT, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–79. 10.1158/2159-8290.CD-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahadeo KM, et al. Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat Rev Clin Oncol. 2019;16:45–63. 10.1038/s41571-018-0075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubin DB, et al. Clinical predictors of neurotoxicity after chimeric antigen receptor T-cell therapy. JAMA Neurol. 2020;77:1536–42. 10.1001/jamaneurol.2020.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Curran KJ, et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood. 2019;134:2361–8. 10.1182/blood.2019001641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santomasso BD, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8:958–71. 10.1158/2159-8290.CD-17-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gust J, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7:1404–19. 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grant SJ, et al. Clinical presentation, risk factors, and outcomes of immune effector cell-associated neurotoxicity syndrome following chimeric antigen receptor T cell therapy: a systematic review. Transplant Cell Ther. 2022;28:294–302. 10.1016/j.jtct.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schuster SJ, et al. Grading and management of cytokine release syndrome in patients treated with tisagenlecleucel in the JULIET trial. Blood Adv. 2020;4:1432–9. 10.1182/bloodadvances.2019001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strati P, et al. Prognostic impact of corticosteroids on efficacy of chimeric antigen receptor T-cell therapy in large B-cell lymphoma. Blood. 2021;137:3272–6. 10.1182/blood.2020008865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nellan A, et al. Improved CNS exposure to tocilizumab after cerebrospinal fluid compared to intravenous administration in rhesus macaques. Blood. 2018;132:662–6. 10.1182/blood-2018-05-846428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balis FM, Lester CM, Chrousos GP, Heideman RL, Poplack DG. Differences in cerebrospinal fluid penetration of corticosteroids: possible relationship to the prevention of meningeal leukemia. J Clin Oncol. 1987;5:202–7. 10.1200/JCO.1987.5.2.202. [DOI] [PubMed] [Google Scholar]
- 59.Labar B, et al. Dexamethasone compared to prednisolone for adults with acute lymphoblastic leukemia or lymphoblastic lymphoma: final results of the ALL-4 randomized, phase III trial of the EORTC Leukemia Group. Haematologica. 2010;95:1489–95. 10.3324/haematol.2009.018580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.•.Gardner RA et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood 134, 2149–2158. 10.1182/blood.2019001463. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study which reported that early intervention with steroids or tocilizumab may reduce the potential development for severe toxicity and have minimal impact on treatment efficacy.
- 61.Chen F, et al. Measuring IL-6 and sIL-6R in serum from patients treated with tocilizumab and/or siltuximab following CAR T cell therapy. J Immunol Methods. 2016;434:1–8. 10.1016/j.jim.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Titov A, et al. The biological basis and clinical symptoms of CAR-T therapy-associated toxicites. Cell Death Dis. 2018;9:897. 10.1038/s41419-018-0918-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang L, et al. Etanercept as a new therapeutic option for cytokine release syndrome following chimeric antigen receptor T cell therapy. Exp Hematol Oncol. 2021;10:16. 10.1186/s40164-021-00209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strati P, et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma. Blood Adv. 2020;4:3123–7. 10.1182/bloodadvances.2020002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi Y, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res. 2006;16:126–33. 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- 66.Yi Y, et al. CRISPR-edited CART with GM-CSF knockout and auto secretion of IL6 and IL1 blockers in patients with hematologic malignancy. Cell Discov. 2021;7:27. 10.1038/s41421-021-00255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kenderian SS, et al. ZUMA-19: A phase 1/2 multicenter study of lenzilumab use with axicabtagene ciloleucel (Axi-Cel) in patients (Pts) with relapsed or refractory large B cell lymphoma (R/R LBCL). Blood. 2020;136:6–7. 10.1182/blood-2020-135988.32614958 [DOI] [Google Scholar]
- 68.Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol. 2012;1:36. 10.1186/2162-3619-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Engel P, et al. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 1995;3:39–50. 10.1016/1074-7613(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 70.Maude SL, et al. Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL. J Clin Oncol. 2016;34:3011–3011. 10.1200/JCO.2016.34.15_suppl.3011. [DOI] [Google Scholar]
- 71.Anagnostou T, Riaz IB, Hashmi SK, Murad MH, Kenderian SS. Anti-CD19 chimeric antigen receptor T-cell therapy in acute lymphocytic leukaemia: a systematic review and meta-analysis. Lancet Haematol. 2020;7:e816–26. 10.1016/S2352-3026(20)30277-5. [DOI] [PubMed] [Google Scholar]
- 72.Locke FL, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20:31–42. 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nastoupil LJ, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. J Clin Oncol. 2020;38:3119–28. 10.1200/JCO.19.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jacobson CA, et al. Axicabtagene ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J Clin Oncol. 2020;38:3095–106. 10.1200/JCO.19.02103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pasquini MC, et al. Post-marketing use outcomes of an anti-CD19 chimeric antigen receptor (CAR) T cell therapy, Axicabtagene ciloleucel (axi-cel), for the treatment of large B cell lymphoma (LBCL) in the United States (US). Blood. 2019;134:764–764. 10.1182/blood-2019-124750. [DOI] [Google Scholar]
- 76.Locke FL, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386:640–54. 10.1056/NEJMoa2116133. [DOI] [PubMed] [Google Scholar]
- 77.Bishop MR, et al. Second-line Tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386:629–39. 10.1056/NEJMoa2116596. [DOI] [PubMed] [Google Scholar]
- 78.Kamdar M, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399:2294–308. 10.1016/S0140-6736(22)00662-6. [DOI] [PubMed] [Google Scholar]
- 79.Perales MA et al. Role of CD19 chimeric antigen receptor T cells in second-line large B cell lymphoma: lessons from phase 3 trials. An Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Transplant Cell Ther 28, 546–559. 10.1016/j.jtct.2022.06.019 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.•.Iacoboni G et al. Real-world evidence of brexucabtagene autoleucel for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv. 10.1182/bloodadvances.2021006922. [DOI] [PMC free article] [PubMed] [Google Scholar]; Recently published real-world analysis of brexi-cel in patients with MCL which serves to validate clinical trial data from ZUMA-2.
- 81.Shah N, Chari A, Scott E, Mezzi K, Usmani SZ. B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia. 2020;34:985–1005. 10.1038/s41375-020-0734-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sanchez E, et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br J Haematol. 2012;158:727–38. 10.1111/j.1365-2141.2012.09241.x. [DOI] [PubMed] [Google Scholar]
- 83.Ghilardi G, et al. Bendamustine is safe and effective for lymphodepletion before tisagenlecleucel in patients with refractory or relapsed large B-cell lymphomas. Ann Oncol. 2022;33:916–28. 10.1016/j.annonc.2022.05.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoon DH, Osborn MJ, Tolar J & Kim CJ Incorporation of immune checkpoint blockade into chimeric antigen receptor T cells (CAR-Ts): combination or built-in CAR-T. Int J Mol Sci 19. 10.3390/ijms19020340 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ceschi A, Noseda R, Palin K, Verhamme K. Immune checkpoint inhibitor-related cytokine release syndrome: analysis of WHO global pharmacovigilance database. Front Pharmacol. 2020;11:557. 10.3389/fphar.2020.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yeku OO, Purdon T, Spriggs DR & Brentjens RJ Chimeric antigen receptor (CAR) T cells genetically engineered to deliver IL-12 to the tumor microenvironment in ovarian cancer. Journal of Clinical Oncology 35. 10.1200/Jco.2017.35.15_Suppl.3050 (2017). [DOI] [Google Scholar]
- 87.Yeku OO, Purdon T, Spriggs DR & Brentjens RJ Interleukin-12 armored chimeric antigen receptor (CAR) T cells for heterogeneous antigen-expressing ovarian cancer. Journal of Clinical Oncology 36. 10.1200/Jco.2018.36.5_Suppl.12 (2018). [DOI] [Google Scholar]
- 88.Koneru M, Purdon TJ, Spriggs D, Koneru S & Brentjens RJ IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology 4, doi:ARTNe994446 10.4161/2162402X.2014.994446 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Choi BD, et al. CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J Immunother Cancer. 2019;7:304. 10.1186/s40425-019-0806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoyos V, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–70. 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Budde LE et al. Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. PLoS One 8, e82742. 10.1371/journal.pone.0082742 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang X, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118:1255–63. 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tasian SK et al. Optimized depletion of chimeric antigen receptor T-cells in murine xenograft models of human acute myeloid leukemia. Blood. 10.1182/blood-2016-08-736041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Diaconu I, et al. Inducible caspase-9 selectively modulates the toxicities of CD19-specific chimeric antigen receptor-modified T cells. Mol Ther. 2017;25:580–92. 10.1016/j.ymthe.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stroncek DF, et al. Myeloid cells in peripheral blood mononuclear cell concentrates inhibit the expansion of chimeric antigen receptor T cells. Cytotherapy. 2016;18:893–901. 10.1016/j.jcyt.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ruella M, et al. Overcoming the immunosuppressive tumor microenvironment of Hodgkin lymphoma using chimeric antigen receptor T cells. Cancer Discov. 2017;7:1154–67. 10.1158/2159-8290.CD-16-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Giavridis T, et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24:731–8. 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Teachey DT, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–79. 10.1158/2159-8290.CD-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gauthier J, et al. Feasibility and efficacy of CD19-targeted CAR-T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood. 2020;135:1650–60. 10.1182/blood.2019002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This review article does not present any original unpublished data. All data presented is associated with the corresponding reference.


