Abstract
Aims
Diabetic ketoacidosis (DKA) is not well characterised in New Zealand. This study is aimed at characterising the change in epidemiology and severity of DKA from 2000 to 2019 at a tertiary hospital in the Waikato region of New Zealand.
Methods
A retrospective clinical data review of all patients admitted to Waikato District Health Board hospitals with DKA was undertaken. Characteristics and severity of DKA were assessed by type of DKA admission (diagnosed at admission, nonrecurrent, and recurrent), ethnicity, social deprivation, intensive care unit (ICU) admission, and length of hospital stay, with linear regression reporting on changes over time.
Results
There were 1254 admissions for DKA (564 individual patients), two-thirds being recurrent events. Nonrecurrent DKA patients were younger, whilst recurrent admissions for DKA were associated with T1D, female gender, greater socioeconomic deprivation, and rural living (all P values < 0.01). DKA admission increased 8-fold between 2000 and 2019, mostly due to an increased number of recurrent events, particularly in Māori and female patients (P < 0.001). ICU admissions increased over time (P < 0.001) whilst length of hospital stay trended down (P = 0.031).
Conclusions
The rise in recurrent DKA is concerning, particularly in youth and indigenous Māori. Healthcare inequities need to be addressed, including adequate access to mental health support to ensure optimal outcomes for all patients with diabetes.
1. Introduction
Diabetic ketoacidosis (DKA) is an acute and potentially fatal metabolic complication of type 1 diabetes (T1D) and type 2 diabetes (T2D) with severe insulin deficiency and pancreatogenic diabetes [1, 2]. DKA may be the first presentation of previously unknown diabetes mellitus or may be a nonrecurrent or recurrent event in those with established diabetes mellitus due to either omission of insulin and/or intercurrent stressors [3]. Although DKA is largely preventable, it is the leading cause of mortality in children with T1D and is associated with long-term sequelae, including cognitive and renal impairment in both children and adults [4–6]. Recurrent presentations of DKA are of particular concern. Risk factors for recurrent DKA include T1D, younger age, low socioeconomic status, suboptimal glycaemic control, alcohol and recreational drug use, insulin pump use, disordered eating, and other mental health disorders [7–9].
Whilst the incidence of DKA as the first presentation of diabetes appears to have remained stable for many years in New Zealand and worldwide, the incidence of DKA in those with established diabetes appears to have significantly increased over the past 20 years [1, 2, 4, 10]. This increase is primarily due to recurrent DKA [1, 10], but there is a significant variation in the incidence of DKA between studies, with many reporting no change in incidence over time [11–16]. These differences appear to be mainly due to differences in the demographics of the populations [14–16], different definitions of DKA [17], lack of differentiation between recurrent DKA and nonrecurrent DKA at diagnosis, and differences in health systems [18, 19].
The Waikato region in New Zealand is a predominantly urban area that comprises approximately 400,000 people, of whom 21% identify as Māori, the Indigenous people of New Zealand [20]. The prevalence of T1D and T2D in the Waikato population is approximately 0.3% [20] and 6.3% [21], respectively. In the Waikato region, as seen internationally, Indigenous Māori and those more socioeconomically deprived with either T1D or T2D have a higher glycated haemoglobin (HbA1c) [20, 21], putting them at greater risk of a DKA event. The demographic breakdown of DKA in a New Zealand paediatric population and the outcomes of DKA in a New Zealand adult population have been well described [13, 16, 22], but the epidemiology of DKA at diagnosis and recurrent DKA across the lifespan in New Zealand has not. Therefore, the aim of this study was to characterise the epidemiology of DKA in the Waikato region and to identify any disparities in the incidence and severity of DKA.
2. Materials and Methods
This study was a retrospective clinical data review of all patients who were admitted to Te Whatu Ora Waikato hospitals (previously Waikato District Health Board) with DKA between 1st January 2000 and 31st December 2019. DKA was defined as pH < 7.3, serum bicarbonate (HCO3−) < 18 mmol/L, ketonaemia (blood beta‐hydroxybutyrate ≥ 0.6 mmol/L), and hyperglycaemia [17]. The following data were collated from the clinical records: date of DKA onset and related hospital admission, age at admission, ethnicity, venous pH and HCO3− levels, admission to the intensive care unit (ICU) (yes/no), length of hospital stay, mortality during admission, and mortality within 12 months. In addition, patient socioeconomic status was determined using the patient's address at the time of admission, and rural/urban locality status was categorised according to Statistics New Zealand, New Zealand's official data agency [23]. Approval for this study was given by the Waikato District Health Board Clinical Audit Support Unit (ref #4161).
2.1. Data Analysis
The types of DKA at admission were categorised into three groups: DKA with diagnosis of diabetes at admission, nonrecurrent DKA (known diabetes with one episode of DKA in the study period), and recurrent DKA (two or more admissions within the study period). The patient characteristics of each group were then reported by gender, ethnicity (Māori vs. non-Māori), age group (≤5, 5-14, 15-24, 25-34, 35-44, and ≥45 years), rurality (rural vs. urban), New Zealand deprivation quintile (1-5, where quintile 5 represents the highest deprivation), type of diabetes (T1D, T2D, and unknown), venous pH levels, HCO3− levels, and length of hospital stay (LOS). For continuous variables, data ware presented as the median and interquartile range (IQR). Ethnicity was grouped as Māori and non-Māori with prioritisation for Māori with multiple ethnicities, and the differences between groups were compared by chi-square test for categorical variables and independent sample t-test for continuous variables. Significance was accepted at a level of P value < 0.05 (P < 0.05).
The total number of DKA admissions and the numbers by type of admission were reported over time. Linear regression analysis was used to examine the effect of time on the number of DKA admissions and to identify the contributions from different types of DKA admissions. Logistic regression analysis was used to estimate the odds ratios and 95% confidence interval (CI) of being admitted to ICU, having recurrent DKA, and dying within 12 months for Māori and non-Māori patients (all as markers of DKA severity). Trends are reported for LOS and ICU admissions over time (DKA at diagnosis, nonrecurrent, and recurrent DKA). The analyses were carried out before and after adjustment for age, gender, NZ deprivation quintile, rurality, year of admission, and types of admissions (not for the regression for having recurrent DKA). For the regression of being admitted to ICU and dying within 12 months, the analysis was performed at hospital admission level. All DKA admissions were included. For the regression of having recurrent DKA, the analysis was performed at patient level. Only the first record for each patient was included, and if the patients had more than one admission, they were classified as having recurrent DKA.
3. Results
During the study period, there were 1254 admissions for DKA in 564 individual patients (Tables 1 and 2). Of these, 156 (12.4%) were diagnosed with diabetes at presentation with DKA, 247 (19.7%) had known diabetes with one episode of DKA in the study period (nonrecurrent DKA), and 851 (67.9%) were recurrent DKA events. Of all DKA admissions, one quarter (26.2%) occurred in Māori patients and 73.8% in non-Māori patients, and the incidence of events was relatively comparable for men (48.3%) and women (51.7%; P = 0.23). The median age at admission was 24 years (IQR: 17–38 years). Patients who presented with DKA as their first presentation of diabetes were younger (48.1% were aged <15 years; P < 0.001), whilst those with recurrent DKA events were more likely to be aged 15–24 years (P < 0.001). Socioeconomic deprivation also influenced the likelihood of a DKA event, with over half of the DKA cases occurring in patients from NZ deprivation quintiles 4 and 5 (Tables 1 and 2). This was consistent regardless of whether this was the first DKA presentation with diabetes or a recurrent DKA event. Not surprisingly, more than 90% of DKA events occurred in those with T1D. Of the 564 patients, approximately two-thirds had only one admission for DKA (n = 370; 65.6%; Table 2) during the study period. A further 116 patients (20.6%) had two or three DKA admissions, and 78 (13.8%) had four or more admissions. Those with four or more DKA admissions were more likely to be 15-24 years of age (Table 2). Overall, a greater number of admissions for DKA were associated with T1D, female gender, greater socioeconomic deprivation, and rural living (all P values < 0.01). Tables 1 and 2 summarize the characteristics of DKA events in each subgroup and the patient characteristics by number of DKA admissions, respectively.
Table 1.
DKA event characteristics by DKA subgroup.
| Factors | 1: DKA with diabetes diagnosis at admission | 2: nonrecurrent DKA | 3: recurrent DKA | Total DKA | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 groups | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | |||||||||
| Gender | ||||||||||||
| Female | 66 | 42.3% | 121 | 49.0% | 461 | 54.2% | 648 | 51.7% | 0.016 | 0.190 | 0.006 | 0.151 |
| Male | 90 | 57.7% | 126 | 51.0% | 390 | 45.8% | 606 | 48.3% | ||||
| Ethnicity | ||||||||||||
| Māori | 34 | 21.8% | 48 | 19.4% | 246 | 28.9% | 328 | 26.2% | 0.005 | 0.566 | 0.068 | 0.003 |
| Non-Māori | 122 | 78.2% | 199 | 80.6% | 605 | 71.1% | 926 | 73.8% | ||||
| Age group | ||||||||||||
| <5 years | 16 | 10.3% | 0 | 1 | 0.1% | 17 | 1.4% | <0.001 | <0.001 | <0.001 | <0.001 | |
| 5-14 years | 59 | 37.8% | 21 | 8.5% | 81 | 9.5% | 161 | 12.8% | ||||
| 15-24 years | 34 | 21.8% | 62 | 25.1% | 363 | 42.7% | 459 | 36.6% | ||||
| 25-34 years | 13 | 8.3% | 30 | 12.1% | 192 | 22.6% | 235 | 18.7% | ||||
| 35-44 years | 14 | 9.0% | 30 | 12.1% | 121 | 14.2% | 165 | 13.2% | ||||
| ≥45 years | 20 | 12.8% | 104 | 42.1% | 93 | 10.9% | 217 | 17.3% | ||||
| Rurality | ||||||||||||
| Rural | 36 | 23.1% | 60 | 24.3% | 192 | 22.6% | 288 | 23.0% | 0.850 | 0.780 | 0.888 | 0.569 |
| Urban | 120 | 76.9% | 187 | 75.7% | 659 | 77.4% | 966 | 77.0% | ||||
| NZ deprivation (quintile)1 | ||||||||||||
| 1 | 20 | 12.8% | 18 | 7.3% | 71 | 8.3% | 109 | 8.7% | 0.327 | 0.365 | 0.301 | 0.389 |
| 2 | 13 | 8.3% | 20 | 8.1% | 96 | 11.3% | 129 | 10.3% | ||||
| 3 | 27 | 17.3% | 38 | 15.4% | 130 | 15.3% | 195 | 15.6% | ||||
| 4 | 36 | 23.1% | 68 | 27.5% | 192 | 22.6% | 296 | 23.6% | ||||
| 5 | 60 | 38.5% | 103 | 41.7% | 362 | 42.5% | 525 | 41.9% | ||||
| Type of diabetes mellitus2 | ||||||||||||
| Type 1 | 140 | 93.3% | 184 | 74.8% | 819 | 96.4% | 1143 | 91.7% | <0.001 | <0.001 | <0.001 | <0.001 |
| Type 2 | 10 | 6.7% | 62 | 25.2% | 31 | 3.6% | 103 | 8.3% | ||||
| pH (median+IQR) | 7.20 | (7.12-7.28) | 7.20 | (7.08-7.27) | 7.20 | (7.08-7.27) | 7.20 | (7.08-7.27) | 0.480 | 0.654 | 0.412 | 0.723 |
| HCO3− (median+IQR) | 12.0 | (8.78-16.73) | 12.0 | (8.00-16.00) | 12.0 | (8.20-16.20) | 12.0 | (8.30-16.20) | 0.860 | 0.954 | 0.881 | 0.920 |
| LOS (days) (median+IQR) | 6.70 | (4.66-8.24) | 3.80 | (1.90-6.84) | 2.70 | (1.77-4.16) | 3.00 | (1.87-5.47) | <0.001 | <0.001 | <0.001 | <0.001 |
| Total | 156 | 12.4% | 247 | 19.7% | 851 | 67.9% | 1254 | 100% | ||||
1Quintile 1 represents the least deprivation, and quintile 5 represents the highest deprivation. 2Six cases of unknown diabetes type. DKA = diabetic ketoacidosis; HCO3− = bicarbonate; IQR = interquartile range; LOS = length of stay.
Table 2.
Patient characteristics by number of diabetic ketoacidosis (DKA) admissions.
| Factors | Total | Only 1 DKA | 2-3 DKAs | 4+ DKAs | P value | |||
|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||
| Female | 282 | 170 | 60.3% | 72 | 25.5% | 40 | 14.2% | 0.01 |
| Male | 282 | 200 | 70.9% | 44 | 15.6% | 38 | 13.5% | |
| Ethnicity | ||||||||
| Māori | 132 | 71 | 53.8% | 41 | 31.1% | 20 | 15.2% | 0.001 |
| Non-Māori | 432 | 299 | 69.2% | 75 | 17.4% | 58 | 13.4% | |
| Age group (at first admission) | ||||||||
| <5 years | 16 | 15 | 12.3% | 1 | 0.8% | 0 | 0.0% | <0.001 |
| 5-14 years | 106 | 62 | 50.8% | 30 | 24.6% | 14 | 11.5% | |
| 15-24 years | 156 | 86 | 55.1% | 30 | 19.2% | 40 | 25.6% | |
| 25-34 years | 76 | 42 | 55.3% | 22 | 28.9% | 12 | 15.8% | |
| 35-44 years | 62 | 42 | 67.7% | 13 | 21.0% | 7 | 11.3% | |
| ≥45 years | 148 | 123 | 83.1% | 20 | 13.5% | 5 | 3.4% | |
| Rurality | ||||||||
| Rural | 164 | 88 | 53.7% | 45 | 27.4% | 31 | 18.9% | <0.001 |
| Urban | 400 | 282 | 70.5% | 71 | 17.8% | 47 | 11.8% | |
| NZ deprivation (quintile)1 | ||||||||
| 1 | 48 | 34 | 70.8% | 5 | 10.4% | 9 | 18.8% | <0.001 |
| 2 | 54 | 31 | 57.4% | 13 | 24.1% | 10 | 18.5% | |
| 3 | 88 | 60 | 68.2% | 20 | 22.7% | 8 | 9.1% | |
| 4 | 146 | 98 | 67.1% | 31 | 21.2% | 17 | 11.6% | |
| 5 | 228 | 147 | 64.5% | 47 | 20.6% | 34 | 14.9% | |
| Type of diabetes mellitus | ||||||||
| Type 1 | 474 | 292 | 61.6% | 106 | 22.4% | 76 | 16.0% | <0.001 |
| Type 2 | 83 | 72 | 86.7% | 9 | 10.8% | 2 | 2.4% | |
| Unknown | 7 | 6 | 85.7% | 1 | 14.3% | |||
| Total | 564 | 370 | 65.6% | 116 | 20.6% | 78 | 13.8% | |
1Quintile 1 represents the least deprivation, and quintile 5 represents the highest deprivation.
Almost one in five patients with recurrent DKA was admitted to ICU (n = 105; 18.6%). Five patients (0.9%) died during their admission (one Māori (recurrent DKA) and 4 non-Māori (two recurrent and two nonrecurrent events)), and a further 35 patients died within the following 12 months (1-year mortality, 7.1%). There was no significant difference between Māori and non-Māori patients being admitted to ICU or dying within 12 months before or after adjustment for age, gender, NZ deprivation quintile, rurality, year of admission, and type of admissions (Table 3).
Table 3.
Odds ratio of ICU admission, recurrent DKA, and mortality within 12 months.
| Factors | Being admitted to ICU | Having recurrent DKA | Dying within 12 months |
|---|---|---|---|
| Age at admission (continuous) | 0.96 (0.94-0.98)∗∗∗ | 0.98 (0.97-0.99)∗∗∗ | 1.08 (1.06-1.10)∗∗∗ |
| Gender | |||
| Female | Reference | Reference | Reference |
| Male | 0.43 (0.23-0.79)∗∗ | 0.68 (0.47-0.98)∗ | 0.90 (0.40-2.02) |
| NZ deprivation quintile1 | |||
| 1 | Reference | Reference | Reference |
| 2 | 0.37 (0.09-1.54) | 1.76 (0.75-4.13) | 1.23 (0.25-6.11) |
| 3 | 0.77 (0.28-2.13) | 1.07 (0.48-2.38) | 0.45 (0.08-2.46) |
| 4 | 0.63 (0.22-1.75) | 1.17 (0.55-2.46) | 0.56 (0.14-2.20) |
| 5 | 0.69 (0.26-1.80) | 1.27 (0.62-2.62) | 0.52 (0.14-1.92) |
| Rurality | |||
| Urban | Reference | Reference | Reference |
| Rural | 2.16 (1.17-3.99)∗ | 1.38 (0.89-2.13) | 0.52 (0.18-1.47) |
| Ethnicity | |||
| Non-Māori | Reference | Reference | Reference |
| Māori | 1.40 (0.74-2.65) | 1.95 (1.27-3.00)∗∗ | 1.56 (0.63-3.86) |
| Year (continuous) | 1.06 (1.00-1.12)∗ | 0.94 (0.91-0.97)∗∗∗ | 1.10 (1.01-1.19) |
| Type of admission | |||
| Non-recurrent | Reference | — | Reference |
| Recurrent | 0.66 (0.34-1.28) | — | 0.52 (0.22-1.21) |
1Quintile 1 represents the least deprivation, and quintile 5 represents the highest deprivation. ∗ < 0.05, ∗∗ < 0.01, and ∗∗∗ < 0.001. DKA = diabetic ketoacidosis; ICU = intensive care unit.
The number of DKA admissions increased 8-fold over time (Figure 1), from 16 cases in 2000 to 129 cases in 2019. The linear regression showed that three quarters of this increase were due to increase in recurrent DKA events, though DKA with diabetes admission and nonrecurrent DKA rates also increased significantly over time (Figure 1). Māori patients were more likely to have recurrent DKA than non-Māori patients, with an unadjusted odds ratio of 1.93 (1.30–2.88) and an adjusted odds ratio of 1.95 (1.27–3.00). Women were also more likely than men to have recurrent DKA events with an odds ratio of 1.61 (1.13–2.28; Table 3). Rural living was not associated with an increased risk of recurrent DKA but was associated with a greater risk of admission to ICU (P < 0.05).
Figure 1.
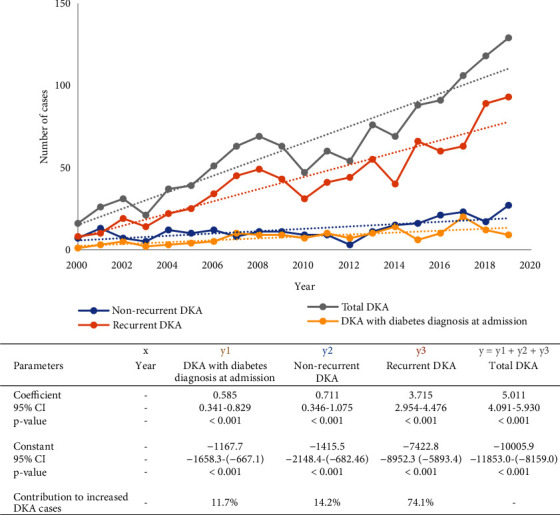
Number of DKA admissions over time and linear regression results. DKA = diabetic ketoacidosis; CI = confidence interval.
Overall, there was no change in median venous pH or mortality over time, though the proportion of patients admitted to ICU trended up between 2000 and 2019 (P < 0.001; OR 1.14 (1.05–1.23)). This was primarily due to an increased proportion of ICU admissions in those diagnosed with DKA at the time of hospital admission (Figure 2(a)). In contrast, mean LOS trended mildly downward over time (Figure 2(b); P = 0.031), Overall, severity of disease did not differ by ethnicity or the level of socioeconomic deprivation.
Figure 2.
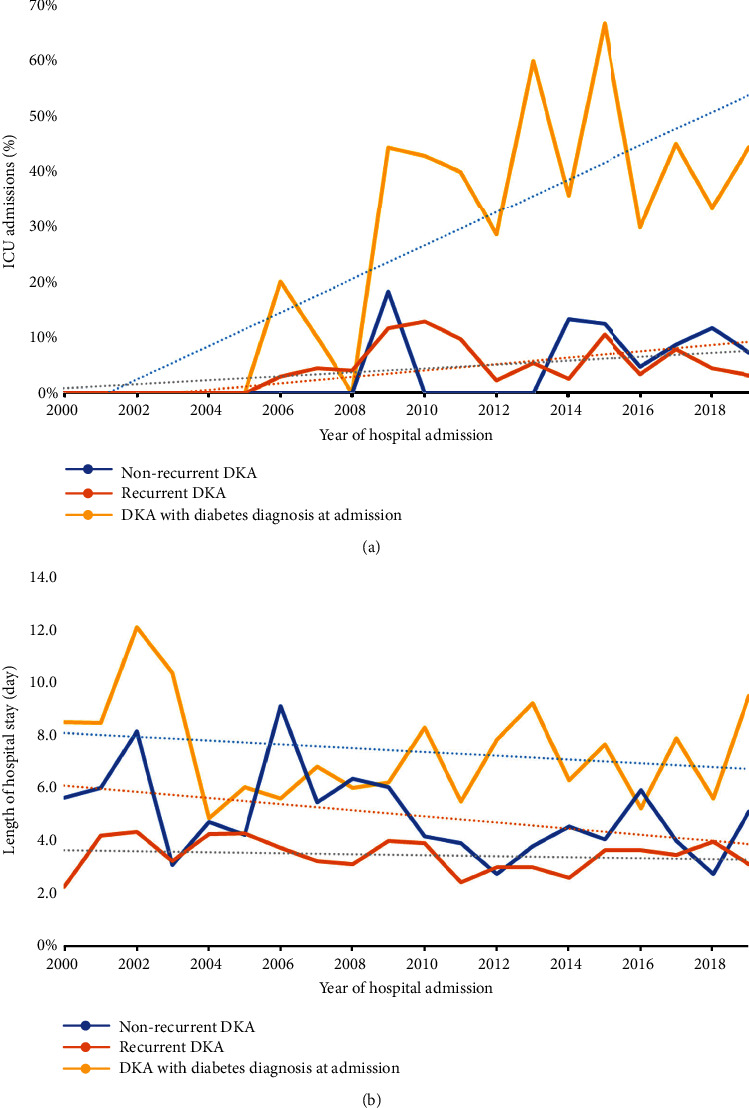
Proportion of intensive care unit (ICU) admissions (a) and mean length of hospital stay (LOS) (b) due to different types of diabetic ketoacidosis (DKA) presentation.
4. Discussion
Our study shows that there has been a dramatic increase in number of presentations of DKA in the Waikato region of New Zealand over time and this has occurred despite the overall number of patients with diabetes (type 1 and type 2 combined) in the region only rising by approximately 6% during this same time period [24]. Importantly, this rise appears to not be due to changes in overall glycaemic control [25] but instead seems to be related to an increase in recurrent DKA events in young adults and/or in those living in areas of higher deprivation. Such findings agree with that presented elsewhere that recurrent DKA associates with emerging adulthood [5, 7, 22], and this is mainly due to a higher overall HbA1c, reduced access to healthcare, and adherence to insulin treatment [7, 22, 26]. Unfortunately, psychosocial screening is not routine for admissions to hospital in New Zealand, though this could provide further insight into these DKA admissions, given the higher rates of low mood and disordered eating in young adults and adolescents with diabetes (particularly T1D) [27]. Through the identification of mental health concerns and diabetes-related distress, appropriate interventions could be introduced, potentially reducing the “recurrent” nature of DKA. Socioeconomic deprivation has also been shown to strongly correlate with a higher incidence of DKA in other large studies [28]. In addition to this, lower health literacy [28] and reduced access to high-quality diabetes care, healthy foods, and diabetes technology (e.g., insulin pumps and continuous glucose monitoring) [29, 30] can all contribute to an increased likelihood of glycaemic dysfunction (and therefore increased DKA risk). The contribution of these factors needs to be urgently evaluated. Further, addressing these inequities needs to be a priority for healthcare in New Zealand if we are to see reductions in the rate of DKA presentations, and it will be interesting to observe the future rates of change for DKA presentations from these population groups following national restructuring of New Zealand health system in 2022.
Importantly, despite the exponential increase seen in recurrent DKA presentations, there has not been any increase in several parameters of DKA severity. Indeed, the mortality rates observed in our study are similar to other national and international studies [4, 31, 32], and LOS appears to have decreased over time, as it has at another tertiary hospital in New Zealand [22]. These findings may be indicative of improvement in initial clinical care but are also representative of a similar mode of healthcare provision between these two centres which may differ from that seen in other countries [11]. However, ICU admissions were either stable or increasing in our cohort which agrees with that report elsewhere [31]. We suggest that in our study, this was likely due to the reduced ability of medical wards to manage mild DKA.
Our data also indicates that the rate of presentation and severity of DKA does not appear to be worse for Māori patients. It has been suggested that this may be due to the fact that most DKA admissions in our study associated with T1D and Māori are only half as likely as non-Māori to be affected by T1D [33]. However, it is encouraging to see that there does not appear to be any inequity in treatment after presentation to hospitals in this region.
The main strength of our study is that we were able to report on 20 years of continuous data from a large, regional hospital with good representation of indigenous Māori, though we do note study limitations. Firstly, our findings may not be representative of all New Zealand because of the different age, ethnic, and rural demographics throughout the country. Secondly, whilst we are able to discuss our data in the context of national data that reports yearly on the number of diabetes patients within our region [24], we are unable to report on how the demographic and obesity rates may have changed between 2000 and 2019 (with regard to age, sex, and ethnicity) and how this may have influenced the results seen. Third, we note that our study lacked both HbA1c data and T1D prevalence data. This makes it difficult to make comparisons to other literature reporting on DKA rates. These factors, collectively, should all be explored in later studies through the use of population/census and clinical/laboratory information. Further, we note the possibility that some patients coded as “nonrecurrent DKA” may have had an additional DKA event outside of the study period and that DKA severity could have been more precisely defined [34]. In particular, the inclusion of admission glucose values would have yielded useful information on the epidemiology of euglycaemic DKA, and this should be explored in further studies.
However, our data do show that there is clearly a very strong need to reduce the risk factors for recurrent DKA in our local population, particularly in young adults and those from lower socioeconomic backgrounds. Based on our findings and those of others, we suggest that it would be useful to screen all patients who are admitted with recurrent DKA for mental health concerns (including substance abuse) and diabetes-related distress. In addition, these patients should receive appropriate input from multidisciplinary team members as soon as possible, including from psychology, dietitian, social, youth and cultural workers, and specialist diabetes services in either the inpatient or outpatient setting.
Acknowledgments
We would like to thank Ms. Danni Parsons for helping with the editing of this manuscript. A summer student scholarship was provided for Ms. Papa by the University of Waikato.
Data Availability
The data used to support the findings of this study are restricted by Te Whatu Ora Health New Zealand in order to protect patient privacy. Data are available from Dr. Ryan Paul for researchers who meet the criteria for access to confidential data.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Authors' Contributions
VP collected and cleaned the clinical data under the supervision of JW, CMR, and JM. LC and RP guided the data analysis (undertaken by CL), and then, VP, LC, and RP prepared the draft manuscript. All authors contributed to the final manuscript, and all approve this work for publication.
References
- 1.Zhong V. W., Juhaeri J., Mayer-Davis E. J. Trends in hospital admission for diabetic ketoacidosis in adults with type 1 and type 2 diabetes in England, 1998–2013: a retrospective cohort study. Diabetes Care . 2018;41(9):1870–1877. doi: 10.2337/dc17-1583. [DOI] [PubMed] [Google Scholar]
- 2.Farsani S. F., Brodovicz K., Soleymanlou N., Marquard J., Wissinger E., Maiese B. A. Incidence and prevalence of diabetic ketoacidosis (DKA) among adults with type 1 diabetes mellitus (T1D): a systematic literature review. BMJ Open . 2017;7(7, article e016587) doi: 10.1136/bmjopen-2017-016587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans K. Diabetic ketoacidosis: update on management. Clinical Medicine . 2019;19(5):396–398. doi: 10.7861/clinmed.2019-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benoit S. R., Zhang Y., Geiss L. S., Gregg E. W., Albright A. Trends in diabetic ketoacidosis hospitalizations and in-hospital mortality—United States, 2000–2014. Morbidity and Mortality Weekly Report . 2018;67(12):362–365. doi: 10.15585/mmwr.mm6712a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poovazhagi V., Senguttuvan P., Padmaraj R. Outcome of acute renal failure in children with diabetic keto acidosis (DKA) Pediatric Oncall Journals . 2011;8(8):63–66. [Google Scholar]
- 6.Jin C. Y., Yu S. W., Yin J. T., Yuan X. Y., Wang X. G. Corresponding risk factors between cognitive impairment and type 1 diabetes mellitus: a narrative review. Heliyon . 2022;8(8, article e10073) doi: 10.1016/j.heliyon.2022.e10073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Degan S., Dubé F., Gagnon C., Boulet G. Risk factors for recurrent diabetic ketoacidosis in adults with type 1 diabetes. Canadian Journal of Diabetes . 2019;43(7):472–476. doi: 10.1016/j.jcjd.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Berger G., Waldhoer T., Barrientos I., et al. Association of insulin-manipulation and psychiatric disorders: a systematic epidemiological evaluation of adolescents with type 1 diabetes in Austria. Pediatric Diabetes . 2019;20(1):127–136. doi: 10.1111/pedi.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franke P. Eating disorders and insulin misuse in people with type 1 diabetes. Journal of Diabetes Nursing. . 2014;18(3):92–98. [Google Scholar]
- 10.Kao K.-T., Islam N., Fox D. A., Amed S. Incidence trends of diabetic ketoacidosis in children and adolescents with type 1 diabetes in British Columbia, Canada. The Journal of Pediatrics . 2020;221:165–173. doi: 10.1016/j.jpeds.2020.02.069. [DOI] [PubMed] [Google Scholar]
- 11.Usher-Smith J., Thompson M., Ercole A., Walter F. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: a systematic review. Diabetologia . 2012;55(11):2878–2894. doi: 10.1007/s00125-012-2690-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hekkala A., Knip M., Veijola R. Ketoacidosis at diagnosis of type 1 diabetes in children in northern Finland: temporal changes over 20 years. Diabetes Care . 2007;30(4):861–866. doi: 10.2337/dc06-2281. [DOI] [PubMed] [Google Scholar]
- 13.Cherubini V., Grimsmann J. M., Åkesson K., et al. Temporal trends in diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes between 2006 and 2016: results from 13 countries in three continents. Diabetologia . 2020;63(8):1530–1541. doi: 10.1007/s00125-020-05152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dabelea D., Rewers A., Stafford J. M., et al. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics . 2014;133(4):e938–e945. doi: 10.1542/peds.2013-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choleau C., Maitre J., Pierucci A. F., et al. Ketoacidosis at diagnosis of type 1 diabetes in French children and adolescents. Diabetes & Metabolism . 2014;40(2):137–142. doi: 10.1016/j.diabet.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Jefferies C., Cutfield S. W., Derraik J. G., et al. 15-year incidence of diabetic ketoacidosis at onset of type 1 diabetes in children from a regional setting (Auckland, New Zealand) Scientific Reports . 2015;5(1):1–7. doi: 10.1038/srep10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhatariya K. K. Defining and characterising diabetic ketoacidosis in adults. Diabetes Research and Clinical Practice . 2019;155, article 107797 doi: 10.1016/j.diabres.2019.107797. [DOI] [PubMed] [Google Scholar]
- 18.Clarke P. M., Glasziou P., Patel A., et al. Event rates, hospital utilization, and costs associated with major complications of diabetes: a multicountry comparative analysis. PLoS Medicine . 2010;7(2, article e1000236) doi: 10.1371/journal.pmed.1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desai D., Mehta D., Mathias P., Menon G., Schubart U. K. Health care utilization and burden of diabetic ketoacidosis in the U.S. over the past decade: a nationwide analysis. Diabetes Care . 2018;41(8):1631–1638. doi: 10.2337/dc17-1379. [DOI] [PubMed] [Google Scholar]
- 20.Chepulis L., Tamatea J. A., Wang C., Goldsmith J., Mayo C. T., Paul R. G. Glycaemic control across the lifespan in a cohort of new Zealand patients with type 1 diabetes mellitus. Internal Medicine Journal . 2021;51(5):725–731. doi: 10.1111/imj.14816. [DOI] [PubMed] [Google Scholar]
- 21.Chepulis L., Morison B., Keenan R., Paul R., Lao C., Lawrenson R. The epidemiology of diabetes in the Waikato region: an analysis of primary care data. Journal of Primary Health Care . 2021;13(1):44–54. doi: 10.1071/HC20067. [DOI] [PubMed] [Google Scholar]
- 22.Braatvedt G., Tekiteki A., Britton H., Wallace J., Khanolkar M. 23 years of managing diabetic ketoacidosis at Auckland Hospital. The New Zealand Medical Journal . 2017;130(1450):p. 16. [PubMed] [Google Scholar]
- 23.Statistics New Zealand. Urban rural. 2021 (generalised) Available from https://datafinder.stats.govt.nz/layer/105158-urban-rural-2021-generalised/
- 24.New Zealand Ministry of Health. Key findings from the 2021 Virtual Diabetes Register. 2021. https://minhealthnz.shinyapps.io/virtual-diabetes-register-web-tool/
- 25.Sandhu S. K., Corbett V. M., Chepulis L., et al. The prevalence of microvascular complications in Waikato children and youth with type 1 diabetes has reduced since 2003. The New Zealand Medical Journal . 2020;133(1510):35–44. [PubMed] [Google Scholar]
- 26.Brandstaetter E., Bartal C., Sagy I., Jotkowitz A., Barski L. Recurrent diabetic ketoacidosis. Archives of Endocrinology and Metabolism . 2019;63(5):531–535. doi: 10.20945/2359-3997000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McClintock J. M., Blackmore T., Chepulis L. M., Fraser S., Paul R. G. The psychological profile of youth and young adults with type 1 diabetes in New Zealand. Pediatric Diabetes . 2022;23(1):150–156. doi: 10.1111/pedi.13289. [DOI] [PubMed] [Google Scholar]
- 28.Kurani S. S., Heien H. C., Sangaralingham L. R., et al. Association of area-level socioeconomic deprivation with hypoglycemic and hyperglycemic crises in US adults with diabetes. JAMA Network Open . 2022;5(1, article e2143597) doi: 10.1001/jamanetworkopen.2021.43597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wheeler B. J., Braund R., Galland B., et al. District health board of residence, ethnicity and socioeconomic status all impact publicly funded insulin pump uptake in New Zealand patients with type 1 diabetes. The New Zealand Medical Journal . 2019;132(1491):78–89. [PubMed] [Google Scholar]
- 30.Isaacs D., Bellini N. J., Biba U., Cai A., Close K. L. Health care disparities in use of continuous glucose monitoring. Diabetes Technology & Therapeutics . 2021;23(Supplement 3) doi: 10.1089/dia.2021.0268. [DOI] [PubMed] [Google Scholar]
- 31.Venkatesh B., Pilcher D., Prins J., Bellomo R., Morgan T. J., Bailey M. Incidence and outcome of adults with diabetic ketoacidosis admitted to ICUs in Australia and New Zealand. Critical Care . 2015;19(1):1–12. doi: 10.1186/s13054-015-1171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shand J. A., Morrow P., Braatvedt G. Mortality after discharge from hospital following an episode of diabetic ketoacidosis. Acta Diabetologica . 2022;59(11):1485–1492. doi: 10.1007/s00592-022-01953-5. [DOI] [PubMed] [Google Scholar]
- 33.Kenealy T. W., Sheridan N. F., Orr-Walker B. J. Six new studies about diabetes: what can we learn that might benefit Māori and Pacific people. New Zealand Medical Journal . 2017;130(1450):8–11. [PubMed] [Google Scholar]
- 34.Kitabchi A. E., Umpierrez G. E., Murphy M. B., et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care . 2001;24(1):131–153. doi: 10.2337/diacare.24.1.131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are restricted by Te Whatu Ora Health New Zealand in order to protect patient privacy. Data are available from Dr. Ryan Paul for researchers who meet the criteria for access to confidential data.


