Abstract
Purpose of Review
To review current literature examining the presence of subclinical micro- and macrovascular alterations in normotensive individuals and their clinical significance in terms of hypertension prediction. Emphasis is placed on alterations that can be detected in peripheral vascular beds using non-invasive, easily applicable methodology, as these are in general easier to capture and evaluate in clinical practice compared to more complex invasive or functional tests.
Recent Findings
Arterial stiffness, increased carotid intima-media thickness, and altered retinal microvascular diameters predict the progression from the normotensive to the hypertensive state. By contrast, there is substantial lack of relevant prospective studies for skin microvascular alterations. Although conclusions regarding causality cannot be safely deduced from available studies, detection of morphological and functional vascular alterations in normotensive individuals emerges as a sensitive indicator of progression to hypertension and hence increased CVD risk.
Summary
An increasing amount of evidence suggests that early detection of subclinical micro- and macrovascular alterations would be clinically useful for the early identification of individuals at high risk for future hypertension onset. Methodological issues and gaps in knowledge need to be addressed before detection of such changes could guide the development of strategies to prevent new-onset hypertension in normotensive individuals.
Keywords: Arterial stiffness, Atherosclerosis, Retinopathy, Albuminuria, Capillary rarefaction, Normotension, Microcirculation, Macrocirculation
Introduction
Cardiovascular disease (CVD) remains the major cause of mortality globally among adults aged 35–70 years [1]. It has been estimated that over 70% of CVD cases and deaths can be attributed to modifiable risk factors, with hypertension exerting the most extensive effects globally. However, marked reductions in deaths from CVDs have been observed in the last couple of decades especially in high-income countries, related to recent advances in prevention and treatment of non-communicable diseases [2, 3]. Comprehensive understanding of the clinical course of CVDs has mitigated the development and implementation of health care policies aiming to control common modifiable CVD risk factors and improve existing preventive treatments.
Endothelial dysfunction is perceived as the earliest precursor of CVD, triggering the cascade of accelerated atherosclerosis, subclinical vascular injury, and subsequently clinically evident CVD manifestations [4]. In this pathophysiological sequel, subclinical vascular damage represents the first alarming sign of probable future CVD that can be easily identified in vivo at a clinical level. As a leading cause of CVD, hypertension affects primarily the vasculature. The term “hypertension-modified organ damage (HMOD)” has been introduced to describe the complications of hypertensive vascular disease, which are listed under the pathologic heading of either cardiac hypertrophy, arteriosclerosis, or arteriolosclerosis [5, 6]. Detection of asymptomatic HMOD has been made easy and affordable through the implementation of appropriate non-invasive tools and is warranted in hypertensive individuals as it may affect therapeutic strategies [5].
However, the wide applicability of such markers of vasculopathy has revealed the presence of micro- and macrovascular alterations in a considerable portion of individuals with normal blood pressure levels as well. Nevertheless, the clinical importance of such changes in normotensive individuals has received much less attention compared to other high CVD risk populations, and their presence is frequently overlooked in clinical practice as many physicians remain unaware of their prognostic significance.
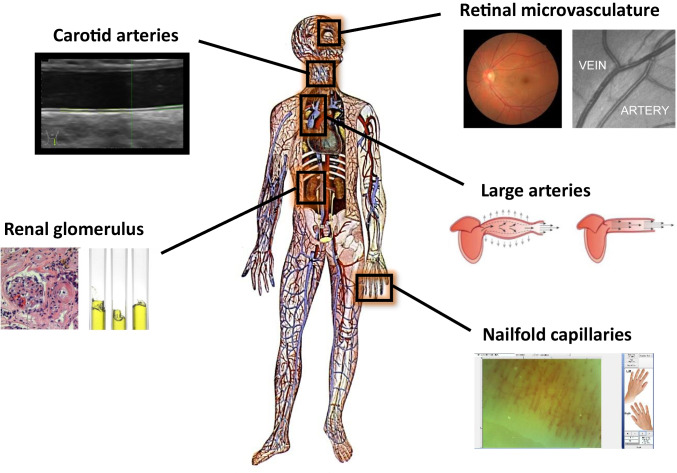
Keeping in mind the pathophysiological and prognostic implementations of early vascular changes, the present review aims to summarize subclinical vascular alterations and their clinical significance in normotensive individuals. We will focus on changes that can be detected non-invasively in peripheral vascular beds as presented in Fig. 1, as these are in general easier to capture in real-world clinical practice compared to more complex invasive or functional tests. Specifically, we will present prospective studies providing evidence of such alterations in normotensive individuals detected by means of non-invasive, easily applicable methodology in the large vessels (aorta, carotid arteries) and the microvasculature (retina, nephrons, dermal capillary network). Their clinical significance will be discussed, with emphasis placed on their prognostic value in terms of hypertension prediction. To this end, a PubMed search was performed to identify relevant articles published in English, using the following medical terms: “arterial stiffness”; “carotid atherosclerosis”; “microalbuminuria”; “retinopathy”; and “capillary rarefaction”.
Fig. 1.
Easily accessible vascular beds for the non-interventional, in vivo study of micro- and macrocirculation. Arterial stiffness and carotid intima-media thickness, secondary to changes in the mechanical and anatomical properties of the arterial wall, are the most widely applied, well-established markers of macroangiopathy. The retina, kidney, and the skin may be used as windows to the heart for the study of microcirculation. While measurement of urinary albumin excretion has long been used as an early indicator of generalized microvascular impairment, methodological advances facilitate the extraction of valuable information on microcirculation from images capturing the retinal microvasculature and the skin capillary network
Arterial Stiffness
Large artery stiffening corresponds to a degradation of the anatomical properties of the arterial wall resulting in a decrease of the high elastin to collagen ratio under the impact of various systemic diseases, particularly those primarily affecting the cardiovascular system [7]. Evaluation of arterial stiffness has been established as a reliable marker of biological age and has been acknowledged as a surrogate marker of CVD. There is indisputable evidence from longitudinal studies in tenths of thousands of individuals to support the incremental prognostic value of arterial stiffness in terms of CVD morbidity and mortality, especially in high-risk groups (coronary artery disease, renal disease, hypertension) but also among low-risk subjects (general population) [8]. Considering the plethora of data, arterial stiffness represents one of the most studied manifestations of early vascular damage in normotensive individuals.
Main prospective studies providing evidence of the prognostic value of increased arterial stiffness using the gold-standard pulse wave velocity (PWV) for future development of hypertension are presented in Table 1. As early as in 1999, the ARIC (Atherosclerosis Risk in Communities Study) [9] showed that in a cohort of 6992 normotensive men and women, arterial elasticity (as estimated by adjusted arterial diameter change, Peterson’s and Young’s elastic modulus, and β stiffness index from ultrasound) was an independent predictor for the development of hypertension over a 6-year follow-up period. A decrease in arterial elasticity by one standard deviation was associated with a 15% increased risk of hypertension, and this association was independent of established risk factors for hypertension and the level of baseline blood pressure. In the Baltimore Longitudinal Study of Aging, increased PWV was an independent predictor of incident hypertension [10]. In particular, there was a 10% increased risk for developing hypertension for every 1 m/s increase in carotid-femoral PWV (cfPWV), suggesting that PWV could help identify high-risk normotensive individuals who could be eligible for interventions aiming at preventing or delaying the onset of hypertension. The Framingham Offspring Study yielded similar results [11]. In a multivariate adjusted regression model, cfPWV (OR: 1.3, 95% CI 1.0–1.6, p = 0.04), augmentation index (OR: 1.7, 95% CI 1.4–2.0, p < 0.001), and forward wave amplitude (OR: 1.6, 95% CI 1.3–2.0, p < 0.001) were independently related to incident hypertension, further supporting the notion that vascular stiffness is a precursor rather than the result of hypertension.
Table 1.
Prospective studies evaluating the prognostic value of PWV for future development of hypertension
| Study | Year published | PWV method | Study population | Number of participants | Time of follow-up | Results |
|---|---|---|---|---|---|---|
| Yambe et al | 2007 | Brachial-ankle | Employees of a single large construction company | 1758 non-hypertensive men (475 with high normal BP) | 2000–2004 | PWV in the highest quartile was significantly predictive of progression to hypertension in individuals with high normal BP |
| Najjar et al | 2008 | Carotid-femoral | Baltimore Longitudinal Study of Aging | 306 non-hypertensives | 4.9 ± 2.5 years | PWV was an independent predictor of incident hypertension |
| Tomiyama et al | 2009 | Brachial-ankle | Employees of a single large construction company (company, headquarters, and offices) | 777 prehypertensive men | 2003–2006 | PWV and BMI are not powerful but significantly independent markers to identify men with prehypertension at high risk for hypertension |
| Satoh et al | 2011 | Brachial-ankle | Male employees of a single local government agency | 2278 non-hypertensives | 3-year follow-up | PWV is a significant and independent predictor of incident hypertension |
| Takase et al | 2011 | Brachial-ankle | Individuals undergoing yearly health check-up in Japan | 2496 non-hypertensives | 733 ± 360 days | PWV is a significant independent predictor of both longitudinal increases in BP and new onset of hypertension |
| Kaess et al | 2012 | Carotid-femoral | Framingham offspring cohort | 1048 non-hypertensives |
1998–2001 2005–2008? |
Higher PWV, AIx, and FWA were associated to incident hypertension |
| Zheng et al | 2015 | Brachial-ankle | Asymptomatic Polyvascular Abnormalities Community Study | 2153 non-hypertensives | An average of 27 months | PWV is an independent predictor of BP progression and incident hypertension |
| Kim et al | 2016 | Brachial-ankle | Korean Genome Epidemiology Study | 1785 non-hypertensives | 4-year follow-up | PWV independently predicts incident hypertension |
| Koivistoinen et al | 2018 | From aortic arch to popliteal artery | Cardiovascular Risk in Young Finns Study | 1183 non-hypertensives | 2007–2011 | PWV directly and independently predicts an increase in BP and the development of hypertension |
| Lee et al | 2019 | Brachial-ankle | Kangbuk Samsung Healthy Study | 10,360 non-hypertensives | 2.17 (1.74–3.63) years |
PWV independently associated with incident hypertension Women were at higher risk |
| Jiang et al | 2020 | Brachial-ankle | Shougang cohort study | 1839 non-hypertensives | 2.4 (2.3–2.4) years | PWV independently and gradually predicted the risk of hypertension and BP progression, modified by the level of SBP at baseline |
Similar results were found in studies using the brachial-ankle pulse wave velocity (baPWV). In more details, in a Korean population of 10,360 young and middle-aged healthy adults, it was found that baPWV was independently associated with incident hypertension, with a stronger risk among females compared to the male population of the study [12]. The independent association between baPWV and incident hypertension was also observed in smaller studies including normotensives [13, 14••, 15–17]. These findings were also confirmed in a Japanese study of 2496 participants (27–84 years) with follow-up of 5215 person-years, in whom baseline value of baPWV was associated not only with new onset of hypertension after adjustment for known risk factors but also with a longitudinal increase in blood pressure in multiple regression analysis [18]. Implementation of baPWV in individuals with high normal blood pressure has further confirmed its predictive value for progression to hypertension, with a 3-year observational period of 524 non-hypertensive individuals showing that assessment of arterial stiffness with baPWV was more reliable for predicting the progression to hypertension in cases of prehypertension compared to individuals with normal and optimal blood pressure [19]. In addition, several studies have demonstrated a positive yet weak association of PWV with future development of hypertension in prehypertensive males [19, 20] and normotensive populations of different race included in the Multi-Ethnic Study of Atherosclerosis [21].
Apart from the gold-standard measurement of PWV, implementation of other markers of arterial stiffness has been suggested to predict the progression to hypertension. In a population of 2512 non-hypertensive individuals, those with aortic inelasticity (as indicated by aortic strain, distensibility, and stiffness index β assessed with echocardiography) were more likely to develop hypertension after 4 years of follow-up. Notably, arterial stiffness remained significantly associated with incident hypertension after adjustment to classical CVD risk factors, both in men and women and in young and old populations [22]. Recently, the cardio-ankle vascular index (CAVI), another marker of arterial stiffness, has also been associated with increased incidence of hypertension. Specifically, a Japanese study in 34,649 normotensives without any CVD showed that increased baseline CAVI elevates the risk of hypertension (HR 1.32 [1.25–1.39] 95% CI) [23].
Carotid Atherosclerosis
Atherosclerosis is a primary pathophysiological process that typically remains silent for several years until the development of clinically evident CVD manifestations [24]. The atherosclerotic burden in apparently healthy people can be easily and non-invasively detected in the carotid arteries using ultrasonography, as a reliable indicator of systemic atherosclerosis. While advanced stages of atherosclerosis include carotid plaque, stenosis, and occlusion, carotid intima-media thickness (cIMT) has been established as a robust marker of subclinical atherosclerosis, especially with values exceeding 1.0 mm which are generally considered as abnormal [25]. Increased cIMT is associated with common CVD risk factors such as current smoking, diabetes, and hypertension and with both prevalent and incident CVD and has been widely used in outcome trials as a surrogate or predictor of CVD outcomes [26•, 27].
Several studies have shown that cIMT independently predicts incident hypertension. In the Multi-Ethnic Study of Atherosclerosis [21], 2512 normotensive participants, with a mean age of 58 years, underwent a high-resolution B-mode ultrasound, in which the cIMT of common and internal carotid arteries was measured. After a follow-up of 4.3 years on average, a significant positive association between increased maximum common cIMT and new-onset hypertension was found. Similar results emerged from a 4-year follow-up study in 1785 non-hypertensive adults, aged 40–69 years [16]. In line with the aforementioned results, another study in 672 normotensives [28] showed that both the average (OR = 1.83, 95% CI: 1.46–2.29, p < 0.0001) and maximum (OR = 1.68, 95% CI: 1.38–2.05, p < 0.0001) values of common carotid artery IMT significantly correlated with incident hypertension. Furthermore, an observational study involving 867 normotensive participants, aged 25 years or older, with a 3-year median follow-up approximately indicated that increased carotid IMT elevated the risk of hypertension by 63%, although a causal relationship between an increased cIMT and the development of hypertension was not established [29]. Nevertheless, findings from two other studies showed that healthy offspring of hypertensives presented increased cIMT compared to healthy decedents of normotensives [30, 31], reinforcing the hypothesis that increased cIMT, even within the normal range, may increase the risk of hypertension.
Urinary Albumin Excretion
Moderately increased albuminuria (MIA), the new terminology for microalbuminuria, defined as urinary albumin excretion between 30 and 300 mg/24 h or urinary albumin/creatinine ratio (UACR) between 30 and 300 mg/g, is a well-recognized non-invasive index of renal microcirculation [32, 33]. The prevalence of MIA in the general population was found to be 7.8% [34]. Increased urinary albumin excretion has been acknowledged as a powerful and independent predictor of prognosis in several disease states, such as diabetes, hypertension [35], and heart failure [36], and has been consistently used as a surrogate endpoint in therapeutic trials [37] for both cardiovascular and renal outcomes. In addition, albuminuria and estimated glomerular filtration rate have been correlated with all-cause and CVD mortality in the general population according to a meta-analysis in 105,872 participants [38].
There is evidence that the presence of MIA was associated with an increased risk for developing hypertension. More specifically, results from the Framingham Offspring Study showed that UACR, even below the threshold for microalbuminuria, was associated with an increased risk for developing hypertension in non-diabetic and non-hypertensive individuals [39]. In this study, a total of 9 different biomarkers were simultaneously measured to conclude that urinary albumin excretion was one of the three markers (along with C-reactive protein and plasminogen activator inhibitor-1) that remained as significant predictors of future incidence of hypertension [39]. More recently, Zhang et al. found that MIA and hypertension are associated in a bidirectional way [40••]. In that longitudinal study, baseline microalbuminuria predicted the risk of incident hypertension (odds ratio = 1.75, p = 0.028), and baseline blood pressure also significantly predicted the risk of microalbuminuria (odds ratios = 1.27 and 1.21 for a per-SD increase in systolic and diastolic blood pressure, respectively). While elevated urinary albumin excretion was more likely to precede hypertension, conclusions regarding causality effects could not be provided.
It should be noted that not only MIA but even a slight increase in UACR, within the normal range, is a risk factor for incident hypertension as shown in several studies including normotensive [41–44] and healthy [45–48] populations. Specifically, in 2016, a 10-year follow-up study [41] involving 9102 normotensive individuals showed that increased UACR, even without exceeding the threshold of 30 mg/g, is an independent risk factor for hypertension (highest UACR quartile HR 1.95 [95% CI 1.51, 2.53]; trend across UACR quartiles p < 0.001). Similarly, the Takahata study [47] established a significant association between elevated UACR, even below the threshold of ≥ 30 mg/g, and incidence of hypertension (OR 2.35, 95% CI 1.28–4.46 for UACR 5–9.9 mg/g and OR 2.78, 95% CI 1.44–5.52 for UACR 10–29.9 vs. UACR 5 mg/g) in 412 participants, free of hypertension, diabetes, renal insufficiency, microalbuminuria (UACR ≥ 30 mg/g), and cardiovascular or renal disease.
Furthermore, MIA has been associated with incidence of CVD events and all-cause mortality in healthy individuals [49, 50]. Analysis of the Framingham study [39] data showed that the presence of microalbuminuria above the median, ≥ 3.9 μg/mg in men and ≥ 7.5 μg/mg in women, was associated with a threefold increased risk of CVD (adjusted HR 2.92, 95% CI 1.57 to 5.44, p < 0.001) and for a marginally insignificant increase in total mortality (adjusted HR 1.75, 95% CI 0.95 to 3.22, p = 0.08) compared to those with a lower-than-average UACR. Furthermore, a 6-year follow-up [49] in 1568 participants, without hypertension, diabetes, or CVD at baseline, suggested that increased UACR, even within the normal range, elevates the mortality risk (HR, 1.55; 95% CI, 1.10 to 2.20; p = 0.014), while UACR at or above the sex-specific median increases the risk of CVD by nearly 3, compared with those with UACR below the median [HR, 2.92; 95% CI, 1.57 to 5.44; p = 0.0007]. Finally, a study in 1318 participants, without a history of diabetes mellitus, hypertension, coronary artery disease, and chronic kidney disease, showed an association between UACR and coronary atherosclerosis, as detected by coronary artery computerized tomography [51].
Retinal Microvascular Alterations
The retina provides an easily accessible window for the study of microcirculation [52]. While clinical fundoscopic evaluation remains the classical method, digital retinal photography can also be applied as a simple and reproducible method to diagnose hypertensive retinopathy. Advanced stages of hypertensive retinopathy have been long considered as an index of HMOD in other organs and systems, such as the kidney. However, the significance of early-stage alterations in the retinal microvasculature is being increasingly recognized in non-hypertensive individuals.
Qualitative fundus evaluation using traditional classification systems may reveal arteriosclerotic changes in the retinal microvasculature. For instance, signs of retinopathy assessed with the Scheie classification in fundus photographs were associated with future onset of hypertension in a huge population of 34,649 normotensive individuals without any CVD, after a mean follow-up period of 3.18 years [23]. However, qualitative evaluation is subjective and requires expertise. On the other hand, digital retinal photography enables the implementation of software to facilitate the quantitative assessment of morphological alterations, such as calculation of arteriolar and venular width, as presented in Fig. 2 [53, 54]. In more details, changes in the caliber of retinal arteries and veins can predict new onset of arterial hypertension. Results from a 10-year follow-up of 2451 normotensive individuals (Beaver Dam Study) showed that, regardless of classic CVD risk factors (age, smoking, lipid levels, diabetes, body mass index, and initial blood pressure levels), individuals with the lowest retinal arteriole-to-venule ratio (AVR) (1st quadrant), compared with those with the highest (4th quadrant), were 80% more likely to develop high blood pressure [55]. This relationship was also confirmed when the AVR was studied as a continuous variable, with a 30% increase in hypertension for each decrease of a standard deviation (SD: 0.07) of the AVR. In addition, the same study showed that the linear relationship of blood pressure values with arteriolar width and AVR was valid for healthy individuals and comprised an independent important prognostic factor in the development of hypertension.
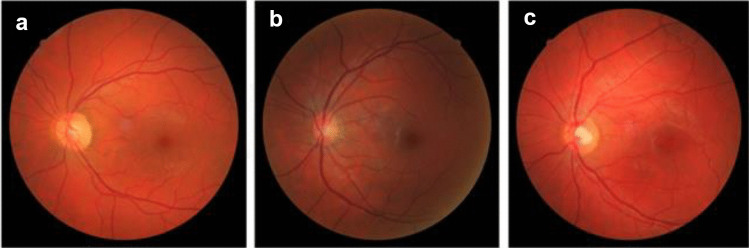
Fig. 2.
Subtle alterations of the retinal arteriolar and venular width can be calculated from digital retinal photography by use of appropriately designed software and have been associated with increased risk of future hypertension onset in multitudinal prospective studies. a Obtained from a 38-year-old normotensive male, arteriolar and venular widths are almost equal corresponding to arteriovenous ratio (AVR) of 1.04. b Obtained from a 32-year-old healthy normotensive female, retinal arteriolar narrowing is evident (AVR 0.69). These changes are remarkably similar to those observed in established hypertension, as shown in c from a 32-year-old hypertensive male (AVR 0.725). Figure obtained and provided by authors (ESH Excellent Center: Hypertension Division of the Third Department of Internal Medicine, Aristotle University of Thessaloniki, Papageorgiou General Hospital, Thessaloniki)
Concordant results emerge from a 3-year follow-up of 5628 normotensives (49 to 73 years old) [56] and a 5-year follow-up of a normotensive Japanese population (Funagata study—1058 participants > 35 years old) [57], as well as from a 7-year follow-up of an older normotensive population (Rotterdam study—1900 participants ≥ 55 years old) [58]. According to these studies, narrowing of the retinal arteries in initially normotensive individuals was significantly associated with an increased incidence of hypertension in the future. In the Multi-Ethnic Study of Atherosclerosis [59] that included 2583 normotensive participants without a history of clinical CVD, not only narrower retinal arteries (referred to as central retinal artery equivalent) but also wider retinal veins (referred to as central retinal vein equivalent) were also identified as independent risk factors for future development of hypertension. Likewise, the meta-analysis by Ding et al. including six population-based studies showed that both narrowing of the retinal arterioles (OR per 20 μm difference in diameter 1.29, 95% CI 1.20–1.39) and venular widening (OR per 20 μm difference in diameter 1.14, 95% CI 1.06–1.23) were significant predictors of future onset of hypertension among 10,229 individuals without prevalent hypertension, diabetes, or CVD [60].
More recent studies have tested the efficacy of novel computerized approaches to predict of hypertension and CVD outcomes. Complementary machine learning-based assessment of the retinal vasculature with phenome-wide and genome-wide analyses was leveraged across 97,895 retinal fundus images from 54,813 UK Biobank participants. Vascular density and fractal dimension as a measure of vascular branching complexity were calculated using convolutional neural networks to segment the retinal microvasculature. Both were significantly associated with a higher risk for incident mortality, hypertension, congestive heart failure, renal failure, type 2 diabetes, sleep apnea, anemia, and multiple ocular conditions epidemiologically, while lower microvascular density was further associated with genetically higher risk for hypertension and diabetes [61••]. Though promising, the advantages of applying deep-learning techniques in the retina need to be counterbalanced over feasibility for incorporation into clinical screening procedures.
Alterations of the Skin Microcirculation
The skin represents an easily accessible vascular bed, and as such, several techniques have been developed for the study of skin microcirculation. For instance, traditional laser Doppler techniques (laser Doppler imaging and laser Doppler flowmetry) that evaluate and quantify red cell flux in small areas of tissue, and more recently the laser speckle contrast imaging, have been applied to document altered microvascular reactivity in systemic diseases such as autoimmune rheumatic disorders and hypertension [62–64, 65•]. Nevertheless, these techniques have not yet been applied in initially normotensive individuals to evaluate prospectively their potential value in terms of CVD risk prediction.
Capillaroscopy is another non-invasive technique that can visualize by use of a stereo microscope the capillary network of the studied organ, usually the skin of the dorsum of the nail of the upper extremities. Nailfold capillaroscopy has been primarily used in rheumatology for the diagnosis and monitoring of autoimmune rheumatic diseases. It can provide valuable information for multiple microcirculatory parameters, including the morphology and density of the capillary network, under the influence of various systemic diseases [66]. Using nailfold capillaroscopy, reduced number of skin capillaries can be detected, which is known as capillary rarefaction (Fig. 3) and represents a common alteration found in hypertensive patients [67], as well as in other high CVD risk populations [68].
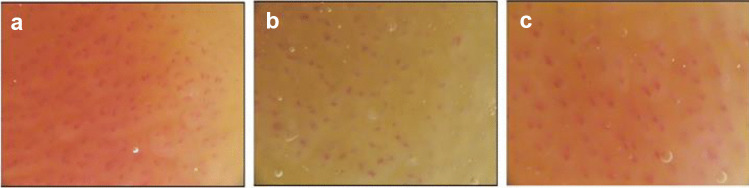
Fig. 3.
Nailfold capillaroscopy provides valuable information on capillary rarefaction as an index of peripheral microangiopathy. Skin capillary network appears dense in a 48-year-old normotensive female in Fig. 1a. By contrast, capillary rarefaction is evident in a 46-year-old normotensive male presented in Fig. 1b, despite the absence of established hypertension, as is the case in Fig. 1c (48-year-old hypertensive male). Figure obtained and provided by authors (ESH Excellent Center: Hypertension Division of the Third Department of Internal Medicine, Aristotle University of Thessaloniki, Papageorgiou General Hospital, Thessaloniki)
Nevertheless, the predictive role of capillary rarefaction for the development of hypertension in normotensive individuals has not been investigated to date with prospective studies. Relevant data come from cross-sectional studies in patients with hypertension [69] and in healthy individuals with a family history of hypertension [70, 71], suggesting that capillary rarefaction may be preexisting and could be partly responsible for the increased peripheral resistance preceding the establishment of arterial hypertension itself. A proposed pathophysiological mechanism underlying capillary rarefaction among normotensives involves metabolic abnormalities. Serne and colleagues [72], using the euglycemic clamp, showed a significant correlation between insulin resistance and capillary density in normotensive individuals (n = 18). This correlation of insulin resistance, however, was not confirmed in a larger (n = 105) group of healthy individuals, using the HOMA index, an indirect and less accurate way of assessing insulin resistance compared to the euglycemic clamp and only fasting glucose correlated with capillary rarefaction [73]. Finally, a study in 66 normotensives examining the potential relationship between impaired metabolic profile and capillary rarefaction reported a significant association between high-density lipoprotein levels and capillary density [74].
Gaps in Knowledge and Future Perspectives
Evaluation of arterial stiffness, carotid atherosclerosis, and retinal and skin microvascular changes is non-invasive, prompt, and feasible in routine clinical practice. Nevertheless, these measures are not routinely applied as part of the general check-up of healthy individuals. First, these methods necessitate expertise and relevant infrastructure, although once obtained consumables are not required. Second, with the exception of arterial stiffness measures and urine albumin excretion (UAE), methodological standardization remains the Achilles’ heel. This is particularly true for cIMT despite several decades of experience, which is therefore not recommended by current European Society of Cardiology guidelines on CVD prevention in clinical practice [75]. While several research centers or groups have developed software for the evaluation of retinal and skin microvascular alterations from digital fundus photographs and nailfold capillaroscopic images, respectively, the need for universal software and protocols emerges as extremely important. Third, the development of diagnostic thresholds remains troublesome especially for retinal and skin microvascular alterations. While cut-off levels have been developed for PWV, cIMT, and UAE to discriminate abnormal values, there is increasing awareness of the linear association with CVD risk even beyond “normal” levels, which may vary further according to age and sex.
Most importantly, the added value of the aforementioned subclinical vascular alterations in predicting future CVD events in low- and intermediate-risk individuals with normal blood pressure levels needs to be clarified in appropriately conducted prospective studies. There is evidence suggesting that accumulation of subclinical vascular alterations may be linked with increased CVD risk, but this needs to be verified in prospective cohort studies [76]. Finally, the implementation of ambulatory blood pressure measurements may be necessary to accurately diagnose normotension and exclude individuals with masked hypertension [77], who may already present signs of HMOD and altered vascular function and morphology [65•, 78].
Conclusion
A growing amount of prospectively obtained data supports that altered morphology and function of the micro- and microvasculature predict the development of new-onset hypertension in normotensive individuals. Specifically, arterial stiffness, increased cIMT and UAE, and altered retinal microvascular diameters predict the progression from the normotensive to the hypertensive state, whereas there is substantial lack of relevant prospective evidence for skin microvascular alterations. Although research reports suggest that such alterations precede and predispose to hypertension, through the remodeling of blood vessels and the increase in vascular resistance, conclusions regarding causality effects cannot be safely driven from available studies. Thus, identification of subtle vascular alterations in normotensive individuals could serve as a sensitive indicator of future hypertension onset and hence an increased CVD risk. It could be reasonably assumed that in these individuals, closer monitoring for the timely diagnosis of hypertension, intensification of lifestyle interventions, and perhaps early initiation of medical treatment would alleviate the global CVD burden. Appropriately designed longitudinal studies are eagerly warranted to delineate the potential role of subtle vascular alterations in primary CVD prevention.
Funding
Open access funding provided by HEAL-Link Greece.
Compliance with Ethical Standards
Conflict of Interest
The authors declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Dagenais GR, Leong DP, Rangarajan S, et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2020. 10.1016/S0140-6736(19)32007-0. [DOI] [PubMed]
- 2.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007. 10.1056/NEJMsa053935. [DOI] [PubMed]
- 3.Joseph P, Leong D, McKee M, et al. Reducing the global burden of cardiovascular disease, part 1. Circ Res. 2017. 10.1161/CIRCRESAHA.117.308903.
- 4.Gkaliagkousi E, Gavriilaki E, Triantafyllou A, Douma S. Clinical significance of endothelial dysfunction in essential hypertension. Curr Hypertens Rep. 2015. 10.1007/s11906-015-0596-3. [DOI] [PubMed]
- 5.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. J Hypertens. 2018. 10.1097/HJH.0000000000001940.
- 6.Perera GA. Hypertensive vascular disease; description and natural history. J Chronic Dis. 1955. 10.1016/0021-9681(55)90019-9. [DOI] [PubMed]
- 7.Gkaliagkousi E, Douma S. The pathogenesis of arterial stiffness and its prognostic value in essential hypertension and cardiovascular diseases. Hippokratia. 2009;13(2):70–75. [PMC free article] [PubMed] [Google Scholar]
- 8.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness. J Am Coll Cardiol. 2010. 10.1016/j.jacc.2009.10.061. [DOI] [PubMed]
- 9.Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M, et al. Arterial stiffness and the development of hypertension. The ARIC study Hypertension. 1999;34(2):201–206. doi: 10.1161/01.HYP.34.2.201. [DOI] [PubMed] [Google Scholar]
- 10.Najjar SS, Scuteri A, Shetty V, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008. 10.1016/j.jacc.2007.10.065.Pulse. [DOI] [PMC free article] [PubMed]
- 11.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D et al. Aortic stiffness, blood pressure progression, and incident hypertension. J Am Med Assoc. 2012. 10.1001/2012.jama.10503.Aortic. [DOI] [PMC free article] [PubMed]
- 12.Lee SJ, Avolio A, Seo DC, et al. Relationship between brachial-ankle pulse wave velocity and incident hypertension according to 2017 ACC/AHA high blood pressure guidelines. J Am Heart Assoc. 2019. 10.1161/JAHA.119.013019. [DOI] [PMC free article] [PubMed]
- 13.Jiang Y, Fan F, Jia J, et al. Brachial-ankle pulse wave velocity predicts new-onset hypertension and the modifying effect of blood pressure in a Chinese community-based population. Int J Hypertens. 2020. 10.1155/2020/9075636. [DOI] [PMC free article] [PubMed]
- 14.•• Koivistoinen T, Lyytikäinen LP, Aatola H, et al. Pulse wave velocity predicts the progression of blood pressure and development of hypertension in young adults. Hypertension. 2018. 10.1161/HYPERTENSIONAHA.117.10368. Inclusion of pulse wave velocity in prediction models improved the incident hypertension risk prediction beyond traditional cardiovascular risk factors, suggesting that pulse wave velocity could be used as a valuable tool towards hypertension risk prediction in young adults. [DOI] [PubMed]
- 15.Satoh H, Saijo Y, Kishi R, Tsutsui H. Brachial-ankle pulse wave velocity is an independent predictor of incident hypertension in Japanese normotensive male subjects. Environ Health Prev Med. 2011. 10.1007/s12199-010-0189-3. [DOI] [PMC free article] [PubMed]
- 16.Kim SH, Kim YH, Kim JS, et al. Target-organ damage and incident hypertension: the Korean genome and epidemiology study. J Hypertens. 2016.; 10.1097/HJH.0000000000000836. [DOI] [PubMed]
- 17.Zheng X, Jin C, Liu Y, et al. Arterial stiffness as a predictor of clinical hypertension. J Clin Hypertens. 2015. 10.1111/jch.12556. [DOI] [PMC free article] [PubMed]
- 18.Takase H, Dohi Y, Toriyama T, et al. Brachial-ankle pulse wave velocity predicts increase in blood pressure and onset of hypertension. Am J Hypertens. 2011.10.1038/ajh.2011.19. [DOI] [PubMed]
- 19.Yambe M, Tomiyama H, Yamada J, et al. Arterial stiffness and progression to hypertension in Japanese male subjects with high normal blood pressure. J Hypertens. 2007. 10.1097/01.hjh.0000254375.73241.e2. [DOI] [PubMed]
- 20.Tomiyama H, Matsumoto C, Yamada J, et al. Predictors of progression from prehypertension to hypertension in Japanese men. Am J Hypertens. 2009. 10.1038/ajh.2009.49. [DOI] [PubMed]
- 21.Peralta CA, Adeney KL, Shlipak MG, et al. Structural and functional vascular alterations and incident hypertension in normotensive adults: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2010. 10.1093/aje/kwp319. [DOI] [PMC free article] [PubMed]
- 22.Dernellis J, Panaretou M. Aortic stiffness is an independent predictor of progression to hypertension in nonhypertensive subjects. Hypertension. 2005. 10.1161/01.HYP.0000157818.58878.93. [DOI] [PubMed]
- 23.Kazuomi Kario, Hiroshi Kanegae, Takamitsu Oikawa KS. Hypertension is predicted by both large and small artery disease. Hypertension. 2019. 10.1161/HYPERTENSIONAHA.118.11800. [DOI] [PubMed]
- 24.Hong YM. Atherosclerotic cardiovascular disease beginning in childhood. Korean Circ J. 2010. 10.4070/kcj.2010.40.1.1. [DOI] [PMC free article] [PubMed]
- 25.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2016. 10.1093/eurheartj/ehw106. [DOI] [PubMed]
- 26.• Song P, Fang Z, Wang H, et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob Heal. 2020. 10.1016/S2214-109X(20)30117-0. This report highlights the global burden of carotid atherosclerosis, which is largely dependant on common cardiovascular risk factors such as smoking, diabetes, and hypertension. [DOI] [PubMed]
- 27.O’Leary DH, Bots ML. Imaging of atherosclerosis: carotid intima-media thickness. Eur Heart J. 2010. 10.1093/eurheartj/ehq185. [DOI] [PubMed]
- 28.Zhang L, Fan F, Qi L, et al. The association between carotid intima-media thickness and new-onset hypertension in a Chinese community-based population. BMC Cardiovasc Disord. 2019. 10.1186/s12872-019-1266-1. [DOI] [PMC free article] [PubMed]
- 29.Takase H, Sugiura T, Murai S, Yamashita S, Ohte N, Dohi Y. Carotid intima-media thickness is a novel predictor of new onset of hypertension in normotensive subjects. Med (United States). 2017. 10.1097/MD.0000000000007710. [DOI] [PMC free article] [PubMed]
- 30.Cuomo S, Gaeta G, Guarini P, et al. Increased carotid intima-media thickness in healthy young subjects with a parental history of hypertension (parental hypertension and vascular health). Heart. 2007. 10.1136/hrt.2006.091769. [DOI] [PMC free article] [PubMed]
- 31.Yildirim A, Kosger P, Ozdemir G, Sahin FM, Ucar B, Kilic Z. Carotid intima-media thickness and elastic properties of aortas in normotensive children of hypertensive parents. Hypertens Res. 2015. 10.1038/hr.2015.49. [DOI] [PubMed]
- 32.Bakris G. Microalbuminuria: prognostic implications. Curr Opin Nephrol Hypertens. 1996. 10.1097/00041552-199605000-00006. [DOI] [PubMed]
- 33.Williams B, Mancia G, Spiering W, et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension ESC/ESH Task Force for the management of arterial hypertension. 2018;36. 10.1097/HJH.0000000000001961.
- 34.Jones CA, Francis ME, Eberhardt MS, et al. Microalbuminuria in the US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2002. 10.1053/ajkd.2002.31388. [DOI] [PubMed]
- 35.Bakris GL. Clinical importance of microalbuminuria in diabetes and hypertension. Curr Hypertens Rep. 2004. 10.1007/s11906-004-0053-1. [DOI] [PubMed]
- 36.Jackson CE, Solomon SD, Gerstein HC, et al. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet. 2009. 10.1016/S0140-6736(09)61378-7. [DOI] [PubMed]
- 37.Redon J, Martinez F. Microalbuminuria as surrogate endpoint in therapeutic trials. Curr Hypertens Rep. 2012. 10.1007/s11906-012-0270-y. [DOI] [PubMed]
- 38.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010. 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed]
- 39.Wang TJ, Evans JC, Meigs JB, et al. Low-grade albuminuria and the risks of hypertension and blood pressure progression. Circulation. 2005. 10.1161/01.CIR.0000158434.69180.2D. [DOI] [PubMed]
- 40.•• Zhang M, Jiang Y, Zhang Q, et al. Bidirectional and temporal association between hypertension and microalbuminuria: a longitudinal study in Chinese adults. J Am Heart Assoc. 2018. 10.1161/JAHA.118.010723. In this logitudinal cohort, a significant bidirectional association was found between microalbuminuria and hypertension, although elevated urinary albumin excretion was more likely to precede hypertension compared to the other direction of the relationship of baseline blood pressure to follow-up urinary albumin. [DOI] [PMC free article] [PubMed]
- 41.Sung KC, Ryu S, Lee JY, et al. Urine albumin/creatinine ratio below 30 mg/g is a predictor of incident hypertension and cardiovascular mortality. J Am Heart Assoc. 2016. 10.1161/JAHA.116.003245. [DOI] [PMC free article] [PubMed]
- 42.Takase H, Sugiura T, Ohte N, Dohi Y. Urinary albumin as a marker of future blood pressure and hypertension in the general population. Med (United States). 2015. 10.1097/MD.0000000000000511. [DOI] [PMC free article] [PubMed]
- 43.Yadav D, Kang DR, Koh SB, Kim JY, Ahn SV. Association between urine albumin-to-creatinine ratio within the normal range and incident hypertension in men and women. Yonsei Med J. 2016. 10.3349/ymj.2016.57.6.1454. [DOI] [PMC free article] [PubMed]
- 44.Park SK, Moon SY, Oh CM, Ryoo JH, Park MS. High normal urine albumin-to-creatinine ratio predicts development of hypertension in Korean men. Circ J. 2014. 10.1253/circj.CJ-13-0745. [DOI] [PubMed]
- 45.Forman JP, Fisher NDL, Schopick EL, Curhan GC. Higher levels of albuminuria within the normal range predict incident hypertension. J Am Soc Nephrol. 2008. 10.1681/ASN.2008010038. [DOI] [PMC free article] [PubMed]
- 46.Jessani S, Levey AS, Chaturvedi N, Jafar TH. High normal levels of albuminuria and risk of hypertension in Indo-Asian population. Nephrol Dial Transplant. 2012. 10.1093/ndt/gfr200. [DOI] [PMC free article] [PubMed]
- 47.Hirayama A, Konta T, Hozawa A, et al. Slight increase in urinary albumin excretion within the normal range predicts incident hypertension in a community-based Japanese population: the Takahata study. Hypertens Res. 2015. 10.1038/hr.2014.117. [DOI] [PubMed]
- 48.Munakata M, Hattori T, Konno S. Relationship between subtle urinary albumin excretion and risk of incident hypertension: modification by glomerular filtration rate. Hypertens Res. 2017. 10.1038/hr.2017.77. [DOI] [PMC free article] [PubMed]
- 49.Ärnlöv J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham heart study. Circulation. 2005;https://doi:10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed]
- 50.Tanaka F, Komi R, Makita S, et al. Low-grade albuminuria and incidence of cardiovascular disease and all-cause mortality in nondiabetic and normotensive individuals. J Hypertens. 2016. 10.1097/HJH.0000000000000809. [DOI] [PubMed]
- 51.Park HE, Heo NJ, Kim M, Choi SY. Significance of microalbuminuria in relation to subclinical coronary atherosclerosis in asymptomatic nonhypertensive, nondiabetic subjects. J Korean Med Sci. 2013. 10.3346/jkms.2013.28.3.409. [DOI] [PMC free article] [PubMed]
- 52.Liew G, Wang JJ, Mitchell P, Wong TY. Retinal vascular imaging: a new tool in microvascular disease research. Circ Cardiovasc Imaging. 2008. 10.1161/CIRCIMAGING.108.784876. [DOI] [PubMed]
- 53.Zabulis, X., Triantafyllou, A., Karamaounas, P., Zamboulis, C., Douma, S. (2014). An image analysis system for the assessment of retinal microcirculation in hypertension and its clinical evaluation. In: Roa Romero, L. (eds) XIII Mediterranean Conference on Medical and Biological Engineering and Computing 2013. IFMBE Proceedings, vol 41. Springer, Cham. 10.1007/978-3-319-00846-2_82.
- 54.Hernandez-Matas C, Zabulis X, Triantafyllou A, Anyfanti P, Argyros AA. Retinal image registration under the assumption of a spherical eye. Comput Med Imaging Graph. 2017. 10.1016/j.compmedimag.2016.06.006. [DOI] [PubMed]
- 55.Wong TY, Shankar A, Klein R, Klein BEK, Hubbard LD. Prospective cohort study of retinal vessel diameters and risk of hypertension. Br Med J. 20040. 10.1136/bmj.38124.682523.55. [DOI] [PMC free article] [PubMed]
- 56.Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar diameter and risk for hypertension. Ann Intern Med. 2004. 10.7326/0003-4819-140-4-200402170-00006. [DOI] [PubMed]
- 57.Tanabe Y, Kawasaki R, Wang JJ, et al. Retinal arteriolar narrowing predicts 5-year risk of hypertension in Japanese people: the Funagata study. Microcirculation. 2010. 10.1111/j.1549-8719.2009.00006.x. [DOI] [PubMed]
- 58.Ikram MK, Witteman JC, Vingerling JR, Breteler MM, Hofman A de JP. Retinal vessel diameters and risk of hypertension: the Rotterdam study. J Hypertens. 2006. 10.1097/HJH.0b013e3283310f7e. [DOI] [PubMed]
- 59.Kawasaki R, Cheung N, Wang JJ, et al. Retinal vessel diameters and risk of hypertension: The Multiethnic Study of Atherosclerosis. J Hypertens. 2009. 10.1097/HJH.0b013e3283310f7e. [DOI] [PMC free article] [PubMed]
- 60.Ding J, Wai KL, McGeechan K, et al. Review: Retinal vascular caliber and the development of hypertension: a meta-analysis of individual participant data. J Hypertens. 2014. 10.1097/HJH.0b013e32836586f4. [DOI] [PMC free article] [PubMed]
- 61.•• Zekavat SM, Raghu VK, Trinder M, et al. Deep learning of the retina enables phenome- and genome-wide analyses of the microvasculature. Circulation. 2022. 10.1161/CIRCULATIONAHA.121.057709. According to this study, assessment of the retinal microvasculature from retinal fundosopy may be utilized in large-scale complementary machine learning-based alogrithms that combine phenome-wide and genome-wide analyses. Deep learning of images may yield new insights into human health and disease through improvements in risk prediction and risk stratification models. [DOI] [PMC free article] [PubMed]
- 62.Margouta A, Anyfanti P, Lazaridis A, et al. Blunted microvascular reactivity in psoriasis patients in the absence of cardiovascular disease , as assessed by laser speckle contrast imaging. Life. 2022. 10.3390/life12111796. [DOI] [PMC free article] [PubMed]
- 63.Dolgyras P, Lazaridis A, Anyfanti P, et al. Microcirculation dynamics in systemic vasculitis: evidence of impaired microvascular response regardless of cardiovascular risk factors. Rheumatology. 2022. 10.1093/rheumatology/keac652. [DOI] [PubMed]
- 64.Koletsos N, Gkaliagkousi E, Lazaridis A, et al. Skin microvascular dysfunction in systemic lupus erythematosus patients with and without cardiovascular risk factors. Rheumatology. 2021. 10.1093/rheumatology/keaa722. [DOI] [PubMed]
- 65.• Lazaridis A, Triantafyllou A, Dipla K, et al. Skin microvascular function, as assessed with laser speckle contrast imaging, is impaired in untreated essential and masked hypertension. Hypertens Res. 2022. 10.1038/s41440-021-00816-w. Altered skin microcirculation dynamics, as assessed with laser speckle contrast imaging coupled with post-occlusive reactive hyperemia, may differentiate untreated essential and masked hypertensives from normotensives. This observation further reinforces the notion of a significant role of microcirculation in the pathogenesis of hypertension. [DOI] [PubMed]
- 66.Anyfanti P, Angeloudi E, Dara A, et al. Nailfold videocapillaroscopy for the evaluation of peripheral microangiopathy in rheumatoid arthritis. Life. 2022. 10.3390/life12081167. [DOI] [PMC free article] [PubMed]
- 67.Triantafyllou A, Anyfanti P, Pyrpasopoulou A, Triantafyllou G, Aslanidis S, Douma S. Capillary rarefaction as an index for the microvascular assessment of hypertensive patients. Curr Hypertens Rep. 2015. 10.1007/s11906-015-0543-3. [DOI] [PubMed]
- 68.Anyfanti P, Gkaliagkousi E, Triantafyllou A, et al. Dermal capillary rarefaction as a marker of microvascular damage in patients with rheumatoid arthritis: association with inflammation and disorders of the macrocirculation. Microcirculation. 2018. 10.1111/micc.12451. [DOI] [PubMed]
- 69.Antonios TFT, Singer DRJ, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in borderline essential hypertension suggests an early structural abnormality. Hypertension. 1999. 10.1161/01.HYP.34.4.655. [DOI] [PubMed]
- 70.Antonios TFT, Rattray FM, Singer DRJ, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in normotensive offspring of individuals with essential hypertension. Heart. 2003. 10.1136/heart.89.2.175. [DOI] [PMC free article] [PubMed]
- 71.Noon JP, Walker BR, Webb DJ, et al. Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J Clin Invest. 1997. 10.1172/JCI119354. [DOI] [PMC free article] [PubMed]
- 72.Serné EH, Gans ROB, Ter Maaten JC, Ter Wee PM, Donker AJM, Stehouwer CDA. Capillary recruitment is impaired in essential hypertension and relates to insulin’s metabolic and vascular actions. Cardiovasc Res. 2001. 10.1016/S0008-6363(00)00198-X. [DOI] [PubMed]
- 73.Irving RJ, Walker BR, Noon JP, Watt GCM, Webb DJ, Shore AC. Microvascular correlates of blood pressure, plasma glucose, and insulin resistance in health. Cardiovasc Res. 2002. 10.1016/S0008-6363(01)00450-3. [DOI] [PubMed]
- 74.Triantafyllou A, Anyfanti P, Triantafyllou G, Zabulis X, Aslanidis S, Douma S. Impaired metabolic profile is a predictor of capillary rarefaction in a population of hypertensive and normotensive individuals. J Am Soc Hypertens. 2016. 10.1016/j.jash.2016.04.007. [DOI] [PubMed]
- 75.Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021. 10.1093/eurheartj/ehab484. [DOI] [PubMed]
- 76.Triantafyllou A, Anyfanti P, Zabulis X, et al. Accumulation of microvascular target organ damage in newly diagnosed hypertensive patients. J Am Soc Hypertens. 2014. 10.1016/j.jash.2014.04.008. [DOI] [PubMed]
- 77.Triantafyllou A, Anyfanti P, Douma S. Retinal arteriolar diameters and incident hypertension in initially normotensive individuals: a masked hypertension effect? J Hypertens. 2014. 10.1097/HJH.0000000000000225. [DOI] [PubMed]
- 78.Triantafyllou A, Doumas M, Anyfanti P, et al. Divergent retinal vascular abnormalities in normotensive persons and patients with never-treated, masked, white coat hypertension. Am J Hypertens. 2013. 10.1093/ajh/hps040. [DOI] [PubMed]