Abstract
In this report we demonstrate the utility of an monoclonal antibody inhibition enzyme-linked immunosorbent assay based on the Mycobacterium leprae 35-kDa protein, purified from the rapidly growing host Mycobacterium smegmatis, for the serodiagnosis of multibacillary leprosy. The assay proved highly specific (97.5%) and sensitive (90%) and compared favorably with two other established methods routinely utilized for leprosy serodiagnosis.
The development of improved specific diagnostic reagents to detect infection with Mycobacterium leprae and to monitor the effectiveness of community control programs is a major priority of leprosy control strategies (1, 5). Ideally, diagnostic reagents should detect all forms of leprosy, ranging from the tuberculoid or paucibacillary (PB) form, characterized by strong cell-mediated immunity (CMI) to M. leprae, to the lepromatous or multibacillary (MB) form, with weak CMI to M. leprae and a high level of antibody formation (2). This implies that a combination of tests will be required for the efficient detection of leprosy, i.e., a specific skin test for the detection of CMI to M. leprae and a serological assay for the detection of anti-M. leprae antibodies. Previous studies have established that assays detecting the monoclonal antibody (MAb) MLO4 epitope of the M. leprae 35-kDa protein using M. leprae sonicate (MLS) are specific and sensitive for the serodiagnosis of leprosy. Antibodies to the 35-kDa protein have been detected in up to 100% of untreated lepromatous patients but are generally absent from the sera of tuberculosis patients and control subjects (7, 10, 12, 14), while levels of anti-35-kDa protein antibodies correlate strongly with the antigenic load and decline with effective chemotherapy (3, 6, 9). One limiting factor of the assay is the requirement for MLS, which must be prepared from M. leprae purified from infected armadillos as M. leprae still cannot be cultivated in vitro. When the gene encoding the M. leprae 35-kDa protein was expressed at high levels in Mycobacterium smegmatis and recombinant product was purified, the antigen exhibited properties suggesting its potential as a leprosy-specific diagnostic tool (15, 16). The protein was specifically recognized by the immune responses of the large majority of leprosy patients tested, while strong delayed-type hypersensitivity was elicited by the recombinant 35-kDa protein in M. leprae-sensitized guinea pigs but not in Mycobacterium tuberculosis- or Mycobacterium bovis BCG-sensitized animals. Furthermore, only the form of the antigen derived from recombinant M. smegmatis, and not that purified from Escherichia coli, retained conformational determinants and was recognized by leprosy sera. Therefore, we have assessed the suitability of the purified recombinant M. leprae 35-kDa protein for the routine serological diagnosis of leprosy.
Sera were obtained from 60 MB and 30 PB leprosy patients, previously untreated, who were diagnosed according to the Ridley-Jopling classification (8) at Anandaban Leprosy Hospital, Kathmandu, Nepal. Sera from 50 clinically well health care workers in Nepal served as the endemic control group. The tuberculosis (TB) group consisted of sera from 15 patients from Nepal with active, smear-positive, radiologically confirmed pulmonary TB (endemic TB) and sera from 15 patients from Royal Prince Alfred Hospital, Sydney, Australia, with culture-proven pulmonary TB, who had not been exposed to leprosy (nonendemic TB).
The M. leprae 35-kDa protein was purified from the sonicate of recombinant M. smegmatis transformed with pWL19 as previously described (15). Antibodies to the 35-kDa protein were detected by three methods. The first was a MAb inhibition enzyme-linked immunosorbent assay (ELISA), as initially described elsewhere (13), using a 10-μg/ml concentration of MLS and the MAb MLO4-peroxidase conjugate supplied by J. Ivanyi (MRC Unit for Tuberculosis and Related Infections, Hammersmith Hospital, London, United Kingdom). The dilution of sera causing 50% inhibition of binding of MLO4 to MLS compared to the maximum binding in the absence of serum (ID50) was calculated, and samples with ID50s greater than 10 were considered positive. Previous studies (10, 12, 14) had confirmed that this level discriminates between leprosy patients and endemic control subjects. The second assay was a similar MAb inhibition assay except that the coating antigen was the purified 35-kDa protein (r35 kDa-MIA). In initial optimization experiments, the protein was tested over a concentration range of 0.1 to 10 μg/ml. All sera were subsequently tested with a 0.5-μg/ml concentration of antigen. The final assay detected antibodies to the 35-kDa protein by direct ELISA, with the purified 35-kDa protein used at a concentration of 10 μg/ml and patients’ sera diluted 1 in 100. Samples with A405s greater than 0.42, which was the mean of 50 control serum samples plus 2 standard deviations, were considered positive. Immunoglobulin M antiphenolic glycolipid-I antibodies (PGL-I) were measured by direct ELISA, with the dissacharide bovine serum albumin glucoconjugate (provided by M. J. Colston, Laboratory for Leprosy and Mycobacterial Research, National Institute for Medical Research, London, United Kingdom) used at a concentration of 250 ng/ml and patients’ sera diluted 1 in 100. Samples with A405s greater than 0.46, which was the mean of 50 control serum samples plus 2 standard deviations, were considered positive.
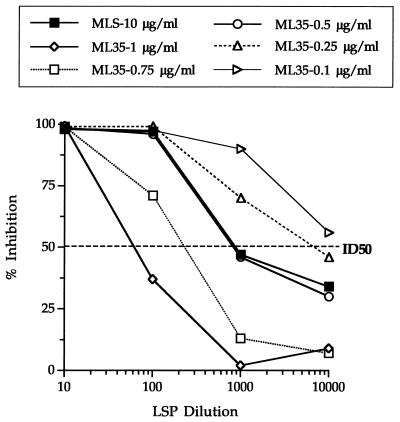
The most suitable concentration of the purified recombinant 35-kDa protein for use in the MLO4 inhibition assay was determined. This was achieved by comparing different concentrations of the 35-kDa protein with a 10-μg/ml concentration of MLS in the inhibition assay by using a pool of sera from 10 previously untreated, lepromatous leprosy patients. As shown in Fig. 1, the titer causing ID50 using the recombinant protein at a concentration of 0.5 μg/ml was closest to the ID50 obtained at 10 μg/ml of MLS. Concentrations greater than 0.5 μg/ml of recombinant protein did not allow sufficient inhibition to provide adequate discrimination between test and control samples, whereas concentrations less than 0.5 μg/ml did not allow sufficient binding of anti-35-kDa protein antibodies.
FIG. 1.
Optimization of the r35 kDa-MIA for the detection of anti-M. leprae 35-kDa protein antibodies. Results are expressed as percentages of inhibition of binding of the MAb MLO4-peroxidase conjugate to the protein compared to the maximum binding in the absence of serum. The serum titer causing 50% inhibition of maximum binding of MAb MLO4 (ID50) is represented by the horizontal dashed line. LSP, pooled sera from 10 lepromatous leprosy patients.
The levels of anti-35 kDa protein and anti-PGL-I antibodies were assayed in the sera of all subjects (Table 1). Assessment of anti-PGL-I antibodies is the most widely used assay for the detection of anti-M. leprae antibodies and thus served as a useful comparison for the efficiency of the 35-kDa protein assays. The inhibition assay utilizing 0.5 μg of the purified M. leprae 35-kDa protein (r35 kDa-MIA) exhibited a high degree of sensitivity for MB leprosy, with 54 of 60 patients (90%) testing seropositive. A lower proportion of the PB leprosy patients (5 of 30, 17%) were seropositive, all with low antibody titers (Table 1). The standard MLS-MLO4 inhibition assay (MLS-MIA) demonstrated similar sensitivities for MB (90%) and PB (17%) leprosy. Mean positive titers were also lower in the PB than the MB group. The direct assay incorporating the 35-kDa protein was less sensitive than the inhibitory assay utilizing the 35-kDa protein for MB leprosy, with 83% of patients demonstrating positive antibody titers, although this difference was not statistically significant. The PGL-I assay detected a lower, although not statistically different, proportion of MB patients (85%), while a slightly higher percentage of PB patients were seropositive (Table 1).
TABLE 1.
Comparison of three serological assays to detect anti-M. leprae 35-kDa protein antibodies and an assay for immunoglobulin M anti-PGL-I antibodies in leprosy and tuberculosis patients and control subjects
| Patient group | n | % Seropositive (mean positive titer or absorbance) bya:
|
|||
|---|---|---|---|---|---|
| MLS-MIA | r35 kDa-MIA | 35 kDa direct ELISA | PGL-I direct ELISA | ||
| Endemic control | 50 | 6 (133) | 2 (76) | 4 (0.200) | 2 (0.211) |
| Endemic TB | 15 | 6.7 (20) | 6.7 (32) | 13.4 (0.243) | 0 (0.173) |
| Nonendemic TB | 15 | 0 (0) | 0 (0) | 0 (0.193) | 0 (0.130) |
| MB | 60 | 90** (1,526) | 90** (1,421) | 83** (0.896) | 85** (1.058) |
| PB | 30 | 17 (188) | 17* (88) | 17 (0.270) | 28** (0.333) |
Values in parentheses for MLS-MIA and r35 kDa-MIA are mean positive titers. Values in parentheses for the direct 35 kDa and PGL-I assays are mean absorbances (A405). Significant differences in the proportions of positive responders compared to endemic controls were determined by chi-square test for each assay. *, P < 0.05; **, P < 0.001.
The relative specificities of all four assays were analyzed by detecting the proportions of seropositive individuals in three control groups: endemic individuals, endemic TB patients, and nonendemic TB patients. These results are represented in Table 1. When the data for all three groups were combined, the r35 kDa-MIA was highly specific (97.5%), with only 2 of 80 control individuals being seropositive. A similar level of specificity was observed for the PGL-I assay (98.8%). Both the direct 35-kDa protein assay and the MLS-MIA showed lower, yet not statistically different, levels of specificity (95%). In all cases, the only positive samples detected were from subjects from regions in which leprosy is endemic (both health workers and TB patients). The positive predictive values of the inhibition assay with the r35-kDa protein assay were 98% for MB and 83% for PB patients, while the negative predictive values were 89% and 66%, respectively, for MB and PB patients.
Monitoring of leprosy prevalence within a community is dependent on the development of tests which specifically detect all forms of leprosy. It is evident that diagnostic tests based exclusively on cell-mediated or humoral immunity to M. leprae will not fulfill such a requirement. It is most likely that a combination of such tests would be required to give wide coverage. This is highlighted by our recent study on the 35-kDa protein of M. leprae (15). The immune systems of over 90% of leprosy patients recognized this antigen, with most individuals making exclusively a cell-mediated or antibody response to the protein, irrespective of the clinical classification of the patient. In this report, we have evaluated the use of the 35-kDa protein purified from a fast-growing mycobacterial species in the serodiagnosis of leprosy. The protein used in the form of a MAb inhibition assay was highly sensitive and specific for detecting MB leprosy and compared favorably with two previously established tests for leprosy serological diagnosis. This is due to the recombinant protein purified from M. smegmatis retaining the structural characteristics of the native antigen (15). Indeed, previous studies comparing structure (15), function (17), and immunogenicity (4, 11) of recombinant proteins purified from mycobacterial host systems have demonstrated considerable advantages over the same protein purified from E. coli expression systems. The obvious benefit of the use of this antigen is its relative abundance and ease of purification compared to MLS or purified PGL-I. Whereas extraction of large quantities of M. leprae is a time-consuming process, recombinant M. smegmatis is a fast-growing organism producing relatively large amounts of recombinant 35-kDa protein (1 to 2 mg/liter of culture). Furthermore, far less protein is required for the r35 kDa-MIA than for the MLS-MIA.
The M. leprae 35-kDa protein is a major and specific target of the cellular immune response to M. leprae, inducing T-cell proliferation and gamma interferon secretion by leprosy patients and contacts but not by M. tuberculosis-infected individuals (15). The protein also elicits M. leprae-specific delayed-type hypersensitivity in mycobacterial-sensitized animals (15). This study shows that the same recombinant antigen can be utilized in a sensitive and specific assay of the humoral response to M. leprae. Thus, a combination of tests based on this single antigen may be of considerable benefit in both the diagnosis of clinical leprosy and the recognition of subclinical leprosy infection. Moreover, this study further illustrates the advantages of utilizing recombinant mycobacterial proteins derived from mycobacterial hosts, suggesting that refinement of such expression systems may prove beneficial for the specific diagnosis of other mycobacterial infections.
Acknowledgments
We are grateful for the assistance of the staff and patients of Anandaban Leprosy Hospital, Kathmandu, Nepal, which is fully supported by The Leprosy Mission International.
This work was supported by the National Health and Medical Research Council of Australia. J.T. was a recipient of an Australian Postgraduate Award and P.R. was a recipient of a University of Sydney Medical Foundation Research Fellowship.
REFERENCES
- 1.Brennan P J, Cho S-N, Klatser P R. Immunodiagnostics, including skin tests. Int J Lepr. 1996;64:S58–S62. [PubMed] [Google Scholar]
- 2.Britton W J. Immunology of leprosy. Trans R Soc Trop Med Hyg. 1993;46:508–514. doi: 10.1016/0035-9203(93)90066-y. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi V, Sinha S, Girder B K, Sengupta U. On the value of sequential serology with a Mycobacterium leprae specific antibody competition ELISA in monitoring leprosy chemotherapy. Int J Lepr. 1991;59:32–40. [PubMed] [Google Scholar]
- 4.Garbe T, Harris D, Vordermeier M, Lathigra R, Ivanyi J, Young D. Expression of the Mycobacterium tuberculosis 19-kilodalton antigen in Mycobacterium smegmatis: immunological analysis and evidence of glycosylation. Infect Immun. 1993;61:260–267. doi: 10.1128/iai.61.1.260-267.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupte M D. Research needs related to epidemiology and control: subclinical infection. Int J Lepr. 1996;64:S29–S37. [PubMed] [Google Scholar]
- 6.Mwatha J, Moreno C, Sengupta U, Sinha S, Ivanyi J. A comparative evaluation of serological assays for lepromatous leprosy. Lepr Rev. 1988;59:195–199. doi: 10.5935/0305-7518.19880024. [DOI] [PubMed] [Google Scholar]
- 7.Parkash O, Chaturvedi V, Girdhar B K, Sengutpta U. A study on performance of two serological assays for diagnosis of leprosy patients. Lepr Rev. 1995;66:26–30. doi: 10.5935/0305-7518.19950004. [DOI] [PubMed] [Google Scholar]
- 8.Ridley D S, Jopling W H. Classification of leprosy according to immunity. A five group system. Int J Lepr. 1966;34:255–273. [PubMed] [Google Scholar]
- 9.Roche P W, Failbus S S, Neupane K D, Theuvenet W J, Britton W J. Serological monitoring of the response to chemotherapy in leprosy patients. Int J Lepr. 1993;61:35–43. [PubMed] [Google Scholar]
- 10.Roche P W, Britton W J, Failbus S S, Williams D, Pradhan H M, Theuvenet W J. Operational value of serological measurements in multibacillary leprosy patients: clinical and bacteriological correlates of antibody responses. Int J Lepr. 1990;58:480–490. [PubMed] [Google Scholar]
- 11.Roche P W, Winter N, Triccas J A, Feng C, Britton W J. Expression of Mycobacterium tuberculosis MPT64 in recombinant M. smegmatis: purification, immunogenicity and application to skin tests for tuberculosis. Clin Exp Immunol. 1996;103:226–232. doi: 10.1046/j.1365-2249.1996.d01-613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinha S, McEntegart A, Girdhar B, Bhatia A S, Sengupta U. Appraisal of two Mycobacterium leprae-specific serological assays for monitoring chemotherapy in lepromatous (LL/BL) leprosy patients. Int J Lepr. 1989;57:24–32. [PubMed] [Google Scholar]
- 13.Sinha S, Sengupta U, Ramu G, Ivanyi J. A serological test for leprosy based on competitive inhibition of monoclonal antibody binding to the MY2a determinant of M. leprae. Trans R Soc Trop Med Hyg. 1983;77:869–871. doi: 10.1016/0035-9203(83)90309-7. [DOI] [PubMed] [Google Scholar]
- 14.Sinha S, Sengupta U, Ramu G, Ivanyi J. Serological survey of leprosy and control subjects by a monoclonal antibody-based immunoassay. Int J Lepr. 1985;53:33–38. [PubMed] [Google Scholar]
- 15.Triccas J A, Roche P W, Winter N, Feng C G, Butlin C R, Britton W J. A 35-kilodalton protein is a major target of the human immune response to Mycobacterium leprae. Infect Immun. 1996;64:5171–5177. doi: 10.1128/iai.64.12.5171-5177.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winter N, Triccas J A, Rivoire B, Pessolani M C V, Eiglmeier K, Hunter S W, Brennan P J, Britton W J. Characterization of the gene encoding the immunodominant 35 kDa protein of Mycobacterium leprae. Mol Microbiol. 1995;16:865–876. doi: 10.1111/j.1365-2958.1995.tb02314.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Lathigra R, Garbe T, Catty C, Young D. Genetic analysis of superoxide dismutase, the 23 kilodalton antigen of Mycobacterium tuberculosis. Mol Microbiol. 1991;5:381–391. doi: 10.1111/j.1365-2958.1991.tb02120.x. [DOI] [PubMed] [Google Scholar]