Abstract
Introduction
Current antiretroviral therapies (ARTs) have improved outcomes for people living with HIV. However, the requirement to adhere to lifelong daily oral dosing may be challenging for some people living with HIV, leading to suboptimal adherence and therefore reduced treatment effectiveness. Treatment with long-acting (LA) ART may improve adherence and health-related quality of life. The objective of this study was to evaluate the cost-effectiveness of cabotegravir + rilpivirine (CAB+RPV) LA administered every 2 months (Q2M) compared with current ART administered as daily oral single-tablet regimens (STRs) from a Spanish National Healthcare System perspective.
Methods
A hybrid decision-tree and Markov state-transition model was used with pooled data from three phase III/IIIb trials (FLAIR, ATLAS, and ATLAS-2M) over a lifetime horizon, with health states defined by viral load and CD4+ cell count. Direct costs (in €) were taken from Spanish public sources from 2021 and several deterministic and probabilistic analyses were carried out. An annual 3% discount rate was applied to both costs and utilities.
Results
Over the lifetime horizon, CAB+RPV LA Q2M was associated with an additional 0.27 quality-adjusted life years (QALYs) and slightly greater lifetime costs (€4003) versus daily oral ART, leading to an incremental cost-effectiveness ratio of €15,003/QALY, below the commonly accepted €30,000/QALY willingness-to-pay threshold in Spain. All scenario analyses showed consistent results, and the probabilistic sensitivity analysis showed cost-effectiveness compared with daily oral STRs in 62.4% of simulations, being dominant in 0.3%.
Conclusion
From the Spanish National Health System perspective, CAB+RPV LA Q2M is a cost-effective alternative compared with the current options of daily oral STR regimens for HIV treatment.
Clinical Trials Registration
ATLAS, NCT02951052; ATLAS-2M, NCT03299049; FLAIR, NCT02938520.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-023-00840-y.
Keywords: Adherence, Antiretroviral therapy, Cabotegravir, Cost-effectiveness, HIV, Injectable, Long-acting, Rilpivirine
Plain Language Summary
Over the past decades, treatments for HIV infection have improved outcomes for people living with HIV. However, most of the treatments available consist of daily oral administration, which may present challenges for some people. These challenges may lead to a less optimal intake of the medicines and, therefore, to a potential reduction of treatment effectiveness. A new long-acting treatment alternative for HIV with two drugs is now available: cabotegravir + rilpivirine long-acting is the first injectable treatment administered in the muscle every 2 months by a healthcare professional. Long-acting injectables may improve treatment administration and health-related quality of life of people living with HIV. This study estimated the cost-effectiveness of cabotegravir + rilpivirine long-acting in Spain compared with daily oral single-tablet treatment for HIV. An economic model using clinical data and Spanish inputs was used to estimate cost-effectiveness and health outcomes over a lifetime. Cabotegravir + rilpivirine long-acting compared with daily oral single-tablet treatment showed an increase in health-related quality of life, leading to a cost-effectiveness ratio of €15,003, below the Spanish willingness-to-pay threshold of €30,000. All different scenarios tested showed consistent results, with cabotegravir + rilpivirine long-acting being cost-effective in 62.4% of the simulations and less costly and more effective in 0.3%. This study demonstrated that, in Spain, cabotegravir + rilpivirine long-acting administered every 2 months is a cost-effective alternative to the current daily oral single-tablet treatment options for HIV.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-023-00840-y.
Key Summary Points
| Why carry out this study? |
| Suboptimal adherence due to daily oral pill burden, stigma, and other challenges is still common in people living with HIV and may lead to reduced treatment adherence and effectiveness and increased onward transmission. |
| Cabotegravir + rilpivirine long-acting, administered every 2 months through an injection by a healthcare professional, is the first long-acting antiretroviral therapy (ART) that may reduce these challenges by omitting daily oral ART intake. |
| In this study, the cost-effectiveness of injectable long-acting cabotegravir + rilpivirine over commonly used, daily oral, single-tablet ART regimen was evaluated in Spain. |
| What was learned from this study? |
| Cabotegravir + rilpivirine long-acting was cost-effective over daily oral single-tablet regimen ART from the Spanish National Healthcare System perspective. |
| Cabotegravir + rilpivirine long-acting could provide a cost-effective alternative treatment for Spanish people living with HIV, particularly for those experiencing challenges associated with daily oral medication (e.g., adherence, pill burden, or stigma), or even for treatment preferences. |
Introduction
In the past decades, advances in antiretroviral therapy (ART) have improved HIV-1 infection treatment, making it a manageable condition [1] and allowing people living with HIV (PLHIV) to increase their life expectancy to a range that approaches that of the general population [2]. While PLHIV’s life expectancies have improved with ART, therapy effectiveness and quality of life may still be reduced in some PLHIV as a result of several challenges associated with lifelong daily oral therapy, such as adherence, pill burden, and stigma [3–5]. By eliminating daily oral dosing, long-acting (LA) injectable ART may relieve PLHIV of some of these challenges [6].
Cabotegravir + rilpivirine (CAB+RPV) is an integrase strand transfer inhibitor and non-nucleoside reverse transcriptase inhibitor, respectively, and the first complete LA injectable ART for HIV-1 treatment [7]. Cabotegravir + rilpivirine is administered via intramuscular injection by a healthcare professional every 2 months (Q2M). Previous studies have demonstrated that Q1M dosing regimens were non-inferior to daily oral ART for maintaining virologic suppression in PLHIV [8, 9] and that Q2M dosing was non-inferior to Q1M dosing [10]. Furthermore, an indirect treatment comparison leveraging results from FLAIR, ATLAS, and ATLAS-2M studies demonstrated that CAB+RPV LA Q2M was non-inferior to daily oral ART [11]. Long-term data from the LATTE-2 (NCT02120352) study showed efficacy and acceptable safety and tolerability of CAB+RPV LA over approximately 5 years of treatment [12]. On the basis of results from these studies, CAB+RPV LA has been approved by the European Medicines Agency for the treatment of HIV-1 infection in adults who are virologically suppressed (HIV-1 RNA < 50 copies/mL) on a stable antiretroviral regimen without present or past evidence of viral resistance to, and no prior virological failure with agents of the non-nucleoside reverse transcriptase inhibitor or integrase inhibitor class [13, 14]. As such, CAB+RPV LA offers an alternative to the daily oral single-tablet regimens (STRs) that are the most commonly used ART alternatives in Spain for treating HIV-1 infection [15].
CAB+RPV LA has demonstrated additional health benefits among PLHIV, such as improved health-related quality of life (HRQoL) [8–10]. Participants in the FLAIR (NCT02938520) and ATLAS (NCT02951052) studies reported higher treatment satisfaction with CAB+RPV LA Q2M compared with daily oral comparators, and participants in the ATLAS-2M (NCT03299049) study reported higher treatment satisfaction with Q2M over Q1M dosing. Additionally, a post hoc analysis on the FLAIR and ATLAS studies revealed a utility advantage of 0.02 associated with CAB+RPV LA vs daily oral options, as a consequence of the improvements shown in HRQoL [16]. Moreover, a multi-criteria decision analysis demonstrated that multidisciplinary experts in Spain perceived CAB+RPV LA efficacy as non-inferior to daily oral ART and that CAB+RPV LA presented superior patient-reported outcome profiles, including high preference for and satisfaction with CAB+RPV LA compared with daily oral ART [17]. Additionally, these experts considered that CAB+RPV LA could be particularly beneficial for some of the PLHIV with low adherence or who were highly affected by HIV-associated stigma. Overall, Spanish multidisciplinary experts believed that CAB+RPV LA would make a valuable alternative to HIV-1 treatment compared with oral STRs. Notably, 91% of these experts thought the CAB+RPV LA regimen aligned with the interests and objectives of the Spanish National Healthcare System.
Few studies have assessed the cost-effectiveness of CAB+RPV LA compared with current standard-of-care daily oral ART. A recent study in a sub-Saharan setting showed that CAB+RPV LA Q1M would be more cost-effective in PLHIV with suboptimal adherence; these results were also supported by a study in a US setting [18, 19]. Additionally, a Canadian study demonstrated CAB+RPV LA Q1M to be the dominant intervention (more effective and less costly) compared with daily oral regimens [20]. Here, the cost-effectiveness of CAB+RPV LA Q2M compared with daily oral STRs was evaluated from the Spanish National Health System perspective.
Methods
Cost-Effectiveness Model
A previously published deterministic hybrid Markov state-transition model was adapted to the Spanish setting in order to evaluate the cost-effectiveness of CAB+RPV [20]. To capture the complexity of HIV treatment management, a traditional Markov process was combined with a decision-tree process, which managed treatment allocation and aggregated results across treatment lines. To differentiate between those discontinuing for virologic and non-virologic reasons, an internal decision process was employed (Table S1 in the supplementary material). The model was designed and implemented in Microsoft Excel for Windows (Microsoft Corp, Redmond, CA, USA). Most model calculations were undertaken within Visual Basic for Applications, and routines coded in Visual Basic for Applications controlled the modeling process around these calculations.
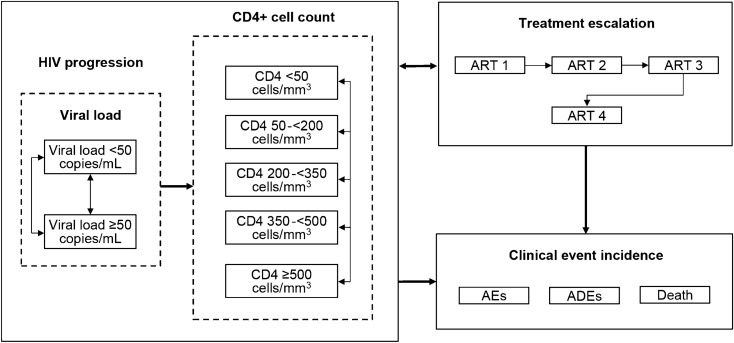
The treatment pathways and health states modeled are depicted in Fig. 1. Health states were based on treatment line, virologic response, and CD4+ cell count, with death as an absorbing state. Participants were subject to the risk of AIDS-defining events and treatment-related adverse events (AEs), but not as explicit health states. Participants were subject to the risk of AIDS-defining events modeled as cyclical (monthly) probabilities. The source publication for AIDS-defining event probability over time stated the risk of AIDS-defining events sometimes rose with increasing CD4+ cell count. The lowest probability by CD4+ cell count was used, so that improving health states did not yield higher probabilities of AIDS-defining events, to replicate known disease progression (Table S2 in the supplementary material). Participants were also subject to the risk of AEs modeled as cyclical (monthly) probabilities while on first-line treatment. However, only AEs related to injection site reactions were modeled as all other AEs were assumed equivalent between intervention and comparators, in line with evidence from the indirect treatment comparison. Participants could discontinue or change from their initial ART line because of virologic failure (failure to maintain HIV RNA < 50 copies/mL), viral rebound (virologic failure after initially achieving suppression), or non-virologic reasons (Fig. S1 in the supplementary material). Detailed descriptions of the model structure, inputs, and validation have been previously published [20].
Fig. 1.
Treatment pathway and within-therapy health states modeled for CAB+RPV LA. AE adverse event, ADE AIDS-defining event, ART antiretroviral regimen, CAB cabotegravir, LA long-acting, RPV rilpivirine
Clinical Parameters
As a result of their similar design, results from ATLAS and FLAIR, two phase III, randomized, multicenter, parallel-group, non-inferiority, open-label trials, were pooled. The pooled analysis established non-inferiority of CAB+RPV LA Q1M compared with continuation on abacavir/dolutegravir/lamivudine in FLAIR or with continuation on current first-line daily oral ART, excluding abacavir/dolutegravir/lamivudine, in ATLAS. Additionally, ATLAS-2M, a similarly designed phase IIIb study, showed non-inferiority of CAB+RPV LA Q2M compared with Q1M dosing [8–10]. Efficacy data from these three trials were used to model the cost-effectiveness of introducing CAB+RPV LA Q2M in virologically suppressed PLHIV compared with daily oral STRs currently recommended in Spain as standard of care (Table S3 in the supplementary material; a list of STR comparators can be found in Table 1). Efficacy of ART was measured by virologic response (HIV RNA ≤ 50 copies/mL) and the average increase in CD4+ cell count. Probabilities of virologic and non-virologic discontinuations were sourced from the literature (Table S4 in the supplementary material). Adverse events were incorporated via monthly, treatment-specific probabilities and were associated with a monthly utility decrement and a per-event cost.
Table 1.
Antiretroviral regimen costs based on list prices in Spain
| Regimen | Net monthly cost (WAC), € | Source |
|---|---|---|
| CAB+RPV LA | 803.14 | BotPlus database [36] |
| Pooled daily oral STRs | 767.52 | |
| Specified oral STR ART | ||
| Atripla, TDF/EFV/FTC | 272.11 | BotPlus database [36] |
| Biktarvy, BIC/FTC/TAF | 906.50 | |
| Dovato, DTG/3TC | 589.91 | |
| Eviplera, FTC/RPV/TDF | 645.31 | |
| Genvoya, EVG/COBI/FTC/TAF | 906.50 | |
| Juluca, DTG/RPV | 752.52 | |
| Odefsey, DTG/FTC/TAF | 645.65 | |
| Stribild, COBI/FTC/TDF/EVG | 906.50 | |
| Symtuza, DRV/COBI/FTC/TAF | 816.22 | |
| Triumeq, DTG/ABC/3TC | 798.28 | |
| Subsequent ART | ||
| Pooled oral ART | 798.87 | BotPlus database [36] |
| Subsequent ART 1 | 798.87 | Assumed same as pooled subsequent treatment |
| Subsequent ART 2 | ||
| Subsequent ART 3 | ||
ABC abacavir, ART antiretroviral therapy, BIC bictegravir, CAB cabotegravir, COBI cobicistat, DRV darunavir, DTG dolutegravir, EFV efavirenz, EVG elvitegravir, FTC emtricitabine, LA long-acting, RPV rilpivirine, STR single-tablet regimen, TAF tenofovir alafenamide, TDF tenofovir disoproxil fumarate, WAC whole acquisition cost, 3TC lamivudine
Healthcare Costs
Based upon a review of prior HIV cost-effectiveness analyses [21], the model included direct costs associated with ART, including administration for injectable treatments and AE treatment, routine healthcare use, and end-of-life care costs. Healthcare costs were informed by published literature (prices from older publications were inflated to 2021 prices using the Spanish Consumer Price Index) or public sources and validated by Spanish experts in HIV management. Costs were applied in the model on either a monthly or per-event basis and were discounted at a rate of 3%. Each treatment was associated with an acquisition cost (Table 1). In the case of CAB+RPV LA, the publicly available drug acquisition cost was applied for the loading dose, then once Q2M starting in the month following the loading dose. For the purpose of modeling the daily oral ART treatment costs, prescriptions were assumed to be fulfilled as normal, and unused tablets were assumed to be wasted; although in real life, all unused tablets might not be wasted. The costs of daily oral STRs were notified, publicly available prices; however, a reduced level of prices was explored through sensitivity analysis. An additional cost derived from the treatments relevant to virologic failure with or without resistance was included. Costs associated with the management of AEs were applied as a per-event cost in the cycle of incidence. Routine healthcare use costs were stratified by the model’s CD4+ cell count health states (< 50, 50–200, 200–350, 350–500, and > 500 cells/mm3) and covered all healthcare resource use needed for the management of PLHIV in each CD4+ cell count health state. End-of-life care costs reflected the additional resource use experienced by PLHIV in the months before death and were applied in the final month of life.
Adherence
In long-term data from CAB+RPV LA studies, the observed adherence rate was 96–98% through week 96 of follow-up [22, 23]. However, the literature indicates that suboptimal adherence to daily oral ART is common [24–29]. Reduced adherence is associated with reduced treatment effectiveness, leading to increased odds of viral rebound and development of ART resistance [26, 30]. One study reported a relationship between virologic suppression at 6 months after ART initiation and medication possession ratio [19]. On the basis of this, a −9.5% adherence-related adjustment was made in the STR group to reduce the probability of virologic suppression and increase the probability of viral rebound relative to 100% adherence with CAB+RPV LA (which was based on results showing 98% and 99% of adherence with the Q2M and Q1M dosing, respectively, in the ATLAS-2M trial) [10]. This base-case adherence assumption on STRs comes from reducing a figure of 25.6% taken from the Simplification With Easier Emtricitabine Tenofovir group [31, 32] by 63% due to evidence of better adherence associated with STRs as compared with open ART combinations [33]. However, a scenario analysis without applying this difference in adherence between CAB+RPV LA and daily oral STRs was also carried out.
HRQoL
To assess HRQoL, values from the model published by Kauf et al. were used [34], which were derived from five open-label studies in ART-experienced individuals and have been widely used in HIV modeling (Fig. S2 in the supplementary material); values for a Spanish HIV population for country-specific health state utility by CD4+ cell count were not available. For quality-adjusted life years (QALYs), a utility advantage of 0.02 was applied for LA treatment versus STRs on the basis of results from a post hoc analysis of HRQoL data from ATLAS and FLAIR studies in which SF-12 data were used to derive SF-6D utility scores via the algorithm reported by Brazier and Roberts [16, 35]. Age-dependent adjustments were added to a patient’s starting age using a smoothing function fitted to the data to extrapolate across all ages.
Statistical Analyses
Base-Case Analysis
To assess the cost-effectiveness of CAB+RPV LA versus daily oral STRs for HIV treatment in treatment-experienced PLHIV in Spain over a lifetime horizon, mean values were applied to all model inputs. Total event incidence and discounted costs, QALYs, and life years (LYs) were estimated per 1000 individuals for each modeled group, in addition to incremental and cost-effectiveness results. Cost-effectiveness was defined in terms of the incremental cost-effectiveness ratio (ICER). The base case accounted for two key benefits of CAB+RPV LA: assumption of adherence benefits with directly administered CAB+RPV LA (100% adherence) and the anticipated benefit of HRQoL with an absence of disutility associated with oral therapy.
Scenario Analysis
To explore the influence of the input parameters on base-case results, several scenario analyses were conducted, including variations in the discount rates (0% and 5%), variations in costs (−20% and +20%), variations in costs by CD4+ cell count category (−20% and +20%), a cost decrease for CD4+ cell count of 201–350 cells/mm3 category (−50%), reduced pharmacological treatment costs of STRs and CAB+RPV LA (−38%), variations in percentage of patients initiating CAB+RPV LA with oral lead-in (from 0 to 10%), and no adherence difference between comparators (100% adherence assigned to daily oral STRs as well).
Deterministic Sensitivity Analysis
To identify parameters that were influential to the modeled results and to assess the relative impact of changes to parameter values on results, several deterministic sensitivity analyses were conducted, including model settings (time horizon, cost and benefits discounts [an annual discount rate of 3% was applied following Spanish recommendations for economic evaluations]) [36], baseline characteristics, health state utilities, costs, oral treatment-related disutility, and efficacy. Each parameter was varied individually with a percentage change from 80% to 120% of the base-case values.
Probabilistic Sensitivity Analysis
To assess the impact of uncertainty in chosen model input values on model results, parameters were varied simultaneously in a probabilistic sensitivity analysis using more than 1000 iterations. Cost and utility inputs were sampled from gamma and beta distributions, respectively, according to the means and standard errors (Table S2 in the supplementary material).
Compliance with Ethics Guidelines
In the case of the present study, this section does not apply, since no patient was involved as it has been only based on published data as inputs to the economic model which provides results based on calculations [8–10].
Results
Base-Case Analysis
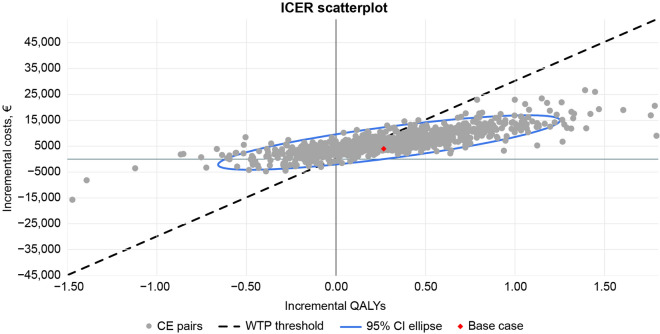
The base-case analysis, with the analysis conducted from the payer’s perspective, reflected a monthly cost for CAB+RPV LA of €803 with a 3% discount applied to costs and outcomes (Table 1). Over the lifetime horizon, CAB+RPV LA was associated with an additional 0.27 QALYs and slightly greater lifetime costs compared with the pooled daily STRs (€239,633 vs €235,629, respectively; cost difference, €4003; Table 2). The ICER of CAB+RPV LA was €15,003/QALY, below the commonly accepted €30,000/QALY willingness-to-pay (WTP) threshold in Spain, resulting in a cost-effective alternative for the Spanish National Health System (Fig. 2, red dot). Disaggregated costs by components are presented in Table 2.
Table 2.
Base-case results of discounted patient-level cost-effectiveness and discounted costs by component for CAB+RPV LA vs daily oral STRs
| Treatment | Total QALYs | Total LYs | Total costs | ICER (Cost/QALY) | ||
|---|---|---|---|---|---|---|
| CAB+RPV LA | 13.51 | 17.99 | 239,632.70 | – | ||
| Pooled oral STRs | 13.25 | 17.89 | 235,629.33 | |||
| Incremental | 0.27 | 0.10 | 4003.37 | 15,002.96 | ||
| Cost component | CAB+RPV LA | Pooled comparator | Incremental cost | |||
|---|---|---|---|---|---|---|
| Health states | 49,613.72 | 51,202.43 | −1588.71 | |||
| 1st line therapy | 90,822.33 | 77,475.06 | 13,347.27 | |||
| 1st line administration | 678.50 | 0 | 678.50 | |||
| Subsequent line | 31,888.54 | 30,881.15 | 1007.39 | |||
| Fourth line | 50,632.20 | 60,025.97 | −9393.77 | |||
| AIDS-defining events | 285.53 | 290.11 | −4.58 | |||
| End of life | 15,653.08 | 15,754.60 | −101.52 | |||
All costs are in euros
CAB cabotegravir, ICER incremental cost-effectiveness ratio, LA long-acting, LY life year, QALY quality-adjusted life year, RPV rilpivirine, STR single-tablet regimen
Fig. 2.
Incremental cost-effectiveness ratio scatterplot for probabilistic sensitivity analysis (gray dots) and base case (red dot) of CAB+RPV LA versus daily oral STRs. CAB cabotegravir, CE cost-effectiveness, CI confidence interval, ICER incremental cost-effectiveness ratio, LA long-acting, RPV rilpivirin, STR single-tablet regimen, QALY quality-adjusted life year, WTP willingness-to-pay
Scenario Analysis
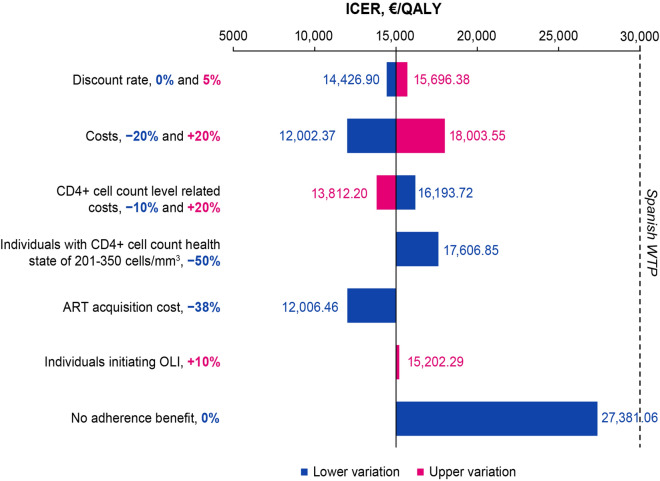
To further explore the influence of selected input parameters on the results of the base-case analysis, variations in discount rates, treatment costs, CD4+ cell count health state-associated costs, decreased ART acquisition costs, increased proportion of PLHIV initiating oral lead-in, and a scenario in which CAB+RPV LA had no adherence benefit relative to oral ART were evaluated. Overall, all scenarios had either further incremental or no impact on the modeled LYs and QALYs from the base-case analysis (Table 3), and all resulted in an ICER value below the Spanish WTP threshold (Fig. 3). Notably, a 0% discount rate, 20% decrease in treatment costs, 20% increase in CD4+ cell count health state-associated costs, and 38% decrease in acquisition costs led to an ICER below the base-case analysis ICER (€15,003).
Table 3.
Main outcomes from the scenario analysis
| Scenarios | CAB+RPV LA | Pooled daily oral STRs | Incremental | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total QALYs | Total LYS | Total costs, € | Total QALYs | Total LYS | Total costs, € | Total QALYs | Total LYS | Total costs, € | ICER, € | |
| Base case | 13.51 | 17.99 | 239,632.70 | 13.25 | 17.89 | 235,629.33 | 0.27 | 0.10 | 4003.37 | 15,002.96 |
| 0% discount rate | 20.60 | 27.83 | 380,816.35 | 20.19 | 27.61 | 374,795.49 | 0.42 | 0.22 | 6020.85 | 14,426.90 |
| 5% discount rate | 10.63 | 14.05 | 184,577.66 | 10.42 | 13.99 | 181,294.72 | 0.21 | 0.06 | 3282.94 | 15,696.38 |
| −20% change in treatment costs | 13.51 | 17.99 | 191,706.16 | 13.25 | 17.89 | 188,503.46 | 0.27 | 0.10 | 3202.70 | 12,002.37 |
| +20% change in treatment costs | 13.51 | 17.99 | 287,559.25 | 13.25 | 17.89 | 282,755.20 | 0.27 | 0.10 | 4804.05 | 18,003.55 |
| −20% change in CD4+ cell count health state-associated costs | 13.51 | 17.99 | 229,709.96 | 13.25 | 17.89 | 225,388.84 | 0.27 | 0.10 | 4321.12 | 16,193.72 |
| +20% change in CD4+ cell count health state-associated costs | 13.51 | 17.99 | 249,555.45 | 13.25 | 17.89 | 245,869.82 | 0.27 | 0.10 | 3685.63 | 13,812.20 |
| 50% decrease in costs for CD4+ cell count health state of 201–350 cells/mm3 | 13.51 | 17.99 | 232,961.21 | 13.25 | 17.89 | 228,263.02 | 0.27 | 0.10 | 4698.19 | 17,606.85 |
| 38% decrease in ART acquisition cost | 13.51 | 17.99 | 160,774.10 | 13.25 | 17.89 | 157,570.31 | 0.27 | 0.10 | 3203.79 | 12,006.46 |
| 10% of participants initiating with OLI (vs 0%) | 13.51 | 17.99 | 239,685.89 | 13.25 | 17.89 | 235,629.33 | 0.27 | 0.10 | 4056.56 | 15,202.29 |
| No adherence benefit for CAB+RPV LA (vs oral ART) | 13.56 | 18.05 | 239,509.69 | 13.37 | 18.05 | 234,371.26 | 0.19 | 0 | 5138.42 | 27,381.06 |
ART antiretroviral therapy, CAB cabotegravir, ICER incremental cost-effectiveness ratio, LA long-acting, LY life year, OLI oral lead-in, RPV rilpivirine, STR single-tablet regimen, QALY quality-adjusted life year
Fig. 3.
Incremental cost-effectiveness ratio results of the scenario analysis. The dashed line represents the Spanish WTP threshold (€30,000). ART antiretroviral therapy, ICER incremental cost-effectiveness ratio, LI oral lead-in, QALY quality-adjusted life year, WTP willingness-to-pay
Deterministic Sensitivity Analysis
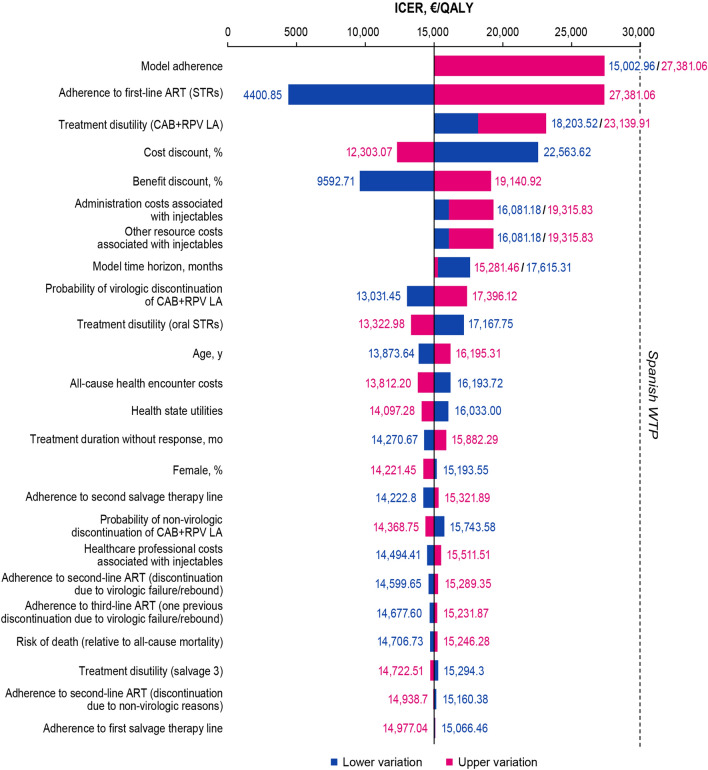
To explore how uncertainty in the individual input parameters used in the model influenced the results of the study, deterministic sensitivity analyses with variations in individual parameters were conducted (Fig. 4). The deterministic sensitivity analyses showed that the ICER was most sensitive to the inclusion or exclusion of modeled adherence (lower vs upper variation, €15,003 vs €27,381) and adherence to the daily oral STRs (lower vs upper variation, €4401 vs €27,381). Other influential parameters were treatment disutility for oral rather than injectable treatment and the cost and benefits discounts (lower vs upper variation, €18,204 vs €23,140; €22,564 vs €12,303; €9,593 vs €19,141; respectively). All scenario analyses showed consistent results, with an ICER of CAB+RPV LA below the Spanish WTP threshold.
Fig. 4.
Incremental cost-effectiveness ratio tornado plot of key influential parameters in the deterministic sensitivity analyses. The dashed line represents the Spanish WTP threshold (€30,000). ART antiretroviral therapy, CAB cabotegravir, ICER incremental cost-effectiveness ratio, LA long-acting, RPV rilpivirine, STR single-tablet regimen, QALY quality-adjusted life year, WTP willingness-to-pay
Probabilistic Sensitivity Analysis
To assess how second-order uncertainty in the model parameters impacted the results of the study, a probabilistic sensitivity analysis was conducted. The mean estimates for lifetime QALYs, LYs, and total costs are presented in Table 4. Compared with pooled daily oral STRs, CAB+RPV LA Q2M resulted in a mean additional total cost of €6416, similar to results from the base-case analysis. Cabotegravir + rilpivirine LA generally resulted in greater QALYs compared with the pooled daily STRs (mean incremental QALYs, 0.30), with 81.4% of model iterations falling above 0 incremental QALYs. Overall, the probabilistic sensitivity analysis showed that CAB+RPV LA was cost-effective versus pooled daily oral STRs in 62.4% of simulations and dominant (both less costly and more effective) in 0.3% of simulations (Fig. 2).
Table 4.
Probability sensitivity analysis main outcomes
| Outcome | CAB+RPV LA Q2M | Pooled daily oral STRs | Incremental |
|---|---|---|---|
| QALYs | 13.577 | 13.281 | 0.296 |
| CD4+ <50 cells/mm3 | 0.052 | 0.054 | −0.002 |
| CD4+ 50–200 cells/mm3 | 0.283 | 0.307 | −0.024 |
| CD4+ 200–350 cells/mm3 | 1.061 | 1.177 | −0.012 |
| CD4+ 350–500 cells/mm3 | 2.493 | 2.699 | −0.206 |
| CD4+ >500 cells/mm3 | 9.780 | 9.349 | 0.431 |
| Treatment disutility | 0.091 | 0.303 | −0.213 |
| AE disutility | 0 | 0 | 0 |
| ADE disutility | 0.001 | 0.001 | 0 |
| LYs | 18.706 | 17.973 | 0.103 |
| Total cost, € | 363,333.31 | 356,917.77 | 6415.54 |
| Health state costs | 172,430.39 | 172,307.73 | 122.65 |
| First-line therapy costs | 102,790.86 | 89,347.23 | 13,443.63 |
| Subsequent-line therapy costs | 27,904.91 | 26,896.86 | 1008.05 |
| Salvage therapy costs | 43,524.46 | 52,404.27 | −8879.81 |
| AE | 66.24 | 0 | 66.24 |
| ADE | 285.27 | 290.00 | −4.73 |
| Societal and death | 15,563.28 | 15,671.68 | −108.40 |
| Cost-effectiveness, € | |||
| QALYs | 21,637.93 | – | – |
| LYs | 62,254.28 | – | – |
AE adverse event, ADE AIDS-defining event, CAB cabotegravir, LA long-acting, LY life year, RPV rilpivirine, STR single-tablet regimen, QALY quality-adjusted life year
Discussion
Cost-effectiveness of CAB+RPV LA Q2M versus daily oral STRs in virologically suppressed PLHIV was evaluated in the Spanish setting. Overall, in accordance with other studies [18–20], CAB+RPV LA Q2M demonstrated cost-effectiveness compared with pooled daily oral STRs. Specifically, this analysis accounted for two important benefits of CAB+RPV LA: assumption of adherence benefits and the absence of a disutility associated with oral therapy.
Although adherence to HIV treatment is a key driver of real-world effectiveness of ART, it is not generally considered in trial-based settings. On the basis of previous studies demonstrating an adherence rate of 97–98% and because CAB+RPV LA is directly administered by a healthcare professional [22, 23], no adherence reduction with CAB+RPV LA was assumed, but a 9.5% reduction in adherence on daily oral ART was assumed. The base-case analysis showed increased QALYs (0.27 QALYs) and an ICER (€15,003) well below the WTP threshold in Spain, demonstrating the cost-effectiveness of CAB+RPV LA Q2M over pooled daily oral STRs. Variations in estimates with lower adherence to oral therapy resulted in greater incremental benefits to CAB+RPV LA, indicating the long-acting therapy was even more cost-effective than in the base-case analysis. Furthermore, when comparing CAB+RPV LA with daily oral STRs and assuming complete adherence to oral therapy, CAB+RPV LA remained more cost-effective than pooled daily oral STRs; although the ICER was higher in this scenario than in the base-case analysis (€27,381 vs €15,003), it was below the Spanish WTP threshold (€30,000). These results support another study in ART-experienced PLHIV that suggested CAB+RPV LA Q1M would be more cost-effective in PLHIV with suboptimal adherence to daily oral ART [18]. Reduced adherence is associated with greater odds of virologic non-suppression, and therefore greater risk of onward transmission [37–39]. Although onward disease transmission was not evaluated in this analysis, it was assessed in a recent cost-effectiveness study in a Canadian setting that showed the benefit of reduced onward disease transmission with CAB+RPV LA since the high adherence demonstrated by the participants considerably increased QALYs; this is similar to results in this present analysis in Spain [20]. However, it should be noted that, in the general population, not all PLHIV with daily oral suboptimal adherence would necessarily demonstrate better adherence with LA treatment.
The other key benefit of CAB+RPV LA analyzed was the absence of a disutility usually associated with daily oral therapy. The need to adhere to lifelong oral ART is a daily reminder of a person’s HIV status and may increase their fear of unwanted HIV disclosure, feelings of self-stigma, or other psychological and emotional challenges, such as anxiety or pill fatigue [4, 40]. Additionally, treatment complications, such as malabsorption and dysphagia, might be faced by PLHIV who have comorbidities [41]. Altogether, these challenges associated with daily oral treatment may result in suboptimal adherence, reduced effectiveness [26], and greater risk of resistance and onward transmission [30], potentially increasing healthcare costs [42]. In contrast, CAB+RPV LA only requires a healthcare visit once every 2 months without a need for PLHIV to store medication, thus decreasing the concern of unintentional HIV status disclosure and reducing the impact of challenges associated with daily oral ART. Therefore, it is reasonable to assume that compared with LA injectables, daily oral treatment is associated with a degree of disutility. This assumption was supported by a post hoc analysis based on patient-reported outcomes in ATLAS and FLAIR studies, in which participants reported improvement in treatment satisfaction and a preference for CAB+RPV LA over daily oral ART [8, 9]. When factoring in the utility advantage of CAB+RPV LA in these analyses, observed increased QALYs and ICER gain resulted in CAB+RPV LA Q2M being cost-effective over pooled daily oral STRs.
Finally, the sensitivity analyses performed further supported these conclusions. Deterministic sensitivity analyses indicated that the ICER was most responsive to the variations of model adherence and to the treatment disutility applied for oral rather than LA injectable treatment. With similar mean incremental QALYs and costs compared with the deterministic analysis, the probabilistic sensitivity analyses demonstrated that the results observed were robust. Importantly, in all the scenarios conducted, CAB+RPV LA Q2M remained under the Spanish WTP threshold and, therefore, was cost-effective. Of note, it is well known that the publicly funded prices of treatments are always lower than those published, so an assumption was made for presenting a sensitivity analysis that shows that the lower the pricing level is, the more cost-effective CAB+RPV LA Q2M would be compared with the base case.
There were some limitations to this study. The adherence level to CAB+RPV LA was assumed at 100% based on clinical trials showing 97–98% adherence, which might not reflect the adherence that would be seen in the general population and did not account for potential patients who would not show up to their appointment; however, in the model removing the adherence advantage of CAB+RPV LA, results demonstrated that CAB+RPV LA was still cost-effective. Additionally, this analysis was carried out in settings in which patients were inclined to participate in the study and receive intramuscular injection, which may not reflect the willingness of the general population, nor the issues some PLHIV might experience in regularly accessing their healthcare professional for injections. Furthermore, as stated above, not all PLHIV with daily oral suboptimal adherence might demonstrate a better adherence with an LA treatment. Therefore, the results observed in regard to adherence should be interpreted with these caveats in mind. Further studies focusing on adherence in real-world settings are warranted. In this study, indirect treatment comparison data were not used, only pooled clinical data from ATLAS, FLAIR, and ATLAS-2M were used; however, depending on the country, this could be considered an advantage as some experts/stakeholders may consider indirect treatment comparison of lower value compared with face-to-face clinical data. Additionally, the model did not fully capture the utility improvement associated with the advantages of LA treatment. Finally, the study was based on Spanish data, comparing CAB+RPV LA with the most common STRs used in Spain, and discounts were applied following Spanish recommendations for economic evaluations; therefore, other ART regimens used might not have been incorporated into this model, and conclusions drawn from these analyses might not apply to other settings in which costs may differ substantially.
Conclusion
Overall, this study demonstrated that CAB+RPV LA Q2M could provide a cost-effective alternative treatment for PLHIV compared with daily oral STRs in Spain. This is particularly true for some subgroups of PLHIV who would benefit the most from LA therapy, such as those with suboptimal oral ART adherence, those with oral treatment-associated problems, or even those with alternative treatment preferences.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Funding
This study, including the journal’s Rapid Service fee, was funded by ViiV Healthcare, Durham, NC, USA.
Editorial Assistance
Editorial assistance was provided under the direction of the authors by Deborah Lew, PhD, and Lauren Bragg, ELS, MedThink SciCom, and funded by ViiV Healthcare.
Author Contributions
Conceptualization: Melanie Schroeder, Victoria Neches, and Laura Amanda Vallejo-Aparicio; Methodology: all authors; Formal analysis and investigation: all authors; Writing: original draft preparation: Melanie Schroeder, Victoria Neches, and Laura Amanda Vallejo-Aparicio; Writing: review and editing: all authors.
Disclosures
Santiago Moreno has received expert honoraria from ViiV Healthcare for participation in the present study; research funding from ViiV Healthcare and Gilead Sciences; support for attending meetings and/or travel from ViiV Healthcare, Gilead Sciences, Janssen Pharmaceuticals, and Merck Sharp & Dohme; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from ViiV Healthcare, Gilead Sciences, Janssen Pharmaceuticals, Merck Sharp & Dohme; and has participated on a data safety monitoring board or advisory board for ViiV Healthcare, Gilead Sciences, Janssen Pharmaceuticals, and Merck Sharp & Dohme. Antonio Rivero has received expert honoraria from ViiV Healthcare for participation in the present study; research funding from ViiV Healthcare and Gilead Sciences; support for attending meetings from Gilead Sciences; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from ViiV Healthcare, Gilead Sciences, Janssen Pharmaceuticals, Merck Sharp & Dohme; and has participated on a data safety monitoring board or advisory board for ViiV Healthcare, Gilead Sciences, Janssen Pharmaceuticals, and Merck Sharp & Dohme. Pere Ventayol has received expert honoraria from ViiV Healthcare for participation in the present study; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Gilead Sciences, ViiV Healthcare, and Boehringer Ingelheim; and has received support for attending meetings and/or travel from Janssen Pharmaceuticals and Sanofi. Vicenç Falcó has received expert honoraria from ViiV Healthcare for participation in the present study; research funding from ViiV Healthcare and Gilead Sciences; consulting fees from ViiV Healthcare; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from ViiV Healthcare, Janssen Pharmaceuticals, and Merck Sharp & Dohme; and support for attending meetings and/or travel from Gilead Sciences. Miguel Torralba has received expert honoraria from ViiV Healthcare for participation in the present study; research funding from ViiV Healthcare; consulting fees from Gilead Sciences and ViiV Healthcare; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Gilead Sciences, ViiV Healthcare, Merck Sharp & Dohme, and Janssen Pharmaceuticals; support for attending meetings and/or travel from Gilead Sciences, ViiV Healthcare, Merck Sharp & Dohme, and Janssen Pharmaceuticals; and has participated on advisory boards for Gilead Sciences and ViiV Healthcare. Melanie Schroeder is an employee of and may hold stocks in ViiV Healthcare. Victoria Neches is an employee of GSK. Laura Amanda Vallejo-Aparicio is an employee of GSK and may hold stock in GSK. Isaac Mackenzie, Matthew Turner, and Cale Harrison are employees of HEOR, which received honoraria from ViiV Healthcare for the development of the study.
Prior Presentation
Data included in this manuscript have been previously presented in part at ISPOR Europe; November 6–9, 2022; Virtual and Vienna, Austria; Poster EE345.
Compliance with Ethics Guidelines
In the case of the present study, this section does not apply, since no patient was involved as it has been only based on published data as inputs to the economic model which provides results based on calculations [8–10].
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study. However, model estimates reports are available under specific query to the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194:11–19. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 2.The Antiretroviral Therapy Cohort Collaboration Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4:e349–e356. doi: 10.1016/S2352-3018(17)30066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arts EJ, Hazuda DJ. HIV-1 antiretroviral drug therapy. Cold Spring Harb Perspect Med. 2012;2:a007161. doi: 10.1101/cshperspect.a007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc. 2013;16(3 Suppl 2):18640. doi: 10.7448/IAS.16.3.18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de los Rios P, Okoli C, Castellanos E, et al. Physical, emotional, and psychosocial challenges associated with daily dosing of HIV medications and their impact on indicators of quality of life: findings from the positive perspectives study. AIDS Behav. 2021;25:961–72. [DOI] [PMC free article] [PubMed]
- 6.Margolis DA, Boffito M. Long-acting antiviral agents for HIV treatment. Curr Opin HIV AIDS. 2015;10:246–252. doi: 10.1097/COH.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabenuva [prescribing information]. Durham, NC: ViiV Healthcare; 2022.
- 8.Swindells S, Andrade-Villanueva JF, Richmond GJ, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med. 2020;382:1112–1123. doi: 10.1056/NEJMoa1904398. [DOI] [PubMed] [Google Scholar]
- 9.Orkin C, Arasteh K, Górgolas Hernández-Mora M, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med. 2020;382:1124–1135. doi: 10.1056/NEJMoa1909512. [DOI] [PubMed] [Google Scholar]
- 10.Overton ET, Richmond G, Rizzardini G, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 48-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet. 2020;396:1994–2005. doi: 10.1016/S0140-6736(20)32666-0. [DOI] [PubMed] [Google Scholar]
- 11.Chounta V, Snedecor SJ, Wu S, Van de Velde N. Indirect comparison of 48-week efficacy and safety of long-acting cabotegravir and rilpivirine maintenance every 8 weeks with daily oral standard of care antiretroviral therapy in participants with virologically suppressed HIV-1-infection. BMC Infect Dis. 2022;22:428. doi: 10.1186/s12879-022-07243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith GHR, Henry WK, Podzamczer D, et al. Efficacy, safety, and durability of long-acting cabotegravir and rilpivirine in adults with human immunodeficiency virus type 1 infection: 5-year results from the LATTE-2 study. Open Forum Infect Dis. 2021;8:ofab439. [DOI] [PMC free article] [PubMed]
- 13.Vocabria. European Medicines Agency. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/vocabria. Accessed 10 Apr 2023.
- 14.Rekambys European Medicines Agency. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/rekambys. Accessed 10 Apr 2023.
- 15.Gesida/National plan on AIDS consensus document regarding antiretroviral treatment in adults infected with the human immunodeficiency virus. Gesida, Ministerio de Sanidad. 2020. http://gesida-seimc.org/wp-content/uploads/2020/07/TAR_GUIA_GESIDA_2020_COMPLETA_Julio.pdf. Accessed 10 Apr 2023.
- 16.Murray M, Antela A, Mills A, et al. Patient-reported outcomes in ATLAS and FLAIR participants on long-acting regimens of cabotegravir and rilpivirine over 48 weeks. AIDS Behav. 2020;24:3533–3544. doi: 10.1007/s10461-020-02929-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calleja-Hernández MÁ, Martinez-Sesmero JM, Vallejo-Aparicio LA, Hernández-Novoa B, Badia X. Contribution of cabotegravir + rilpivirine long-acting for the treatment of HIV-1 infection. Farm Hosp. 2022;46:208–214. [PubMed] [Google Scholar]
- 18.Phillips AN, Bansi-Matharu L, Cambiano V, et al. The potential role of long-acting injectable cabotegravir-rilpivirine in the treatment of HIV in sub-Saharan Africa: a modelling analysis. Lancet Glob Health. 2021;9:e620–e627. doi: 10.1016/S2214-109X(21)00025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross EL, Weinstein MC, Schackman BR, et al. The clinical role and cost-effectiveness of long-acting antiretroviral therapy. Clin Infect Dis. 2015;60:1102–1110. doi: 10.1093/cid/ciu1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker B, Ward T, Hayward O, et al. Cost-effectiveness of the long-acting regimen cabotegravir plus rilpivirine for the treatment of HIV-1 and its potential impact on adherence and viral transmission: a modelling study. PLoS ONE. 2021;16:e0245955. doi: 10.1371/journal.pone.0245955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauskopf J. A methodological review of models used to estimate the cost effectiveness of antiretroviral regimens for the treatment of HIV infection. Pharmacoeconomics. 2013;31:1031–1050. doi: 10.1007/s40273-013-0098-6. [DOI] [PubMed] [Google Scholar]
- 22.Mascolini M. Adherence to long-acting CAB and RPV unjections through 96 weks of maintenance therapy in LATTE-2/Good adherence to injected cabotegravir and rilpivirine in 230 LATTE-2 participants. Presented at 22nd International AIDS Conference 2018; July 23–27, 2018.
- 23.Teichner P, Wu S, Zhang F, et al. Long-term patient adherence and management of treatment interruptions with long-acting injectables cabotegravir + rilpivirine for maintenance therapy in phase IIb/III studies. Presented at IDWeek 2020; Oct 21–22, 2020; Virtual. Poster 1029.
- 24.Sutton SS, Ahuja D, Magagnoli J. What is the effect of pill burden on adherence to HIV antiretroviral therapy? JAAPA. 2016;29:16–17. doi: 10.1097/01.JAA.0000502870.89587.b6. [DOI] [PubMed] [Google Scholar]
- 25.Chesney MA. Factors affecting adherence to antiretroviral therapy. Clin Infect Dis. 2000;30(Suppl 2):S171–S176. doi: 10.1086/313849. [DOI] [PubMed] [Google Scholar]
- 26.Bezabhe WM, Chalmers L, Bereznicki LR, Peterson GM. Adherence to antiretroviral therapy and virologic failure: a meta-analysis. Medicine (Baltimore) 2016;95:e3361. doi: 10.1097/MD.0000000000003361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glass TR, Sterne JA, Schneider MP, et al. Self-reported nonadherence to antiretroviral therapy as a predictor of viral failure and mortality. AIDS. 2015;29:2195–2200. doi: 10.1097/QAD.0000000000000782. [DOI] [PubMed] [Google Scholar]
- 28.Zhang S, Rust G, Cardarelli K, Felizzola J, Fransua M, Stringer HG., Jr Adherence to highly active antiretroviral therapy impact on clinical and economic outcomes for Medicaid enrollees with human immunodeficiency virus and hepatitis C coinfection. AIDS Care. 2015;27:829–835. doi: 10.1080/09540121.2015.1021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Connor JL, Gardner EM, Esser S, et al. A simple self-reported adherence tool as a predictor of viral rebound in people with viral suppression on antiretroviral therapy. HIV Med. 2016;17:124–132. doi: 10.1111/hiv.12284. [DOI] [PubMed] [Google Scholar]
- 30.Churchill D, Waters L, Ahmed N, et al. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015. HIV Med. 2016;17(Suppl 4):s2–104. doi: 10.1111/hiv.12426. [DOI] [PubMed] [Google Scholar]
- 31.Soler Soneira M, Olmedo Lucerón C, Sánchez-Cambronero Cejudo L, Cantero Gudino E, Limia Sánchez A. [The cost of vaccination throughout life in Spain]. Rev Esp Salud Publica. 2020;94. [PubMed]
- 32.Cooper V, Moyle GJ, Fisher M, et al. Beliefs about antiretroviral therapy, treatment adherence and quality of life in a 48-week randomised study of continuation of zidovudine/lamivudine or switch to tenofovir DF/emtricitabine, each with efavirenz. AIDS Care. 2011;23:705–713. doi: 10.1080/09540121.2010.534433. [DOI] [PubMed] [Google Scholar]
- 33.Altice F, Evuarherhe O, Shina S, Carter G, Beaubrun AC. Adherence to HIV treatment regimens: systematic literature review and meta-analysis. Patient Prefer Adherence. 2019;13:475–490. doi: 10.2147/PPA.S192735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kauf TL, Roskell N, Shearer A, et al. A predictive model of health state utilities for HIV patients in the modern era of highly active antiretroviral therapy. Value Health. 2008;11:1144–1153. doi: 10.1111/j.1524-4733.2008.00326.x. [DOI] [PubMed] [Google Scholar]
- 35.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42:851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 36.BotPlus. https://botplusweb.portalfarma.com/. Accessed 10 Apr 2023.
- 37.Nachega JB, Marconi VC, van Zyl GU, et al. HIV treatment adherence, drug resistance, virologic failure: evolving concepts. Infect Disord Drug Targets. 2011;11:167–174. doi: 10.2174/187152611795589663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin M, Del Cacho E, Codina C, et al. Relationship between adherence level, type of the antiretroviral regimen, and plasma HIV type 1 RNA viral load: a prospective cohort study. AIDS Res Hum Retroviruses. 2008;24:1263–1268. doi: 10.1089/aid.2008.0141. [DOI] [PubMed] [Google Scholar]
- 39.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 40.Shubber Z, Mills EJ, Nachega JB, et al. Patient-reported barriers to adherence to antiretroviral therapy: a systematic review and meta-analysis. PLoS Med. 2016;13:e1002183. doi: 10.1371/journal.pmed.1002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki T, Hara N, Osa M, et al. Efficacy of switching to dolutegravir plus rilpivirine, the small-tablet regimen, in patients with dysphagia: two case reports. J Pharm Health Care Sci. 2017;3:23. doi: 10.1186/s40780-017-0093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunn K, Lafeuille MH, Jiao X, et al. Risk factors, health care resource utilization, and costs associated with nonadherence to antiretrovirals in Medicaid-insured patients with HIV. J Manag Care Spec Pharm. 2018;24:1040–1051. doi: 10.18553/jmcp.2018.17507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study. However, model estimates reports are available under specific query to the authors.