Abstract
Background and hypothesis: Motor abnormalities are predictive of psychosis onset in individuals at clinical high risk (CHR) for psychosis and are tied to its progression. We hypothesize that these motor abnormalities also disrupt their speech production (a highly complex motor behavior) and predict CHR individuals will produce more variable speech than healthy controls, and that this variability will relate to symptom severity, motor measures, and psychosis-risk calculator risk scores. Study design: We measure variability in speech production (variability in consonants, vowels, speech rate, and pausing/timing) in N = 58 CHR participants and N = 67 healthy controls. Three different tasks are used to elicit speech: diadochokinetic speech (rapidly-repeated syllables e.g., papapa…, pataka…), read speech, and spontaneously-generated speech. Study results: Individuals in the CHR group produced more variable consonants and exhibited greater speech rate variability than healthy controls in two of the three speech tasks (diadochokinetic and read speech). While there were no significant correlations between speech measures and remotely-obtained motor measures, symptom severity, or conversion risk scores, these comparisons may be under-powered (in part due to challenges of remote data collection during the COVID-19 pandemic). Conclusion: This study provides a thorough and theory-driven first look at how speech production is affected in this at-risk population and speaks to the promise and challenges facing this approach moving forward.
Subject terms: Biomarkers, Psychosis, Human behaviour
Introduction
Individuals with psychosis exhibit motor abnormalities (e.g., tremors, rigidity, dyskinesia, soft-signs) and recent work has suggested that these behaviors may also represent sensitive prognostic indicators during the prodromal period1–4. In addition, motor signs can be objectively measured, in contrast to other symptom domains which are often subject to observer/rater bias2,5. However, motor assessments frequently require significant expertise, as well as time-intensive analyses and/or cumbersome instrumentation2,6–9. In this work, we explore one potential solution, examining the feasibility of using the physical properties of speech to measure motor abnormalities. Speech is a highly complex motor behavior, involving very fine-tuned movements that, when distorted in even subtle ways, can produce easily observable acoustic consequences (e.g., millimeter differences in placement/movement and millisecond differences in timing/coordination can substantially change the speech acoustics)10.
Because basal ganglia and cerebellar circuits modulate motor function and are also implicated in leading models of psychosis11–13, there is good reason to believe that motor signs may be an early and sensitive biomarker4. Indeed, of the identified early vulnerability markers seen in children that develop adult psychosis, motor abnormalities may be the most common14. For example, a myriad of motor behavior domains have been demonstrated to predict infants/children that ultimately develop adult schizophrenia including: delays in achieving motor milestones15, neuromotor deficits and involuntary movements16, and neurological soft signs17. One study comparing childhood video tapes of schizophrenia patients with childhood videos of their healthy siblings as well as healthy community controls, found that the pre-schizophrenia children showed a higher rate of motor abnormalities and delays18. In a similar study, Schiffman and colleagues19 examined video-taped social interactions of 11–13 year old children who later developed schizophrenia and observed that a high occurrence of movement abnormalities distinguished the pre-schizophrenia children from matched controls. In adolescence, neuromaturational factors and environmental stressors can exacerbate underlying vulnerabilities in the motor and dopamine system20, leading to other outward manifestations in this age group, including spontaneous dyskinesias (i.e., spontaneous jerking and irregular ballistic movements)21. Indeed, among high-risk groups (i.e., those showing a low level of symptoms) these particular motor behaviors increase in frequency and severity as a function of development and increased disease burden, are associated with increased attenuated positive symptoms22,23 and strongly predict conversion to psychosis24–26. As not all at-risk individuals go on to develop a psychotic disorder, this is highly relevant27. Irrespective of medication (i.e., the motor abnormalities are present in neuroleptic naïve samples), these spontaneous jerking movements in the head, face, lips, and torso can continue to emerge during the adolescent prodromal period, until onset, when they remain a key clinical feature of the illness28. At least one cross-sectional study suggests that with advanced age, all patients with schizophrenia will eventually develop these behaviors29. We point readers to recent review articles for more discussion of motor function in the prodromal syndrome2,27,30–34.
Previous work has examined speech production in schizophrenia/psychosis35–44. This work has been promising, but results are mixed. A recent meta-analysis found three speech measures (speech rate, pause duration, and proportion spoken time) differentiated clinical and control groups (individuals with schizophrenia had slower speech rates, longer pauses, and lower speaking proportions), but only one (pause duration) showed a large effect43. In addition, the meta-analysis reported differences in results depending on the speech task used, generally finding that more cognitively or socially demanding tasks (e.g., free speech or dialogues) resulted in larger effects. However, this prior work has generally not examined individuals at clinical high-risk (CHR), nor has it focused on motor abnormalities. It also has largely pursued a data-driven approach. Our work pursues a hypothesis-driven approach, studying acoustic measures that are predicted to be disrupted by motor abnormalities in speech produced by individuals at clinical-high-risk.
We hypothesize that disruptions to motor control will impact control over vocal articulators (e.g., tongue, lips), leading to more variable speech in CHR participants when compared to healthy controls (HC), analogous to what has been observed in speech disorders45–47. Furthermore, if these speech measures reflect motor abnormalities, we would expect that increased variability in speech productions should relate to other measures of motor abnormalities (e.g., finger-tapping, as a test of convergent validity), worse symptom severity (as a test of clinical validity), and higher risk of conversion to psychosis (as a test of predictive validity). To systematically examine the conditions under which motor difficulties are observed, we elicit speech in highly-controlled samples that are specifically designed to measure motor difficulties (diadochokinetic speech), read speech, as well as more free-form, naturalistic speech which closely resembles everyday speech.
Results
We present results by speech task. We focused on acoustic speech measures that have been extremely well-studied and can be reliably measured automatically (which allows us to study greater quantities of speech). The main text focuses on (i) variability in the voice-onset-time of voiceless (in English: p,t,k) and voiced (in English, b,d,g) stop consonants, i.e., the primary acoustic measure of stop consonants, defined as the duration between the release of the consonant and the onset of the following vowel, (ii) variability in vowel durations, and (iii) variability in speech rates. We discuss the remaining speech measures we studied (including variability in vowel formants and variability in pausing/timing)48–53 in Table 1 and Supplementary Materials 1.3.
Table 1.
Summary of the studied speech measures by speech task.
| Diadochokinetic-AMR | Diadochokinetic-SMR | Read | Spontaneous | ||
|---|---|---|---|---|---|
| Consonant production measures | |||||
| CoV of voiceless stop VOTs | CHR | 0.31 (0.09) | 0.39 (0.1) | 0.43 (0.1) | 0.4 (0.09) |
| HC | 0.27 (0.1) | 0.35 (0.09) | 0.39 (0.07) | 0.4 (0.07) | |
| p | 0.05 | 0.02 | 0.03 | 0.73 | |
| CoV of voiced stop VOTs | CHR | 0.7 (0.26) | 0.66 (0.26) | ||
| HC | 0.71 (0.26) | 0.58 (0.31) | |||
| p | 0.67 | 0.18 | |||
| Speech rate measures | |||||
| CoV of speech rate | CHR | 0.07 (0.04) | 0.1 (0.05) | 0.2 (0.05) | 0.43 (0.45) |
| HC | 0.05 (0.04) | 0.08 (0.04) | 0.18 (0.06) | 0.37 (0.32) | |
| p | <0.01 | 0.01 | 0.04 | 0.52 | |
| Vowel production measures | |||||
| CoV of vowel durations | CHR | 0.2 (0.09) | 0.43 (0.12) | 0.56 (0.05) | 0.74 (0.11) |
| HC | 0.17 (0.09) | 0.4 (0.15) | 0.56 (0.06) | 0.73 (0.09) | |
| p | 0.13 | 0.18 | 0.42 | 0.72 | |
| Formant dispersion 20% | CHR | 158.63 (86.4) | 211.7 (85.72) | 341.76 (53.41) | 357.52 (48.52) |
| HC | 128.85 (64.57) | 185.02 (75.8) | 332.5 (48.44) | 362.32 (58.29) | |
| p | 0.03 | 0.08 | 0.33 | 0.77 | |
| Change in formant dispersion 20–50% | CHR | 14.59 (21.02) | 20.33 (20.06) | −22.07 (14.82) | −4.5 (16.36) |
| HC | 10.68 (20.01) | 19.41 (19.17) | −21.99 (15.2) | −2.06 (17.79) | |
| p | 0.33 | 0.81 | 0.98 | 0.48 | |
| Overlap between vowel categories | CHR | 0.8 (5.91) | 0 (0) | ||
| HC | 0 (0) | 0 (0) | |||
| p | 0.64 | 0.45 | |||
| Timing/pausing measures | |||||
| CoV of syllable durations | CHR | 0.21 (0.09) | 0.39 (0.1) | ||
| HC | 0.17 (0.08) | 0.36 (0.13) | |||
| p | 0.11 | 0.1 | |||
| CoV of intersyllable durations | CHR | 0.42 (0.25) | 0.9 (0.27) | ||
| HC | 0.34 (0.18) | 0.82 (0.23) | |||
| p | 0.05 | 0.17 | |||
| Number of pauses | CHR | 0.1 (0.03) | 0.15 (0.05) | ||
| HC | 0.09 (0.03) | 0.14 (0.07) | |||
| p | 0.13 | 0.4 | |||
For each speech measure/speech task combination, the table provides descriptive statistics [mean (standard deviation)] by group (CHR vs. HC), as well the p-value corresponding to the CHR vs. HC group difference test. Blank cells indicate that the speech measure in question was not calculated for the speech task in question. Measures are bolded if they show a significant CHR vs. HC group difference and italicized if just above significance. CoV coefficient of variation, VOT voice-onset-time.
N.B.: One CHR participant was identified as clinical high-risk in-remission and another participant had a 7-months’ gap between their clinical interview and speech tasks. Supplementary Materials 2.10 includes analyses without these two participants; the results are qualitatively similar to the analyses of the full dataset reported below.
Diadochokinetic speech tasks
Participants first completed a diadochokinetic speech task, in which they produced particular syllable types as quickly and as accurately as possible54,55. This task consisted of two trial types that we analyze separately: Alternating Motion Rate (AMR) trials, in which participants repeated a single target syllable 15 times (e.g., pa-pa-pa…, ta-ta-ta…, ka-ka-ka…) and Sequential Motion Rate (SMR) trials, in which they repeated sequences of three syllables 10 times (e.g., pa-ta-ka…, ka-ta-pa…).
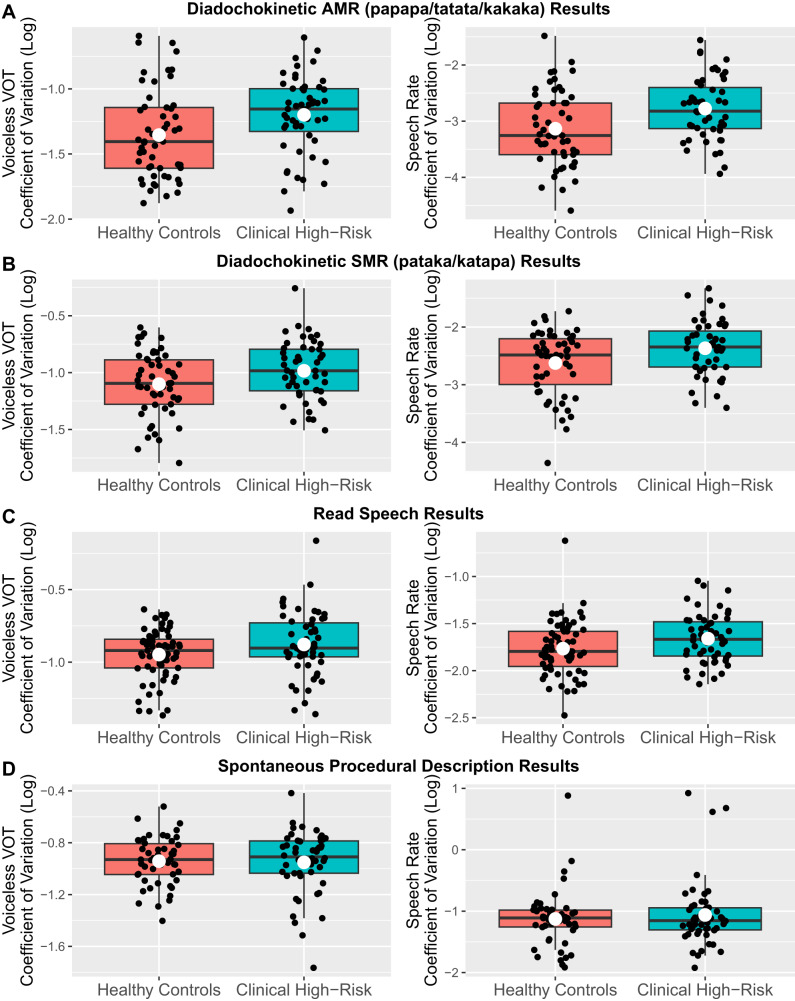
Out of the seven speech measures we studied (Table 1), we found evidence that CHR individuals produced more variable voiceless stop consonant voice-onset-times than HC—near significantly in AMR trials ( = 0.09, s.e. = 0.05, t = 1.95, p = 0.054; Fig. 1A) and significantly in SMR trials ( = 0.12, s.e. = 0.05, t = 2.45, p = 0.016; Fig. 1B). CHR individuals also produced more variable speech rates than HC in both AMR and SMR trials (AMR: = 0.36, s.e. = 0.12, t = 2.98, p = 0.004; SMR: = 0.26, s.e. = 0.1, t = 2.52, p = 0.013; with one exception, all other speech measures showed no significant effects). However, these two measures generally did not correlate with SIPS scores, finger-tapping, or risk scores (results in Table 2 and Supplementary Materials 2.1).
Fig. 1. CHR vs. HC group differences in consonant and speech rate variability across the four studied speech tasks.
CHR individuals produce more variable consonants (left plot in each row) and speech rates (right plot in each row) compared to controls in A diadochokinetic-AMR, B diadochokinetic-SMR, and C read speech, but not D spontaneous speech. Each black dot is one participant; the white dot is the average across participants.
Table 2.
Correlations between speech measures that show CHR vs. HC group differences and non-speech motor/clinical/risk validation measures. Note that the spontaneous speech task is not included as we did not observe significant group differences in that task.
| Diadochokinetic-AMR | Diadochokinetic-SMR | Read Speech | ||||
|---|---|---|---|---|---|---|
| Voiceless VOT CoV | Speech rate CoV | Voiceless VOT CoV | Speech rate CoV | Voiceless VOT CoV | Speech rate CoV | |
| SIPS positive total | r = 0.1 (p = 0.79) | r = 0.19 (p = 0.19) | r = 0.08 (p = 0.84) | r = 0.07 (p = 0.62) | r = 0.28 (p = 0.12) | r = 0.07 (p = 0.62) |
| SIPS negative total | r = 0.23 (p = 0.28) | r = 0.07 (p = 0.64) | r = 0.22 (p = 0.32) | r = 0.18 (p = 0.21) | r = 0.17 (p = 0.48) | r = 0.05 (p = 0.7) |
| SIPS disorganized total | r = 0.07 (p = 0.9) | r = 0.26 (p = 0.08) | r = 0.28 (p = 0.16) | r = 0.13 (p = 0.37) | r = 0.2 (p = 0.38) | r = 0.02 (p = 0.89) |
| SIPS G3 (motor) | r = 0.15 (p = 0.62) | r = 0.11 (p = 0.48) | r = 0.2 (p = 0.39) | r = 0.06 (p = 0.68) | r = 0.26 (p = 0.2) | r = 0.11 (p = 0.45) |
| Finger-tapping CoV (dominant hand) | r = 0.06 (p = 0.92) | r = 0.08 (p = 0.6) | r = 0.08 (p = 0.87) | r = 0.09 (p = 0.54) | r = 0.17 (p = 0.48) | r = 0.11 (p = 0.47) |
| Finger-tapping CoV (non-dominant hand) | r = 0.15 (p = 0.62) | r = 0.29 (p = 0.05) | r = 0.05 (p = 0.96) | r = 0.1 (p = 0.51) | r = 0.18 (p = 0.49) | r = 0.39 (p = 0.01) |
| SIPS-RC risk score | r = 0.4 (p = 0.02) | r = 0.07 (p = 0.65) | r = 0.32 (p = 0.1) | r = 0.09 (p = 0.53) | r = 0.13 (p = 0.66) | r = 0.1 (p = 0.51) |
Read speech
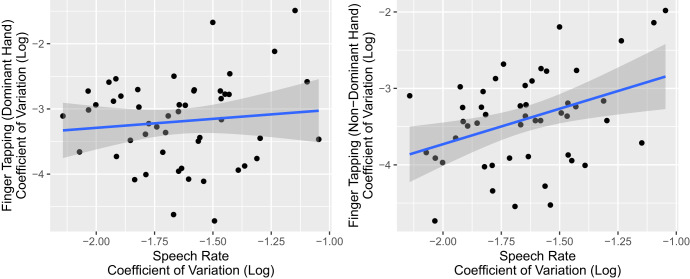
Participants then read a standardized passage aloud at a comfortable pace (full text in Supplementary Materials 1.1). As in the diadochokinetic speech task, we found that CHR individuals produced more variable voiceless stop consonant voice-onset-times ( = 0.08, s.e. = 0.04, t = 2.23, p = 0.028) and speech rates ( = 0.11, s.e. = 0.05, t = 2.1, p = 0.038) than HC (Fig. 1C; all other speech measures showed no significant effects). Variation in speech rate (but not consonant voice-onset-time) was significantly positively correlated with another motor measure, variability in finger-tapping rate in the non-dominant hand ( = 0.93, s.e. = 0.32, t = 2.86, p = 0.006), but not in the dominant hand ( = 0.28, s.e. = 0.37, t = 0.74, p = 0.465; Fig. 2). However, these measures did not correlate with clinical or risk measures (results in Table 2 and Supplementary Materials 2.2).
Fig. 2. Correlation between variability in speech rate and variability in finger-tapping rate in the read speech task.
We observe a significant positive relationship between variability in speech rate and variability in finger-tapping rates in the non-dominant hand (left plot), but not the dominant hand (right plot). Each point represents one participant; the line of best-fit is shown, with shaded regions showing standard errors of the regression fit.
Spontaneous speech
Finally, we elicited spontaneous speech, by asking participants to describe how to make a peanut butter and jelly sandwich for ~2 min. In contrast to the diadochokinetic and read speech samples, we found that none of the speech measures differed by group status in spontaneous speech (Fig. 1D), including the two measures impacted in the previous tasks: variability in voiceless consonant voice-onset-time ( = −0.01, s.e. = 0.04, t = −0.34, p = 0.732) and variability in speech rate ( = 0.06, s.e. = 0.1, t = 0.64, p = 0.524). Because none of the acoustic speech tasks showed significant results (Supplementary Materials 2.3), we did not test for associations with non-speech motor/clinical/risk measures.
In-person vs. remote results
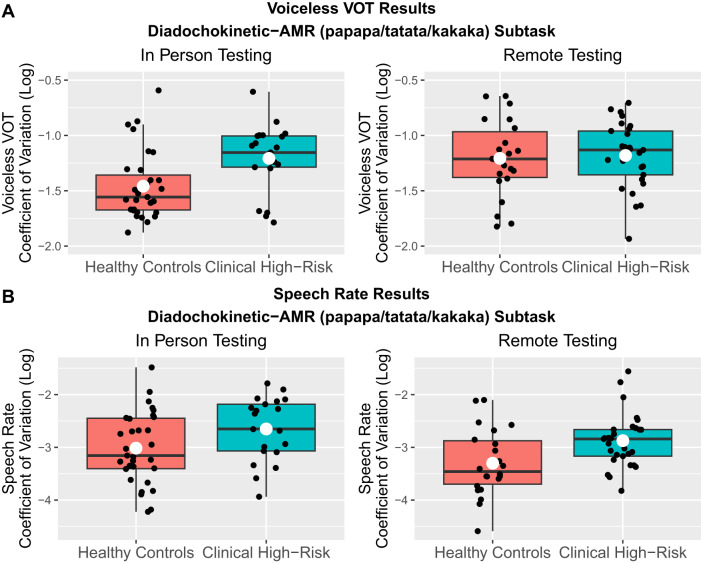
Because data collection occurred between 2019–2022, our study had to be adapted to the remote format partway through due to the COVID-19 pandemic (see Methods for details). In post-hoc analyses, we tested whether results differed between participants tested in-person (N = 70) vs. remotely (N = 52), focusing on the measures and tasks that showed group differences in our primary analyses (Fig. 3 and S20–S21). Full results are presented in Supplementary Materials 2.7, but we generally observed smaller group differences in consonant (voice-onset-time) variability in the remote subgroup relative to the in-person group. This seemed to be driven by greater variability in the remotely-recorded control group relative to the in-person control group. For speech rate, however, the in-person and remote subgroups showed qualitatively similar patterns, except in the diadochokinetic-SMR subtask, where we again observed a reduction in CHR vs. HC group differences when tested remotely.
Fig. 3. Comparison of results between in-person vs. remote testing conditions.
A Group differences in consonant variability only emerged in in-person (left plot), not remote (right plot) testing conditions. B Group differences in speech rate variability were qualitatively similar across the in-person and remote subgroups (example plots from diadochokinetic-AMR speech). Each black dot represents one participant; the white dot represents the average value across participants.
Unpacking why we did not see a relationship with clinical/motor symptoms
Contrary to our predictions, we found that the speech measures that showed CHR vs. HC group differences did not correlate with motor, clinical, or risk measures. We ran several additional exploratory analyses in an attempt to unpack this surprising finding.
Past work has shown that some linguistic measures are highly correlated with sociodemographic factors56–58. To verify this was not the case for the speech measures we studied, we ran regressions predicting demographic factors (age, sex, race, native language) from the speech measures that significantly differed between the high-risk and healthy control groups. We found no significant relationships, suggesting that the observed group differences were not accounted for by demographic factors (see Supplementary Materials 2.8).
We then tested whether the non-speech (motor/clinical/risk) measures correlated with one another as we would expect based on previous work7,59. They did not. In our sample, individuals with greater motor abnormalities (measured by finger-tapping) did not have worse overall symptoms or higher risk of conversion scores (see Supplementary Materials 2.9 for results). This suggests we did not have sufficient power to detect motor abnormalities. Indeed, past work has found a = 0.37 correlation between finger tapping speed and total negative symptoms in the CHR group7. Assuming the effect size is similar for finger-tapping variability and speech variability, which we measure here, a post-hoc power analysis suggests that, in the best case scenario (i.e., without a midway shift to remote testing), we would need a sample size of N = 56 (with = 0.05 and = 0.9) to detect this effect size, whereas our analysis had sample sizes ranging from N = 47–5160.
Discussion
We find evidence that individuals at clinical high-risk for psychosis produce more variable speech - in particular, more variable consonant voice-onset-times and speech rates—than healthy controls in two of the three speech types we study. However, contrary to predictions, we found that increased speech variability did not correlate with non-speech motor measures, symptom severity, or conversion risk scores. Follow-up analyses suggest that these comparisons may have been underpowered and, in particular, affected by a midway shift from in-person to remote testing. This theory-driven analysis provides a thorough first look at how speech production is affected in the CHR population and speaks to the promise and challenges facing this approach to measuring motor symptoms.
Not all aspects of speech are affected and not in all contexts
Our findings converge with Parola et al.’s43 meta-analysis, which found that other aspects of speech rate were one of the three strongest acoustic factors differentiating groups (n.b. they did not study voice-onset-time or speech rate variability). This stands in notable contrast to past findings in the field, which generally showed mixed results across studies (i.e., the particular speech measures that differed between groups differed depending on the study and speech samples; see discussion in Hitczenko et al.61). We believe this consistency across speech tasks and convergence with past studies reflects the benefits of adopting a theory-driven approach when studying highly variable speech signals.
That being said, most of the speech measures did not show group differences. In particular, we failed to find effects of variability of voiced consonant voice-onset-times (b,d,g), which likely reflects motor control demands. Specifically, English voiceless consonants involve more motor coordinating/timing than voiced consonants, as the vocal folds need to be suppressed for a specific amount of time62–67. We also failed to find the expected effect for vowels. This is less expected, but one possibility is that it may reflect the more precise articulatory and timing requirements for stop consonants, which are overall much shorter than vowels.
In addition to variable results across measures, group differences only appeared in some of the speech tasks: diadochokinetic-AMR, diadochokinetic-SMR, and read speech, but not spontaneous speech. This may reflect the degree to which different tasks present challenges to speech articulation. Diadochokinetic speech involves unnatural rapid repetition of syllables, while the passage participants read includes many low frequency words (e.g., Aristotle, bow, refraction). Indeed, qualitatively, participants often remarked on the difficult aspects of these tasks, or produced disfluent speech. These sorts of targeted, more challenging speech tasks may be necessary for detecting the impact of motor disruptions on speech articulation. Another possibility is that this simply reflects statistical power; the spontaneous task was shorter, and the content was more variable, which may have washed out subtle differences between groups.
In addition, there was some evidence of differences between in-person and remote participants. Healthy control individuals generally had more variable speech measures when tested remotely vs. in-person, reducing our ability to detect group differences. There has been substantial recent interest in developing remote options for all manner of clinical assessment (e.g., to reach individuals who are medically underserved68), but this result reveals that these approaches need to be carefully developed and validated. In the case of speech measures specifically, our results likely reflect issues in an analysis pipeline that was developed to analyze speech recorded in controlled laboratory conditions. More broadly, expanding access to such assessments requires developing analysis methods that are robust to variation in testing and recording conditions.
Finally, when group differences did emerge, they were subtle. In particular, the distributions over speech measures between the CHR and HC subgroups overlapped substantially. Predicting group membership from individual speech measures yielded categorization accuracy rates between 60-65%, which is typically considered inadequate (see Supplementary Materials 2.6 for categorization analyses)69,70. It is important to stress that these measures are not diagnostic on their own (after all, speech is affected by a large number of interacting factors, only one of which is motor abilities). Nonetheless, the fact that we observe group differences and above-chance categorization rates supports the notion that theoretically-motivated speech measures, in conjunction with other sources of information, could be useful for diagnostics down the line, and future work should continue to study this possibility.
Speech measures did not correlate with motor, clinical, or conversion risk variables
While the observed group differences provide converging support that speech/motor symptoms are observed very early in the progression of psychosis, the biggest challenge facing these speech measures is that they mostly did not correlate with clinical/motor/risk variables. This could reflect insufficient power. We had non-speech motor symptoms for ~50 CHR participants, which would only let us detect effect sizes of ~0.39 or higher ( = 0.05; = 0.9). Exploratory analyses studying the relationship between motor and clinical measures in our sample (Supplementary Materials 2.9) suggest that our particular sample and measures may have been insufficient to detect the typical clinical-high-risk motor profile, which would also weaken our ability to detect speech-motor relationships. Relatedly, the clinical-high-risk participants in our sample all had a relatively low risk of conversion (i.e., risk scores of 10.1% or lower), so there may not have been enough variability in clinical status in our sample to detect significant effects between speech measures and risk/symptom severity scores. Finally, we had to adapt our data collection procedure partway through to adhere to pandemic-related restrictions, including shortening the finger-tapping task and collecting speech samples remotely (participants were mailed audio recorders to their homes). While these changes were unavoidable, they reduced our power (e.g., by reducing the number of finger-tapping observations) and may have affected reliability, by introducing noise into our measures. We provided extensive guidelines, but ultimately had limited control over the participants’ environment (e.g., how noisy it was) and equipment (e.g., keyboard). Beyond practical task differences, the pandemic also may have had a substantial effect on individuals’ mental health, further increasing variability71,72.
Nonetheless, even though the speech measures did not correlate with clinical/motor variables, the fact that they differed by clinical status (CHR vs. HC), which is assigned based on clinical interview, means that, on some level, these measures must be related to symptomatology. In addition, the speech differences we observe could reflect motor abnormalities that are not captured by previously-developed measures (e.g., finger-tapping). In this case, we would not expect to see a correlation between the speech measures and previously-developed measures, but the speech measures would nonetheless be clinically informative. In sum, these differences are worthy of further investigation to understand what these speech measures reflect and how they can help researchers/clinicians.
Recommendations for future approaches
Based on our results, future studies should prioritize difficult speech tasks that specifically target the speech feature of interest (e.g., diadochokinetic speech involving both voiceless/voiced consonants and a variety of vowels; sustained phonation tasks). An additional benefit of the more targeted measures is that they are easily transferable across other languages (many languages have the diadochokinetic speech syllables), which will be critical for establishing the validity and generalizability of these measures across populations73.
It would also be informative to systematically vary the phonetic (and other types of) complexity of the speech stimuli used (as in Kuruvilla-Dugdale et al.74), in order to systematically test whether more difficult speech stimuli better reveal the subtle differences in motor performance between clinical-high-risk and healthy participants, and are more sensitive to clinical severity/risk. Future studies should also study other motor measures that have been shown to capture motor/cerebellar abnormalities in early psychosis (e.g., pursuit rotor procedural learning tasks75, in which participants track a moving target with a computer mouse, or postural sway tasks9,76, in which participants’ balance is evaluated in various standing conditions). Because many existing motor tasks are difficult to adapt to remote testing, the COVID-19 pandemic limited the motor measures we could collect from our participants, but these tasks tap into distinct components of motor control (timing, motor learning, coordination, etc.), and determining which (if any) of them correlate with the speech measures we study will be important.
Finally, the clinical-high-risk group is heterogeneous and future work should identify and study well-motivated subgroups26,77,78. This is important because speech is affected by motor abilities, but also many other factors. For example, past work has often studied speech as a window into negative symptoms43. Even within the motor domain, it is possible that several motor networks may be impacted in this population (e.g., some individuals may show increased motor variability, while others may exhibit catatonia, or a reduction in movement variability/increase in rigidity)4,59,79, and that numerous distinct motor signs may be present in the same individuals26. While the motor deficits we focus on here should result in more speech variability, researchers adopting other focuses may predict that individuals will exhibit less speech variability. Competing effects of this sort could obscure a clear relationship between speech and symptoms. To address this issue, future work could collect a larger clinical-high-risk sample and identify subgroups (e.g., one that primarily shows negative symptoms, one that primarily shows increased motor variability, one that shows increased motor rigidity) and test whether they show different speech profiles in accordance with their different symptom profiles.
Overall, however, while many questions remain, the present work provides a solid foundation for future work investigating the insights that speech production can provide for understanding the mechanisms impacted in individuals at clinical-high-risk for psychosis.
Methods
Participants
N = 122 participants (N = 56 CHR; N = 66 HC) provided speech data, though not everybody provided data for all three tasks. The data of two CHR participants were excluded: one dropped out of the study and the other was later determined to have been erroneously classified as high-risk. This left N = 104 (N = 51 CHR; N = 53 HC) diadochokinetic speech samples, N = 120 (N = 55 CHR; N = 65 HC) read speech samples, and N = 100 (N = 50 CHR; N = 50 HC) spontaneous speech samples. The Structured Interview for Prodromal Syndromes (SIPS) was used to determine the clinical status of each participant (CHR vs. HC)80. See Table 3 for participant demographics.
Table 3.
Summary of participant demographic information.
| Clinical high-risk (CHR) | Healthy controls (HC) | |
|---|---|---|
| N | 56 (In-Person: 26; Remote: 30) | 66 (In-Person: 44; Remote: 22) |
| Sex (% Female) | 60.7% (In-Person: 50%; Remote: 70%) | 62.1% (In-Person: 59.1%; Remote: 68.2%) |
| Age (SD) | 21.8 (2.8) (In-Person: 21.5 (2.4); Remote: 22.1 (3.1)) | 21.7 (3.2) (In-Person: 21.2 (3); Remote: 22.7 (3.5)) |
| Race |
44.6% White; 19.6% Black; 17.9% Asian; 8.9% Central/South American; 1.8% Native Hawaiian or Pacific Islander; 7.2% Multiracial (1.8% First Nations & White; 1.8% Black & White; 3.6% Asian & White) (In-Person: 38.5% White; 30.8% Black; 19.2% Asian; 11.5% Central/South American; Remote: 50% White; 10% Black; 16.7% Asian; 6.7% Central/South American; 3.3% Native Hawaiian or Pacific Islander; 13.2% Multiracial (3.3% First Nations & White; 3.3% Black & White; 6.7% Asian & White)) |
45.5% White; 9.1% Black; 28.8% Asian; 3% First Nations; 13.6% Multiracial (1.5% First Nations & White; 1.5% Black & White; 3% Asian & White; 7.6% not reported) (In-Person: 50% White; 13.6% Black; 25% Asian; 2.3% First Nations; 9.1% Multiracial (not reported); Remote: 36.4% White; 36.4% Asian; 4.5% First Nations; 22.7% Multiracial (4.5% First Nations & White; 4.5% Black & White; 9.1% Asian & White; 4.5% not reported)) |
| Ethnicity | 26.8% Hispanic; 73.2% Not Hispanic (In-Person: 23.1% Hispanic; Remote: 30% Hispanic) | 10.6% Hispanic; 89.4% Not Hispanic (In-Person: 11.4% Hispanic; Remote: 9.1% Hispanic) |
| First Language | 62.5% English; 17.9% Other; 19.6% Not reported (In-Person: 73.1% English; 26.9% Other; Remote: 53.3% English; 10% Other; 36.7% Not reported | 80.3% English; 18.2% Other; 1.5% Not reported (In-Person: 81.8% English; 15.9% Other; 2.3% Not reported; Remote: 77.3% English; 22.7% Other) |
Speech tasks
Working one-on-one with an experimenter, participants provided three speech samples, recorded via a Zoom H2n portable audio recorder (44.1 kHz sample rate; 16-bit recording; X/Y recording configuration; no compression/limiting or low-cut filtering was used). Participants were seated 16 inches from the recorder and worked with the experimenter to ensure proper audio/gain levels prior to recording.
Diadochokinetic speech task
Participants first completed a diadochokinetic speech task, commonly-used to examine speech motor abilities, in which they were asked to produce particular syllable types as quickly and accurately as possible54,55. They first produced 12 Alternating Motion Rate (AMR) trials, repeating a target syllable 15 times (two trials each of: pa-pa-pa…, ta-ta-ta…, ka-ka-ka…, ba-ba-ba…, da-da-da…, ga-ga-ga…). They then produced 20 Sequential Motion Rate (SMR) trials, producing sequences of three syllables 10 times each per trial (10 trials each of pa-ta-ka… and ka-ta-pa…).
Read speech task: Rainbow passage
Participants then read aloud the Rainbow Passage at a comfortable pace (passage in Supplementary Materials 1.1)81. The passage is commonly-used for eliciting read speech, as it is phonetically balanced (covering all English speech sounds) and emotionally neutral. The Rainbow Passage has quite a few low-frequency words (e.g., “Aristotle”, “refraction”), so it is relatively difficult to read. This speech task allows us to precisely control the speech content, while eliciting a more naturalistic speaking style than diadochokinetic speech.
Spontaneous procedural description task: Peanut butter and jelly
Finally, we elicited spontaneous speech, by asking participants to describe how to make a peanut butter and jelly sandwich for ~2 min. Unlike the other tasks, the speech content differed between participants (though many words overlapped: e.g., peanut, butter, knife). Such procedural description tasks are less emotionally and cognitively demanding than personal narratives while still generating a large volume of speech82.
Speech measures
At a high-level, for each participant, for each speech sample, we estimated how variable (i) their consonant productions, (ii) their vowel productions, (iii) their speech rates, and (iv) their pausing/timing were using semi-automated methods83–86. Semi-automated methods greatly increase the amount of speech we can study, as extracting speech measures by-hand is extremely time-consuming, and ensure that our measurements are consistent and replicable (analysis code is available at github.com/khitczenko/chr_speech; the National Institute of Mental Health Data Archive provides de-identified clinical, risk, and demographic information). As a result, we focused on speech measures that have been extremely well-studied and can be reliably measured automatically. The main text focuses on (i) variability in the voice-onset-time of stop consonants (in English: p,t,k,b,d,g), or the time that elapses between the release of the consonant and the onset of the following vowel, (ii) variability in the duration of vowels, and (iii) variability in speech rates (calculated at the syllable level). We discuss the remaining speech measures we studied48–53 in Table 1 and Supplementary Materials 1.3.
For the diadochokinetic speech samples, we used DDKtor86 (https://github.com/MLSpeech/DDKtor) - a deep neural network model specifically trained to match human annotations of diadochokinetic speech - to automatically obtain the onset and offset (and thus, duration) of each stop consonant, vowel, and syllable given hand-selected windows of analysis which corresponded to the individual diadochokinetic trials. For the read and spontaneous speech, we first used the Montreal Forced Aligner85 (https://github.com/MontrealCorpusTools/Montreal-Forced-Aligner) to automatically align a transcript we created for each speech sample to its audio. The Montreal Forced Aligner uses a pronunciation dictionary and a trained acoustic model (specifically, a GMM-HMM model triphone model trained from MFCC features) to provide the onset and offset (and thus, duration) of each vowel, consonant, and syllable produced. We then applied AutoVOT87,88—a discriminative learning algorithm trained to match human voice-onset-time measurements - to the aligner output to obtain even more reliable onsets and offsets for the stop consonant voice-onset-times. Finally, we used the FastTrack software84 (https://github.com/santiagobarreda/FastTrack) to automatically obtain measurements of the first and second formant for each vowel that was output either from DDKtor (for diadochokinetic speech) or from the Montreal Forced Aligner (for read and spontaneous speech). FastTrack uses linear predictive coding to systematically identify candidate formant analyses for each vowel, from which it selects one winning analysis based on the smoothness of the predicted formant contours. We followed all recommendations provided by the creators of these tools (see Supplementary Materials 1.2 for full details).
These automated tools have been evaluated in the context of previous work and are highly reliable. The DDKtor software matches human annotations of diadochokinetic segment duration with correlations of r = 0.85-0.90 and matches human annotation of diadochokinetic speech rate with correlations of r = 0.94–0.9786. The Montreal Forced Aligner has an average phone boundary error of ~20 ms, across both isolated word productions and conversational speech, comparable to human interrater reliability85. For the AutoVOT software, ~90% of its predicted voice-onset-times are within 10–15 ms of gold-standard human annotation, again paralleling interrater reliability rates87,88. Finally, FastTrack has an average error of ~20 Hz and 98.9% of vowels have errors of less than 5% of the human-annotated value84. Overall, these tools perform comparably to human annotators and, when applied to our speech samples specifically, result in valid measurements that match expected average values (Supplementary Materials 1.4).
We measure variability using coefficients of variation, which control for potential differences in means, calculated as follows89:
All measures are log-transformed in the analyses (as they tend to be skewed right, due to lower bounds at 0).
Variability in consonant duration
We focused on syllable-initial stop consonants (in English: p, t, k, b, d, g) that precede vowels (e.g., the bolded sounds in “passerby”, “pulp”, “peanut”, but not the “p” in “prism”), as they have easily-measurable acoustics45,90. In the diadochokinetic speech tasks, we restricted our analysis even further to only include voiceless stop consonants (in English: p, t, k), as the automated tool we use for this task has only been validated for this subset. We used the voice-onset-time duration of each consonant to calculate: (i) the coefficient of variation over voiceless stop (p,t,k) consonants (all speech samples) and (ii) the coefficient of variation over voiced stop (b,d,g) consonants (read and spontaneous speech only).
Variability in vowel duration
We focused on vowels that bear primary stress (e.g., only the bolded sounds: “element”, “awaken”, “analysis”) as they have easily-measurable acoustics. We used each relevant vowel’s duration to calculate the coefficient of variation across vowel tokens in each speech sample.
Variability in speech rate
Speech rate was calculated as the number of syllables participants produced per second. For diadochokinetic speech, we calculated the speech rate of each individual trial and calculated the coefficient of variation across all trials produced. For read and spontaneous speech, we calculated the speech rate of delimited phrases, defined as any spoken interval between silences of at least 150 ms91. We then calculated the coefficient of variation across all produced phrases.
Non-speech validation measures
We used symptom severity measures, non-speech motor measures, and risk measures to establish the clinical, convergent, and predictive validity of the speech measures.
Clinical utility: Symptomatology
We assessed symptom severity with SIPS scores80. We focused particularly on the positive symptoms, negative symptoms, and disorganized symptoms totals to study how broadly clinically useful the speech measures are. In addition, we looked at the individual item G3 (“Motor Difficulties”—i.e., have you noticed any clumsiness, awkwardness, or lack of coordination in your movements?) to provide convergent validity of our speech measures as measuring motor difficulties.
Convergent validity: Finger-tapping scores
Participants also completed a computerized finger-tapping task, a well-established neuropsychological measure of motor deficits7,59,92–98. This is an ideal task because it taps into broad motor network function, including motor timing, which is often affected in motor speech disorders, has been found to be sensitive to mechanisms driving psychosis, and is readily amenable to reliable and valid in-person and remote assessments7,26,92,99–110. In this task, participants are instructed to press the spacebar with their index finger as quickly as possible for 10 s. They complete three trials per hand. Motivated by previous work59,111 and to parallel our speech measures, we study the coefficient of variation in number of taps across trials, calculated separately for the dominant and non-dominant hands.
Predictive validity: SIPS risk calculator
Finally, we use the SIPS-RC risk calculator112 to calculate a probability estimate of each participant’s risk of conversion to psychosis within one year (from SIPS and General Functioning scores109). SIPS-RC scores can range from 0.4% to 46.9%, but range from 0.8% to 10.1% in our sample.
Adaptations to remote testing
Data collection occurred between 2019–2022, and our study had to be adapted to the remote format partway through due to the COVID-19 pandemic.
We adapted speech data collection, by mailing participants the same Zoom H2n recorders that had been used in the lab prior to the pandemic and having an experimenter administer the tasks over Zoom (tele-conferencing software). Similarly, all clinical interviews were conducted over Zoom beginning March 2020. Finally, the finger-tapping task, which, prior to March 2020, was collected in-lab as part of the Penn computerized neurocognitive battery113 and included 5 trials per hand was adapted into a shortened, online version, where participants only completed 3 trials per hand. To equate these measures, only the first 3 trials from each in-person participant’s task were used.
Analyses
We run separate analyses for each speech measure in each speech sample type114–119. First, to test our prediction that CHR individuals exhibit more variability in their speech productions relative to controls, we run a linear regression predicting each speech measures (separately) from group status (CHR vs. HC). For durational speech measures, we control for averaged speech rate, by including it as an additional predictor in the regression. Next, for each speech measure that significantly differentiates clinical status, we test its clinical/convergent/predictive validity, by running separate linear regressions predicting each validation measure from each speech measure, within the CHR group only. We use an alpha level of = 0.05 for all statistical tests.
Supplementary information
Supplementary Materials for "Speech characteristics yield important clues about motor function. Speech variability in individuals at clinical high-risk for psychosis"
Acknowledgements
This work was supported by the National Institutes of Health (R21 MH119677 to M.G. and V.A.M.). Thank you to Emily Cibelli for assistance developing the speech protocol, to Cameron Martinez, Denise Zou, Solmi Park, Maksim Giljen, Gabrielle Olson, Juston Osborne, and Kate Damme for assistance in data collection/compilation, and to the editor and reviewers for their helpful feedback.
Data availability
All speech measure data and analysis code used in this study are available at github.com/khitczenko/chr_speech. The National Institute of Mental Health Data Archive provides the de-identified clinical, risk, and demographic information.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41537-023-00382-9.
References
- 1.Cuesta MJ, et al. Motor abnormalities in first-episode psychosis patients and long-term psychosocial functioning. Schizophr. Res. 2018;200:97–103. doi: 10.1016/j.schres.2017.08.050. [DOI] [PubMed] [Google Scholar]
- 2.van Harten PN, Walther S, Kent JS, Sponheim SR, Mittal VA. The clinical and prognostic value of motor abnormalities in psychosis, and the importance of instrumental assessment. Neurosci. Biobehav. Rev. 2017;80:476–487. doi: 10.1016/j.neubiorev.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Walther S, Strik W. Motor symptoms and schizophrenia. Neuropsychobiology. 2012;66:77–92. doi: 10.1159/000339456. [DOI] [PubMed] [Google Scholar]
- 4.Mittal VA, Bernard JA, Northoff G. What can different motor circuits tell us about psychosis? An RDoC perspective. Schizophr. Bull. 2017;43:949–955. doi: 10.1093/schbul/sbx087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dean DJ, Teulings HL, Caligiuri M, Mittal VA. Handwriting analysis indicates spontaneous dyskinesias in neuroleptic naïve adolescents at high risk for psychosis. J. Vis. Exp. 2013 doi: 10.3791/50852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osborne KJ, Kraus B, Lam PH, Vargas T, Mittal VA. Contingent negative variation blunting and psychomotor dysfunction in schizophrenia: A systematic review. Schizophr. Bull. 2020;46:1144–1154. doi: 10.1093/schbul/sbaa043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damme KSF, Osborne KJ, Gold JM, Mittal VA. Detecting motor slowing in clinical high risk for psychosis in a computerized finger tapping model. Eur. Arch. Psychiatry Clin. Neurosci. 2020;270:393–397. doi: 10.1007/s00406-019-01059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean DJ, Samson AT, Newberry R, Mittal VA. Motion energy analysis reveals altered body movement in youth at risk for psychosis. Schizophr. Res. 2018;200:35–41. doi: 10.1016/j.schres.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernard JA, et al. Cerebellar networks in individuals at ultra high-risk of psychosis: Impact on postural sway and symptom severity. Hum. Brain Mapp. 2014;35:4064–4078. doi: 10.1002/hbm.22458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kent RD. Research on speech motor control and its disorders: A review and prospective. J. Commun. Disord. 2000;33:391–428. doi: 10.1016/S0021-9924(00)00023-X. [DOI] [PubMed] [Google Scholar]
- 11.Andreasen NC, Paradiso S, O’Leary DS. "Cognitive Dysmetria" as an integrative theory of schizophrenia: A dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr. Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 12.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: Version III—the final common pathway. Schizophr. Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Northoff G, Hirjak D, Wolf RC, Magioncalda P, Martino M. All roads lead to the motor cortex: Psychomotor mechanisms and their biochemical modulation in psychiatric disorders. Mol. Psychiatry. 2021;26:92–102. doi: 10.1038/s41380-020-0814-5. [DOI] [PubMed] [Google Scholar]
- 14.Erlenmeyer-Kimling L, et al. Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychoses: The New York high-risk project. Am. J. Psychiatry. 2000;157:1416–1422. doi: 10.1176/appi.ajp.157.9.1416. [DOI] [PubMed] [Google Scholar]
- 15.Filatova S, et al. Early motor developmental milestones and schizophrenia: A systematic review and meta-analysis. Schizophr. Res. 2017;188:13–20. doi: 10.1016/j.schres.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Kindler J, et al. Abnormal involuntary movements are linked to psychosis-risk in children and adolescents: Results of a population-based study. Schizophr. Res. 2016;174:58–64. doi: 10.1016/j.schres.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 17.Rosso IM, et al. Childhood neuromotor dysfunction in schizophrenia patients and their unaffected siblings: A prospective cohort study. Schizophr. Bull. 2000;26:367–378. doi: 10.1093/oxfordjournals.schbul.a033459. [DOI] [PubMed] [Google Scholar]
- 18.Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr. Bull. 1994;20:441–451. doi: 10.1093/schbul/20.3.441. [DOI] [PubMed] [Google Scholar]
- 19.Schiffman J, et al. Childhood motor coordination and adult schizophrenia spectrum disorders. Am. J. Psychiatry. 2009;166:1041–1047. doi: 10.1176/appi.ajp.2009.08091400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howes OD, et al. Molecular imaging studies of the striatal dopaminergic system in psychosis and predictions for the prodromal phase of psychosis. Br. J. Psychiatry. 2007;191:s13–s18. doi: 10.1192/bjp.191.51.s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mittal VA, Dhruv S, Tessner KD, Walder DJ, Walker EF. The relations among putative biorisk markers in schizotypal adolescents: Minor physical anomalies, movement abnormalities, and salivary cortisol. Biol. Psychiatry. 2007;61:1179–1186. doi: 10.1016/j.biopsych.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 22.Mittal VA, et al. Movement abnormalities and the progression of prodromal symptomatology in adolescents at risk for psychotic disorders. J. Abnorm. Psychol. 2007;116:260–267. doi: 10.1037/0021-843X.116.2.260. [DOI] [PubMed] [Google Scholar]
- 23.Mittal VA, Neumann C, Saczawa M, Walker EF. Longitudinal progression of movement abnormalities in relation to psychotic symptoms in adolescents at high risk of schizophrenia. Arch. Gen. Psychiatry. 2008;65:165–171. doi: 10.1001/archgenpsychiatry.2007.23. [DOI] [PubMed] [Google Scholar]
- 24.Mittal VA, Walker EF. Movement abnormalities predict conversion to Axis I psychosis among prodromal adolescents. J. Abnorm. Psychol. 2007;116:796–803. doi: 10.1037/0021-843X.116.4.796. [DOI] [PubMed] [Google Scholar]
- 25.Mittal VA, et al. Markers of basal ganglia dysfunction and conversion to psychosis: Neurocognitive deficits and dyskinesias in the prodromal period. Biol. Psychiatry. 2010;68:93–99. doi: 10.1016/j.biopsych.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dean DJ, Walther S, Bernard JA, Mittal VA. Motor clusters reveal differences in risk for psychosis, cognitive functioning, and thalamocortical connectivity: Evidence for vulnerability subtypes. Clin. Psychol. Sci. 2018;6:721–734. doi: 10.1177/2167702618773759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mittal VA, Walther S. As motor system pathophysiology returns to the forefront of psychosis research, clinical implications should hold center stage. Schizophr. Bull. 2019;45:495–497. doi: 10.1093/schbul/sby176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pappa S, Dazzan P. Spontaneous movement disorders in antipsychotic-naive patients with first-episode psychoses: A systematic review. Psychol. Med. 2009;39:1065–1076. doi: 10.1017/S0033291708004716. [DOI] [PubMed] [Google Scholar]
- 29.Quinn J, et al. Vulnerability to involuntary movements over a lifetime trajectory of schizophrenia approaches 100%, in association with executive (frontal) dysfunction. Schizophr. Res. 2001;49:79–87. doi: 10.1016/S0920-9964(99)00220-0. [DOI] [PubMed] [Google Scholar]
- 30.Walther S, Mittal VA. Why we should take a closer look at gestures. Schizophr. Bull. 2016;42:259–261. doi: 10.1093/schbul/sbv229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mittal V. A., Walker E. F. Movement abnormalities: A putative biomarker of risk for psychosis. In (ed Ritsner M. S.) The Handbook of Neuropsychiatric Biomarkers, Endophenotypes and Genes: Neuropsychological Endophenotypes and Biomarkers. (Springer, Netherlands, Dordrecht) 10.1007/978-1-4020-9464-4_17 (2009).
- 32.Mittal VA, Wakschlag LS. Research Domain Criteria (RDoC) grows up: Strengthening neurodevelopmental investigation within the RDoC framework. J. Affect. Disord. 2017;216:30–35. doi: 10.1016/j.jad.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pieters LE, Nadesalingam N, Walther S, van Harten PN. A systematic review of the prognostic value of motor abnormalities on clinical outcome in psychosis. Neurosci. Biobehav. Rev. 2022;132:691–705. doi: 10.1016/j.neubiorev.2021.11.027. [DOI] [PubMed] [Google Scholar]
- 34.Moberget T, Ivry RB. Prediction, psychosis, and the cerebellum. Biol. Psychiatry. 2019;4:820–831. doi: 10.1016/j.bpsc.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arevian AC, et al. Clinical state tracking in serious mental illness through computational analysis of speech. PLoS ONE. 2020;15:e0225695. doi: 10.1371/journal.pone.0225695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernardini F, et al. Associations of acoustically measured tongue/jaw movements and portion of time speaking with negative symptom severity in patients with schizophrenia in Italy and the United States. Psychiatry Res. 2016;239:253–258. doi: 10.1016/j.psychres.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 37.Cohen AS, Alpert M, Nienow TM, Dinzeo TJ, Docherty NM. Computerized measurement of negative symptoms in schizophrenia. J. Psychiatr. Res. 2008;42:827–836. doi: 10.1016/j.jpsychires.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen AS, Mitchell KR, Docherty NM, Horan WP. Vocal expression in schizophrenia: Less than meets the ear. J. Abnorm. Psychol. 2016;125:299–309. doi: 10.1037/abn0000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Compton MT, et al. The aprosody of schizophrenia: Computationally derived acoustic phonetic underpinnings of monotone speech. Schizophr. Res. 2018;197:392–399. doi: 10.1016/j.schres.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Covington MA, et al. Phonetic measures of reduced tongue movement correlate with negative symptom severity in hospitalized patients with first-episode schizophrenia-spectrum disorders. Schizophr. Res. 2012;142:93–95. doi: 10.1016/j.schres.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lozano-Goupil J, Raffard S, Capdevielle D, Aigoin E, Marin L. Gesture-speech synchrony in schizophrenia: A pilot study using a kinematic-acoustic analysis. Neuropsychologia. 2022;174:108347. doi: 10.1016/j.neuropsychologia.2022.108347. [DOI] [PubMed] [Google Scholar]
- 42.Martínez-Sánchez F, et al. Can the acoustic analysis of expressive prosody discriminate schizophrenia? Span. J. Psychol. 2015;18:E86. doi: 10.1017/sjp.2015.85. [DOI] [PubMed] [Google Scholar]
- 43.Parola A, Simonsen A, Bliksted V, Fusaroli R. Voice patterns in schizophrenia: A systematic review and Bayesian meta-analysis. Schizophr. Res. 2020;216:24–40. doi: 10.1016/j.schres.2019.11.031. [DOI] [PubMed] [Google Scholar]
- 44.Rapcan V, et al. Acoustic and temporal analysis of speech: A potential biomarker for schizophrenia. Med. Eng. Phys. 2010;32:1074–1079. doi: 10.1016/j.medengphy.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 45.Pascal A, et al. Voice onset time in aphasia, apraxia of speech and dysarthria: A review. Clin. Linguist. Phon. 2000;14:131–150. doi: 10.1080/026992000298878. [DOI] [Google Scholar]
- 46.Goberman AM, Coelho C. Acoustic analysis of Parkinsonian speech I: Speech characteristics and L-Dopa therapy. NeuroRehabilitation. 2002;17:237–246. doi: 10.3233/NRE-2002-17310. [DOI] [PubMed] [Google Scholar]
- 47.Kent RD, Kim Y‐J. Toward an acoustic typology of motor speech disorders. Clin. Linguist. Phon. 2003;17:427–445. doi: 10.1080/0269920031000086248. [DOI] [PubMed] [Google Scholar]
- 48.Sichlinger L, Cibelli E, Goldrick M, Mittal VA. Clinical correlates of aberrant conversational turn-taking in youth at clinical high-risk for psychosis. Schizophr. Res. 2019;204:419–420. doi: 10.1016/j.schres.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stanislawski ER, et al. Negative symptoms and speech pauses in youths at clinical high risk for psychosis. npj Schizophr. 2021;7:1–3. doi: 10.1038/s41537-020-00132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hillenbrand J, Getty LA, Clark MJ, Wheeler K. Acoustic characteristics of American English vowels. J. Acoust. Soc. Am. 1995;97:3099–3111. doi: 10.1121/1.411872. [DOI] [PubMed] [Google Scholar]
- 51.McCloy DR, Wright RA, Souza PE. Talker versus dialect effects on speech intelligibility: A symmetrical study. Lang. Speech. 2015;58:371–386. doi: 10.1177/0023830914559234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie X, Myers E. LIFG sensitivity to phonetic competition in receptive language processing: A comparison of clear and conversational speech. J. Cogn. Neurosci. 2018;30:267–280. doi: 10.1162/jocn_a_01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niziolek CA, Kiran S. Assessing speech correction abilities with acoustic analyses: Evidence of preserved online correction in persons with aphasia. Int. J. Speech Lang. Pathol. 2018;20:659–668. doi: 10.1080/17549507.2018.1498920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ackermann H, Hertrich I, Hehr T. Oral diadochokinesis in neurological dysarthrias. Folia Phoniatr. Logop. 1995;47:15–23. doi: 10.1159/000266338. [DOI] [PubMed] [Google Scholar]
- 55.Fletcher SG. Time-by-count measurement of diadochokinetic syllable rate. J. Speech Hear. Res. 1972;15:763–770. doi: 10.1044/jshr.1504.763. [DOI] [PubMed] [Google Scholar]
- 56.Hitczenko K., Cowan H., Mittal V., Goldrick M. Automated coherence measures fail to index thought disorder in individuals at risk for psychosis. In Proceedings of the Seventh Workshop on Computational Linguistics and Clinical Psychology: Improving Access. Online: Association for Computational Linguistics; 2021:129–150.
- 57.Hitczenko K, Cowan HR, Goldrick M, Mittal VA. Racial and ethnic biases in computational approaches to psychopathology. Schizophr. Bull. 2022;48:285–288. doi: 10.1093/schbul/sbab131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palaniyappan L. More than a biomarker: Could language be a biosocial marker of psychosis. npj Schizophr. 2021;7:42. doi: 10.1038/s41537-021-00172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Damme KSF, Schiffman J, Ellman LM, Mittal VA. Sensorimotor and activity psychosis-risk (SMAP-R) scale: An exploration of scale structure with replication and validation. Schizophr. Bull. 2021;47:332–343. doi: 10.1093/schbul/sbaa138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 61.Hitczenko K, Mittal VA, Goldrick M. Understanding language abnormalities and associated clinical markers in psychosis: The promise of computational methods. Schizophr. Bull. 2021;47:344–362. doi: 10.1093/schbul/sbaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bortolini U, Zmarich C, Fior R, Bonifacio S. Word-initial voicing in the productions of stops in normal and preterm Italian infants. Int. J. Pediatr. Otorhinolaryngol. 1995;31:191–206. doi: 10.1016/0165-5876(94)01091-B. [DOI] [PubMed] [Google Scholar]
- 63.Kewley-Port D, Preston MS. Early apical stop production: A voice onset time analysis. J. Phon. 1974;2:195–210. doi: 10.1016/S0095-4470(19)31270-7. [DOI] [Google Scholar]
- 64.Kong E., Beckman M. E. & Edwards J. Fine-grained phonetics and acquisition of Greek voiced stops. In Proceedings of the XVIth International Congress of Phonetic Sciences. Saarbücken: University of Saarlandes, 6–10 (2007).
- 65.Kessinger RH, Blumstein SE. Effects of speaking rate on voice-onset time in Thai, French, and English. J. Phon. 1997;25:143–168. doi: 10.1006/jpho.1996.0039. [DOI] [Google Scholar]
- 66.Gavino M. F. & Goldrick M. Consequences of mixing and switching languages for retrieval and articulation. Bilingualism10.1017/S1366728922000682 (2022).
- 67.Goldrick M, Vaughn C, Murphy A. The effects of lexical neighbors on stop consonant articulation. J. Acoust. Soc. Am. 2013;134:EL172–EL177. doi: 10.1121/1.4812821. [DOI] [PubMed] [Google Scholar]
- 68.Sharp IR, Kobak KA, Osman DA. The use of videoconferencing with patients with psychosis: A review of the literature. Ann. Gen. Psychiatry. 2011;10:14. doi: 10.1186/1744-859X-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010;5:1315–1316. doi: 10.1097/JTO.0b013e3181ec173d. [DOI] [PubMed] [Google Scholar]
- 70.Hosmer D. W., Lemeshow S. & Sturdivant R. Assessing the fit of the model. In Applied Logistic Regression. (John Wiley & Sons, Ltd., 2013) 153–225. https://onlinelibrary.wiley.com/doi/abs/10.1002/9781118548387.ch5.
- 71.Brown E, et al. The potential impact of COVID-19 on psychosis: A rapid review of contemporary epidemic and pandemic research. Schizophr. Res. 2020;222:79–87. doi: 10.1016/j.schres.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Druss BG. Addressing the COVID-19 pandemic in populations with serious mental illness. JAMA Psychiatry. 2020;77:891–892. doi: 10.1001/jamapsychiatry.2020.0894. [DOI] [PubMed] [Google Scholar]
- 73.Parola, A. et al. Speech disturbances in schizophrenia: Assessing cross-linguistic generalizability of NLP automated measures of coherence. Schizophr. Res. 10.1016/j.schres.2022.07.002 (2022). [DOI] [PubMed]
- 74.Kuruvilla DM, Salazar M, Zhang A, Mefferd AS. Detection of articulatory deficits in Parkinson’s disease: Can systematic manipulations of phonetic complexity help. J. Speech Lang. Hear. Res. 2020;63:2084–2098. doi: 10.1044/2020_JSLHR-19-00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dean DJ, et al. Cerebellar morphology and procedural learning impairment in neuroleptic-naive youth at ultrahigh risk of psychosis. Clin. Psychol. Sci. 2014;2:152–164. doi: 10.1177/2167702613500039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dean DJ, et al. Increased postural sway predicts negative symptom progression in youth at ultrahigh risk for psychosis. Schizophr. Res. 2015;162:86–89. doi: 10.1016/j.schres.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta T, Cowan HR, Strauss GP, Walker EF, Mittal VA. Deconstructing negative symptoms in individuals at clinical high-risk for psychosis: Evidence for volitional and diminished emotionality subgroups that predict clinical presentation and functional outcome. Schizophr. Bull. 2021;47:56–63. doi: 10.1093/schbul/sbaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mittal VA, Walker EF, Strauss GP. The COVID-19 pandemic introduces diagnostic and treatment planning complexity for individuals at clinical high risk for psychosis. Schizophr. Bull. 2021;47:1518–1523. doi: 10.1093/schbul/sbab083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Masucci MD, Lister A, Corcoran CM, Brucato G, Girgis RR. Motor dysfunction as a risk factor for conversion to psychosis independent of medication use in a psychosis-risk cohort. J. Nerv. Ment. Dis. 2018;206:356–361. doi: 10.1097/NMD.0000000000000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller TJ, et al. Symptom assessment in schizophrenic prodromal states. Psychiatr. Q. 1999;70:273–287. doi: 10.1023/A:1022034115078. [DOI] [PubMed] [Google Scholar]
- 81.Fairbanks G. Voice and Articulation Drillbook. (Addison-Wesley Educational Publishers, 1960).
- 82.Fromm D. A., Forbes M., Holland A. & MacWhinney B. PWAs and PBJs: Language for Describing A Simple Procedure. http://aphasiology.pitt.edu/2491/ (2013).
- 83.Adi Y., Keshet J., Dmitrieva O. & Goldrick M. Automatic measurement of voice onset time and prevoicing using recurrent neural networks. in Interspeech (ISCA, 2016) 3152–3155. 10.21437/Interspeech.2016-893.
- 84.Barreda S. Fast Track: Fast (nearly) automatic formant-tracking using Praat. Linguist. Vanguard. 10.1515/lingvan-2020-0051 (2021).
- 85.McAuliffe M., Socolof M., Mihuc S., Wagner M. & Sonderegger M. Montreal Forced Aligner: Trainable Text-Speech Alignment Using Kaldi. in Interspeech (ISCA, 2017) 498–502. 10.21437/Interspeech.2017-1386.
- 86.Segal, Y. et al. DDKtor: Automatic Diadochokinetic Speech Analysis. In: Proc. Annual Conference of the International Speech Communication Association, INTERSPEECH. Vol 2022-September, 4611–4615. 10.21437/Interspeech.2022-311 (2022).
- 87.Sonderegger M, Keshet J. Automatic discriminative measurement of voice onset time. J. Acoust. Soc. Am. 2012;132:3965–3979. doi: 10.1121/1.4763995. [DOI] [PubMed] [Google Scholar]
- 88.Keshet J., Sonderegger M. & Knowles T. AutoVOT: A tool for automatic measurement of voice onset time using discriminative structured prediction. https://github.com/mlml/autovot/ (2014). [DOI] [PubMed]
- 89.Whiteside SP, Dobbin R, Henry L. Patterns of variability in voice onset time: A developmental study of motor speech skills in humans. Neurosci. Lett. 2003;347:29–32. doi: 10.1016/S0304-3940(03)00598-6. [DOI] [PubMed] [Google Scholar]
- 90.Lisker L, Abramson AS. A cross-language study of voicing in initial stops: Acoustical measurements. WORD. 1964;20:384–422. doi: 10.1080/00437956.1964.11659830. [DOI] [Google Scholar]
- 91.Stuart-Smith J, Sonderegger M, Rathcke T, Macdonald R. The private life of stops: VOT in a real-time corpus of spontaneous Glaswegian. Lab. Phonol. 2015;6:505–549. doi: 10.1515/lp-2015-0015. [DOI] [Google Scholar]
- 92.Gur RC, et al. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: Standardization and initial construct validation. J. Neurosci. Methods. 2010;187:254–262. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saykin AJ, et al. Normative neuropsychological test performance: Effects of age, education, gender and ethnicity. Appl. Neuropsychol. 1995;2:79–88. doi: 10.1207/s15324826an0202_5. [DOI] [PubMed] [Google Scholar]
- 94.Spencer RMC, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science. 2003;300:1437–1439. doi: 10.1126/science.1083661. [DOI] [PubMed] [Google Scholar]
- 95.Carroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia as measured by a repetitive finger tapping task. Brain Cognit. 2009;71:345–353. doi: 10.1016/j.bandc.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Da Silva FN, et al. More than just tapping: Index finger-tapping measures procedural learning in schizophrenia. Schizophr. Res. 2012;137:234–240. doi: 10.1016/j.schres.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Osborne KJ, Walther S, Shankman SA, Mittal VA. Psychomotor slowing in schizophrenia: Implications for endophenotype and biomarker development. Biomark. Neuropsychiatry. 2020;2:100016. doi: 10.1016/j.bionps.2020.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rund BR, et al. Neurocognition and duration of psychosis: A 10-year follow-up of first-episode patients. Schizophr. Bull. 2016;42:87–95. doi: 10.1093/schbul/sbv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.D’Reaux RA, Neumann CS, Rhymer KN. Time of day of testing and neuropsychological performance of schizophrenic patients and healthy controls. Schizophr. Res. 2000;45:157–167. doi: 10.1016/S0920-9964(99)00196-6. [DOI] [PubMed] [Google Scholar]
- 100.Gur RC, et al. Neurocognitive performance in family-based and case-control studies of schizophrenia. Schizophr. Res. 2015;163:17–23. doi: 10.1016/j.schres.2014.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Becker HE, et al. Neurocognitive functioning before and after the first psychotic episode: Does psychosis result in cognitive deterioration. Psychol. Med. 2010;40:1599–1606. doi: 10.1017/S0033291710000048. [DOI] [PubMed] [Google Scholar]
- 102.Dean DJ, Mittal VA. Spontaneous parkinsonisms and striatal impairment in neuroleptic free youth at ultrahigh risk for psychosis. npj Schizophr. 2015;1:1–6. doi: 10.1038/npjschz.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dickson H, et al. Cognitive impairment among children at-risk for schizophrenia. J. Psychiatr. Res. 2014;50:92–99. doi: 10.1016/j.jpsychires.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 104.Dickson H, Laurens KR, Cullen AE, Hodgins S. Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psychol. Med. 2012;42:743–755. doi: 10.1017/S0033291711001693. [DOI] [PubMed] [Google Scholar]
- 105.Gschwandtner U, et al. Fine motor function and neuropsychological deficits in individuals at risk for schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2006;256:201–206. doi: 10.1007/s00406-005-0626-2. [DOI] [PubMed] [Google Scholar]
- 106.Niendam TA, et al. The course of neurocognition and social functioning in individuals at ultra high risk for psychosis. Schizophr. Bull. 2007;33:772–781. doi: 10.1093/schbul/sbm020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gur RC, et al. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001;25:766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- 108.Wüthrich F, et al. Test–retest reliability of a finger-tapping fMRI task in a healthy population. Eur. J. Neurosci. 2023;57:78–90. doi: 10.1111/ejn.15865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Niendam TA, et al. Neurocognitive performance and functional disability in the psychosis prodrome. Schizophr. Res. 2006;84:100–111. doi: 10.1016/j.schres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 110.Damme KSF, et al. Motor sequence learning and pattern recognition in youth at clinical high-risk for psychosis. Schizophr. Res. 2019;208:454–456. doi: 10.1016/j.schres.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dean DJ, et al. Longitudinal assessment and functional neuroimaging of movement variability reveal novel insights into motor dysfunction in clinical high risk for psychosis. Schizophr. Bull. 2020;46:1567–1576. doi: 10.1093/schbul/sbaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang T, et al. Prediction of psychosis in prodrome: Development and validation of a simple, personalized risk calculator. Psychol. Med. 2019;49:1990–1998. doi: 10.1017/S0033291718002738. [DOI] [PubMed] [Google Scholar]
- 113.Moore TM, Reise SP, Gur RE, Hakonarson H, Gur RC. Psychometric properties of the Penn Computerized Neurocognitive Battery. Neuropsychology. 2015;29:235–246. doi: 10.1037/neu0000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, Vienna, Austria, 2021). https://www.R-project.org/.
- 115.Aust F., Barth M. papaja: Prepare Reproducible APA Journal Articles with R Markdown. https://github.com/crsh/papaja (2022).
- 116.Wickham H. ggplot2: Elegant Graphics for Data Analysis. (Springer-Verlag New York, 2016). https://ggplot2.tidyverse.org.
- 117.Wickham H., François R., Henry L. & Müller K. dplyr: A Grammar of Data Manipulation. https://CRAN.R-project.org/package=dplyr (2022).
- 118.Kassambara A. ggpubr: ’ggplot2’ Based Publication Ready Plots. https://CRAN.R-project.org/package=ggpubr (2020).
- 119.Wickham H. stringr: Simple, Consistent Wrappers for Common String Operations. https://CRAN.R-project.org/package=stringr (2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials for "Speech characteristics yield important clues about motor function. Speech variability in individuals at clinical high-risk for psychosis"
Data Availability Statement
All speech measure data and analysis code used in this study are available at github.com/khitczenko/chr_speech. The National Institute of Mental Health Data Archive provides the de-identified clinical, risk, and demographic information.