Abstract
Multiplex amplification of femA and mecA genetic determinants allowed an early and rapid identification of methicillin-resistant Staphylococcus aureus (MRSA) in endotracheal aspirates of mechanically ventilated patients. femA and/or mecA amplification and bacteriological results were concordant in 57 of 60 samples. In all three discrepant cases, complementary bacteriological tests confirmed the presence of MRSA first identified by molecular analysis. These results underline the value and rapidity of this molecular diagnosis for MRSA infection and control surveillance in intensive care units. Rapid MRSA detection is expected to have a significant clinical impact not only on patient outcome but also on the costs for isolation and treatment.
The frequencies of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant coagulase-negative staphylococcus (MR-CNS) infections are a worldwide concern, especially in intensive care units (ICU) (4, 12, 16). Risk factors include prolonged tracheal intubation and mechanical ventilation in critically ill patients (28, 30). In these patients, MRSA-mediated nosocomial pulmonary infections are associated with a high mortality and morbidity (1, 11, 23). Mechanisms of bacterial colonization and airway inoculation include aspiration of secretions contaminated with the pathogenic organism (10). MRSA can be acquired endogenously or during hospitalization by cross-contamination from colonized health care workers or other chronically infected patients (21, 22). Therefore, rapid and specific detection of MRSA colonization in upper and lower respiratory tracts is of paramount importance for appropriate therapeutic management and patient isolation. Unfortunately, conventional MRSA diagnosis in tracheal aspirates is adversely affected by several factors: the time required for proper bacterial identification and an accurate susceptibility test, frequent colonization of the upper airways by gram-negative bacilli (6), potential growth inhibition of staphylococcal species by gram-negative bacteria (18), great variability of growth conditions for MRSA (27), and prior antibiotic treatment, which may reduce the sensitivity of microorganism identification (7).
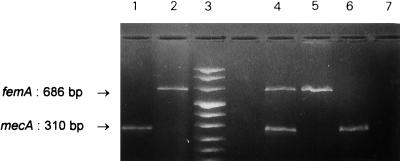
In staphylococcal species, resistance to methicillin and other β-lactam antibiotics is primarily mediated by the overproduction of an additional altered penicillin-binding protein (PBP2a) (13). The mecA gene, the structural determinant encoding PBP2a, is highly conserved among the methicillin-resistant species but is absent from susceptible strains, making it a useful molecular marker of β-lactam resistance in all staphylococci (24, 25). Another chromosomal element, femA, which cooperates with mecA for the expression of β-lactam resistance, appears to be a unique feature of S. aureus (3, 26, 29). We recently validated in vitro a multiplex PCR where coamplification of both determinants clearly distinguished susceptible (lacking mecA) from resistant (mecA+) staphylococci, as well as distinguishing S. aureus (femA+) from coagulase-negative staphylococci (lacking femA) (29) (Fig. 1). Although very attractive, such a molecular identification still awaited clinical evaluation. In this study, we prospectively assessed the value of the multiplex assay for endotracheal aspirates (ETA). After family consent, samples were collected from mechanically ventilated patients during and a few months after an outbreak of MRSA infections.
FIG. 1.
Differential identification of staphylococcal species and methicillin-resistant strains. DNA fragments resulting from the amplification of each gene were separated by electrophoresis on agarose gel. Lanes 1 and 2, amplification of femA and mecA determinants with primers F1/F2 and M1/M2, respectively; lane 3, molecular weight markers (values [in base pairs] in order from top to bottom are 1,114, 900, 692, 501/486, 404, 320, 242, and 190); lanes 4 to 7, simultaneous amplification of both markers from MRSA (lane 4; amplification of both determinants) and MSSA (lane 5; amplification of femA alone) DNA and from MR-CNS (lane 6; amplification of mecA alone) and MS-CNS (lane 7; no amplification) DNA.
(This study was presented at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, October 1997 [28a].)
A total of 48 patients, treated in four contiguous ICU, were included in the study, 33 of them being assessed over a period of 3 weeks during the outbreak and 15 being assessed a few months afterward. ETA were collected as follows. Five milliliters of sterile physiological saline was first instilled via the tracheal tube. After two or three artificial respiratory cycles, up to 3 ml of fluid was gently aspirated into a sterile tube via a sterile flexible cannula connected to a vacuum pump. Sixty duplicate ETA were collected for both microbiological examination and multiplex PCR analysis. Specimens for molecular analysis were either prepared immediately or maintained for a maximum of 24 h at 4°C until processed. Conventional identification of species and susceptibility tests were performed by disk diffusion testing with oxacillin in accordance with the National Committee for Clinical Laboratory Standards criteria described previously (15, 20). For the multiplex PCR assay, clinical specimens were homogenized in 5 ml of TE buffer (20 mM Tris HCl [pH 8.0], 10 mM EDTA) containing 2% (wt/vol) sodium dodecyl sulfate. The homogenate (1.5 ml) was then centrifuged for 5 min at 7,500 × g. The cellular pellet was washed once with TE buffer, lysed in the presence of 1% (vol/vol) Triton X-100 and 50 μg of lysostaphin (Sigma Chemical Co., St. Louis, Mo.), and incubated for 15 min at 37°C. Lysis was completed by adding 100 μg of proteinase K (Boehringer, Mannheim, Germany). The lysate was incubated for another 15 min at 55°C and 5 min at 95°C. It was centrifuged at 4,000 × g for 5 min. In order to purify bacterial DNA, 200 μl of the supernatant was then filtered on a NucleoSpin C+T column (Macherey-Nagel, Düren, Germany) and eluted with 200 μl of sterile H2O, according to the manufacturer’s protocol. Two different amounts of the DNA suspension (2 and 20 μl) were subjected to multiplex PCR amplification as previously described (29). Primers used were 5′-TGGCTATCGTGTCACAATCG-3′ and 5′-CTGGAACTTGTTGAGCAGAG-3′ for mecA and 5′-CTTACTTACTGGCTGTACCTG-3′ and 5′-ATGTCGCTTGTTATGTGC-3′ for femA, yielding 310- and 686-bp fragments, respectively (Fig. 1). The 40 PCR cycles were carried out in a model 2400 thermocycler (Perkin-Elmer, Foster City, Calif.) as follows: denaturation at 92°C for 20 s, annealing at 58°C for 20 s, and DNA extension at 72°C for 20 s, with increments of 2 s per cycle for the denaturation and extension segments. Amplified, ethidium bromide-stained DNA fragments were visualized after electrophoresis on agarose gel.
Molecular and conventional tests were performed in different laboratories, and results were compared (Table 1). In the multiplex amplification, identical results were obtained with either 2 or 20 μl of the DNA suspension. A perfect correlation between genotypic and phenotypic analyses was found for 57 ETA. The mecA and femA determinants were amplified from every ETA containing MRSA (n = 25), as determined by standard bacteriological methods. Single femA or mecA signals were found in specimens containing either methicillin-susceptible S. aureus (MSSA) (n = 10) or MR-CNS (n = 6), respectively. On the other hand, no signal was obtained from ETA colonized with gram-negative bacteria (n = 5) or methicillin-susceptible CNS (MS-CNS) (n = 6) and from five ETA containing normal pharyngeal flora.
TABLE 1.
Bacteriological and molecular results for ETAa
| No. of ETA | Identification
|
|
|---|---|---|
| Phenotypic (bacteriological tests) | Genotypic (multiplex PCR strategy) | |
| Correlations (n = 57) | ||
| 25 | MRSA | femA+/mecA+ |
| 10 | MSSA | femA+/no mecA |
| 6 | MR-CNS | no femA/mecA+ |
| 6 | MS-CNS | no femA/no mecA |
| 5 | Gram-negative bacteria | no femA/no mecA |
| 5 | Normal flora | no femA/no mecA |
| Discrepancies (n = 3) | ||
| 1 | Gram-negative bacteriab | femA+/mecA+ |
| 2 | No MRSAc | femA+/mecA+ |
The 25 MRSA-positive ETA were found in 15 of 33 patients during the outbreak; the 5 ETA containing normal flora were collected from three patients. All other results correspond to one ETA per patient.
P. aeruginosa coinfection.
MRSA nasal carriage.
Of note, discrepancies were found in three cases where femA and mecA markers were both amplified in ETA but no bacteriological evidence of staphylococci was found in the corresponding culture. One of the three cultures was massively positive for Pseudomonas aeruginosa. However, further identification on hyperselective medium (mannitol-salt-agar), prompted by the discrepant molecular result, identified MRSA in this ETA. For the other two cases, bacteriological controls revealed MRSA nasal carriages at the time of ETA specimen collection. Successive cultures repeated over the next few days also identified MRSA in ETA.
These results extend our previous in vitro data (29). They emphasize the sensitivity and specificity of the multiplex PCR strategy for detecting MRSA in ETA. The entire procedure can be completed in less than 6 h, either on the day of sample collection or the next day. This is quicker than conventional identification and susceptibility tests (48 to 72 h). Interestingly, the current data suggest that MRSA molecular detection is valuable for samples coinfected by fast-growing gram-negative bacteria such as P. aeruginosa, a potential cause of false-negative results by standard methods. Such a rapid and accurate MRSA identification may be applied to other sites such as nares, sputum, and wounds.
Accordingly, we can expect this method to have a positive economic impact. Effective but expensive barrier isolations are indeed recommended to control MRSA, especially for patients at high risk for MRSA carriage (transfer from a nursing home or other chronic care facilities and hospitals) (2, 5, 14). On the other hand, empiric glycopeptide treatment has been officially recommended for nosocomial pneumonia with risk factors (2), because delayed administration of adequate antibiotic therapy is associated with a greater risk of hospital mortality (reviewed in reference 17). Whereas the cost per sample of a conventional culture appears to be in the same range as that for our molecular assay (14) (data not shown), rapid same-day bacterial identification and susceptibility testing by a molecular method would prevent unnecessary isolation procedures and inappropriate empiric glycopeptide treatment, both of which are responsible for high additional costs (8, 14, 19). Moreover, rapid testing has been shown to have a major impact on care and outcome (i.e., resulting in a lower mortality rate, fewer laboratory studies, and shorter stays in the ICU, etc.) (9).
Acknowledgments
The project was funded by JSM-R&D-T2, the Joint Staff section of the Belgian Army supporting research and development (grant G95/01). P.V. was supported in part by a grant from Eli Lilly Benelux.
We thank J. Lünzer (Macherey-Nagel) for the generous gift of NucleoSpin C+T kits and Anne Vandenberghe for so enthusiastically and carefully collecting the endotracheal samples. We also thank M. Philippe, Departement of Clinical Chemistry, for stimulating discussions and providing laboratory facilities.
REFERENCES
- 1.Al Ujayli B, Nafziger D A, Saravolatz L. Pneumonia due to Staphylococcus aureus infection. Clin Chest Med. 1995;16:111–120. [PubMed] [Google Scholar]
- 2.American Thoracic Society. Hospital-acquired pneumonia in adults: diagnosis, assessment of severity, initial antimicrobial therapy, and preventive strategies. A consensus statement. Am J Respir Crit Care Med. 1996;153:1711–1725. doi: 10.1164/ajrccm.153.5.8630626. [DOI] [PubMed] [Google Scholar]
- 3.Berger-Bachi B, Barberis-Maino L, Strassle A, Kayser F H. FemA, a host-mediated factor essential for methicillin resistance in Staphylococcus aureus: molecular cloning and characterization. Mol Gen Genet. 1989;219:263–269. doi: 10.1007/BF00261186. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg L H, Klugman K P. Control of methicillin-resistant Staphylococcus aureus bacteraemia in high-risk areas. Eur J Clin Microbiol Infect Dis. 1994;13:82–85. doi: 10.1007/BF02026131. [DOI] [PubMed] [Google Scholar]
- 5.Casewell M W. New threats to the control of methicillin-resistant Staphylococcus aureus. J Hosp Infect. 1995;30:465–471. doi: 10.1016/0195-6701(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 6.Cazzadori A, Di-Perri G, Vento S, Bonora S, Fendt D, Rossi M, Lanzafame M, Mirandola F, Concia E. Etiology of pneumonia following isolated closed head injury. Respir Med. 1997;91:193–199. doi: 10.1016/s0954-6111(97)90038-x. [DOI] [PubMed] [Google Scholar]
- 7.Chastre J, Fagon J Y, Laner C. Procedures for the diagnosis of pneumonia in ICU patients. Intensive Care Med. 1992;18:S10–S17. doi: 10.1007/BF01752971. [DOI] [PubMed] [Google Scholar]
- 8.Doebelling B N, Wenzel R P. The direct costs of universal precautions in a teaching hospital. JAMA. 1990;264:2083–2087. [PubMed] [Google Scholar]
- 9.Doern G V, Vautour R, Gaudet M, Levy B. Clinical impact of rapid in vitro susceptibility testing and bacterial identification. J Clin Microbiol. 1994;32:1757–1762. doi: 10.1128/jcm.32.7.1757-1762.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estes R J, Meduri G U. The pathogenesis of ventilator-associated pneumonia. I. Mechanisms of bacterial transcolonization and airway inoculation. Intensive Care Med. 1995;21:365–383. doi: 10.1007/BF01705418. [DOI] [PubMed] [Google Scholar]
- 11.Fagon J Y, Chastre J, Vuagnat A, Trouillet J L, Novara A, Gibert C. Nosocomial pneumonia and mortality among patients in intensive care units. JAMA. 1996;275:866–869. [PubMed] [Google Scholar]
- 12.Fridkin S K, Welbel S F, Weinstein R A. Magnitude and prevention of nosocomial infections in the intensive care unit. Infect Dis Clin N Am. 1997;11:479. doi: 10.1016/s0891-5520(05)70366-4. [DOI] [PubMed] [Google Scholar]
- 13.Hackbart C, Chambers H. Methicillin-resistant staphylococci: genetics and mechanisms of resistance. Antimicrob Agents Chemother. 1989;33:991–999. doi: 10.1128/aac.33.7.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jernigan J A, Clemence M A, Stott G A, Titus M G, Alexander C H, Palumbo C M, Farr B M. Control of methicillin-resistant Staphylococcus aureus at a university hospital: one decade later. Infect Control Hosp Epidemiol. 1995;146:686–696. doi: 10.1086/647042. [DOI] [PubMed] [Google Scholar]
- 15.Kloos W E, Lambe D W., Jr . Staphylococcus. In: Balows A, Hausler W J Jr, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C: American Society for Microbiology; 1991. pp. 222–237. [Google Scholar]
- 16.Kloos W E, Bannerman T L. Update of clinical significance of coagulase-negative staphylococci. Clin Microbiol Rev. 1994;7:117–140. doi: 10.1128/cmr.7.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kollef M H. The importance of initial antibiotic selection in ventilator-associated pneumonia. In: Vincent J L, editor. Yearbook of intensive care and emergency medicine 1998. Berlin, Germany: Springer-Verlag; 1998. pp. 300–309. [Google Scholar]
- 18.Machan Z A, Pitt T L, White W, Watson D, Taylor G W, Cole P J, Wilson R. Interaction between Pseudomonas aeruginosa and Staphylococcus aureus: description of an anti-staphylococcal substance. J Med Microbiol. 1991;34:213–217. doi: 10.1099/00222615-34-4-213. [DOI] [PubMed] [Google Scholar]
- 19.Marshall J C, Evans D C. Antimicrobial therapy for ICU-acquired infection: time for reappraisal. In: Vincent J L, editor. Yearbook of intensive care and emergency medicine 1998. Berlin, Germany: Springer-Verlag; 1998. pp. 283–291. [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. 5th ed. M2-A5. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1994. [Google Scholar]
- 21.Phillips L G, Heggers J P, Robson M C. Burn and trauma units as source of methicillin-resistant Staphylococcus aureus. Burn Care Rehabil. 1992;13:293–297. doi: 10.1097/00004630-199203000-00023. [DOI] [PubMed] [Google Scholar]
- 22.Pujol M, Pena C, Pallares R, Ariza J, Ayats J, Dominguez M A, Gudiol F. Nosocomial Staphylococcus aureus bacteremia among nasal carriers of methicillin-resistant and methicillin-susceptible strains. Am J Med. 1996;100:509–516. doi: 10.1016/s0002-9343(96)00014-9. [DOI] [PubMed] [Google Scholar]
- 23.Rello J, Torres A, Riccart M, Valles J, Gonzalez J, Artigas A, Rodriguez-Roisin R. Ventilator-associated pneumonia by Staphylococcus aureus. Comparison of methicillin-resistant and methicillin-sensitive episodes. Am J Respir Crit Care Med. 1994;150:1545–1549. doi: 10.1164/ajrccm.150.6.7952612. [DOI] [PubMed] [Google Scholar]
- 24.Ryffel C, Tesch W, Birch-Machin I, Reynolds P E, Barberis-Maino L, Kayser F H, Berger-Bachi B. Sequence comparison of mecA genes isolated from methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. Gene. 1990;94:137–138. doi: 10.1016/0378-1119(90)90481-6. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki E, Hiramatsu K, Yokota T. Survey of methicillin-resistant clinical strains of coagulase-negative staphylococci for mecA gene distribution. Antimicrob Agents Chemother. 1992;36:429–434. doi: 10.1128/aac.36.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unal S, Hoskins J, Flokowitsch J E, Wu C Y E, Preston D A, Skatrud P L. Detection of methicillin-resistant staphylococci by using polymerase chain reaction. J Clin Microbiol. 1992;30:1685–1691. doi: 10.1128/jcm.30.7.1685-1691.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unal S, Werner K, DeGirolami P, Barsanti F, Eliopoulos G. Comparison of tests for detection of methicillin-resistant Staphylococcus aureus in a clinical microbiology laboratory. Antimicrob Agents Chemother. 1994;38:345–347. doi: 10.1128/aac.38.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valles L, Leon C, Alvarez-Lerma F. Nosocomial bacteremia in critically ill patients: a multicenter study evaluating epidemiology and prognosis. Clin Infect Dis. 1997;24:387–395. doi: 10.1093/clinids/24.3.387. [DOI] [PubMed] [Google Scholar]
- 28a.Vannuffel P, Bouyer M, Laterre P-F, Gigi J, Vandenberghe A, Vandercam B, Reynaert M, Gala J-L. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Rapid and specific identification of MRSA in endotracheal aspirates from mechanically ventilated ICU patients, abstr. D-25; p. 87. [Google Scholar]
- 29.Vannuffel P, Gigi J, Ezzedine H, Vandercam B, Delmee M, Wauters G, Gala J-L. Specific detection of methicillin-resistant Staphylococcus species by multiplex PCR. J Clin Microbiol. 1995;33:2864–2867. doi: 10.1128/jcm.33.11.2864-2867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent J L, Bihari D J, Suter P M, Bruining H A, White J, Nicolas-Chanoin M H, Wolff M, Spencer R C, Hemmer M. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA. 1995;274:639–644. [PubMed] [Google Scholar]