Abstract
The vacA genotypes s1,m1 and s1,m2 were detected in 44 and 30% of Helicobacter pylori isolates, respectively, from patients with gastric mucosa-associated lymphoid tissue lymphoma, compared to 26 and 56% of isolates, respectively, from individuals with gastritis. The vacA s1 genotype was significantly associated with, but not predictive of, the presence of vacuolating cytotoxin activity.
Helicobacter pylori is linked to development of gastric mucosa-associated lymphoid tissue (MALT) lymphoma (3, 10); however, the underlying pathogenetic mechanisms are unclear. Hussell et al. demonstrated that proliferation of lymphoma cells and production of tumor-specific immunoglobulin were stimulated by H. pylori and that this effect is dependent on H. pylori-specific T cells (7). Recently, the vacA subtype s1 was suggested as a marker for more virulent strains (1).
We determined the frequency of vacA subtypes and cytotoxin activity in H. pylori isolates from 27 patients (12 male and 15 female patients; median age, 60 years) with low-grade gastric MALT lymphoma (stage EI1) compared with H. pylori isolates from 27 age- and sex-matched symptomatic individuals with simple gastritis and no history of peptic ulcer or gastric malignancy.
H. pylori was cultured under standard conditions and identified by Gram stain morphology and biochemical testing.
Genomic DNA extraction was performed as previously described (5). Allelic regions of the vacA gene were PCR amplified in an automated thermal cycler (Perkin-Elmer) under previously published conditions (5) and visualized in 1% agarose gels stained with ethidium bromide.
For determination of cytotoxin activity, H. pylori cells were grown for 48 to 72 h in BBFKS–8% Dent liquid. Culture supernatants were centrifuged and sterilely filtered with a 0.22-μm-pore-size Millex-GV filter (Millipore, Eschborn, Germany) and tested for vacuolating cytotoxin activity with HeLa (ATCC CCL 2) and Vero (ATCC CCL 81) cells under standard conditions. After inoculation on 96-well microtiter plates with a density of 2 × 104 cells per well, serial dilutions (1:2 to 1:8) of H. pylori culture supernatants were inoculated onto the coated plates and incubated in a humid atmosphere with 5% CO2 at 37°C. After 24 h, the grade of vacuolation was determined by inverse microscopy (100× to 200×). Cell lines were considered cytotoxin positive if vacuolation was observed in more than 50% of cells (positive control, H. pylori ATCC 49503; negative control, Tx30a).
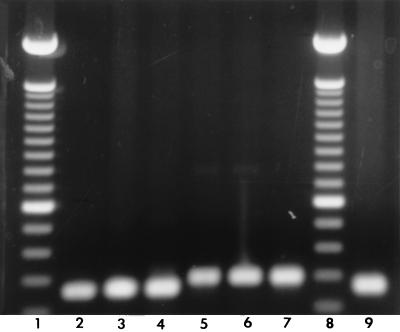
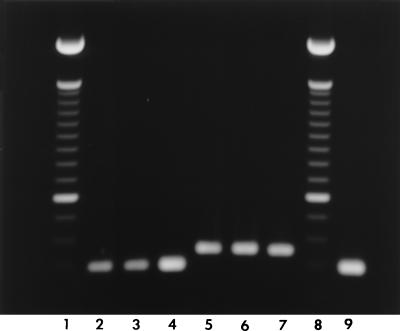
PCR amplification revealed a single band of the expected size for either the vacA s1 or s2 type and for either the vacA m1 or m2 type for all H. pylori strains investigated (Fig. 1 and 2). There was a high prevalence of vacA s1 (78%) in H. pylori strains from both study groups (Table 1). s1,m1 was numerically more common in H. pylori isolates from patients with MALT lymphoma than in those from patients with histologic gastritis (44 versus 26%, respectively; P = 0.08).
FIG. 1.
One percent agarose gel electrophoresis of the 259-bp (lanes 2 to 4) and the 286-bp (lanes 5 to 7) PCR products for the vacA s1 and s2 genotypes, respectively. Lanes 1 and 8, 100-bp ladder; lane 9, H. pylori ATCC 49503 (s1).
FIG. 2.
One percent agarose gel electrophoresis of the 290-bp (lanes 2 to 4) and the 352-bp (lanes 5 to 7) PCR products for the vacA m1 and m2 genotypes, respectively. Lanes 1 and 8, 100-bp ladder; lane 9, H. pylori ATCC 49503 (m1).
TABLE 1.
Frequency of vacA genotypes in H. pylori strains from patients with MALT lymphoma or simple gastritis
| Genotype | No. of isolates (%) from patient group
|
||
|---|---|---|---|
| MALT (n = 27) | Gastritis (n = 27) | Total (n = 54) | |
| s1,m1 | 12 (44) | 7 (26) | 19 (35) |
| s1,m2 | 8 (30) | 15 (56) | 23 (42) |
| s2,m2 | 7 (26) | 5 (18) | 12 (23) |
| s2,m1 | 0 (0) | 0 (0) | 0 (0) |
Among the 42 H. pylori strains containing vacA s1, 16 (38%) exhibited cytotoxic activity in one of the two cell lines. None of the strains containing s2 exhibited cytotoxic activity with either cell line (P < 0.05). Only five strains (25%) from patients with low-grade gastric MALT lymphoma containing the s1 genotype showed cytotoxin activity. Of interest, of the 16 toxin-positive (Tox+) strains, 13 (81.3%) were Tox+ in Vero cells but only 8 (50%) were Tox+ in HeLa cells, suggesting that use of a single cell line may significantly underestimate the actual frequency of cytotoxin activity in H. pylori strains. There was no significant difference between the vacA s1,m1 and s1,m2 genotypes with respect to cytotoxin activity.
Although the vacA gene is thought to be present in all H. pylori strains, cytotoxin is expressed by only approximately 50% (4). The presence of cytotoxic activity has been suggested as a marker for strains with enhanced virulence acting either directly via cytotoxic action or indirectly via an increased inflammatory and immune response. vacA genotype s1 has been associated with enhanced activity of the vacuolating cytotoxin and with a greater degree of gastric inflammation (2).
In this study, the vacA s1 genotype was identified in about 75% of H. pylori strains from patients with low-grade gastric MALT lymphoma and in about the same proportion in strains from the control group, suggesting that the vacA s1 genotype is commonly present in H. pylori isolated from German patients. Interpretation and analysis of the role of putative H. pylori virulence factors have been hampered by the fact that considerable geographic variation of strains has been demonstrated, such that findings from one region may not be confirmed in another (9). Preliminary studies regarding the frequency of vacA genotypes in different patient populations of various geographic regions are available. Mendes et al. reported a higher prevalence of the s1 genotype in patients with peptic ulcers and in those with gastric carcinoma than in patients with simple gastritis (8). Studies performed in the United States and in the United Kingdom found no significant differences in the frequency of vacA s1 in strains from peptic ulcer patients compared with strains from those with simple gastritis (6, 11). These data suggest that the frequency of the vacA s1 genotype in isolates causing different diseases is dependent on the most prevalent genotype in a particular population or geographic region, such that the associations of the vacA genotype and different gastroduodenal diseases are inconsistent and spurious.
The failure of the s1 genotype to be always associated with cytotoxic activity shows that, while the s1 genotype may be necessary for the expression of vacuolating cytotoxin, its presence cannot be used as a surrogate for the presence of cytotoxin-positive H. pylori. Overall, cytotoxin activity was found in a minority of H. pylori strains obtained from patients with low-grade gastric MALT lymphoma, suggesting that cytotoxicity plays little if any role in the pathogenesis of this H. pylori-related disease. Importantly, we found that vacuolating cytotoxin activity was detected more frequently in Vero cells than in HeLa cells, showing that the use of a single cell line may underestimate the frequency of cytotoxic activity in H. pylori strains. It has been suggested that, because of the problem of geographic variation in the presence and disease associations of putative H. pylori virulence factors, disease-specific associations should be evaluated in isolates from different geographic regions before any claim of a possible association is made (9).
REFERENCES
- 1.Atherton J C, Cao P, Peek R M, Tummuru M K R, Blaser M J, Cover T L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 2.Atherton J C, Peek R M, Tham K T, Cover T L, Blaser M J. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology. 1997;112:92–99. doi: 10.1016/s0016-5085(97)70223-3. [DOI] [PubMed] [Google Scholar]
- 3.Bayerdörffer E, Neubauer A, Rudolph B, Thiede C, Lehn N, Eidt S, Stolte M the MALT Lymphoma Study Group. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. Lancet. 1995;345:1591–1594. doi: 10.1016/s0140-6736(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 4.Cover T L, Blaser M J. Purification and characterization of the vacuolating cytotoxin from Helicobacter pylori. J Biol Chem. 1992;267:10570–10575. [PubMed] [Google Scholar]
- 5.Go M F, Chan K Y, Versalovic J, Koeuth T, Graham D Y, Lupski J R. Cluster analysis of Helicobacter pylori genomic DNA fingerprints suggests gastroduodenal disease-specific associations. Scand J Gastroenterol. 1995;30:640–646. doi: 10.3109/00365529509096306. [DOI] [PubMed] [Google Scholar]
- 6.Go, M. F., S. M. Small, and D. Y. Graham. 1996. Lack of an association of vacA genotype and duodenal ulcer disease. Gut 39(Suppl. 2):A54.
- 7.Hussell T, Isaacson P G, Crabtree J E, Spencer J. Helicobacter pylori specific tumour infiltrating T cells provide contact dependent help for the growth of malignant B cells in low grade gastric lymphoma of mucosa-associated lymphoid tissue. J Pathol. 1996;178:122–127. doi: 10.1002/(SICI)1096-9896(199602)178:2<122::AID-PATH486>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 8.Mendes, E. N., D. M. M. Queiroz, G. A. Rocha, A. M. R. Oliveira, and C. A. Oliveira. 1996. VacA genotypes in patients with gastritis, peptic ulcer and gastric carcinoma. Gut 39(Suppl. 2):A68.
- 9.Miehlke S, Kibler K, Kim J G, Figura N, Small S M, Graham D Y, Go M F. Allelic variation in Helicobacter pylori cagA gene from Korea compared to the United States. Am J Gastroenterol. 1996;91:1322–1325. [PubMed] [Google Scholar]
- 10.Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 11.Stephens, J. C., A. M. Folwell, R. A. Swann, and B. J. Rathbone. 1996. H. pylori cagA status, vacA genotypes and gastroduodenal disease. Gut 39(Suppl. 2):A71.