Abstract
Background
Insulin resistance (IR) is a pathophysiologic hallmark of type 2 diabetes and associated with the presence of chronic kidney disease (CKD). Experimental studies suggest that endothelin-1 increases IR. We assessed the association between IR and cardio-renal outcomes and the effect of the selective endothelin receptor antagonist atrasentan on IR in patients with type 2 diabetes and CKD.
Methods
We used data from the RADAR and SONAR trials that recruited participants with type 2 diabetes and CKD [eGFR 25–75 mL/min/1.73 m², urine albumin-to-creatinine ratio of 300–5000 mg/g]. IR was calculated using the homeostatic model assessment (HOMA-IR). The association between HOMA-IR and the pre-specified cardio-renal outcomes was assessed using multivariable Cox proportional hazards regression, and effects of atrasentan on HOMA-IR by a linear mixed effect model.
Results
In the SONAR trial, each log-unit increase in HOMA-IR was associated with an increased risk of the composite cardio-renal outcome [hazard ratio 1.32 (95%CI 1.09,1.60; p = 0.004)], kidney outcome [hazard ratio 1.30 (95%CI 1.00,1.68; p-value = 0.048)], and the kidney or all-cause mortality outcome [hazard ratio 1.25 (95%CI 1.01,1.55; p-value = 0.037)]. After 12 weeks treatment in the RADAR trial (N = 123), atrasentan 0.75 mg/day and 1.25 mg/day compared to placebo reduced HOMA-IR by 19.1 (95%CI -17.4, 44.3) and 26.7% (95%CI -6.4, 49.5), respectively. In the SONAR trial (N = 1914), atrasentan 0.75 mg/day compared to placebo reduced HOMA-IR by 9.6% (95%CI 0.6, 17.9).
Conclusions
More severe IR is associated with increased risk of cardio-renal outcomes. The endothelin receptor antagonist atrasentan reduced IR.
Trial registration
RADAR trial (Reducing Residual Albuminuria in Subjects With Diabetes and Nephropathy With AtRasentan): NCT01356849.
SONAR trial (The Study Of Diabetic Nephropathy With AtRasentan) NCT01858532.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-023-01964-8.
Keywords: Insulin resistance, Atrasentan, Endothelin receptor antagonist, Chronic kidney disease, Type 2 diabetes
Background
Insulin resistance (IR) is a pathophysiological hallmark of type 2 diabetes with reduced insulin sensitivity being detectable up to 5 years prior to the diagnosis of type 2 diabetes [1].
The pathophysiological processes associated with IR can lead to kidney impairment prior to the diagnosis of type 2 diabetes, as increased IR is associated with both the presence of microalbuminuria and chronic kidney disease (CKD) in subjects without diabetes [2–4]. Moreover, in the general population and in newly diagnosed patients with type 2 diabetes, a higher IR is independently associated with a faster rate of glomerular filtration rate decline [5–7].
Binding of endothelin-1 (ET-1) to the endothelin A (ETA) receptor induces glomerular/tubular dysfunction, inflammation, and fibrosis in both diabetic and non-diabetic kidneys [8]. There appears to be a reciprocal association between ET-1 and IR resulting in a vicious circle of increased organ damage. In vitro research demonstrated that intracellular pathways involved in insulin-mediated glucose uptake in adipocytes and vascular smooth muscles cells are disrupted by ET-1 [9, 10]. Conversely, IR increases renal ET-1 levels and ETA receptor expression [11, 12]. In clinical studies, IR increased in response to administration of exogenous ET-1 in healthy volunteers supporting the close relation between ET-1 and IR [13].
Selective endothelin receptor A antagonists (ERA) have been shown to slow the progression of CKD. Low dose atrasentan, an ERA, decreased albuminuria and reduced the risk of major kidney outcomes in patients with type 2 diabetes and CKD [14, 15]. A small study demonstrated that administration of an ERA reduces IR in obese patients with coronary artery disease [16]. In addition, a small observational study reported a significant reduction in HbA1c in patients with pulmonary hypertension using ERA [17]. In the RADAR dose-finding study, the ERA atrasentan reduced HbA1c compared to placebo in patients with type 2 diabetes and CKD possibly mediated by improvements in IR [18]. However, because of the small sample size and short follow-up, these results are prone to chance findings and can only be interpreted as hypothesis generating. Larger studies with longer follow-up are thus required to provide more robust evidence about the magnitude and time-course of the effects of ERA treatment on IR and glycemic control.
Therefore, the aims of this study were firstly to assess the association between baseline IR and clinical outcomes in a large well characterized cohort of patients already diagnosed with type 2 diabetes and CKD. Secondly, we assessed whether the ERA atrasentan reduces IR and HbA1c in patients with type 2 diabetes and CKD.
Methods
Study design and patient population
This study is a post-hoc analysis of the RADAR and SONAR clinical trials. The study design and primary results of both trials have been published before [14, 15, 19, 20]. In short, the Reducing Residual Albuminuria in Subjects With Diabetes and Nephropathy With AtRasentan (RADAR) trial was a randomized double-blind phase 2b clinical trial recruiting 153 patients with type 2 diabetes, urinary albumin-to-creatinine ratio [UACR] ≥ 300 - ≤3500 mg/g, and an estimated glomerular filtration rate (eGFR) of ≥ 30 - ≤75 mL/min/1.73m2 receiving maximum tolerated labeled dose of renin-angiotensin-system (RAS) inhibitors. Patients were randomly assigned to 12 weeks treatment with atrasentan 0.75 mg/day, atrasentan 1.25/mg day or matched placebo. The primary efficacy endpoint of the trial was the change in UACR from baseline to week 12 [18].
The Study Of Diabetic Nephropathy With AtRasentan (SONAR) trial recruited patients between 18 and 85 years old with type 2 diabetes, UACR ≥ 300 - < 5000 mg/g, and eGFR of ≥ 25 - < 75 mL/min per 1.73 m [2]. After screening and run-in to optimize RAS inhibitor treatment, eligible participants proceeded to a 6-week open-label enrichment period, during which all patients received 0.75 mg/day atrasentan. Atrasentan responders were defined as patients with ≥ 30% reduction in UACR who did not have substantial fluid retention (defined as an increase in body weight of ≥ 3 kg and a B-type natriuretic peptide (BNP) increase ≥ 300 pg/mL), and who did not have an increase in serum creatinine of more than 0·5 mg/dL and 20% from baseline. After six weeks enrichment, 2648 responders and 1020 non-responders without overt signs of fluid retention were randomly assigned to continue 0.75 mg/day atrasentan or to transition to placebo [15, 20]. The RADAR and SONAR trials were designed and conducted in accordance with national regulatory and ethical guidelines and are registered with ClinicalTrials.gov (NCT01356849 and NCT01858532).
Insulin resistance
Insulin resistance was calculated using the homeostatic model assessment (HOMA-IR): [(fasting plasma insulin [FPI] × fasting plasma glucose [FPG])/22.5] [21]. Blood samples for measurement of insulin and glucose were taken while patients were in a fasted state. Insulin and glucose were measured at the start and end of the 6-week enrichment period, randomization, at 1 and 12 months post-randomization, and annually thereafter. In RADAR, HOMA-IR was determined in 123 patients (80.4% of the overall cohort). In SONAR, 1102 patients were available for analysis of the association between HOMA-IR at baseline of the enrichment phase and long-term outcomes. At randomization, HOMA-IR was assessed in 1914 patients. In this subgroup, the effect of atrasentan versus placebo was assessed on HOMA-IR and HbA1c over time.
Endpoints
We investigated four cardio-renal clinical endpoints: (1) the composite cardiovascular (CV)-kidney endpoint defined as time from randomization to first occurrence of a sustained doubling of serum creatinine, end-stage kidney disease ([ESKD] defined as eGFR < 15 mL/min/1.73 m [2], need for chronic dialysis, renal transplantation), CV death, non-fatal myocardial infarction (MI) or non-fatal stroke; [20] (2) the composite kidney or all-cause mortality endpoint defined as time from randomization to first occurrence of doubling of serum creatinine from baseline (confirmed by 30-day serum creatinine), ESKD or death; (3) the composite kidney endpoint which was defined as time to doubling of serum creatinine from baseline (confirmed by 30-day serum creatinine) or ESKD; and (4) the CV composite endpoint defined as time to CV death, non-fatal MI and non-fatal stroke.
Statistics
Summary statistics were used to describe the demographic and clinical characteristics of patients included in the IR analysis and the randomized RADAR and SONAR trial populations. We log-transformed HOMA-IR, UACR and brain natriuretic peptide (BNP) values before analysis to take into account their skewed distribution. Cox proportional hazards regression was used to assess the association between baseline HOMA-IR and the relative hazard of the CV-kidney outcomes. In addition, HOMA-IR in tertiles were categorized and estimated the hazard ratio using the lower tertile as a common reference for the middle and upper tertiles. Three regression models were used: (1) a baseline model with treatment, age and sex as covariates; (2) the baseline model adding eGFR, log(UACR), body mass index (BMI) and systolic- and diastolic blood pressure as additional covariates; and (3) the final model that included all aforementioned covariates plus hemoglobin, insulin use, CV disease history and BNP. All covariates were measured at baseline of the enrichment phase before patients were exposed to atrasentan.
We used the RADAR and SONAR trials for determination of the effect of atrasentan on IR. Analysis of co-variance was used in RADAR to estimate the effect of atrasentan 0.75 mg/day and 1.25 mg/day compared to placebo on HOMA-IR. An unpaired t-test was performed to compare the natural logarithm of HOMA-IR between start of the open-label enrichment phase and the randomization visit. A linear mixed effect model was used to assess the effect of atrasentan compared to placebo on changes in HOMA-IR from randomization. The model included treatment, visit and interactions between treatment and visit as categorical fixed effects. An unstructured variance–covariance matrix was used to allow for correlations and general patterns of standard deviations across the repeated outcome measurements. Linear mixed models were used to assess whether the effect of atrasentan compared to placebo on IR was consistent across subgroups by baseline age, sex, eGFR, UACR, BMI and insulin by including a fixed effect for the subgroup and three-way interaction between treatment, visit and subgroup. All analysis were performed with the software package ‘R’, version 4.2.0. (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient disposition and baseline characteristics
Baseline characteristics of RADAR participants with available HOMA-IR data (N = 123) are described in Additional file 1: Table S1. Out of 3668 patients randomized in the SONAR trial, 1102 (30.0%) were included in the analysis of the association between baseline IR and long-term cardio-renal outcomes. Reasons for exclusion were unavailable insulin measurement at baseline of enrichment (n = 2329), insulin measurements taken while not fasting (n = 186) and missing information on other covariates (n = 51). Across progressively higher IR tertiles, patients in the highest tertile had a higher BMI, higher hemoglobin and hematocrit at baseline, were more likely to be of female sex, and more like to use insulin and statins (Table 1). Baseline characteristics were generally representative of the overall SONAR trial population (Additional file 2: Table S2).
Table 1.
Demographic and clinical characteristics at the start of the enrichment phase according to tertiles of Insulin Resistance
| Characteristics | Tertile 1 (n = 366) | Tertile 2 (n = 369) | Tertile 3 (n = 367) | p-value for trend |
|---|---|---|---|---|
| HOMA-IR | 2.3 [0.0-3.9] | 5.9 [3.9–10.3] | 19.6 [10.4–286.0] | NA |
| Age, years | 63.1 (9.4) | 64.4 (8.6) | 63.6 (8.2) | 0.445 |
| Sex, n (%) | ||||
| Women | 72 (19.7%) | 114 (30.9%) | 99 (27.0%) | 0.002 |
| Men | 294 (80.3%) | 255 (69.1%) | 268 (73.0%) | |
| Race, n (%) | ||||
| Asian | 166 (45.4%) | 139 (37.7%) | 108 (29.4%) | < 0.001 |
| Black | 13 (3.6%) | 29 (7.9%) | 21 (5.7%) | |
| Other | 27 (7.4%) | 12 (3.3%) | 12 (3.3%) | |
| White | 160 (43.7%) | 189 (51.2%) | 226 (61.6%) | |
| Body weight (kg) | 76.2 (15.5) | 83.3 (18.0) | 92.0 (21.7) | < 0.001 |
| BMI, kg/m2 | 27.6 (4.6) | 30.2 (5.6) | 32.7 (6.3) | < 0.001 |
| Blood pressure (mmHg) | ||||
| Systolic | 136.6 (16.2) | 137.5 (16.2) | 137.7 (14.6) | 0.338 |
| Diastolic | 75.6 (9.7) | 75.5 (10.3) | 76.6 (9.3) | 0.162 |
| eGFR, ml/min 1.73m2 | 42.4 (12.9) | 41.2 (12.0) | 41.4 (12.2) | 0.311 |
| UACR, mg/g | 888 [514–1773] | 815 [469–1657] | 881 [452–1570] | 0.258 |
| Haemoglobin, g/L | 125.9 (17.3) | 128.8 (17.0) | 131.2 (17.5) | < 0.001 |
| BNP, pg/mL | 51 [27–95] | 46 [23–87] | 44 [26–86] | 0.294 |
| Hematocrit, L/L | 0.38 (0.05) | 0.39 (0.05) | 0.40 (0.05) | < 0.001 |
| CVD history, n (%) | 75 (18.8%) | 82 (21.9%) | 84 (22.2%) | 0.308 |
| Insulin use, n (%) | 136 (37.2%) | 260 (70.5%) | 304 (82.8%) | < 0.001 |
| Diuretic use, n (%) | 282 (77.0%) | 309 (83.7%) | 290 (79.0%) | 0.066 |
| Statin use, n (%) | 272 (74.3%) | 299 (81.0%) | 301 (82.0%) | 0.020 |
Abbreviations: HOMA-IR = Homeostatic Model Assessment for Insulin Resistance; BMI = body mass index; eGFR = estimated glomerular filtration rate; UACR = urine albumin creatinine ratio; BNP = B-type natriuretic peptide; CVD = cardiovascular disease
Note: HOMA-IR: median (min and max values per tertile); UACR and BNP: median (interquartile range); all other numerical values: mean (standard deviation); Regarding p-value for difference: for continues variables linear regression was performed with the variable of interest as dependent variable and IR tertile as numerical covariate to assess the presence of a significant trend across tertiles. For categorical variables a chi-square test was performed to assess the presence of a significant difference in distribution across tertiles
Long term outcomes
During follow-up, 103, 86, 62, and 43 participants experienced a CV-kidney, kidney or all-cause mortality, kidney, and CV endpoints, respectively. Cox proportional hazard regression with adjustment for patient demographics, randomized treatment and cardiovascular risk markers, including eGFR, UACR, BNP and CV disease history showed that each log increament in HOMA IR was significantly associated with a higher risk of the CV kidney, kidney or all-cause mortality and kidney composite outcomes with corresponding HRs per log increment baseline HOMA-IR in the fully adjusted model of 1.32 (95%CI 1.09,1.60, p = 0.004); 1.25 (95%CI 1.01, 1.55, p = 0.037); 1.30 (95%CI 1.00-1.68, p = 0.048); 1.34 (95%CI 0.99, 1.81, p = 0.060); for the CV-kidney, kidney or all-cause mortality, kidney, and CV outcomes, respectively (Table 2). Repeating the analyses for participants not using insulin at baseline (n = 402) yielded similar results as our main analyses (Additional file 3: Table S3).
Table 2.
Association between baseline HOMA-IR and long-term cardio-renal outcomes
| Outcome | n/N events (%) | Model 1 | p value | Model 2 | p value | Model 3 | p value |
|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||||
| Cardiorenal outcomes | |||||||
| Tertile 1 | 27/339 (8.0%) | (reference) | (reference) | (reference) | |||
| Tertile 2 | 37/332 (11.1%) | 1.31 (0.79–2.17) | 0.289 | 1.41 (0.84–2.39) | 0.197 | 1.74 (1.01–3.01) | 0.046 |
| Tertile 3 | 39/328 (11.9%) | 1.44 (0.88–2.35) | 0.149 | 1.64 (0.96–2.81) | 0.072 | 2.29 (1.28–4.09) | 0.005 |
| p value for trend across tertiles | NA | 1.19 (0.94–1.51) | 0.154 | 1.27 (0.98–1.65) | 0.076 | 1.49 (1.12–1.97) | 0.006 |
| per log unit increase | NA | 1.13 (0.95–1.35) | 0.163 | 1.19 (0.99–1.43) | 0.069 | 1.32 (1.09–1.60) | 0.004 |
| Renal composite or all-cause mortality | |||||||
| Tertile 1 | 23/343 (6.7%) | (reference) | (reference) | (reference) | |||
| Tertile 2 | 29/340 (8.5%) | 1.20 (0.69–2.10) | 0.511 | 1.25 (0.70–2.24) | 0.453 | 1.40 (0.76–2.56) | 0.278 |
| Tertile 3 | 34/333 (10.2%) | 1.48 (0.87–2.51) | 0.149 | 1.61 (0.89–2.90) | 0.116 | 2.06 (1.09–3.88) | 0.026 |
| p value for trend across tertiles | NA | 1.22 (0.93–1.58) | 0.145 | 1.27 (0.95–1.70) | 0.110 | 1.44 (1.05–1.97) | 0.023 |
| per log unit increase | NA | 1.12 (0.93–1.36) | 0.240 | 1.16 (0.95–1.43) | 0.151 | 1.25 (1.01–1.55) | 0.037 |
| Renal composite | |||||||
| Tertile 1 | 16/350 (4.6%) | (reference) | (reference) | (reference) | |||
| Tertile 2 | 23/346 (6.6%) | 1.38 (0.72–2.64) | 0.331 | 1.46 (0.72–2.93) | 0.293 | 1.87 (0.90–3.88) | 0.093 |
| Tertile 3 | 23/344 (6.7%) | 1.48 (0.78–2.81) | 0.235 | 1.51 (0.71–3.18) | 0.281 | 2.49 (1.11–5.60) | 0.028 |
| p value for trend across tertiles | NA | 1.20 (0.88–1.64) | 0.244 | 1.21 (0.84–1.74) | 0.305 | 1.55 (1.05–2.30) | 0.029 |
| per log unit increase | NA | 1.09 (0.87–1.37) | 0.443 | 1.11 (0.87–1.43) | 0.406 | 1.30 (1.00-1.68) | 0.048 |
| CV composite | |||||||
| Tertile 1 | 12/354 (3.4%) | (reference) | (reference) | (reference) | |||
| Tertile 2 | 15/354 (4.2%) | 1.22 (0.57–2.62) | 0.609 | 1.42 (0.65–3.11) | 0.385 | 1.61 (0.71–3.66) | 0.258 |
| Tertile 3 | 16/351 (4.6%) | 1.34 (0.63–2.84) | 0.445 | 1.61 (0.72–3.62) | 0.249 | 1.88 (0.78–4.51) | 0.160 |
| p value for trend across tertiles | NA | 1.15 (0.80–1.67) | 0.448 | 1.26 (0.85–1.88) | 0.253 | 1.35 (0.88–2.07) | 0.167 |
| per log unit increase | NA | 1.17 (0.89–1.52) | 0.256 | 1.25 (0.95–1.66) | 0.116 | 1.34 (0.99–1.81) | 0.060 |
Note:
Model 1 covariates: age, sex, treatment assignment (Atrasentan or Placebo)
Model 2 covariates: age, sex, treatment assignment (Atrasentan or Placebo), race, BMI, eGFR, log(UACR), SBP, DBP
Model 3 covariates: age, sex, treatment assignment (Atrasentan or Placebo), race, BMI, eGFR, log(UACR), SBP, DBP, hemoglobin, insulin use, cardiovascular disease history, log(BNP)
Effect of atrasentan on insulin resistance
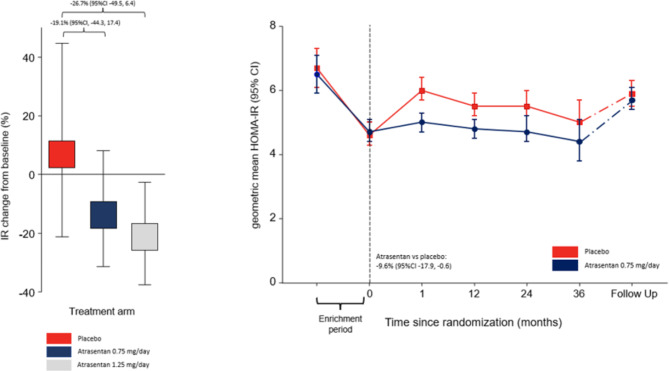
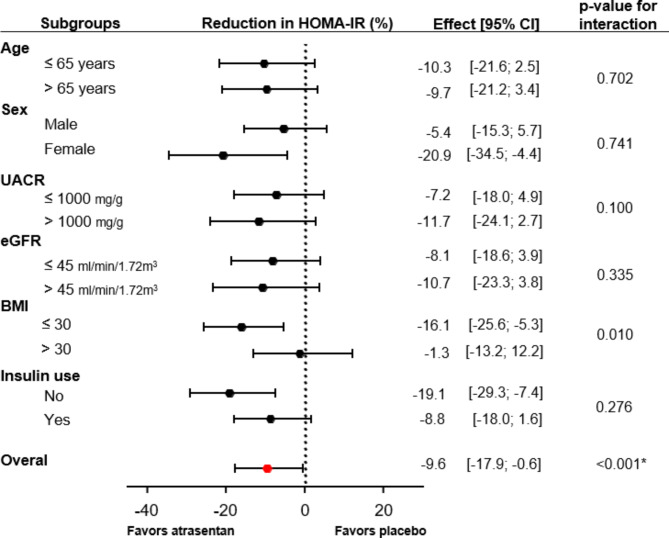
In the placebo group (N = 26) of the RADAR trial, HOMA-IR slightly increased by 7.8% (95% CI -20.3, 45.7) after 12 weeks follow-up, while HOMA-IR decreased in patients randomized to atrasentan 0.75 mg/day (N = 47) and 1.25 mg/day (N = 50) by -12.9% (95%CI -30.5, 9.2) and − 21.0% (95%CI -36.5, -1.7%), respectively, resulting in a difference with placebo of -19.1% (95%CI -44.3, 17.4; p = 0.266) and − 26.7% (95%CI -49.5, 6.4; p = 0.102), respectively (Fig. 1A). In the SONAR trial, geometric mean HOMA-IR at the start of the 6-week enrichment period was 6.8 units (95%CI 6.4, 7.1) which decreased to 4.7 units (95%CI 4.4, 4.9; p vs. baseline < 0.0001) after 6-weeks treatment with 0.75 mg/day atrasentan. One month after randomization, HOMA-IR increased in the placebo group from 4.6 units (95%CI 4.3, 5.0) to 5.5 units (95%CI 5.1, 5.9, p < 0.001), corresponding to a 21.5% (95%CI 16.5, 26.9) increase. HOMA-IR remained at 4.8 units (95%CI 4.4–5.2) in the atrasentan group corresponding to a 2.3% (95%CI -1.7, 7.0) change (Fig. 1B). During the remainder of the double-blind treatment period, patients randomized to atrasentan had a significantly lower HOMA-IR compared to patients on placebo resulting in a between-group difference of -9.6% [95%CI -17.9, -0.6, p < 0.001]) (Fig. 1B). Six weeks following study drug discontinuation, HOMA-IR levels were similar between the placebo and atrasentan group (Fig. 1B). The effect of atrasentan compared to placebo was consistent in subgroup analyses by age, sex, baseline UACR, baseline eGFR and use of insulin (Fig. 2). Among patients with baseline BMI < 30 kg/m2 the effect of atrasentan was more pronounced compared to those with a BMI > 30 kg/m2 (p for interaction 0.010; Fig. 2). During the double-blind phase, atrasentan treated patients had a significantly lower HbA1c compared to placebo treated patients resulting in a between-group difference in HbA1c of 0.18% (95%CI 0.05,0.31, p-value < 0.001) (Additional file 4: Supplementary Fig. 1).
Fig. 1.
Atrasentan reduces insulin resistance. Panel A: Change from baseline in HOMA-IR in the RADAR trial. Panel B: Geometric mean HOMA-IR values per study visit in the SONAR trial. During the enrichment period of the SONAR trial all patients received atrasentan 0.75 mg/day. Enrichment results of patients subsequently randomized to continue atrasentan or to transition to placebo are presented separately
Fig. 2.
Treatment effect of atrasentan on HOMA-IR during the SONAR double-blind phase. Note: * p-value for atrasentan versus placebo for all patients combined averaged over visits
Discussion
In patients with type 2 diabetes and CKD at high risk of kidney failures and CV events, more severe IR was independently associated with a higher risk of kidney and CV outcomes. In these high-risk patients, low dose atrasentan reduced IR by approximately 10% with consistent effects irrespective of baseline kidney function or IR.
To our knowledge, this is the first prospective study that demonstrates that higher IR is independently associated with a higher risk of cardio-renal outcomes in patients with type 2 diabetes mellitus and CKD. Previous cross-sectional studies already demonstrated an association between IR and kidney disease, but there are very few prospective studies [2–4]. Zhang et al. found that among subjects with normal glucose tolerance, those in the highest HOMA-IR quartile had a 50% increased risk of developing microalbuminuria [5]. In the general population, an increase in HOMA-IR during a 6 year baseline period was associated with an increased risk of adverse kidney outcomes during subsequent 6 years follow-up (n = 5347) [7]. However, these studies did not include patients with type 2 diabetes and CKD. We found a higher level of IR to be associated with an increased risk of decreased kidney function in a well-characterized international cohort of patients with type 2 diabetes and CKD. Our findings support a prior study on a new classification of diabetes which demonstrated that newly diagnosed patients with type 2 diabetes and preserved kidney function and characterized by severe insulin resistance had a higher risk of developing CKD compared to those not characterized by high insulin resistance [6]. Both cardiovascular events as well as kidney events contributed to the increased risk of developing the cardiovascular composite outcome, a prespecified adjudicated outcome in the SONAR trial. Our findings suggest that even after development of overt nephropathy, the presence of IR is associated with a higher risk of adverse clinical outcomes.
Using both the RADAR and SONAR trials allowed for a comprehensive characterization of the effect of atrasentan on HOMA-IR as the trials complemented each other in various ways. The RADAR trial was a relatively small dose-finding trial with a placebo comparison to generate the hypothesis that atrasentan reduces IR. The small sample size of the RADAR may have resulted in an overestimation of the effect size [22, 23]. The results of the larger SONAR confirm the beneficial effect of atrasentan on IR with a more robust and precise effect estimate. Additionally, while IR reduced in the atrasentan group after a relatively short follow-up period of 12 weeks in RADAR, SONAR confirmed that these initial effects are sustained over more than 2 years of follow-up. Moreover, SONAR demonstrated that the reduction in IR is reversible among participants who switched from atrasentan to placebo at randomization further supporting a true pharmacologic effect. Finally, HbA1c also reduced with atrasentan in the SONAR trial as previously observed in RADAR [18].
The effects of atrasentan on IR were consistent in most examined subgroups. It is not entirely clear why the effect of atrasentan on IR was more pronounced among participants with a lower BMI. It is possible that the higher percentage of patients on insulin in the high BMI group may influence the HOMA-IR measurement although in a subgroup analysis the effect of atrasentan was not different in those using and non-using insulin. It could also be possible that pharmacokinetic effects of atrasentan have played a role since a prior study reported an association between higher body weight (and BMI) and lower atrasentan exposure [24].
The key strength of this analysis was that the data were derived from two randomized controlled trials which were conducted to high standards and enrolled a broad internationally representative patient cohort with type 2 diabetes and CKD. The multiple IR measurements over time allowed for a comprehensive evaluation of the effect of atrasentan. This study also has limitations, the most obvious being that insulin measurements were only available for a subset of SONAR participants. In addition, although HOMA-IR is a widely used feasible method for the assessment of insulin resistance, considerable random variability in HOMA-IR levels exists [25, 26]. Although some studies reported that HOMA-IR provides a valid estimate of insulin sensitivity in patients with type 2 diabetes, other studies reported that HOMA-IR may not be a reliable predictor of insulin resistance compared to the ‘gold standard’ euglycemic clamp technique in certain populations such as older patients with poorly controlled diabetes [25, 27, 28]. Also, the kidneys play an important role in the clearance of insulin and impaired kidney function could have influenced insulin levels in addition to underlying metabolic disturbances related to type 2 diabetes. Insulin treatment may have affected the IR assessments as well, but all blood samples were taken in fasted states during which we would not expect patients to use short acting insulin. The model adjusted for use of insulin, in addition we performed a separate analysis of patients not treated with insulin, which did not influence the findings. Finally, because this is a post-hoc analysis, we cannot exclude chance findings.
Conclusions
In conclusion, IR is associated with a higher risk of developing cardio-kidney outcomes in patients with type 2 diabetes and CKD. The ERA atrasentan reduces IR, a finding which was consistent across most patient subgroups. Whether the reduction in IR contributes to the long-term kidney protective effects of atrasentan requires further study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional File 1: Table S1 with patient characteristics of the RADAR trial.
Additional File 2: Table S2 with baseline characteristics of SONAR trial population for long-term outcomes.
Additional File 3: Table S3 with Association between HOMA-IR and long-term cardio-renal outcomes in participants not using insulin.
Additional File 4: Figure S1 with HbA1c during the SONAR trial for patients with a HbA1c measurement at randomization.
Acknowledgements
The authors thank dr. Niels Jongs for his advice on the statistical analysis for this manuscript.
Abbreviations
- BMI
Body mass index
- BNP
B-type natriuretic peptide
- CKD
Chronic kidney disease
- CV
Cardiovascular
- eGFR
Estimated glomerular filtration rate
- ERA
Endothelin receptor A antagonists
- ESKD
End-stage kidney disease
- ET-1
Endothelin-1
- ETA
Endothelin A receptor
- FPG
Fasting plasma glucose
- FPI
Fasting plasma insulin
- HOMA-IR
Homeostatic model assessment
- IR
Insulin resistance
- MI
Myocardial infarction
- RADAR
Reducing Residual Albuminuria in Subjects With Diabetes and Nephropathy With AtRasentan
- RAS
Renin-angiotensin-system
- SONAR
The Study Of Diabetic Nephropathy With AtRasentan
- UACR
Urinary albumin-to-creatinine ratio
Authors’ contributions
J.D.S. and H.J.L.H. collected the data, analyzed the results, and wrote and edited the manuscript. All authors reviewed the manuscript.
Funding
This study was conducted in the framework of the IMI BEAt-DKD program. The BEAt-DKD project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 115974. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation program and EFPIA.
Data Availability
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided after review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement by the corresponding author and the SONAR Steering Committee. Data requests can be submitted at any time.
Declarations
Ethics approval and consent to participate
The RADAR and SONAR trials were designed and conducted in accordance with national regulatory and ethical guidelines. All subjects signed an informed consent.
Consent for publication
All authors gave the consent for the publication of the article.
Conflict of interest
J.D. Smeijer has nothing to disclose. D.E. Kohan has served as a consultant for AbbVie, AstraZeneca, Chinook Therapeutics and Travere Therpeutics. P. Rossing has received research support and personal fees from AstraZeneca and Novo Nordisk, and personal fees from Astellas, AbbVie, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, Mundipharma, Sanofi, and Vifor; all fees are given to Steno Diabetes Center Copenhagen. S.T. participates on a steering committee for Bayer Fidelio/Figaro studies, and speaker’s bureau with Servier and Pfizer. R. Correa Rotter served on advisory boards for Boehringer and AstraZeneca and has been a speaker for AstraZeneca, Boehringer Ingelheim, AbbVie, Takeda, Amgen, and Janssen. A. Liew has served as a consultant and member of advisory boards for Alnylam Pharmaceuticals, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer-Ingelheim, Chinook Therapeutics, Dimerix Limited, Eledon Pharmaceuticals, George Clinical, GlaxoSmithKline, Kira Pharmaceuticals, Prokidney, Otsuka Pharmaceuticals and Visterra Inc, Zai Lab Co. Ltd; has received Speaker’s honorarium from AstraZeneca, Baxter Healthcare, Boehringer-Ingelheim, Chinook Therapeutics and Otsuka Pharmaceuticals; and has served as a member of Data Safety and Monitoring Committee for Dimerix Limited and Zai Lab Co. Ltd. S.C.W. Tang has served as a consultant for Eledon Pharmaceuticals and Travere Therapeutics, has received speaker honoraria from AstraZeneca, Bayer AG, Boehringer-Ingelheim, Kyowa Kirin and Novartis, and serves on the Executive Committee of KDIGO. D. de Zeeuw served on advisory boards and/or speaker for Bayer, Boehringer Ingelheim, Fresenius, Mitsubishi-Tanabe, Travere Pharmaceuticals; Steering Committees and/or speaker for AbbVie and Janssen; Data Safety and Monitoring Committees for Bayer. Honoraria paid to Institution and consultant/speaker. R.T. Gansevoort has nothing to disclose. W. Ju has nothing to disclose. H.J.L.Heerspink is supported by a VIDI (917.15.306) grant from the Netherlands Organisation for Scientific Research and has served as a consultant for AbbVie, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Pharma, Fresenius, Gilead, Janssen, Merck, Mundipharma, Mitsubishi Tanabe, and Retrophin; and has received grant support from AbbVie, AstraZeneca, Boehringer Ingelheim, and Janssen.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Trajectories of glycemia, insulin sensitivity and insulin secretion preceding the diagnosis of type 2 diabetes: the Whitehall II study. Lancet. 2009;373:2215. doi: 10.1016/S0140-6736(09)60619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parvanova AI, Trevisan R, Iliev IP, Dimitrov BD, Vedovato M, Tiengo A, et al. Insulin resistance and Microalbuminuria A Cross-Sectional, Case-Control Study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes. 2006;55:1456–62. doi: 10.2337/db05-1484. [DOI] [PubMed] [Google Scholar]
- 3.Cheng HT, Huang JW, Chiang CK, Yen CJ, Hung KY, Wu KD. Metabolic syndrome and insulin resistance as risk factors for development of chronic kidney Disease and Rapid decline in renal function in Elderly. J Clin Endocrinol Metab. 2012;97:1268–76. doi: 10.1210/jc.2011-2658. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Muntner P, Hamm LL, Fonseca V, Batuman V, Whelton PK, et al. Insulin resistance and risk of chronic kidney disease in nondiabetic US adults. J Am Soc Nephrol. 2003;14:469–77. doi: 10.1097/01.ASN.0000046029.53933.09. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Lee ET, Howard B, Best LG, Umans JG, Yeh J, et al. Insulin resistance, Incident Cardiovascular Diseases, and decreased kidney function among nondiabetic american IndiansThe strong heart study. Diabetes Care. 2013;36:3195–200. doi: 10.2337/dc12-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahlqvist E, Storm P, Käräjämäki A, Martinell M, Dorkhan M, Carlsson A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6:361–9. doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 7.Yang S, Kwak S, Song YH, Han SS, Lee HS, Kang S, et al. Association of longitudinal trajectories of insulin resistance with adverse renal outcomes. Diabetes Care. 2022;45:1268–75. doi: 10.2337/dc21-2521. [DOI] [PubMed] [Google Scholar]
- 8.Kohan DE, Pollock DM. Endothelin antagonists for diabetic and non-diabetic chronic kidney disease. Br J Clin Pharmacol. 2013;76:573–9. doi: 10.1111/bcp.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang ZY, Zhou QL, Chatterjee A, Feener EP, Myers MG, White MF, et al. Endothelin-1 modulates insulin signaling through phosphatidylinositol 3-kinase pathway in vascular smooth muscle cells. Diabetes. 1999;48:1120–30. doi: 10.2337/diabetes.48.5.1120. [DOI] [PubMed] [Google Scholar]
- 10.Lee YC, Juan CC, Fang VS, Hsu YP, Lin SH, Kwok CF, et al. Evidence that endothelin-1 (ET-1) inhibits insulin-stimulated glucose uptake in rat adipocytes mainly through ETA receptors. Metabolism. 1998;47:1468–71. doi: 10.1016/S0026-0495(98)90071-3. [DOI] [PubMed] [Google Scholar]
- 11.Sarafidis PA, Bakris GL, Review Insulin and endothelin: an interplay contributing to hypertension development? J Clin Endocrinol Metab. 2007;92:379–85. doi: 10.1210/jc.2006-1819. [DOI] [PubMed] [Google Scholar]
- 12.Piatti PM, Monti LD, Conti M, Baruffaldi L, Galli L, Phan C, et al. Hypertriglyceridemia and Hyperinsulinemia are potent inducers of Endothelin-1 release in humans. Diabetes. 1996;45:316–21. doi: 10.2337/diab.45.3.316. [DOI] [PubMed] [Google Scholar]
- 13.Ottosson-Seeberger A, Lundberg JM, Alvestrand A, Ahlborg G. Exogenous endothelin-1 causes peripheral insulin resistance in healthy humans. Acta Physiol Scand. 1997;161:211–20. doi: 10.1046/j.1365-201X.1997.00212.x. [DOI] [PubMed] [Google Scholar]
- 14.de Zeeuw D, Coll B, Andress D, Brennan JJ, Tang H, Houser M, et al. The Endothelin Antagonist Atrasentan lowers residual Albuminuria in patients with type 2 Diabetic Nephropathy. J Am Soc Nephrol. 2014;25:1083–93. doi: 10.1681/ASN.2013080830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heerspink HJL, Parving HH, Andress DL, Bakris G, Correa-Rotter R, Hou FF, et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;393:1937–47. doi: 10.1016/S0140-6736(19)30772-X. [DOI] [PubMed] [Google Scholar]
- 16.Ahlborg G, Shemyakin A, Böhm F, Gonon A, Pernow J. Dual endothelin receptor blockade acutely improves insulin sensitivity in obese patients with insulin resistance and coronary artery disease. Diabetes Care. 2007;30:591–6. doi: 10.2337/dc06-1978. [DOI] [PubMed] [Google Scholar]
- 17.Stapel JR, Speed JS, Clemmer JS. Endothelin antagonism reduces hemoglobin A1c in patients with pulmonary hypertension. Can J Physiol Pharmacol. 2022;100:828–33. doi: 10.1139/cjpp-2022-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Zeeuw D, Coll B, Andress D, Brennan JJ, Tang H, Houser M, et al. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol. 2014;25:1083–93. doi: 10.1681/ASN.2013080830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heerspink HJL, Andress DL, Bakris G, Brennan JJ, Correa-Rotter R, Hou FF, et al. Baseline characteristics and enrichment results from the SONAR trial. Diabetes Obes Metab. 2018;20:1829–35. doi: 10.1111/dom.13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heerspink HJL, Andress DL, Bakris G, Brennan JJ, Correa-Rotter R, Dey J, et al. Rationale and protocol of the study of diabetic nephropathy with AtRasentan (SONAR) trial: a clinical trial design novel to diabetic nephropathy. Diabetes Obes Metab. 2018;20:1369–76. doi: 10.1111/dom.13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985 28:7 1985; 28: 412–419. [DOI] [PubMed]
- 22.Hwang TJ, Carpenter D, Lauffenburger JC, Wang B, Franklin JM, Kesselheim AS. Failure of investigational drugs in late-stage Clinical Development and Publication of Trial results. JAMA Intern Med. 2016;176:1826–33. doi: 10.1001/jamainternmed.2016.6008. [DOI] [PubMed] [Google Scholar]
- 23.De Martini D. Empowering phase II clinical trials to reduce phase III failures. Pharm Stat. 2020;19:178–86. doi: 10.1002/pst.1980. [DOI] [PubMed] [Google Scholar]
- 24.Koomen J, Stevens J, Bakris G, Correa-Rotter R, Hou FF, Kitzman DW, et al. Inter-individual variability in atrasentan exposure partly explains variability in kidney protection and fluid retention responses: a post hoc analysis of the SONAR trial. Diabetes Obes Metab. 2021;23:561–8. doi: 10.1111/dom.14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarafidis PA, Lasaridis AN, Nilsson PM, Pikilidou MI, Stafilas PC, Kanaki A et al. Validity and reproducibility of HOMA-IR, 1/HOMA-IR, QUICKI and McAuley’s indices in patients with hypertension and type II diabetes. Journal of Human Hypertension 2007 21:9 2007; 21: 709–716. [DOI] [PubMed]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Katsuki A, Sumida Y, Urakawa H, Gabazza EC, Murashima S, Morioka K, et al. Neither Homeostasis Model Assessment nor quantitative insulin sensitivity check Index can predict insulin resistance in Elderly patients with poorly controlled type 2 diabetes Mellitus. J Clin Endocrinol Metab. 2002;87:5332–5. doi: 10.1210/jc.2002-020486. [DOI] [PubMed] [Google Scholar]
- 28.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional File 1: Table S1 with patient characteristics of the RADAR trial.
Additional File 2: Table S2 with baseline characteristics of SONAR trial population for long-term outcomes.
Additional File 3: Table S3 with Association between HOMA-IR and long-term cardio-renal outcomes in participants not using insulin.
Additional File 4: Figure S1 with HbA1c during the SONAR trial for patients with a HbA1c measurement at randomization.
Data Availability Statement
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided after review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement by the corresponding author and the SONAR Steering Committee. Data requests can be submitted at any time.