Abstract
Introduction:
Patients with classical congenital adrenal hyperplasia (CAH) have prenatal and postnatal hormonal imbalances. To characterize the ontogeny of reported brain and behavior changes in older children with CAH, we aimed to study brain structure in infants with CAH compared to healthy controls.
Methods:
We performed neuroimaging in 16 infants with classical CAH due to 21-hydroxylase deficiency [8 males, gestational age 38.2 ± 1.7 weeks, post-conceptional age (PCA) 42.2 ± 3.0 weeks] and 14 control infants (9 males, gestational age 38.5 ± 1.8 weeks, PCA 42.5 ± 2.4 weeks) utilizing 3-Tesla magnetic resonance imaging. Regional brain volumes were adjusted for PCA and sex, along with an additional adjustment for total brain volume (TBV), for group comparisons by regression analyses [mean, 95% confidence interval (CI)]. The degree to which each brain region was differentiated between CAH and control infants was examined by relaimpo analyses, adjusting for all other brain regions, PCA, and sex.
Results:
Infants with CAH had significantly smaller thalamic volumes [8606 mm3, 95% CI (8209, 9002)] compared to age-matched control infants [9215 mm3, 95% CI (8783, 9647); β = −609; p = 0.02], which remained smaller after further adjustment for TBV. Upon further adjustment for TBV, the temporal lobe was larger in infants with CAH [66817 mm3, CI (65957, 67677)] compared to controls [65616 mm3, CI (64680, 66551); β = 1202, p = 0.03]. The brain regions most differentiated between CAH vs controls were the thalamus (22%) and parietal lobe (10%).
Conclusions:
Infants with CAH exhibit smaller thalamic regions from early life, suggesting a prenatal influence on brain development in CAH. Thalamic emergence at 8–14 weeks makes the region particularly vulnerable to changes in the intrauterine environment, with potential implications for later maturing brain regions. These changes may take time to manifest, meriting longitudinal study through adolescence in CAH.
Keywords: congenital adrenal hyperplasia, 21-hydroxylase deficiency, brain development, magnetic resonance imaging, newborn, pediatrics
INTRODUCTION
Congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency is the most common primary adrenal insufficiency in children, affecting 1 in 14,000–18,000 live births in the severe classic form [1]. Patients with CAH also have adrenal androgen overproduction due to disrupted steroidogenesis, which begins as early as week 7 of gestation with the development of the adrenal gland. Thus, fetuses with CAH experience disruptions to the intrauterine hormonal environment that persist throughout pregnancy until postnatal treatment is started. These early alterations are known to impact long-term neurodevelopment in fetuses by affecting early brain developmental trajectories [2–4].
Youth and adults with CAH are known to be at higher risk for behavioral problems, learning disabilities and neuropsychological co-morbidities [5–8]. These findings coincide with the discovery of neurological changes in brain structure and organization, identified using magnetic resonance imaging (MRI) in youth and adults with CAH. Brain structural differences in patients with CAH include smaller volumes of the whole brain, prefrontal cortex, and components of the limbic system (regional volumes of the amygdala, hippocampus, and precuneus) compared to controls [9–12]. Additionally, adult women with CAH exhibit changes to white matter microstructure associated with structural connectivity and brain organization which are correlated with exogenous glucocorticoid exposure [11]. Youth with CAH also exhibit white matter microstructure abnormalities on MRI [13]. However, the ontogeny of these neurological changes and their association with intrauterine environmental and hormonal disruptions in CAH remain unknown.
Brain development during the first 1000 days, from conception to 2 years old, sets the foundation for neurocognitive, behavioral and psychological development across the lifespan [14]. During this period, brain development follows a precise and synchronous timetable that when disrupted, sets the brain on an adverse neurodevelopmental trajectory in years thereafter [15]. Animal models of CAH show that activation effects of prenatal androgens and cortisol deficiency are associated with reduced brain volumes early in life, particularly in the limbic regions. These brain alterations are correlated with deficient cognitive and behavioral performance later in life. As well, animal models of CAH show that cognitive and memory impairments are the result of aberrant brain structure and organization in early life [16]. Despite established evidence of aberrant neurological differences in these vulnerable children, there is surprisingly limited data on the origins of these neurological aberrations and their contribution to the reported neurodevelopmental sequelae in CAH.
In this paper, we aim to study brain structural differences between infants with and without CAH due to 21-hydroxylase deficiency. Using MRI-based volumetric analysis, we compared both global and regional volumes of various brain structures between infants with CAH and age-matched typically developing infants. Based on the timing of hormonal dysregulation and prior evidence, we hypothesized that infants with CAH would show reduced volumes in the deep gray and limbic regions of the brain.
METHODS
Study Population
This cross-sectional study protocol was reviewed and approved by the Institutional Review Board at Children’s Hospital Los Angeles, approval number CHLA-15–00001. Participants were recruited from the CHLA CAH Comprehensive Care Center and Altamed General Pediatrics Clinic at CHLA. Written informed consent was obtained from parents or legal guardians in accordance with The Code of Ethics of the World Medical Association. Infants were identified through state newborn screening and met criteria for confirmed 21-hydroxylase deficiency by either biochemical testing or genotyping of CYP21A2. Healthy control infants were born between 34 and 42 weeks gestation and had no known significant health conditions. The participants’ parents were 18 years of age or older, English- or Spanish-speaking, and able to complete questionnaires and surveys regarding the maternal pregnancy history and their child’s health. No mothers were treated prenatally with dexamethasone.
There were 15 infants with the salt-wasting form of CAH due to 21-hydroxylase deficiency and one infant with simple-virilizing form. Serum testosterone, androstenedione, and 17-hydroxyprogesterone levels were obtained at the time of the study visit. All infants were started on hydrocortisone, fludrocortisone, and salt at diagnosis, with the days on treatment recorded.
MRI Acquisition
MRI data were obtained using a 3-Tesla MRI scanner (Philips Achieva, Andover, MA) using a 32-channel neurovascular coil. A bundle-and-feed, non-sedating protocol was utilized prior to the scan, where the infant participant was fed, changed and then swaddled in a blanket [17]. Each participant was then secured in an infant vacuum full-body splint (MedVac, Med-X Products, Littleton, NC) and positioned supine in the scanner with sandbags adjacent to their sides and feet to simulate containing touch. Each participant had two layers of ear protection and headphones placed over the ear to transmit lullabies at a low volume via bone conduction. MRI scans were thus performed without sedation during natural sleep and movement was minimized. If motion artifacts were detected during the scan, sequences were repeated a second time. High-resolution T1-weighted images were acquired with a sagittal reconstruction (TR = 2000 ms, TE = 4.6 ms, inversion time = 1000 ms, flip angle = 8°, with isotropic 1 mm3).
Volumetric Analysis
Volumetric imaging analyses were conducted using an atlas-based approach, using age-matched templates from the ALBERTs infant atlases [18]. Before automated segmentation, 3D T1-weighted anatomical images were automatically skull-stripped using FSL’s automated brain extraction tool and then edited manually using ITK-SNAP [19] (shown in Figure 1). The skull-stripped T1 images were then inverted into pseudo-T2-weighted images to match the imaging modality of the ALBERTs atlases. The inverted images were bias-corrected and then run through automated segmentation on NeBSS [20] which uses an atlas-based expectation maximization to assign segmentation labels for the brain [18]. The 50 subregions were then refined into the following larger structural regions of interest (ROIs): frontal lobe, parietal lobe, occipital lobe, temporal lobe, limbic region, cerebellum, thalamus, lateral ventricles, corpus callosum, and brain stem. Segmented brain maps were reviewed and manually edited using ITK-SNAP. Lastly, MR brain volumes were calculated by multiplying the number of voxels (3D pixels) within each region of interest with the volume of one voxel obtained by the MRI scanner. We also quantified total brain volumes (TBV) in all infants.
Figure 1. Acquisition and segmentation of brain structural images in infants with CAH and age-matched controls for volumetric analyses.
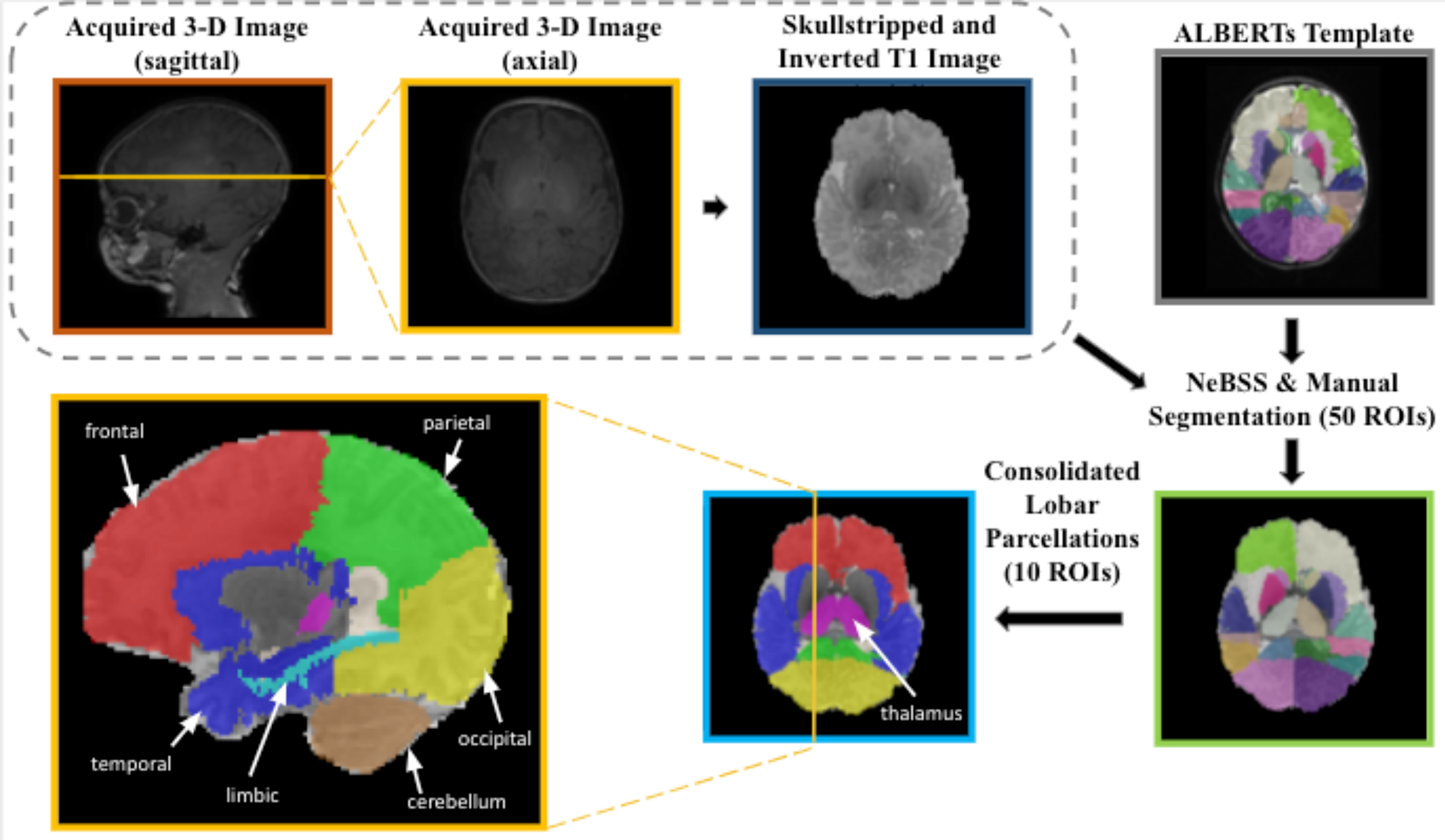
Prior to automated segmentation, 3D T1-weighted anatomical images were automatically skull-stripped (FSL) and then edited manually (ITK-SNAP) and inverted into pseudo-T2-weighted images to match the imaging modality of the ALBERTs atlases. The images were then bias-corrected and automatically segmented (NeBSS) into 50 brain tissue types. These 50 subregions were then refined into larger structural regions of interest (ROIs).
Statistical Analysis
All data analyses were performed in R v4.1.1 [21]. We began by examining the distributions of brain ROIs and demographic variables between CAH and control infants. All brain volume measurements appeared to be normally distributed with no outliers. We examined the differences in brain region volumes between CAH and control infants through a series of linear regression models. The first set of models were adjusted for post-conceptional age and sex, and the second set additionally adjusted for total brain volume. The estimated marginal brain volume means with 95% confidence intervals (CI) were computed, as well as p-values for the effect of CAH status. While both gestational age and PCA were recorded for infants, there was a high degree of correlation between these two measures (r = 0.57, p < 0.001). Therefore, we adjusted only for PCA in regression models.
We used relaimpo analysis (Relative Importance of Regressors in Linear Models; R libraries relaimpo, v2.2–3) [22] to compute the percentage of the variance (r2lmg or percent variance) explained by each predictor on the outcome in our regression model [23]. Relaimpo analyses examined the degree to which each brain region was differentiated between CAH and control infants, adjusting for all other brain regions, PCA, and sex.
We additionally examined the relationships between hormone levels at diagnosis and medication dosing at the time of imaging on brain region volumes in participants with CAH. We used linear regression, adjusting for PCA, sex, and days on medication treatment.
RESULTS
Demographic Differences by CAH Status
In this study, 16 infants with CAH [8 males, gestational age 38.2 ± 1.74 weeks, post-conceptional age (PCA) 42.2 ± 3.0 weeks] and 14 healthy controls (9 males, gestational age 38.5 ± 1.8 weeks, PCA 42.5 ± 2.4 weeks) underwent brain imaging via MRI (Table 1). Infants were all born between 34 and 42 weeks gestation. Imaging was performed between 0.7–8.1 weeks post-birth for the CAH group and 1.3–9.4 weeks post-birth for the healthy control group. There were no significant differences between the groups in sex, gestational age (age at birth), or PCA (gestational age at birth + age at imaging). The CAH infants had an average serum testosterone level of 289.1 ± 277.7 ng/dL (10.03 ± 9.6 nmol/L), serum androstenedione level of 3674.6 ± 3699.8 ng/dL (128.2 ± 129.1 nmol/L), and serum 17-hydroxyprogesterone (17-OHP) level of 29080.4 ± 14325.2 ng/dL (506.6 ± 338.3 nmol/L) (Table 1).
Table 1.
Study Participant Characteristics for CAH and Control Infants
| CAH (n = 16) | Control (n = 14) | Group Difference, p-value | |
|---|---|---|---|
| Age at MRI (weeks) | |||
| Gestational Age | 38.2 ± 1.74 | 38.5 ± 1.8 | 0.83a |
| Post-conceptional Age | 42.2 ± 3.0 | 42.5 ± 2.4 | 0.88a |
| Biological sex (n) | Female, 8 Male, 8 | Female, 5 Male, 9 | 0.68b |
| Hispanic/ Latino (n; %) | 6; 37.5% | 7; 50% | 0.79b |
| Race (n; %) | |||
| White | 14; 87.5% | 5; 35.7% | 0.001b |
| Asian | 2; 12.5% | 2; 14.3% | |
| CAH Form (n) | |||
| Salt-wasting | 15 | - | - |
| Simple-virilizing | 1 | ||
| Glucocorticoid dose (mg/m2/day) | 35.2 ± 16.4 | - | - |
| Fludrocortisone dose (mg/day) | 0.12 ± 0.04 | ||
| NaCl dose (mEq/day) | 4.27 ± 0.70 | ||
| CAH Analytes | |||
| Newborn screen 17-OHP (ng/dL) | 29080.4 ± 14325.2 | - | - |
| Newborn screen 17-OHP [nmol/L] | [881.1 ± 434.1] | ||
| Confirmatory serum 17-OHP (ng/dL) | 16719.8 ± 11164.5 | ||
| Confirmatory serum 17-OHP [nmol/L] | [506.6 ± 338.3] | ||
| Highest newborn 17-OHP (ng/dL) | 28861.9 ± 13996.9 | ||
| Highest newborn 17-OHP [nmol/L] | [874.5 ± 424.1] | ||
| Confirmatory serum testosterone (ng/dL) | 289.1 ± 277.7 | ||
| Confirmatory serum testosterone [nmol/L] | [10.03 ± 9.6] | ||
| Confirmatory serum androstenedione (ng/dL) | 3674.6 ± 3699.8 | ||
| Confirmatory serum androstenedione [nmol/L] | [128.2 ± 129.1] | ||
| Plasma Renin Activity (ng/mL/hr and μg/L/hr) | 50.1 ± 75.4 |
Mean ± SD
Wilcoxon sign-rank test;
Chi-square test
There were no significant differences in demographic characteristics between CAH and control infants, including gestational age (p = 0.83), PCA (p = 0.88), biological sex (p = 0.68), and Hispanic/Latino ethnicity (p = 0.79). For race, there was a higher proportion of Caucasian infants in the CAH group than in the control group (87.5% vs 35.7%, p = 0.001; Table 1).
Radiological Findings
A radiologist reviewed all scans for incidental findings of gross abnormalities. Two infants with CAH who had incidental findings noted on their brain scans. One patient had a right frontal venous angioma. The other patient had blood in the right lateral ventricle and one punctate white-matter lesion. We ensured that these findings were manually corrected during image analyses.
Brain Volumes and CAH Status
There were no differences in TBV between CAH and control infants, controlling for PCA and sex. Additionally, there were no differences between left and right hemisphere volumes (p > 0.05 for all). Therefore, we proceeded with examining the total (i.e., combined left and right) brain region volumes for group comparisons (Table 2). The thalamus was significantly smaller in infants with CAH [8606 mm3, 95% CI (8209, 9002)] compared to controls [9215 mm3, CI (8783, 9647); β = −609, p = 0.02; Table 2], adjusting for PCA and sex. After further adjusting for TBV (in addition to PCA and sex), the thalamus remained smaller in infants with CAH [8646 mm3, CI (8373, 8918)] compared to controls [9223 mm3, CI (8927, 9519); β = −577, p = 0.002)].
Table 2.
Brain Volume Comparisons between CAH and Control Infants
| CAH | Controls | ||||
|---|---|---|---|---|---|
| Region | Volume (mm3) | 95% CI | Volume (mm3) | 95% CI | |
| TBV | Raw | 390672 | (371064, 410281) | 393126 | (371747, 414506) |
| Frontal | Raw | 120,293 | (113015, 127571) | 119,799 | (111864,127734) |
| TBV Adjusted† | 121,273 | (120164,122381) | 120,006 | (118799, 121212) | |
| Parietal | Raw | 74,717 | (70077, 79357) | 76,307 | (71248, 81366) |
| TBV Adjusted† | 75,335 | (74356, 76314) | 76,438 | (75373, 77503) | |
| Occipital | Raw | 51,438 | (47980, 54895) | 52,294 | (48524, 56063) |
| TBV Adjusted | 51,890 | (50918, 52863) | 52,389 | (51331, 53448) | |
| Temporal | Raw | 66,293 | (62355, 70231) | 65,505 | (61211, 69799) |
| TBV Adjusted * | 66,817 | (65957, 67677) | 65,616 | (64680, 66551) | |
| Limbic | Raw | 19,572 | (18254, 20890) | 19,634 | (18197, 21071) |
| TBV Adjusted | 19,743 | (19325, 20160) | 19,670 | (19216, 20125) | |
| Cerebellum | Raw | 26,404 | (24715, 28093) | 26,936 | (25095, 28777) |
| TBV Adjusted | 26,574 | (25418, 27730) | 26,972 | (25714, 28230) | |
| Thalamus | Raw * | 8,606 | (8209, 9002) | 9,215 | (8783, 9647) |
| TBV Adjusted ** | 8,646 | (8373, 8918) | 9,223 | (8927, 9519) | |
| LV | Raw | 5,399 | (4590, 6209) | 4,766 | (3883, 5649) |
| TBV Adjusted | 5,451 | (4721, 6181) | 4,777 | (3982, 5571) | |
| Corpus Callosum | Raw | 2,597 | (2386, 2808) | 2,566 | (2336, 2796) |
| TBV Adjusted | 2,620 | (2496, 2744) | 2,571 | (2436, 2706) | |
| Brainstem | Raw | 6,044 | (5717, 6371) | 6,207 | (5851, 6564) |
| TBV Adjusted | 6,075 | (5837, 6314) | 6,214 | (5955, 6473) | |
Abbreviations: CAH, congenital adrenal hyperplasia; CI, confidence interval; TBV, total brain volume; LV, lateral ventricles
All regressions (Raw, TBV Adjusted) were adjusted for post-conceptional age and sex.
0.05 < p < 0.10;
p < 0.05;
p < 0.01
As well, after adjusting tissue volumes for TBV, the temporal lobe was larger [66817 mm3, CI (65957, 67677)] in infants with CAH compared to controls [65616 mm3, CI (64680, 66551); β = 1202, p = 0.03]. While not significant, the parietal lobe was trending toward significance as smaller in infants with CAH (β = −1103, p = 0.08) and the frontal lobe was trending toward significance as larger (β = 1267, p = 0.08 Table 2).
The brain regions that were most differentiated between CAH vs control infants were the thalamus (percent variance = 22%), followed by the parietal lobe (percent variance = 10%; shown in Figure 2) as examined by relaimpo analyses.
Figure 2. Relaimpo analysis to examine the degree to which each brain region was differentiated between CAH and control infants.
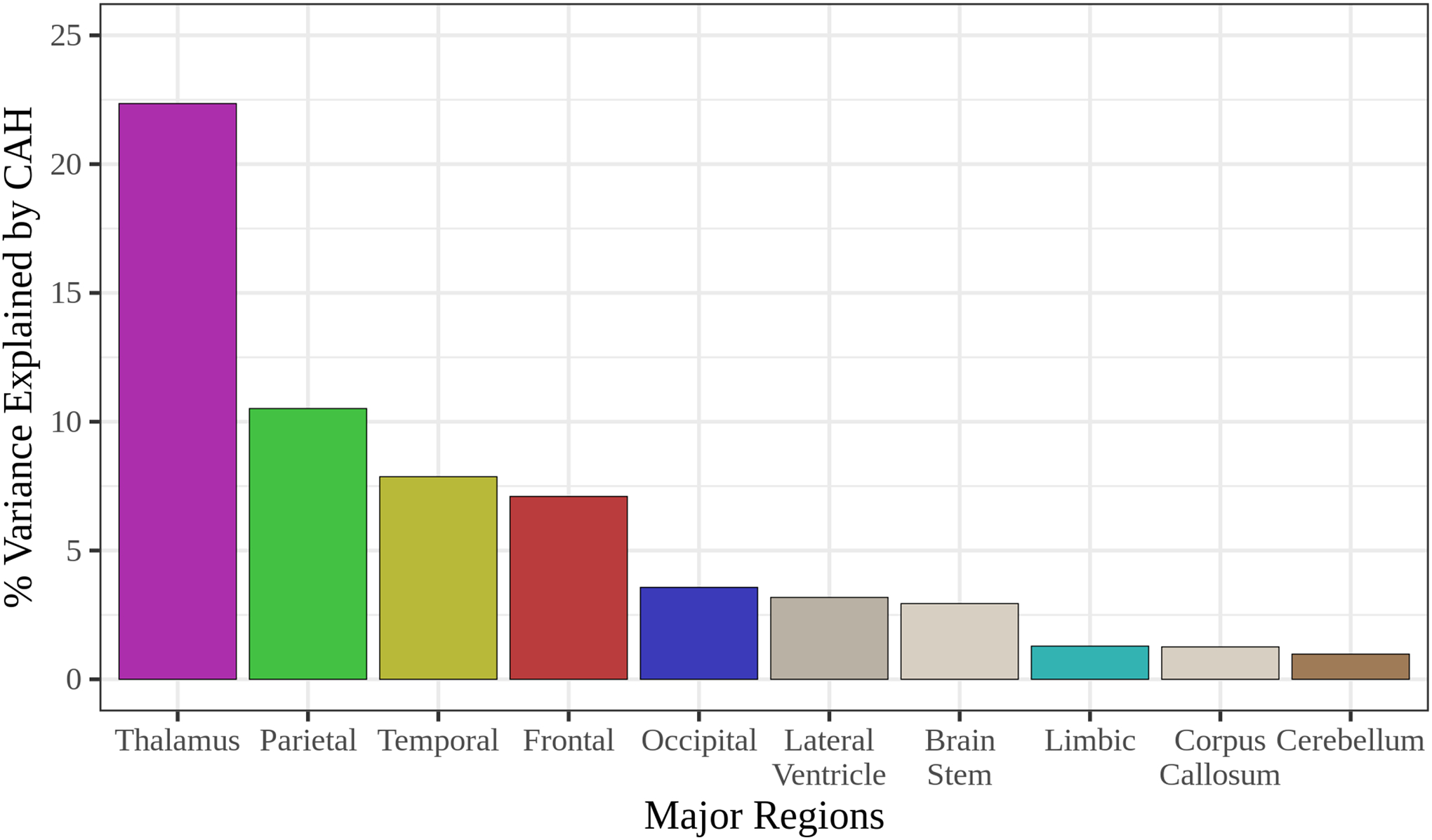
Relative Importance of Regressors in Linear Models (relaimpo) analyses were performed, adjusting for post-conceptional age and sex. The brain regions most differentiated between CAH and control infants were the thalamus (percent variance = 22%) and the parietal lobe (percent variance = 10%).
Key Brain Volumes and Clinical Features in CAH
We examined the relationships between hormone levels at diagnosis and medication dosing at the time of imaging on brain region volumes, adjusting for PCA, sex, and days of treatment, in infants with CAH (N = 16; Supplementary Table 1). We did not find any associations between hormone levels at diagnosis and brain volumes. However, we did observe that higher levels of androstenedione [β = −0.10, CI (−0.20, 0.00), p = 0.09] were approaching significance with lower thalamic volumes.
DISCUSSION
The main finding of our study is that infants with classical CAH due to 21-hydroxylase deficiency exhibit reduced thalamic volumes compared to healthy, age-matched infants. Gestational timing of the active emergence and differentiation of the thalamic nuclei at 8–14 weeks makes the thalamus particularly vulnerable to changes in the intrauterine environment [24]. Our findings support that the extent of thalamic volume reduction could be associated with the severity of CAH in these infants, with a smaller thalamus potentially indicative of specific vulnerabilities to thalamic circuits related to sensory and motor connectivity as the brain matures [25]. Disrupted normative growth patterns in the thalamus, together with those seen in the temporal lobe, could indicate disruptions to sensory-motor network function and integration, and could underlie behavioral problems reported in youth and adults with CAH. We know from other disease models of preterm [26, 27], epileptic [28], and low birth-weight infants [29] that smaller thalamic volumes can persist into late childhood and are a reliable biomarker of poor neurocognitive outcomes. Differences in adjusted temporal lobe volumes could indicate altered brain organization in infants with CAH, with a potential association with intrauterine exposure to excess androgens. Notably, unaffected male infants have been found to exhibit higher adjusted temporal volumes compared to unaffected female infants [30] and increases in adjusted temporal lobe volumes are known to be related to poor cognitive outcomes later in life [31]. It would be worthwhile to study further the relationship between early brain differences and later developmental changes in children with CAH.
Early aberrations, such as those observed in the thalamus and temporal lobe, could impact the maturational trajectory of other gray matter regions. Although we did not find any differences in total brain, hippocampus, amygdala, or prefrontal cortex volumes between infants with and without CAH, these structural differences have been noted in older children and adults with CAH [9–12, 32] and merit longitudinal follow-up of the infants in this study. It is possible that most of these brain regions mature later in infancy and childhood and have not yet manifested by early infancy. For example, aberrations in regions such as the prefrontal cortex emerge later in maturation and may not yet exist. Additionally, studies in other disease models have also shown an association between reduced thalamic volume and increased risk of white matter abnormalities [33, 34]. Youth with CAH have affected white matter microstructure that could lead to cognitive impairment, emotional dysregulation, and executive function deficits [11, 13, 32]. However, associations with reduced thalamic volume during infancy and white matter microstructural deficits in later life have yet to be investigated in CAH. Some of these changes may have been programmed in utero while others may result from a complex interplay between multiple brain regions during newborn development. Furthermore, some of the later changes reported in older children with CAH may be confounded due to effects of postnatal hormonal replacements. The interplay between these early structural discrepancies (e.g., in the thalamus), ongoing developmental changes, and postnatal hormone therapy may underlie the various neurological abnormalities reported in patients with CAH.
There were some limitations to this study. There was a relatively small sample size due to the rarity of this condition (studied at a single center), which limited the detectable effect size in our group comparisons, as well as precluded the analysis of sex differences. A larger sample size would help to confirm the relationship between hormone levels at diagnosis and disruptions to the development of key brain regions. Thus, future multicenter studies are merited to determine the differential effect of sex and hormone levels on brain development in infants with CAH. As well, multicenter studies would allow for a broader range of disease severity of patients (i.e., more infants with simple-virilizing CAH). The lack of myelination in the neonatal period precluded any meaningful measurements of white matter at this age, even though discrepancies in brain structural connectivity have been reported in older children with CAH [9–12, 30] and would be interesting to assess during infancy. Finally, the cross-sectional study design did not allow for longitudinal observation from infancy through adolescence, to follow the trajectory of abnormal maturation changes in CAH.
Our study has established the earliest evidence of neurostructural abnormalities in infants with CAH. This can carry potential implications for later effects on structural and functional development that are seen in older children and adults with CAH. Our findings suggest that regional structural development is affected during the prenatal period and may manifest as additional changes later in life. Longitudinal study from infancy through adolescence will be critical to further understand how early differences and postnatal treatments can affect developmental trajectories in CAH.
Supplementary Material
Acknowledgments
We gratefully thank the participants and their families. We would also like to acknowledge the CHLA Children’s Imaging Research Program; Altamed General Pediatrics Clinic at CHLA, Norma Martinez, Heather Ross, and Christina Koppin for their assistance with recruitment and data collection; as well as CARES Foundation and the Abell Foundation for their ongoing philanthropic support of the CHLA CAH Comprehensive Care Center.
Conflict of Interest Statement
M.S.K. receives unrelated research funding from Neurocrine Biosciences, Spruce Biosciences, Adrenas Therapeutics, and Diurnal. M.E.G. receives unrelated research support from Novo Nordisk, Adrenas Therapeutics, Neurocrine Biosciences, and Spruce Biosciences. M.E.G. serves on advisory boards or as a consultant for Adrenas Therapeutics, Ascendis, Eton Pharmaceuticals, Novo Nordisk, and Pfizer; serves on data safety monitoring boards for Ascendis; serves as an adjudication committee member for ICON Clinical Research, LLC/Aeterna Zentaris; and receives royalties from McGraw-Hill and UpToDate.
Funding Sources
This study was supported by The Saban Research Institute at CHLA and its Imaging Core Pilot Program (MSK), CARES Foundation (MEG and MSK), and The Abell Foundation (MEG). MSK is supported by the National Institutes of Health NIH/NICHD K23HD084735 and R03HD101718. VR is supported by NIH/NHLBI K01HL153942 and the Saban Research Institute Research Career Development Award. JLW is supported by K23HD099309. This work was supported by grants UL1TR001855 and UL1TR000130 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Statement of Ethics
This study was approved by the Institutional Review Board at Children’s Hospital Los Angeles, approval number CHLA-15-00001. Written informed consent was obtained from parents or legal guardians in accordance with The Code of Ethics of the World Medical Association.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
REFERENCES
- 1.Speiser PW, et al. , Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab, 2018. 103(11): p. 4043–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis EP and Narayan AJ, Pregnancy as a Period of Risk, Adaptation, and Resilience for Mothers and Infants. Dev Psychopathol, 2020. 32(5): p. 1625–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faa G, et al. , Fetal Programming of Neuropsychiatric Disorders. Birth Defects Res C Embryo Today, 2016. 108(3): p. 207–223. [DOI] [PubMed] [Google Scholar]
- 4.Godfrey KM and Barker DJ, Fetal Programming and Adult Health. Public Health Nutr, 2001. 4(2B): p. 611–24. [DOI] [PubMed] [Google Scholar]
- 5.Athanasiadis L, Psychological Evaluation of Patients with Congenital Adrenal Hyperplasia (CAH), in Fertility and Reproductive Outcomes in Different Forms of Congenital Adrenal Hyperplasia. 2021, Springer, Cham. p. 141–155. [Google Scholar]
- 6.Sewell R, et al. , Behavioral Health Diagnoses in Youth with Differences of Sex Development or Congenital Adrenal Hyperplasia Compared with Controls: A PEDSnet Study. J Pediatr, 2021. 239: p. 175–181.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harasymiw LA, Grosse SD, and Sarafoglou K, Attention-Deficit/Hyperactivity Disorder Among US Children and Adolescents With Congenital Adrenal Hyperplasia. J Endocr Soc, 2020. 4(12): p. bvaa152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kung KTF, et al. , Emotional and behavioral adjustment in 4 to 11-year-old boys and girls with classic congenital adrenal hyperplasia and unaffected siblings. Psychoneuroendocrinology, 2018. 97: p. 104–110. [DOI] [PubMed] [Google Scholar]
- 9.Herting MM, et al. , Brain Differences in the Prefrontal Cortex, Amygdala, and Hippocampus in Youth with Congenital Adrenal Hyperplasia. J Clin Endocrinol Metab, 2020. 105(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van’t Westeinde A, et al. , Altered Gray Matter Structure and White Matter Microstructure in Patients with Congenital Adrenal Hyperplasia: Relevance for Working Memory Performance. Cereb Cortex, 2020. 30(5): p. 2777–2788. [DOI] [PubMed] [Google Scholar]
- 11.Webb EA, et al. , Quantitative Brain MRI in Congenital Adrenal Hyperplasia: In Vivo Assessment of the Cognitive and Structural Impact of Steroid Hormones. J Clin Endocrinol Metab, 2018. 103(4): p. 1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merke DP, et al. , Children with classic congenital adrenal hyperplasia have decreased amygdala volume: potential prenatal and postnatal hormonal effects. J Clin Endocrinol Metab, 2003. 88(4): p. 1760–5. [DOI] [PubMed] [Google Scholar]
- 13.Cotter DL, et al. , White Matter Microstructural Differences in Youth with Classical Congenital Adrenal Hyperplasia. J Clin Endocrinol Metab, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cusick S and Georgieff M. The First 1,000 days of Life: The Brain’s Window of Opportunity. 2013. [cited 2022; Available from: https://www.unicef-irc.org/article/958-the-first-1000-days-of-life-the-brains-window-of-opportunity.html].
- 15.Rajagopalan V, et al. , Local Tissue Growth Patterns Underlying Normal Fetal Human Brain Gyrification Quantified In Utero. J Neurosci, 2011. 31(8): p. 2878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maheu FS, et al. , Steroid Abnormalities and the Developing Brain: Declarative Memory for Emotionally Arousing and Neutral Material in Children with Congenital Adrenal Hyperplasia. Psychoneuroendocrinology, 2008. 33(2): p. 238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong SZ, Zhu M, and Bulas D, Techniques for Minimizing Sedation in Pediatric MRI. J Magn Reson Imaging, 2019. 50(4): p. 1047–1054. [DOI] [PubMed] [Google Scholar]
- 18.Gousias IS, et al. , Magnetic Resonance Imaging of the Newborn Brain: Automatic Segmentation of Brain Images into 50 Anatomical Regions. PLoS One, 2013. 8(4): p. e59990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yushkevich PA, et al. , User-guided 3D Active Contour Segmentation of Anatomical Structures: Significantly Improved Efficiency and Reliability. Neuroimage, 2006. 31(3): p. 1116–28. [DOI] [PubMed] [Google Scholar]
- 20.Ceschin R, et al. NeBSS: Semi-Automated Parcellation of Neonatal Structural Brain MRI. 2016. DOI: doi: 10.20944/preprints201612.0060.v1. [DOI] [Google Scholar]
- 21.Team, R.C., A language and environment for statistical computing. 2021, R Foundation for Statistical Computing: Vienna, Austria. [Google Scholar]
- 22. https://cran.r-project.org/web/packages/relaimpo/index.html .
- 23.Gröemping U, Relative Importance for Linear Regression in R: The Package relaimpo. J Stat Softw, 2006. 17(1): p. 1–27. [Google Scholar]
- 24.Mojsilović J and Zecević N, Early Development of the Human Thalamus: Golgi and Nissl Study. Early Hum Dev, 1991. 27(1–2): p. 119–44. [DOI] [PubMed] [Google Scholar]
- 25.Toulmin H, et al. , Specialization and Integration of Functional Thalamocortical Connectivity in the Human Infant. Proc Natl Acad Sci U S A, 2015. 112(20): p. 6485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boardman JP, et al. , A Common Neonatal Image Phenotype Predicts Adverse Neurodevelopmental Outcome in Children Born Preterm. Neuroimage, 2010. 52(2): p. 409–14. [DOI] [PubMed] [Google Scholar]
- 27.Cayam-Rand D, et al. , Interaction between Preterm White Matter Injury and Childhood Thalamic Growth. Ann Neurol, 2021. 90(4): p. 584–594. [DOI] [PubMed] [Google Scholar]
- 28.Yoong M, et al. , Cognitive Impairment in Early Onset Epilepsy is Associated with Reduced Left Thalamic Volume. Epilepsy Behav, 2018. 80: p. 266–271. [DOI] [PubMed] [Google Scholar]
- 29.Bjuland KJ, et al. , Brain Volumes and Cognitive Function in Very-low-birth-weight (VLBW) Young Adults. Eur J Paediatr Neurol, 2014. 18(5): p. 578–90. [DOI] [PubMed] [Google Scholar]
- 30.Lehtola SJ, et al. , Associations of age and sex with brain volumes and asymmetry in 2–5-week-old infants. Brain Struct Funct, 2019. 224(1): p. 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young JM, et al. , Resilience and Vulnerability: Neurodevelopment of Very Preterm Children at Four Years of Age. Front Hum Neurosci, 2020. 14: p. 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nass R, et al. , Magnetic Resonance Imaging in the Congenital Adrenal Hyperplasia Population: Increased Frequency of White-matter Abnormalities and Temporal Lobe Atrophy. J Child Neurol, 1997. 12(3): p. 181–6. [DOI] [PubMed] [Google Scholar]
- 33.Wisnowski JL, et al. , Reduced Thalamic Volume in Preterm Infants is Associated with Abnormal White Matter Metabolism Independent of Injury. Neuroradiology, 2015. 57(5): p. 515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ball G, et al. , The Effect of Preterm Birth on Thalamic and Cortical Development. Cereb Cortex, 2012. 22(5): p. 1016–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.


