Abstract
Objective
Neuropsychological evidence revealed language impairment in children with benign epilepsy with centrotemporal spikes (BECTS). This study investigates language function using task-activated fMRI.
Methods
We conducted a language task fMRI study on three groups on a 3.0T MRI scanner, including a new onset drug naïve group (NODN-BECTS, n=11, age=9.6±1.6), an established epilepsy with medication-treated group (Med-BECTS, n=17, age=10.7±2.2) and a healthy control group (HC, n=18, age=10.8±1.7). We use MATLAB14 and SPM12 to pre-process and analyze the data. A one-sample t-test was used to identify task-related brain activation changes in each group, based on the general linear model (GLM). And, then two sample t-test was performed to compare different activated regions between groups. In addition, scores on the most recent Mandarin school exams were acquired to examine and contrast extra-scanner language performance.
Results
Statistical results show that some language-related brain regions (such as the left superior frontal gyrus and cerebellar vermis) were additionally activated in the NODN-BECTS group compared with the HC group. Compared with NODN-BECTS and HC groups, decreased activations were found in language-related regions in the Med-BECTS group, including the left insula, superior and middle frontal gyri, and bilateral middle occipital gyri. On the Mandarin school exams, the average score for HC was 87.3±8.2, NODN was 84.8±7.8, and Med was 78.2±13.2. There was a trend toward statistical significance between the Med and the HC (p = 0.074) as well as NODN (p = 0.092) groups. No statistically significant differences were found between the HC and the NODN-BECTS groups.
Significance
Language task fMRI reveals additional areas of activation in new onset BECTS compared to healthy controls which may be compensatory in nature. Antiseizure medications (ASMs) and/or longer duration of BECTS additionally appears to affect language-related regions and reduce their functional ability.
Keywords: benign epilepsy with centrotemporal spikes, cognition, language impairment, functional magnetic resonance imaging
Introduction
Benign epilepsy with centro-temporal spikes (BECTS) is the most common type of idiopathic childhood focal epilepsy, characterized by abnormal nocturnal epileptiform spike activity originating in the rolandic or sensorimotor cortex, without a significant lesion. BECTS accounts for about 15% of children with epilepsy, with the incidence of 6.2–21 per 100,000 children,1 and it accounts for about 15–24% in China. It is a typical age-dependent syndrome, the age of onset between 3 and 13 years old, and the epileptic seizures can subside between 15 and 17 years old.2 The classic electroencephalogram (EEG) shows frequent blunt high-voltage centro-temporal spikes (CTS), often followed by slow waves activated by sleep and tend to spread or shift from side to side.
Traditionally, BECTS has been considered a benign syndrome with a favorable prognosis because of the self-limiting nature of the disease and the widely held belief that cognitive abilities will be preserved. However, a growing literature has suggested that BECTS can be associated with abnormalities in cognition, particularly language and language-dependent abilities, including expressive and receptive verbal and written language, IQ, visuomotor abilities, reading disorders and executive dysfunction.3–7 However, others have noted that the cognitive correlates may be even more widespread. For example, Malfait et al concluded that some children in the BECTS group had weakness across multiple cognitive domains (below 25th percentile rank) including 53% for vocabulary, 47% for verbal fluency, 40% for working memory, and 40% for reading comprehension.8 A recent systematic review and meta-analysis of cognition in this syndrome reported with spread and nonfocal cognitive impairments.9 But little research has focused on the brain functional differences between new-onset drug naïve BECTS patients and BECTS patients on medications.
Recently, some studies had found that anti-seizure medications (ASMs) could negatively or positively affect a patient’ s cognition. Adam Strzelczyk noted that topiramate and zonisamide might be associated with negative effects in some aspects of cognition; cannabidiol, fenfluramine, levetiracetam, brivaracetam and lamotrigine might have some positive effects, while the remaining ASMs did not appear to have a detrimental effect. They also claimed that there was enormous heterogeneity in cognitive, behavioural and developmental impairments that was complex and could change naturally over time; there was a lack of standardized instruments for evaluating these outcomes in developmental and epileptic encephalopathies, with a reliance on subjective evaluations by proxy (caregivers).10. Therefore, there is a great need to develop a tool that can objectively evaluate the effects of ASMs on the cognitive function of patients. In another study, Vincenzo Crunelli announced that attention deficits could be detected before the epilepsy diagnosis, might persist even when seizures were pharmacologically controlled and were aggravated by valproic acid monotherapy.11 Thus, in addition to the disease itself, ASMs do affect cognitive function in children with epilepsy.
Furthermore, neuroimaging data including cortical thickness and volume showed a higher than expected abnormalities12,13 in different cortical and subcortical regions.
Functional magnetic resonance imaging (fMRI) studies reported that blood oxygen-level dependent (BOLD) fMRI activities were influenced by epileptiform activities.14–16
In our previous study, we applied regional homogeneity (ReHo) analysis to the resting state fMRI data and detected changes of resting state networks in children with BECTS comparing with healthy controls. The ReHo method evaluates the synchronization between the time-series of a voxel and its neighboring voxels.17,18 Our previous findings showed that children with BECTS had increased synchronization in language activation regions and decreased synchronization in default mode network regions compared to healthy controls.19
There are many factors that potential influence cognitive dysfunction (especially language dysfunction) in children with BECTS, such as age of seizure onset, type of seizure, duration of disease, influence of antiseizure medications and EEG patterns. To date, knowledge of how these factors affect brain activation and brain networks in children with BECTS is limited. A recent study has shown that children with BECTS exhibited a greater range of language network activation on task BOLD-fMRI examinations compared to healthy controls.8
Nevertheless, whether and to what extent ASMs affect the activation of language networks remains an important question. Therefore, in the present study, we investigated extra-scanner language performance using a standardized school-based test and the corresponding changes in language activation map and reorganization patterns using a language activation task fMRI, comparing a new-onset drug-naïve BECTS group (NODN group), children in chronic BECTS group treated with ASMs (Med group), and healthy controls (HC group).
Methods
Participants
Participants included 46 right-handed Chinese-speaking children, aged 8–12 years, including 11 children with new-onset drug naïve BECTS (NODN group), Table 1), 17 with established BECTS treated by ASMs (Med group), and 18 healthy controls (HC group). Children with BECTS were recruited from the Shenzhen Children’s Hospital, Guangdong, China, and met the following inclusion criteria: (1) Confirmed diagnosis of BECTS that included: a. EEG showing classic centrotemporal spikes arising from a normal background; b. Clinical history of at least one seizure that was consistent with the diagnosis of BECTS; c. No additional neurological condition. d. Age between 6 and 13 years old. e. Written informed assent from each enrolled child as appropriate by age and consent from a parent or legal guardian. (2) The exclusion criteria were: a. Seizures or syndromes inconsistent with BECTS; b. Any structural changes or pathologic abnormality of cerebrum revealed by magnetic resonance imaging (MRI); c. Other neurological or neuropsychiatric disorders, such as cerebral palsy, attention deficit hyperactivity disorder, or neurometabolic diseases.
Table 1.
Clinical Characteristics in BECTS Patients and HC
| Groups | Numbers | Gender (M/F) | Age (m ± std in Years) | Onset Age (Years) | Duration (Years) | Seizure’s Hemispheric Localization (L/R) | Mandarin Scores |
|---|---|---|---|---|---|---|---|
| HC | 18 | 11/7 | 10.8±1.7 | – | – | – | 87.3±8.2 |
| NODN | 11 | 7/4 | 9.6±1.6 | 8.5±2.0 | 0.7±0.4 | 4/7 | 84.8±7.8 |
| MED | 17 | 9/8 | 10.7±2.2 | 9.3±1.6 | 2.4±1.3 | 9/8 | 78.2±13.2 |
Abbreviations: m, mean; std, standard deviation; HC, healthy controls; NODN, BECTS patients without drugs; MED, BECTS patients with antiseizure medications treatment; M/F, male/female; L, left hemisphere; R, right hemisphere.
Patients were diagnosed by the pediatric neurologists from the neurology department in Shenzhen Children’s Hospital, and the healthy controls were recruited from nearby primary schools in Shenzhen. In addition, Mandarin was the first language of all participants. At the time of recruitment, all participants were screened by an MRI scanner and those with abnormal structure of brain were excluded. We also collected their mid-term and final Mandarin test scores which represented their language ability (an academic achievement test considered related to language ability, in which lower scores represented lower language ability, with a total raw score of 100). The mean scores were calculated for each group, this providing a stable metric of extra-scanner performance. Seizure types for BECTS patients include tonic seizures, clonic seizures, atonic seizures, tonic-clonic seizures, and focal seizures with a loss awareness. EEG results differed from patients to patients, including temporal (middle or posterior usually) spikes, sharp slow wave singly or frequently, continuous emission during sleep, left and right side out of sync, etc. This study was approved by the Health Science Institutional Review Board of University of Wisconsin-Madison (No.2012–0376).
Medication Usage
Twelve of 17 BECTS patients in MED group took one antiseizure medication (lamotrigine n=6; topiramate n=1; oxcarbazepine n=3; levetiracetam n=1; valproate n=1), the other 5 patients took two or three ASMs (lamotrigine+valproate, valproate +levetiracetam). The average medication period was 2.42±1.33 years. There were no other forms of treatment in progress.
Task fMRI Design
All participants in each group underwent a language task—Name Pictures Silently. The total scanning time was approximately 20 minutes, including functional and structural acquisition. During the task, all the participants were instructed to name the pictures displayed on the screen silently in their mind.
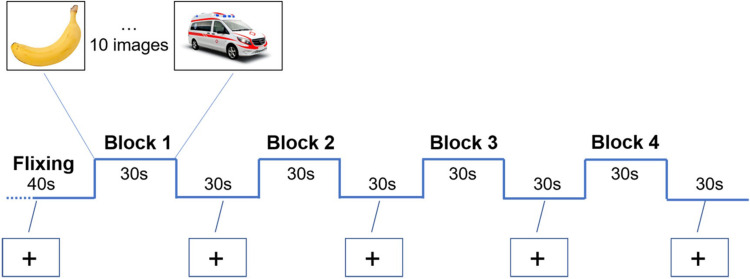
The block design mode was used for the fMRI language task (Figure 1). Four blocks made up the entire scan session. Each block contained a 30-second silent naming task (10 pictures in each block, 3s per picture), followed by a 30-second rest baseline, in which participants were instructed to focus on a fixation cross for 30s between blocks. The rest baseline before the first task block was set to 40 seconds, because the first 5 time points (10 seconds) of fMRI data would be discarded due to the instability of the initial MRI signal. The video projector projected the task stimuli onto the screen above the participant’s head, and the participant could watch them through a mirror attached to the MRI head coil. Before the test, participants were trained to name pictures silently to ensure that they could fully understand the procedures.
Figure 1.
Task fMRI experimental block design.
MR Data Acquisition
Structural and functional brain imaging data were acquired on a Signa Excite 1.5T MR imaging system (General Electric, Fairfield, USA) and a standard head coil. Foam pads were used to reduce head motion and scanner noise. All participants are required to remain still, open their eyes, and listen to instructions to complete the language task. The language task fMRI data were acquired by using an echo planar imaging (EPI) sequence with the following parameters: Repetition time (TR) = 2000ms, echo time (TE) = 30ms, flip angle = 90, 26 axial slices, thickness/skip = 4/0mm, in-plane resolution = 64 × 64, 190 volumes.
Structural images include axial T2WI (TR = 2300ms, TE = 108ms, FoV read = 230mm, 17 axial slices, voxel size = 0.6×0.6×6.0mm3), axial FLAIR (TR = 9000ms, TE = 134ms, FOV read = 230mm, 17 axial slices, voxel size = 0.7×0.7×6.0mm3) and T1 3D-MPRAGE (TR = 2300ms, TE = 2.26ms, FOV read = 256mm, 176 sagittal slices, voxel size = 1.0×1.0×1.0mm3).
Data Preprocessing
Data were preprocessed and analyzed by using MATLAB13 (MathWorks, Sherborn, MA) and Statistic Parametric Mapping (SPM12, http://www.fil.ion.ucl.ac.uk/spm/). Due to the instability of the initial MRI signal, the first 5 time points (10 seconds) of the fMRI data of each subject were discarded to allow the longitudinal magnetization to reach a stable state, and allow the participants to adapt to the scanning environment. Subsequently, the remaining 180 volume images were preprocessed. The preprocessing of the fMRI datasets included realignment, slice timing, co-registration, normalization and smooth. Specifically, all EPI data were spatial normalized to a standard template of MNI (Montreal Neurological Institute). A 4-mm full-width at half-maximum (FWHM) isotropic Gaussian kernel was used to smooth the normalized data to reduce noises. All those subjects with head motion greater than 3.0 mm or 3◦ in any of the six parameters (x, y, z, pitch, roll, yaw) were excluded. Seven participants in NODN group, 5 in MED group and 4 in HC were excluded due to strenuous head movement, 46 participants in three groups were included finally.
First-Level and Second-Level Analysis
In order to identify task-related activation changes in each group, one sample t-test was performed in SPM on the results of the first-level general linear model (GLM) containing specific functions of the experimental conditions, including age and gender as covariates.
A two-sample t-test was performed to compare the different activated regions between groups. A cluster of >54 voxels with p-value <0.05 (FDR corrected) was considered statistically significant. Age and gender were included as covariates.
Results
Within Group
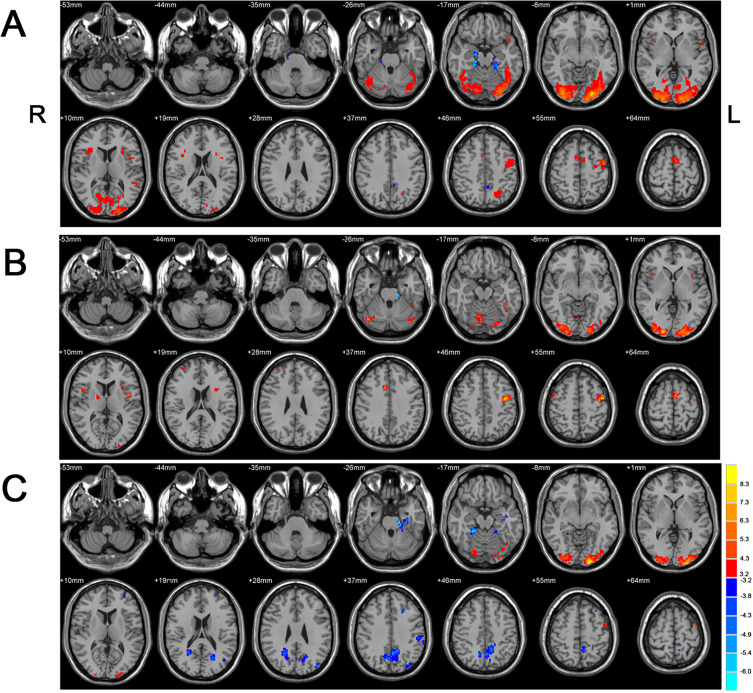
Based on a general linear model, the results from one-sample t-test showed different activated patterns and intensity of signals across the three groups, as shown in Figure 2. In the HC group, significantly activations were found in language-related regions during language task BOLD-fMRI scanning, including the left middle and inferior frontal gyrus, bilateral lingual, occipital gyri, bilateral cerebellar crus 1 as shown in Figure 2A. In NODN group, activation map showed similar (slightly decreased) activations in language areas, visual and cerebellar regions that had been seen in the HC group. Additionally identified were some new activation regions in NODN group compared with HC, including the right middle frontal gyrus in Figure 2B. In the Med group, we found decreased activation in language and visual areas (bilateral cuneus and precuneus) compared to the NODN group, as shown in Figure 2C.
Figure 2.
One-sample t-test results in different groups (p<0.005, 10 voxel). (A) Healthy control group. (B) New onset drug naïve group (NODN group). (C) Medication group (Med).
Abbreviations: L, left; R, right.
Between Groups
NODN BECTS versus HC
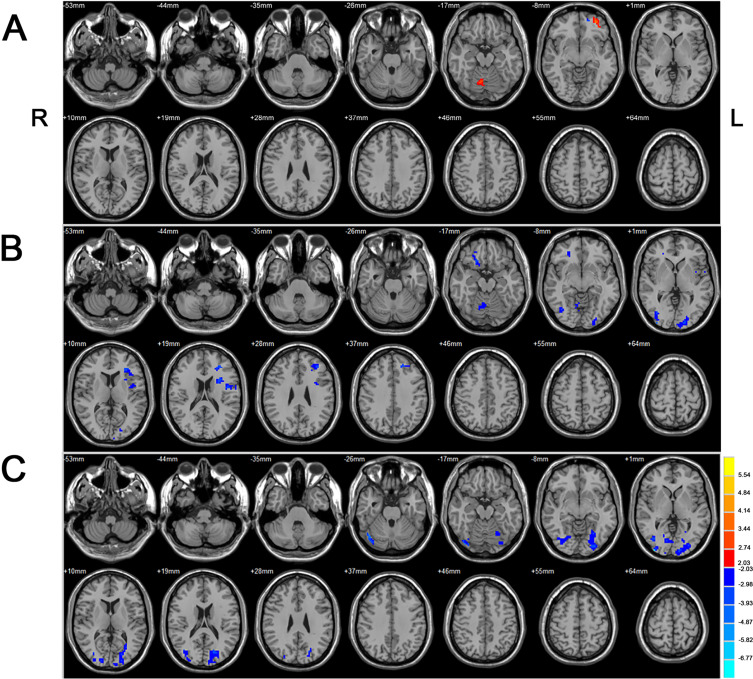
Two Sample t-test showed increased areas of activation on the left frontal lobe and vermis of the cerebellum in the NODN group compared to HC. Additionally, decreased activations were seen in left middle frontal and inferior gyri and insula in NODN group. Figure 3 and Table 2 show the difference in activation in brain regions between the groups.
Figure 3.
Two Sample t-test results between different groups (p<0.05, voxel>54, corrected by FDR Correction). (A) New-onset drug-naïve BECTS (NODN) versus HC. Warm colors indicate NODN> HC. (B) Chronic BECTS group treated with antiseizure medications (Med) versus New-onset drug-naïve BECTS (NODN). Cool colors indicate Med < NODN. (C) Chronic BECTS group treated with antiseizure medications (Med) versus HC. Cool colors indicate Med < HC.
Abbreviations: L, left; R, right.
Table 2.
Statistically Different Brain Regions in the Two Groups of BECTS Patients
| Brian Regions | H | Peak MNI Coordinate | Cluster Voxel | Peak t Value | |||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| 1 | Rolandic_Oper, Insula | L | −30 | 12 | 18 | 162 | −4.2128 |
| 2 | Middle Frontal Gyrus, Superior Frontal Gyrus | L | −27 | 36 | 15 | 153 | −4.2617 |
| 3 | Cuneus, Occipital_Mid, Occipital_Inf_ | L | −18 | −96 | −3 | 111 | −4.0186 |
| 4 | Cerebellum Posterior Lobe | R | 9 | −63 | −18 | 80 | −2.9898 |
| 5 | Frontal_Sup_Orb_R, Middle Frontal Gyrus | R | 18 | 24 | −15 | 70 | −3.9907 |
| 6 | Middle Occipital Gyrus | R | 36 | −96 | 0 | 68 | −5.3142 |
Notes: Negative t value indicates smaller t value in Med BECTS group. P<0.05, minimum voxel=54, corrected by FDR correction.
Abbreviations: MNI, Montreal Neurological Institute; t, the t-value in T-test; H, hemisphere; L, left; R, right.
Med BECTS versus NODN BECTS
Compared to the NODN group, there are decreased activations in language regions (left superior and middle frontal gyri), visual network regions (left cuneus and precuneus) in the Med group. Figure 3 shows the difference in activation in brain regions between the groups.
Med BECTS versus HC
Compared to the HC group, there was decreased activations in language, regions (left superior and middle frontal gyri), visual network regions (bilateral cuneus and precuneus), bilateral cerebellum, and left sensori-motor regions in the Med group. Figure 3 show the difference in activation in brain regions between the groups.
Mandarin School Exam Performance
The average Mandarin school exams score of the HC group was 87.3±8.2, NODN group 84.8±7.8, and Med group 78.2±13.2. There was no statistically significant difference between the HC vs NODN, p = 0.687 groups. There was a trend towards significance in the NODN vs MED, p = 0.092 as well as the HC vs MED, p = 0.074 groups.
Discussion
The patterns of language activation were seen in the HC group reflect findings noted in prior studies. In the NODN group, we identified increased activation in the left middle frontal lobe and cerebellar vermis compared with the HC group. Additionally, decreased activations were seen in left middle frontal and inferior gyri and insula in NODN group. This pattern of findings suggests that the language activation regions in NODN group is reorganized early in the course of the disorder, independent of ASM effects, and there are new areas that may represent compensation for areas of decreased activation in these subjects. This is consistent with other studies which have shown increased activation in middle frontal gyrus of patients with epilepsy, compared to HC.20–22 Other studies have shown the existence of an extended language activation map in BECTS. For example, Datta et al23 found an extended language network during an oral fMRI language task, involving activations in right middle temporal gyrus, left fusiform gyrus and right inferior prefrontal gyrus. In summary, these results indicate a considerable altered and reorganized language network in drug naïve BECTS patients with areas potentially playing a compensatory role to maintain language function.
When comparing NODN group with MED group, the latter exhibited decreased activation in language (left superior and middle frontal gyri) and visual (left cuneus and precuneus) network regions. Similarly, when comparing HC with the MED group, the latter exhibited decreased activated in language (left superior and middle frontal gyri), visual regions (bilateral cuneus and precuneus), as well as the cerebellum, and sensorimotor regions. These findings indicate that ASMs and/or duration of BECTS may lead to reduction in language and other network functions. Research by Unnikrishnan et al22 showed intrauterine exposure to phenobarbitone and valproate impaired language development in CWE, with effects persisting into the second decade. Hamed23 reviewed the evidence that long-term treatment with some antiseizure medications (ASMs) [e.g. carbamazepine, phenytoin, valproate, lamotrigine, gabapentin, vigabatrin and oxcarbazepine] (even in therapeutic drug doses) might result in tinnitus, phonophobia, sensorineural hearing loss, dizziness, ataxia, disequilibrium, imbalance, nystagmus, abnormalities in saccadic and pursuit eye movements and delayed conduction within the cochlea, auditory nerve and brainstem auditory pathways evidenced by abnormalities in brainstem auditory evoked potentials and nystagmography recordings indicating auditory and central and/or peripheral vestibular dysfunctions. These evidences might support the opinion that ASMs could lead to brain functional impairments.
Research by Lillywhite et al24 showed that when a high level of cognition is required, the difference in functional activation patterns between the BECTS group and the healthy controls was related to poor performance. They also hypothesized that language impairment in BECTS is a regional rather than a global issue.24 However, studies had found that BECTS patients and HC participants had significant differences in language fMRI laterality indices.22 They believed that, given that there were no obvious behavioral differences between the two groups, these differences in function activation may be compensatory in nature.
Participants also completed two school-based Mandarin examinations that provided insight into their extra-scanner language status. The mean of two administration of the test (midterm and final) was used which provided a stable performance metric. The results showed no significant difference between the NODN group and HC group, but with a trend towards significance when comparing the controls as well as NODN groups to the Med group. Thus, it seems that the language reorganization in the NODA group appears to be adequately helping to compensate/maintain language while in the MED group the network changes are no longer.
Limitation
The sample size of this study is small, and although we have done our best to control the variables, continuing to expand the sample size will increase the confidence in the results even more. Therefore, in the next steps, we will continue to collect patients and seek multi-center collaboration.
Conclusions
FMRI language activation map responses in patients with new onset drug naïve BECTS (NODN) differed from healthy control (HC) participants. When performing an fMRI language task, some language-related activation regions in the NODA group were increased and suggestive of compensatory activation, inferring reorganization of language function.
When compared with the NODN and HC groups, the MED group exhibited decreased activation in multiple brain areas, perhaps indicating that the effects of ASM, the duration of BECTS, or their combination might have a detrimental impact on the patient’s language activation map and function.
BECTS was traditionally regarded as a “benign” syndrome, mainly because of its self-limiting nature in adolescence and good prognosis after the initial period of seizures.25,26 Nevertheless, additional research has demonstrated that BECTS patients harbor various cognitive and functional impairments in adulthood,27 especially language function.6,23,28 Our study also found that this disease, as well as ASMs could have an major influence on children’s neuropsychological development.
Funding Statement
This project was supported by RSNA Seed Grant (No. RSD1214), Sanming Project of Medicine in Shenzhen (No. SZSM202011005), Sciences and Technology Project of Shenzhen (No. JCYJ20220530155805012), Natural Science Foundation of Guangdong Province (No. 2022A1515011427), Guangdong High-level Hospital Construction Fund (ynkt2021-zz47).
Ethical Statement
This study was performed in line with the principles of the Declaration of Helsinki.
Disclosure
None of the authors has any conflicts of interest to declare for this work.
References
- 1.Wirrell EC. Benign epilepsy of childhood with centrotemporal spikes. Epilepsia. 1998;39(4):S32–41. doi: 10.1111/j.1528-1157.1998.tb05123.x [DOI] [PubMed] [Google Scholar]
- 2.Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30(4):389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x [DOI] [PubMed] [Google Scholar]
- 3.Zanaboni MP, Varesio C, Pasca L, et al. Systematic review of executive functions in children with self-limited epilepsy with centrotemporal spikes. Epilepsy Behav. 2021;123:108254. doi: 10.1016/j.yebeh.2021.108254 [DOI] [PubMed] [Google Scholar]
- 4.Bedoin N, Ferragne E, Lopez C, Herbillon V, De Bellescize J, Des PV. Atypical hemispheric asymmetries for the processing of phonological features in children with rolandic epilepsy. Epilepsy Behav. 2011;21(1):42–51. doi: 10.1016/j.yebeh.2011.02.026 [DOI] [PubMed] [Google Scholar]
- 5.Ebus SC, Overvliet GM, Arends JB, Aldenkamp AP. Reading performance in children with rolandic epilepsy correlates with nocturnal epileptiform activity, but not with epileptiform activity while awake. Epilepsy Behav. 2011;22(3):518–522. doi: 10.1016/j.yebeh.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 6.Overvliet GM, Besseling RM, van der Kruijs SJ, et al. Clinical evaluation of language fundamentals in Rolandic epilepsy, an assessment with CELF-4. Eur J Paediatr Neurol. 2013;17(4):390–396. doi: 10.1016/j.ejpn.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 7.Nicolai J, Aldenkamp AP, Arends J, Weber JW, Vles JS. Cognitive and behavioral effects of nocturnal epileptiform discharges in children with benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav. 2006;8(1):56–70. doi: 10.1016/j.yebeh.2005.08.016 [DOI] [PubMed] [Google Scholar]
- 8.Malfait D, Tucholka A, Mendizabal S, et al. fMRI brain response during sentence reading comprehension in children with benign epilepsy with centro-temporal spikes. Epilepsy Res. 2015;117:42–51. doi: 10.1016/j.eplepsyres.2015.08.009 [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Zhang J, Wang F, et al. Surface-based morphometry study of the brain in benign childhood epilepsy with centrotemporal spikes. Ann Transl Med. 2020;8(18):1150. doi: 10.21037/atm-20-5845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strzelczyk A, Schubert-Bast S. Psychobehavioural and cognitive adverse events of anti-seizure medications for the treatment of developmental and epileptic encephalopathies. CNS Drugs. 2022;36(10):1079–1111. doi: 10.1007/s40263-022-00955-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crunelli V, Lőrincz ML, McCafferty C, et al. Clinical and experimental insight into pathophysiology, comorbidity and therapy of absence seizures. Brain. 2020;143(8):2341–2368. doi: 10.1093/brain/awaa072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim EH, Yum MS, Shim WH, Yoon HK, Lee YJ, Ko TS. Structural abnormalities in benign childhood epilepsy with centrotemporal spikes (BCECTS). Seizure. 2015;27:40–46. doi: 10.1016/j.seizure.2015.02.027 [DOI] [PubMed] [Google Scholar]
- 13.Pardoe HR, Berg AT, Archer JS, Fulbright RK, Jackson GD. A neurodevelopmental basis for BECTS: evidence from structural MRI. Epilepsy Res. 2013;105(1–2):133–139. doi: 10.1016/j.eplepsyres.2012.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Detre JA. Clinical applicability of functional MRI. J Magn Reson Imaging. 2006;23(6):808–815. doi: 10.1002/jmri.20585 [DOI] [PubMed] [Google Scholar]
- 15.Salek-Haddadi A, Diehl B, Hamandi K, et al. Hemodynamic correlates of epileptiform discharges: an EEG-fMRI study of 63 patients with focal epilepsy. Brain Res. 2006;1088(1):148–166. doi: 10.1016/j.brainres.2006.02.098 [DOI] [PubMed] [Google Scholar]
- 16.Mankinen K, Long XY, Paakki JJ, et al. Alterations in regional homogeneity of baseline brain activity in pediatric temporal lobe epilepsy. Brain Res. 2011;1373:221–229. doi: 10.1016/j.brainres.2010.12.004 [DOI] [PubMed] [Google Scholar]
- 17.Liu D, Yan C, Ren J, Yao L, Kiviniemi VJ, Zang Y. Using coherence to measure regional homogeneity of resting-state FMRI signal. Front Syst Neurosci. 2010;4:24. doi: 10.3389/fnsys.2010.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang L, Zuo XN. Regional homogeneity: a multimodal, multiscale neuroimaging marker of the human connectome. Neuroscientist. 2016;22(5):486–505. doi: 10.1177/1073858415595004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng H, Ramos CG, Nair VA, et al. Regional homogeneity (ReHo) changes in new onset versus chronic benign epilepsy of childhood with centrotemporal spikes (BECTS): a resting state fMRI study. Epilepsy Res. 2015;116:79–85. doi: 10.1016/j.eplepsyres.2015.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swanson SJ, Sabsevitz DS, Hammeke TA, Binder JR. Functional magnetic resonance imaging of language in epilepsy. Neuropsychol Rev. 2007;17(4):491–504. doi: 10.1007/s11065-007-9050-x [DOI] [PubMed] [Google Scholar]
- 21.Friederici AD, Gierhan SM. The language network. Curr Opin Neurobiol. 2013;23(2):250–254. doi: 10.1016/j.conb.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 22.Scharff C, Friederici AD, Petrides M. Neurobiology of human language and its evolution: primate and non-primate perspectives. Front Evol Neurosci. 2013;5:1. doi: 10.3389/fnevo.2013.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Datta AN, Oser N, Bauder F, et al. Cognitive impairment and cortical reorganization in children with benign epilepsy with centrotemporal spikes. Epilepsia. 2013;54(3):487–494. doi: 10.1111/epi.12067 [DOI] [PubMed] [Google Scholar]
- 24.Lilly White LM, Saling MM, Harvey AS, et al. Neuropsychological and functional MRI studies provide converging evidence of anterior language dysfunction in BECTS. Epilepsia. 2009;50(10):2276–2284. doi: 10.1111/j.1528-1167.2009.02065.x [DOI] [PubMed] [Google Scholar]
- 25.Loiseau P, Duché B. Benign childhood epilepsy with centrotemporal spikes. Cleve Clin J Med. 1989;56(1):S17–22. doi: 10.3949/ccjm.56.s1.17 [DOI] [PubMed] [Google Scholar]
- 26.Camfield CS, Camfield PR. Rolandic epilepsy has little effect on adult life 30 years later: a population-based study. Neurology. 2014;82(13):1162–1166. doi: 10.1212/WNL.0000000000000267 [DOI] [PubMed] [Google Scholar]
- 27.Danielsson J, Petermann F. Cognitive deficits in children with benign rolandic epilepsy of childhood or rolandic discharges: a study of children between 4 and 7 years of age with and without seizures compared with healthy controls. Epilepsy Behav. 2009;16(4):646–651. doi: 10.1016/j.yebeh.2009.08.012 [DOI] [PubMed] [Google Scholar]
- 28.Overvliet GM, Aldenkamp AP, Klinkenberg S, Vles JS, Hendriksen J. Impaired language performance as a precursor or consequence of Rolandic epilepsy. J Neurol Sci. 2011;304(1–2):71–74. doi: 10.1016/j.jns.2011.02.009 [DOI] [PubMed] [Google Scholar]