Abstract
Variability in sleep duration and cardiovascular health have been infrequently investigated, particularly among reproductive-age women. We examined these associations across the menstrual cycle among a cohort of 250 healthy premenopausal women, aged 18-44 years. The BioCycle study (New York, 2005–2007) collected cardiovascular biomarkers (serum high- and low-density lipoprotein (HDL, LDL), total cholesterol, triglycerides, and C-reactive protein (CRP)) at key time points along the menstrual cycle (follicular, ovulatory, and luteal phases). Women also recorded sleep duration in daily diaries. From these data, we computed L-moments, robust versions of location, dispersion, skewness, and kurtosis. We fitted linear mixed models with random intercepts and inverse probability weighting to estimate associations between sleep variability and cardiovascular biomarkers, accounting for demographic, lifestyle, health, and reproductive factors. Sleep dispersion (any deviation from mean duration) was associated with lower mean LDL for nonshift workers and non-White women. Skewed sleep duration was associated with higher mean CRP and lower mean total cholesterol. Sleep durations with extreme short and long bouts (kurtosis) were associated with a lower mean HDL, but not mean CRP, LDL, or triglycerides. Sleep duration modified associations between sleep dispersion and LDL, HDL, and total cholesterol. Even in young and healthy women, sleep duration variability could influence cardiovascular health.
Keywords: cholesterol, C-reactive protein, lipids, menstrual cycle, sleep, sleep duration, sleep variability, triglycerides
Abbreviations
- BMI
body mass index
- CRP
C-reactive protein
- CVD
cardiovascular disease
- HDL
high-density lipoprotein
- hs-CRP
high-sensitivity C-reactive protein
- LDL
low-density lipoprotein
Cardiovascular diseases (CVDs) are the leading cause of mortality in the United States among both women and men (1, 2). Yet CVD-related deaths are higher among women than men and among Black vs. White adults (3, 4). Despite significant progress in the diagnosis and treatment of CVD, its public health burden remains significant.
Identification of modifiable risk factors for CVD, such as high cholesterol or inflammation biomarkers in their preclinical stages could create opportunities for earlier prevention of later morbidity and mortality as well as improved disease management (5). Indeed, lipids are considered among the most informative biomarkers for CVD risk and severity (6). In addition to lipids, C-reactive protein (CRP), an inflammatory biomarker, was found to provide improved identification of at-risk individuals (7, 8). Sex-specific prevalence of CVD risk factors has been reported, with higher levels of CRP and total cholesterol in women overall, and Black women in particular, compared with White men (9–11). The differential CVD risk profiles in women and observed variation in CVD biomarkers across the menstrual cycle (12, 13) position premenopausal women as a unique population for study of cyclic fluctuations in biomarkers and related factors that are associated with CVD risk (14).
Adequate sleep duration is key to optimal health across the lifespan (15). In women and men, short and long sleep duration (<7 hours and >9 hours per night, respectively) have been associated with CVD risk (16). Women who work in shifts and non-White women are particularly vulnerable to insufficient sleep duration (17, 18). Conversely, sufficient sleep duration, 7–9 hours per night, is linked to better cardiovascular health in adult populations (16, 19, 20). Furthermore, short or long sleep durations have been shown to be associated with higher levels of triglycerides and lower levels of serum high-density lipoprotein (HDL) (21, 22). Although sleep duration has been examined frequently in relation to cardiovascular health, most studies have assessed a single time point or infrequent sleep measures, often years apart. Prospective, women-focused investigations of sleep duration and its daily covariation with CVD biomarkers are scant. Recent findings have suggested that sleep duration changes along the menstrual cycle (23); however, whether changes in sleep duration influence fluctuation in CVD biomarkers remain unknown. Therefore, we utilized data from the BioCycle study to examine associations between daily sleep patterns and variation in key biomarkers of CVD in a cohort of premenopausal women. The BioCycle study employs an unusual, microlongitudinal study design, (i.e., a measurement-intense design over a relatively shorter time interval of 2 menstrual cycles).
METHODS
Study population
The BioCycle study was a microlongitudinal cohort study of 259 healthy, regularly menstruating women aged 18–44 years (24), recruited in 2005–2007 from western New York. The sample was diverse, with 59% White women, 20% Black women, and 21% of American Indian, Hispanic, Asian Indian, Chinese or other Asian, and Pacific Islander decent. Women who used oral contraceptives within 3 months of enrollment, intrauterine devices within 12 months, vitamin, and mineral supplements or prescription medications were excluded. Also excluded, women who were pregnant or breastfed less than 6 months before enrollment; had a diagnosis of polycystic ovary syndrome, recent history of infections, or chronic medical conditions; and who had a self-reported body mass index (BMI) at screening of <18 or >35 (calculated as weight (kg)/height (m)2). Other exclusion criteria were a history of chronic diseases. The complete list of exclusion criteria has been described previously (24).
The BioCycle study utilized a measurement-intense design across 2 menstrual cycles, with clinic visits at 8 time points along the menstrual cycle, including the follicular and luteal phases. Overall, 259 women completed 4,028 visits, of which 95% had at least 13 visits. Fasting morning blood samples were collected up to 8 times per cycle at the following time points: second day of menstruation, mid and late follicular phase, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) surges, predicted ovulation, and early, mid, and late luteal phase (24). To capture key hormonal phases during the menstrual cycle, timing of specimen collection (blood and urine) was supported with the use of home fertility monitors (Clearblue Easy Fertility Monitor; Inverness Medical, Waltham, Massachusetts). Monitor indications of low, high, and peak fertility were used in combination with a calendar to time midcycle visits, with other visits scheduled according to an algorithm that took each woman’s reported cycle length history into consideration (25).
Sleep diaries
Considered as the gold standard for self-reported sleep data (26), diaries collect prospective information at the participants’ natural environment and closer to the timing of the reported event (27). Sleep data were collected daily via dairies using the following question: “Please write in the total number of hours and minutes you slept last night plus any time you napped today. For example, if you went to bed at 11pm last night and slept until 7am this morning and took a half-hour nap after lunch today your response would be: 8 hours 30 minutes.” Overall, 255 women contributed 13,038 sleep diary entries across 2 menstrual cycles, which reflected a 90% completion rate.
Patterns of sleep duration
Person-specific sleep-duration variability across 2 menstrual cycles was estimated using a novel mathematical approach that captured deviations in sleep duration of any magnitude, and in the positive and negative directions in relation to average sleep duration. We computed robust versions of location, dispersion, skewness, and kurtosis of nightly sleep duration using linear combinations of order statistics (L-moments, summarized in Table 1). Frequently utilized to describe the shape of distributions, the L-moments are a system of mathematical measures that are robust to extreme values and yield accurate estimates in unbalanced samples (28).
Table 1.
Geometric Mean Baseline Cardiovascular Biomarker Levels According to Sociodemographic, Health, and Lifestyle Characteristics Among Reproductive-Age Women in the BioCycle Study, New York, 2005–2007
| Overall Sample (n = 259) | CRP a , b (n = 255) | LDL a (n = 255) | HDL a (n = 255) | Triglycerides a (n = 255) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sociodemographic orLifestyle Characteristic | No. | % | Mean | 95% CI | Mean | 95% CI | Mean | (95% CI) | Mean | 95% CI |
| Age group, years | ||||||||||
| 18–22 | 107 | 41 | 0.7 | 0.55, 0.83 | 92.9 | 88.4, 97.6 | 48.7 | 46.5, 51.0 | 52.5 | 48.5, 56.9 |
| 23–29 | 68 | 26 | 0.7 | 0.56, 0.97 | 98.3 | 93.2, 103.6 | 48.5 | 46.0, 51.1 | 47.7 | 43.3, 52.6 |
| 30–39 | 51 | 20 | 1.2 | 0.87, 1.56 | 101.4 | 94.7, 108.5 | 48.6 | 45.8, 51.6 | 59.8 | 52.6, 68.0 |
| ≥40 | 33 | 13 | 1.2 | 0.85, 1.66 | 111.8 | 100.8, 124.0 | 50.9 | 46.9, 55.3 | 58.1 | 49.5, 68.2 |
| Race/ethnicity | ||||||||||
| White | 154 | 59 | 1.0 | 0.81, 1.12 | 98.5 | 94.6, 102.5 | 50.4 | 48.4, 51.9 | 55.7 | 52.5, 59.7 |
| Black | 51 | 20 | 0.7 | 0.50, 1.06 | 97.5 | 89.1, 105.6 | 47.5 | 44.3, 50.9 | 43.4 | 39.3, 48.4 |
| Otherc | 54 | 21 | 0.6 | 0.46, 0.79 | 98.5 | 92.8, 104.6 | 46.5 | 44.3, 49.4 | 55.1 | 48.9, 61.6 |
| Education | ||||||||||
| Less than high school | 33 | 13 | 1.2 | 0.72, 1.43 | 102.0 | 92.1, 113.0 | 45.7 | 41.6, 50.2 | 59.6 | 51.5, 69.1 |
| High school or higher | 226 | 87 | 0.8 | 0.69, 0.93 | 97.6 | 94.5, 100.9 | 49.3 | 47.9, 50.8 | 52.3 | 49.5, 55.4 |
| Marital status | ||||||||||
| Married | 66 | 25 | 1.1 | 0.87, 1.45 | 103.8 | 97.3, 110.8 | 51.3 | 48.5, 54.4 | 55.7 | 50.4, 61.6 |
| Unmarried | 193 | 75 | 0.7 | 0.64, 0.87 | 96.3 | 93.0, 99.8 | 48.1 | 46.6, 49.7 | 52.3 | 49.2, 55.7 |
| Smoking status | ||||||||||
| Nonsmoker | 249 | 96 | 0.8 | 0.72, 0.94 | 98.2 | 95.1, 101.4 | 48.8 | 47.4, 50.3 | 53.0 | 50.3, 55.9 |
| Current smoker | 10 | 4 | 1.0 | 0.40, 2.25 | 96.9 | 81.4, 115.2 | 50.4 | 43.8, 57.9 | 56.1 | 35.7, 88.1 |
| Body mass indexd | ||||||||||
| <25.0 | 167 | 65 | 0.6 | 0.51, 0.68 | 95.6 | 92.3, 99.0 | 50.0 | 48.3, 51.7 | 50.2 | 47.1, 53.4 |
| 25.0–29.9 | 66 | 25 | 1.4 | 1.08, 1.81 | 101.7 | 94.6, 109.3 | 47.3 | 44.7, 50.1 | 58.7 | 52.8, 65.3 |
| ≥30.0 | 26 | 10 | 2.2 | 1.56, 3.16 | 108.0 | 95.3, 122.3 | 45.8 | 40.9, 51.3 | 61.8 | 51.1, 74.8 |
| Physical activitye | ||||||||||
| Low | 25 | 10 | 0.8 | 0.48, 1.46 | 95.7 | 84.0, 108.9 | 48.2 | 43.4, 53.4 | 50.6 | 43.6, 58.8 |
| Moderate | 92 | 35 | 0.7 | 0.60, 0.92 | 102.6 | 98.1, 107.4 | 48.9 | 46.7, 51.1 | 54.8 | 50.2, 59.9 |
| High | 142 | 55 | 0.9 | 0.74, 1.06 | 95.7 | 91.6, 99.9 | 49.0 | 47.2, 51.0 | 52.5 | 48.8, 56.5 |
| Shift work | ||||||||||
| Yes | 86 | 36 | 0.8 | 0.75, 1.07 | 94.7 | 89.5, 100.2 | 49.8 | 47.2, 52.6 | 54.1 | 49.1, 59.6 |
| No | 156 | 64 | 0.9 | 0.60, 0.96 | 99.9 | 95.9, 104.1 | 48.5 | 46.7, 50.4 | 52.8 | 49.2, 56.7 |
| Daily caffeine intake, mgf | ||||||||||
| <31 | 82 | 33 | 0.7 | 0.58,0.93 | 100.4 | 95.8,105.3 | 43.4 | 41,6, 45.4 | 55.7 | 50.8, 60.9 |
| 32–114 | 83 | 34 | 0.8 | 0.64, 1.00 | 95.8 | 90.2, 101.7 | 52.1 | 49.6, 54.7 | 50.0 | 46.1, 54.3 |
| 117–955 | 82 | 33 | 1.0 | 0.78, 1.27 | 98.2 | 92.8, 103.9 | 51.3 | 49.0, 53.8 | 53.9 | 48.7, 9.6 |
| CES-D scoreg | ||||||||||
| <16 | 229 | 92 | 0.9 | 0.46, 1.59 | 99.1 | 95.9, 102.4 | 49.1 | 47.6, 50.6 | 53.7 | 50.8, 56.7 |
| ≥16 | 19 | 8 | 0.8 | 0.72, 0.95 | 87.2 | 76.8, 99.2 | 48.3 | 43.6, 53.6 | 46.3 | 37.6, 56.9 |
Abbreviations: CES-D, Center for Epidemiologic Studies Depression Scale; CI, confidence interval; CRP, C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
a Geometric mean.
b CRP ≤ 8.42 mg/L. This CRP level indicates absence of clinically evident inflammation.
c Other = American Indian, Asian Indian, Chinese/other Asian, Pacific Islander, or unspecified.
d Body mass index was calculated as weight (kg)/height (m)2.
e The International Physical Activity Questionnaire.
f Tertiles of mean caffeine at baseline.
g CES-D score ≥16 indicates identifies individuals at risk for clinical depression.
The first L-moment, location, is identical to a person-specific mean sleep duration along the 2 menstrual cycles. The second L-moment, dispersion, measures the spread of sleep duration entries around woman’s mean sleep duration, focusing on deviations of any magnitude that fall on either side of the person-specific mean. This measure is equivalent to the mean absolute deviation. The third L-moment, skewness, captures asymmetry or unbalance in sleep duration in which the moderate-to-large deviations from the woman’s mean sleep duration tend to occur only on one side of the mean, (i.e., the tendency of a woman to have occasional deviations in sleep duration that are primarily above or primarily below her reported mean sleep duration). Larger negative or positive values correspond to shorter or longer sleep, respectively. Finally, the fourth L-moment, kurtosis, characterizes person-specific large deviations in sleep duration that occur on either side of its mean. To distinguish dispersion and kurtosis, note that dispersion is sensitive to deviations (in hours) of sleep duration of all sizes. In our data, women with very low dispersion had nearly identical sleep duration every night, while women with high dispersion had frequent deviations of up to 7 hours from their mean, occurring in both directions. Kurtosis in sleep duration reflects large deviations from mean sleep duration, and is less sensitive to small deviations. Women with high kurtosis in our data had a few nights with very long sleep and a few nights with very short sleep (up to 7 hours different from their mean value) but did not necessarily have variable sleep on other nights. All together, these moments provide informative, person-specific measures of the differential roles of small and large deviations in sleep duration and deviations in the positive and negative directions from the mean sleep duration.
Biomarkers of cardiovascular disease
Biomarkers of CVD were measured in serum in each of the clinic visits over 2 menstrual cycles. Fasting blood samples were collected and processed at each visit according to standardized procedures and stored frozen at −80C until samples were sent for measurement. CRP, an inflammatory biomarker, has been found to provide improved identification of individuals at risk for CVD in women (29). High-sensitivity CRP (hs-CRP) allows the detection of concentrations within healthy reference levels (30). The IMMULITE 2000 platform (Siemens Medical Solutions, Malvern, Pennsylvania) was used for assessment of hs-CRP, with a chemiluminescent immunoassay that is sensitive to 0.3 mg/L and meets guidelines from the American Heart Association and the Centers for Disease Control and Prevention as the most sensitive assay for the prediction of vascular disease, relative to traditional assays for circulating CRP levels (31, 32).
Assessment of the serum lipid profile was conducted using a Beckman LX20 automated chemistry analyzer (Beckman, Brea, California) for serum HDL cholesterol, triglycerides, and total cholesterol (<5% total coefficient of variation for all assays). Serum low-density lipoprotein (LDL) cholesterol was computed using the formula and procedures suggested by Friedewald et al. (33).
Covariates
Demographic, health, lifestyle, and occupation information was collected by questionnaires and diaries. Women reported their age, race/ethnicity, and work information (number of jobs, rotating or shift work) in questionnaires at enrollment. Ethnicity categories included American Indian, Hispanic, Asian Indian, Chinese or other Asian descent, and Pacific Islander. The International Physical Activity Questionnaire was also administered at baseline to assess physical activity in the prior 7 days (34). Baseline BMI was calculated from height and weight data recorded by study personnel using calibrated scales and standardized assessment procedures.
Alcohol and caffeine intake were recorded in daily diaries and were included as time-varying confounders in statistical models. The average number of alcoholic and caffeinated beverages in the 2 days prior to each clinic visit were estimated from the following 2 questions: “Please write in the number of alcoholic drinks you had today (one drink is equivalent to one can of beer, one glass of wine, or one shot of liquor). Enter ‘0’ if none.” and “Please write in the total number of cups of caffeinated coffee you consumed today [i.e. hot or iced coffee (instant or brewed)]. One cup is based on a serving of 8 fluid oz, a larger cup should be counted as more than 1 (i.e., a 16 oz cup is 2 cups). Enter ‘0’ if none.” Fasting serum estradiol levels were also considered as a time-varying confounder and were measured in serum collected at each study visit by radioimmunoassay at the Kaleida Health Center for Laboratory Medicine (Buffalo, New York) (<10% total coefficient of variation). Overall, there were 3,770 assessments of estradiol levels available for analysis.
Statistical analysis
Descriptive statistics procedures were used to estimate the geometric mean of each biomarker across categories of demographic, lifestyle, and health characteristics at baseline. Plots of the 2 women with most extreme sleep duration patterns were created to illustrate women with low and high location, dispersion, skewness, and kurtosis. We examined intercorrelations among the 4 L-moments and found overall low correlation, with the exception of moderate correlation between L-dispersion and L-kurtosis.
To examine the influence of variability in sleep duration on patterns of cardiovascular biomarkers across 2 menstrual cycles, we fitted linear mixed models with random intercepts and inverse probability weighting (marginal structural models) (35). We modeled associations between 3 summary measures of overall sleep variability and geometric mean concentrations of CVD biomarkers. Specifically, dispersion, skewness, and kurtosis were considered separately as exposures that captured distinct facets of variable diary-reported sleep durations across the menstrual cycle, while cardiovascular biomarkers were analyzed as repeated outcomes. Each biomarker was log-transformed, and effect estimates were interpreted as percent differences in geometric means after applying the formula: ((exp(β) estimate) − 1) × 100. These models accounted for repeated assessment of cardiovascular biomarkers at up to 16 time points across 2 menstrual cycles.
We estimated inverse probability weights to account for baseline and time-varying confounders and mediators relevant to each model. Fixed confounders that are associated with both sleep duration and biomarkers of CVD included age, race, BMI, physical activity, shift work, and mean sleep duration.
As time-varying covariates, we included alcohol, caffeine, and estradiol. Regression models, with 2 random effects for cycle and cycle day, were first fitted with the overall sample. We then conducted sensitivity analyses by restricting the sample to women who did not report shift work and to non-White women to account for potentially distinct sleep-wake patterns attributed to sleep health disparities. Finally, to examine potential interactions between mean sleep duration and dispersion, we added a product term for mean sleep duration and dispersion of sleep duration to weighted and adjusted regression models.
RESULTS
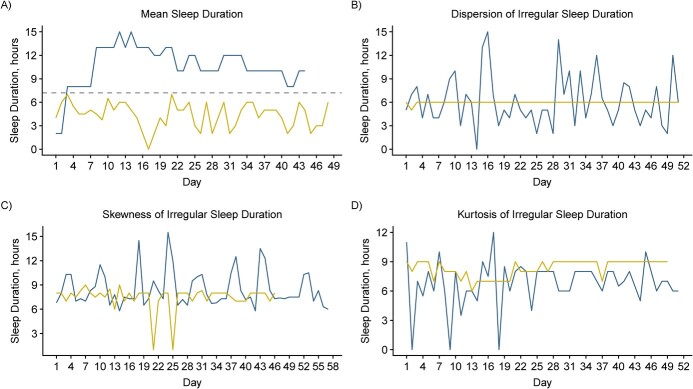
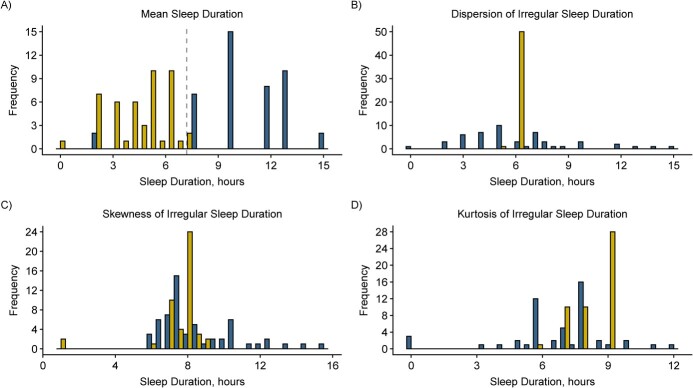
The average age of women was 27 years with mean BMI of 24, and 75% were nulliparous. Table 1 presents mean levels of CRP, LDL, HDL, total cholesterol, and triglycerides according to sociodemographic and lifestyle characteristics. Person-specific averages of reported sleep duration from daily diaries ranged between 4.4 and 10.6 hours, with an overall mean of 7.2 hours, representing recommended sleep duration across menstrual cycles for many but not all women. The dispersion of sleep duration ranged between 0.02 to 1.78 hours and its mean was 0.74. (Table 2) On average, sleep duration was minimally skewed in the overall sample, but positive and negative skewness was apparent for some women. Figures 1 and 2 describe patterns and distributions of sleep duration for a distinct pair of women with extreme values of mean, dispersion, skewness, and kurtosis.
Table 2.
Descriptive Statistics of Linear Moments Representing Facets of Irregular Sleep Duration of Women in the BioCycle Study, New York, 2005–2007
| All Women (n = 255) | Non-White a (n = 154) | Shift Work (n = 94) | |||||
|---|---|---|---|---|---|---|---|
| L-Moment | Description | Mean | Median (Range) | Mean | Median (Range) | Mean | Median (Range) |
| L-location | Mean sleep duration | 7.2 | 7.3 (4.4, 10.6) | 7.1 | 7.2 (4.4, 10.6) | 7.0 | 7.0 (5.0, 8.8) |
| L-dispersion | All magnitude deviations from mean sleep duration, on each side of the mean | 0.74 | 0.69 (0.02, 1.78) | 0.77 | 0.78 (0.02, 1.52) | 0.82 | 0.80 (0.02, 1.78) |
| L-skewness | Unbalanced sleep duration, moderate-large deviations on one side of the mean | −0.005 | 0.003 (−1.0, 0.95) | −0.02 | −0.013 (−1.0, 0.95) | −0.01 | 0.003 (−1.0, 0.32) |
| L-kurtosis | Large deviations in sleep duration with extreme short and long sleep bouts | 0.19 | 0.16 (−0.04, 1.0) | 0.20 | 0.15 (−0.04, 1.0) | 0.19 | 0.15 (0.02, 1.0) |
a Self-reported race/ethnicity; non-White = African American, American Indian, Asian Indian, Chinese/other Asian, any Pacific Islander, or unspecified.
Figure 1.
Patterns of sleep duration for a distinct pair of women with extreme values of mean, dispersion, skewness, and kurtosis in the BioCycle study, New York, 2005–2007. A) The blue line represents sleep duration above the recommended daily nocturnal sleep duration, indicated by the dotted line, while the yellow line represents sleep duration below the recommended nocturnal sleep duration. B) The blue and yellow lines represent high and low variability in sleep duration, respectively. C) The blue line represents skewness in sleep duration that is above the woman’s mean sleep duration; the yellow line represents skewness in sleep duration that is below the woman’s reported mean sleep duration. D) The blue and yellow lines represent high and low kurtosis in sleep duration, respectively.
Figure 2.
Distributions of sleep duration for a distinct pair of women with extreme values of mean, dispersion, skewness, and kurtosis in the BioCycle study, New York, 2005–2007.
Variability in sleep duration and cardiovascular biomarkers
Both unadjusted and adjusted associations between patterns of sleep duration and average changes in levels of cardiovascular biomarkers along the menstrual cycle are presented in Table 3. These associations were obtained from models adjusting for baseline age, race/ethnicity, BMI, shift work, and physical activity, and accounted for time-varying estradiol levels at the prior clinic visit across the menstrual cycle. Overall, none of the cardiovascular biomarkers were influenced by dispersion in sleep duration, which captured deviations of all magnitudes from mean sleep duration, on each side of the mean. However, skewed sleep duration—that is, unbalanced distribution of sleep duration with frequent short or long hours—was positively associated with CRP levels and negatively associated with total cholesterol levels. Specifically, a 1-unit increase of skewness in sleep duration was associated with a higher CRP level (99%) and a lower (11%) total cholesterol level. Lower LDL level influenced by increased skewness of sleep duration approached statistical significance: −14.4% (95% CI: −27.2, 0.6). Women with positively skewed sleep tended to have lower total cholesterol levels, while women with negatively skewed sleep tended to have higher levels of total cholesterol. Positive skewness occurs when a woman has a small number of nights with much longer sleep than her long-run mean. Negative skewness occurs when a woman has a small number of days with much shorter sleep than her long-run mean. We found no relationships between skewed sleep duration and HDL or triglycerides levels. Kurtosis in sleep duration, describing large variability in sleep duration with extreme short or long bouts, was associated with lower HDL levels, −17.1% (95% CI: −31.1,−0.2) but did not influence CRP, LDL, total cholesterol, or triglycerides levels.
Table 3.
Percent Change in Concentrations of Cardiovascular Biomarkers in Relation to Irregularity in Sleep Duration of Women in the BioCycle Study, New York, 2005–2007
| CRP | HDL | LDL | Total Cholesterol | Triglycerides | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sleep Pattern | % Change a | 95% CI | % Change a | 95% CI | % Change a | 95% CI | % Change a | 95% CI | % Change a | 95% CI |
| Dispersion | ||||||||||
| Unadjusted | −4.4 | −35.0, 40.4 | 2.0 | −7.0, 11.8 | −13.7 | −21.9, −4.6 | −7.0 | −13.0, −0.67 | −1.1 | −14.9, 14.9 |
| Overallb,c | 8.5 | −24.1, 54.9 | 7.8 | −2.4, 19.0 | −7.0 | −16.5, 3.6 | −0.6 | −7.4, 6.6 | 5.1 | −10.3, 23.3 |
| No shift workd,e | 27.6 | −24.1, 114.6 | 8.1 | −5.3, 23.5 | −13.1 | −24.8, −0.4 | −4.1 | −12.9, 5.6 | 4.7 | −15.0, 28.9 |
| Non-Whitef,g | −0.7 | −43.1, 73.5 | 24.7 | 8.2, 43.0 | −19.4 | −30.9, −6.0 | −4.2 | −13.8, 6.4 | 0.5 | −20.5, 27.0 |
| Skewness | ||||||||||
| Unadjusted | 81.0 | −4.1, 241.6 | −7.5 | −20.6, 7.8 | −16.1 | −29.1, −0.8 | −11.6 | −20.9, −1.3 | −0.88 | −22.8, 27.2 |
| Overallb,c | 99.3 | 17.2, 238.9 | −7.1 | −20.2, 8.1 | −14.4 | −27.2, 0.6 | −10.9 | −19.9, −1.0 | 1.2 | −20.6, 28.9 |
| No shift workd,e | 81.7 | −10.2, 267.8 | −7.9 | −23.5, 10.8 | −16.5 | −31.7, 1.9 | −13.4 | −24.1, −1.2 | −7.2 | −30.4, 23.7 |
| Non-Whitef,g | 126.7 | 3.1, 398.2 | −3.8 | −21.8, 18.4 | −14.7 | −32.0, 7.0 | −8.7 | −21.4, 6.0 | 10.3 | −20.9, 53.7 |
| Kurtosis | ||||||||||
| Unadjusted | 1.8 | −52.7, 119.3 | −13.5 | −28.1, 4.1 | 12.6 | −8.3, 38.2 | 2.3 | −11.7, 18.5 | 5.9 | −21.7, 43.1 |
| Overallb,c | −1.4 | −50.0, 93.8 | −17.1 | −31.1, −0.2 | 5.9 | −13.3, 29.5 | −2.4 | −15.6, 12.7 | 4.1 | −22.6, 40.1 |
| No shift workd,e | 26.4 | −48.3, 209.2 | −16.9 | −34.0, 4.5 | 10.2 | −14.3, 41.9 | −0.4 | −16.7, 19.0 | 5.4 | −26.3, 50.7 |
| Non-Whitef,g | 6.2 | −54.6, 148.5 | −25.4 | −39.5, −8.0 | 7.7 | −15.6, 37.5 | −5.3 | −21.3, 13.8 | 2.2 | −28.2, 45.3 |
Abbreviations: CI, confidence interval; CRP, C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
a Results from linear mixed models with inverse probability weighting; effect estimates represent the percent change in biomarker concentrations in response to 1-unit change in corresponding sleep irregularity measure.
b Models adjusted for age, race, body mass index, physical activity, shift work, caffeine and alcohol intake, and biomarker and estrogen levels at the prior clinic visit.
c Sample size n = 240 for overall adjusted analyses.
d Women who work shifts were excluded; models adjusted for age, race, body mass index, physical activity, caffeine and alcohol intake, and biomarker and estrogen levels at the prior clinic visit.
e Sample size n = 154 for women who did not report shift work.
f Models excluded White women; models adjusted for mean sleep duration, age, body mass index, shift work, physical activity, caffeine and alcohol intake, and estrogen levels at the prior clinic visit.
g Sample size n = 94 for non-White women.
Among non-White women, we observed significant relationships between dispersion in sleep duration and changes in both LDL and HDL levels. Similar associations were observed among women who did not report shift work. Specifically, a 1-hour increase in dispersion of sleep duration suggested a lower mean LDL level among non-White women (−19.4%, 95% CI: −30.9, −6.0). Higher mean HDL levels (24.7%, 95% CI: 8.2, 43.0) were also apparent in non-White women with each 1-hour increase in dispersion of sleep duration. A positive relationship was apparent between skewness in sleep duration and CRP levels in non-White women; an increase in skewness of sleep duration was associated with 127% higher CRP levels (126.7%, 95% CI: 3.1, 398.2). An increase in extreme short and long sleep bouts, measured by kurtosis, showed lower mean HDL levels in non-White women (−25.4%, 95% CI: −39.5, −8.0%). However, mean CRP, LDL, and triglycerides levels were not associated with both extremes of sleep duration bouts.
Women who did not report shift work had significant associations between dispersion in sleep duration and changes in both LDL and HDL levels, such that each 1-hour increase in sleep dispersion was associated with lower LDL levels (−13.1%, 95% CI: −24.8, −0.4). Further, within this subsample of women who did not report shift work (64%), skewed sleep duration was associated with mean total cholesterol levels and weakly associated with mean LDL levels. Specifically, positively skewed sleep was associated with a 13% lower cholesterol levels and 17% lower LDL. (Table 3).
Interactions between variability in sleep duration and mean sleep duration
We found statistically significant interactions between mean sleep duration and its dispersion in relation to LDL, HDL, and total cholesterol. Specifically, when mean sleep duration was sufficient (e.g., 8 hours), a 2-hour increase in dispersion of sleep duration was associated with (33%) lower LDL levels. However, when mean sleep duration was insufficient (e.g., 5 hours), an increase of 2 hours in sleep dispersion was associated with 43% higher LDL levels (data not shown).
DISCUSSION
In this unique cohort of healthy, premenopausal women, we utilized a novel approach that summarizes person-specific facets of variability in sleep duration ranging from small deviations to extreme bouts of short and long sleep relative to mean sleep duration. We estimated variability in daily sleep duration and examined its associations with key CVD biomarkers while accounting for hormonal fluctuations across 2 menstrual cycles. In these data, women reported an average of 7.2 hours of sleep, which falls within the range of the recommended sleep duration of 7–9 hours per night. However, the amount of reported sleep was variable and ranged from 4.4 and 10.6 hours per night. As expected, we found that mean LDL, HDL, and total cholesterol were susceptible to changes in response to some variability in sleep duration. These findings remained consistent after adjustment for menstrual cycle variations and relevant confounders that could influence both sleep duration patterns and fluctuations in CVD biomarkers.
Overall, dispersed sleep duration was associated with higher HDL levels and lower LDL levels in non-White women. We also found lower LDL levels among women who did not report shift work. Positively skewed sleep (occasional values falling substantially above the person-specific mean) was associated with lower levels of LDL and total cholesterol, while higher levels of LDL and total cholesterol were observed in women with negatively skewed sleep duration, below the mean. Extreme short and long sleep bouts were associated with lower HDL levels among all women, as well as in non-White women. Furthermore, mean sleep duration moderated associations between dispersed sleep duration, LDL, HDL, and total cholesterol. Specifically, dispersed sleep was associated with lower levels of LDL and total cholesterol, and higher HDL levels, in women with sufficient sleep duration. However, dispersed sleep duration in women with insufficient sleep had a negative impact on these CVD biomarkers. These findings indicate that even among healthy and young women, variability in sleep duration could have an influence on cardiovascular biomarkers. Moreover, plausible interrelations among sleep, reproductive hormones, and cardiovascular health were also suggested (36).
Variability in sleep duration is known to disrupt natural circadian rhythms and may result in altered physiological functions. Indeed, variations in sleep duration are emerging as determinants of health, well-being, and longevity (37). Associations between variability in sleep duration and CVD biomarkers are uncommon. Several studies have examined the influence of variability in sleep duration on cardiovascular risk factors, suggesting higher odds of metabolic syndrome, weight gain, and obesity in adults (38–42). However, most reports focused on adiposity outcomes, and only few considered a metabolic syndrome risk, a composite score of CVD risk factors that includes HDL and triglycerides levels. Furthermore, these studies have predominantly assessed variability in sleep duration using the standard deviation (SD) of mean sleep quantity recorded on actigraphy over 7 nights.
A cross-sectional study of adults and older adults associated variability (SD) in sleep duration based on a 7-day actigraphic sleep duration with lower HDL and higher triglycerides and fasting glucose. A follow-up of 6 years, among those who were not classified in the metabolic syndrome group, suggested that higher variability in sleep duration was weakly associated with higher triglycerides levels but was not associated with HDL (43). In a cross-sectional study of 51 healthy college students, lower HDL was also linked to variability in sleep duration (SD) (44). A recent review reported consistent links between variability in sleep duration and cardiometabolic risk factors, including obesity, metabolic syndrome, and poor glycemic control (45). This review suggests that a 1-hour increased variability in sleep duration, measured by SD, is associated with higher risk of excessive weight, metabolic syndrome, type 2 diabetes, and poor glycemic control.
Variability in sleep duration has been also examined in relation to inflammatory markers in a cohort of 392 nurses, of whom 92% were women. While variability in sleep duration, assessed either using 7-day actigraphy or daily sleep diaries, was associated with interleukin-6, it was not associated with CRP (46). However, in a sample of 532 adults with 60% women, positive associations were observed between sleep inconsistency and inflammatory biomarkers. Utilizing 7-day actigraphy data, inconsistency in sleep behaviors was estimated across multiple domains, including time in bed, nocturnal awakening, and sleep onset latency. Findings suggested links between sleep inconsistency and increased levels of inflammatory markers (i.e., CRP, interleukin-6, and fibrinogen). A stratified analysis uncovered sex-specific relationships, significant for women but not men (47).
Strengths and limitations
The present study has several strengths. While sex differences in sleep patterns and CVD risk factors have been examined, women-focused research on sleep and, even more so, the role of the reproductive cycle in these analyses remain rare. This study collected daily sleep data in addition to up to 16 person-specific samples of CVD biomarkers at key hormonally defined time points along 2 menstrual cycles. In comparison with prior studies, these rich data provide far greater texture in sleep patterns and cardiovascular health in premenopausal, healthy women. Second, the BioCycle study employed a microlongitudinal design—a measurement-intense design over a relatively short time interval of 2 menstrual cycles. This unusual design allowed examination of within- and between-woman dynamic covariation of sleep duration, without aggregation of data over long periods.
The implementation of 2 advanced statistical methods, the L-moments and inverse probability weights, allowed us to distinguish among 3 facets of sleep duration variability in relation to CVD biomarkers and to account for relevant fixed and time-varying confounders and mediators. However, this study has limitations as well. Despite using sleep diaries, the gold standard of self-reported sleep duration (26), we cannot rule out measurement error in sleep duration as recorded by these women in their daily diaries. Self-report sleep duration could be consistently misreported in comparison with objectively measured sleep duration (48). Future studies could take advantage of actigraphy, which provides objective assessments over multiple days in the home setting. Exclusions of women with a BMI of <18 or >35 and premenopausal women with abnormal length of menstrual cycle (<21 or >35 days) limit the generalizability of our findings to women with severe obesity or during perimenopause, a phase marked by hormonal fluctuations and sleep disturbances. While alcohol was included as a time-varying covariate, we did not account for recreational drug use as its impact on cardiovascular function remains poorly understood. Information on sleep aids use was not available, although insomnia symptoms were uncommon among these women. Assessment of caffeine intake relied solely on daily coffee consumption and did not consider other caffeinated beverages. Finally, obstructive sleep apnea data were not collected. However, as BMI is a strong risk factor for obstructive sleep apnea, adjustment for BMI in our models may have avoided some of the risk for multicollinearity.
Conclusions
This microlongitudinal study of premenopausal women found that variability in sleep duration was predominantly associated with lower mean LDL, HDL, and total cholesterol, but not CRP or triglycerides. Although CVD biomarkers vary along the menstrual cycle, the observed sleep-related variations in CVD biomarkers persisted even after controlling for estradiol levels. These data suggest that variability in sleep duration could influence biomarkers of CVD in young, healthy women. Future examination of variation in sleep timing among young women is warranted.
ACKNOWLEDGMENTS
Author affiliations: Department of Nutritional Sciences, School of Public Health, University of Michigan, Ann Arbor, Michigan, United States (Galit Levi Dunietz, Ana Baylin); Division of Sleep Medicine, Department of Neurology, University of Michigan, Ann Arbor, Michigan, United States (Galit Levi Dunietz, Ronald D. Chervin, Louise M. O’Brien); Department of Statistics, University of Michigan, Ann Arbor, Michigan, United States (Kerby Shedden, Xiru Lyu); Division of Cancer Epidemiology and Genetics, Metabolic Epidemiology Branch, National Cancer Institute, National Institutes of Health, Rockville, Maryland, United States (Kara A. Michels, Joshua R. Freeman); Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, Michigan, United States (Ana Baylin); Department of Epidemiology and Environmental Health, School of Public Health and Health Professions, University at Buffalo, Buffalo, New York, United States (Jean Wactawski-Wende); Department of Biostatistics, Epidemiology and Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States (Enrique F. Schisterman, Sunni L. Mumford); and Epidemiology Branch, Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Rockville, Maryland, United States (Sunni L. Mumford).
G.L.D. was supported by a Mentored Research Scientist Development Award from the National Heart, Lung, and Blood Institute (award K01 HL144914). This work was supported in part by the Intramural Research Program of the National Institutes of Health (authors K.A.M., J.R.F., E.F.S., S.L.M.). The BioCycle study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (contracts HHSN275200403394C HHSN275201100002I, and Task 1 HHSN27500001).
Data will be made available to the editors of the Journal for review or query upon request.
We thank the BioCycle participants for their extraordinary commitment to the study and the BioCycle investigators and staff who devoted their time and energy to the success of the BioCycle study.
Some findings from this work were presented at the SLEEP conference, June 4–8, 2022, Charlotte, North Carolina.
The study sponsor played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation and review of the manuscript; and decision to submit the manuscript for publication.
Conflict of interest: none declared.
REFERENCES
- 1. Kochanek KD, Murphy SL, Xu J, et al. Mortality in the United States, 2013. Hyattsville, MD: National Center for Health Statistics. 2014. (NCHS Data Brief, no. 178.). https://www.cdc.gov/nchs/products/databriefs/db178.htm. Accessed May 10, 2022. [PubMed]
- 2. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blauwet LA, Redberg RF. The role of sex-specific results reporting in cardiovascular disease. Cardiol Rev. 2007;15(6):275–278. [DOI] [PubMed] [Google Scholar]
- 4. Tajeu GS, Safford MM, Howard G, et al. Black-White differences in cardiovascular disease mortality: a prospective US study, 2003–2017. Am J Public Health. 2020;110(5):696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roberts LD, Gerszten RE. Toward new biomarkers of cardiometabolic diseases. Cell Metab. 2013;18(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Holten TC, Waanders LF, de Groot PG, et al. Circulating biomarkers for predicting cardiovascular disease risk; a systematic review and comprehensive overview of meta-analyses. PLoS One. 2013;8(4):e62080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. [DOI] [PubMed] [Google Scholar]
- 8. Koenig W, Sund M, Fröhlich M, et al. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men. Circulation. 1999;99(2):237–242. [DOI] [PubMed] [Google Scholar]
- 9. Peters SAE, Muntner P, Woodward M. Sex differences in the prevalence of, and trends in, cardiovascular risk factors, treatment, and control in the United States, 2001 to 2016. Circulation. 2019;139(8):1025–1035. [DOI] [PubMed] [Google Scholar]
- 10. Cartier A, Côté M, Lemieux I, et al. Sex differences in inflammatory markers: what is the contribution of visceral adiposity? Am J Clin Nutr. 2009;89(5):1307–1314. [DOI] [PubMed] [Google Scholar]
- 11. Khera A, McGuire DK, Murphy SA, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46(3):464–469. [DOI] [PubMed] [Google Scholar]
- 12. Schisterman EF, Mumford SL, Sjaarda LA. Failure to consider the menstrual cycle phase may cause misinterpretation of clinical and research findings of cardiometabolic biomarkers in premenopausal women. Epidemiol Rev. 2014;36(1):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Michels KA, Wactawski-Wende J, Mills JL, et al. Folate, homocysteine and the ovarian cycle among healthy regularly menstruating women. Hum Reprod. 2017;32(8):1743–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agarwala A, Michos ED, Samad Z, et al. The use of sex-specific factors in the assessment of Women's cardiovascular risk. Circulation. 2020;141(7):592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramar K, Malhotra RK, Carden KA, et al. Sleep is essential to health: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2021;17(10):2115–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Covassin N, Singh P. Sleep duration and cardiovascular disease risk: epidemiologic and experimental evidence. Sleep Med Clin. 2016;11(1):81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cappuccio FP, Miller MA. Sleep and cardio-metabolic disease. Curr Cardiol Rep. 2017;19(11):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kingsbury JH, Buxton OM, Emmons KM. Sleep and its relationship to racial and ethnic disparities in cardiovascular disease. Curr Cardiovasc Risk Rep. 2013;7(5):387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoevenaar-Blom MP, Spijkerman AMW, Kromhout D, et al. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN study. Sleep. 2011;34(11):1487–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cappuccio FP, Cooper D, D'Elia L, et al. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–1492. [DOI] [PubMed] [Google Scholar]
- 21. Smiley A, King D, Harezlak J, et al. The association between sleep duration and lipid profiles: the NHANES 2013–2014. J Diabetes Metab Disord. 2019;18(2):315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaneita Y, Uchiyama M, Yoshiike N, et al. Associations of usual sleep duration with serum lipid and lipoprotein levels. Sleep. 2008;31(5):645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Michels KA, Mendola P, Schliep KC, et al. The influences of sleep duration, chronotype, and nightwork on the ovarian cycle. Chronobiol Int. 2020;37(2):260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wactawski-Wende J, Schisterman EF, Hovey KM, et al. BioCycle study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol. 2009;23(2):171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Howards PP, Schisterman EF, Wactawski-Wende J, et al. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle study. Am J Epidemiol. 2009;169(1):105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mallinson DC, Kamenetsky ME, Hagen EW, et al. Subjective sleep measurement: comparing sleep diary to questionnaire. Nat Sci Sleep. 2019;11:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bolger N, Davis A, Rafaeli E. Diary methods: capturing life as it is lived. Annu Rev Psychol. 2003;54(1):579–616. [DOI] [PubMed] [Google Scholar]
- 28. Hosking JRM. L-moments: analysis and estimation of distributions using linear combinations of order statistics. J R Stat Soc Series B Stat Methodol. 1990;52(1):105–124. [Google Scholar]
- 29. Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–1565. [DOI] [PubMed] [Google Scholar]
- 30. Rifai N, Ballantyne CM, Cushman M, et al. Point: high-sensitivity C-reactive protein and cardiac C-reactive protein assays: is there a need to differentiate? Clin Chem. 2006;52(7):1254–1256. [DOI] [PubMed] [Google Scholar]
- 31. Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. [DOI] [PubMed] [Google Scholar]
- 32. Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363–369. [DOI] [PubMed] [Google Scholar]
- 33. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 34. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. [DOI] [PubMed] [Google Scholar]
- 35. Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dunietz GL, Vanini G, Shannon C, et al. Associations of plasma hypocretin-1 with metabolic and reproductive health: two systematic reviews of clinical studies. Sleep Med Rev. 2020;52:101307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mazzotti DR, Guindalini C, Moraes WADS. Human longevity is associated with regular sleep patterns, maintenance of slow wave sleep, and favorable lipid profile. Front Aging Neurosci. 2014;6:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang T, Redline S. Cross-sectional and prospective associations of Actigraphy-assessed sleep regularity with metabolic abnormalities: the Multi-Ethnic Study of Atherosclerosis. Diabetes Care. 2019;42(8):1422–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kobayashi D, Takahashi O, Shimbo T, et al. High sleep duration variability is an independent risk factor for weight gain. Sleep Breath. 2013;17(1):167–172. [DOI] [PubMed] [Google Scholar]
- 40. Häusler N, Marques-Vidal P, Haba-Rubio J, et al. Association between actigraphy-based sleep duration variability and cardiovascular risk factors—results of a population-based study. Sleep Med. 2020;66:286–290. [DOI] [PubMed] [Google Scholar]
- 41. Xu X, Conomos MP, Manor O, et al. Habitual sleep duration and sleep duration variation are independently associated with body mass index. Int J Obes (Lond). 2018;42(4):794–800. [DOI] [PubMed] [Google Scholar]
- 42. Makarem N, Zuraikat FM, Aggarwal B, et al. Variability in sleep patterns: an emerging risk factor for hypertension. Curr Hypertens Rep. 2020;22(2):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang T, Mariani S, Redline S. Sleep irregularity and risk of cardiovascular events: the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2020;75(9):991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoopes EK, Berube FR, D’Agata MN, et al. Sleep duration regularity, but not sleep duration, is associated with microvascular function in college students. Sleep. 2021;44(2):zsaa175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zuraikat FM, Makarem N, Redline S, et al. Sleep regularity and cardiometabolic heath: is variability in sleep patterns a risk factor for excess adiposity and glycemic dysregulation? Curr Diab Rep. 2020;20(8):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Slavish DC, Taylor DJ, Dietch JR, et al. Intraindividual variability in sleep and levels of systemic inflammation in nurses. Psychosom Med. 2020;82(7):678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dzierzewski JM, Donovan EK, Kay DB, et al. Sleep inconsistency and markers of inflammation. Front Neurol. 2020;11:1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dunietz GL, Jansen EC, Hershner S, et al. Parallel assessment challenges in nutritional and sleep epidemiology. Am J Epidemiol. 2021;190(6):954–961. [DOI] [PMC free article] [PubMed] [Google Scholar]