Abstract
Objectives
This study aims to investigate the effects of a combination of soy isoflavones, 8-prenylnaringenin (8-PN), and melatonin in postmenopausal women suffering from moderate-to-severe hot flashes (HFs).
Methods
A multicenter, prospective, open-label study enrolled 44 postmenopausal women suffering from moderate-to-severe HFs (≥ 5 daily or ≥ 35 weekly) to receive 54.4 mg standardized soy isoflavones (including 24.5 mg genistein and 16.3 mg daidzein), 100 µg 8-PN, and 1 mg melatonin once daily for 12 weeks. The primary clinical outcomes included changes in health-related quality of life (HRQoL) scores (Menopause-Specific QoL questionnaire [MENQoL] and Cervantes Scale) and HFs following 4 and 12 weeks of treatment. Other analyses included treatment adherence, acceptability, tolerability, and safety.
Results
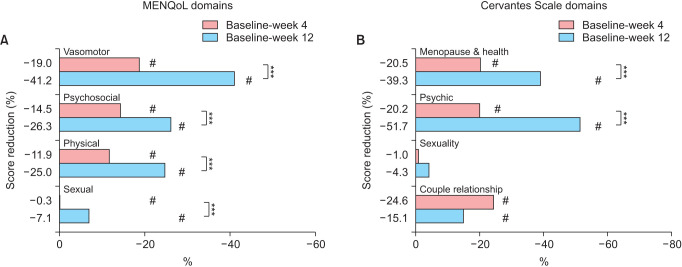
All of the four domains of MENQoL questionnaire significantly improved at 4 weeks (P < 0.05) and 12 weeks (P < 0.001), affecting significantly the vasomotor, psychosocial, and physical spheres (41.2%, 26.3%, and 25.0%; 12 weeks improvements, respectively). Similarly, in the menopause (39.3%) and psychic (51.7%) domains (both P < 0.05 at 12 weeks), the global score of the Cervantes Scale significantly increased at 4 weeks (18.6%) and 12 weeks (35.4%). Accordingly, moderate-to-severe HFs significantly decreased at 4 weeks compared to baseline (41.7% reduction) and further reduced at 12 weeks (76.5%), including the total number of episodes.
Conclusions
Food supplements containing soy isoflavones, 8-PN, and melatonin showed an early and progressive benefit for reducing clinically significant HFs and for improving HRQoL across all domains, favorably affecting postmenopausal women’s overall well-being.
Keywords: Hot flashes, Isoflavones, Menopause, 8-Prenylnaringenin, Quality of life
INTRODUCTION
Menopause, referring to permanent cessation of menstruation resulting from a decrease in estrogen and progesterone levels, marks the end of fertility in women. During perimenopause and postmenopause, women often experience vasomotor symptoms (VMS) such as hot flashes (HFs), but also anxiety, sleep disturbances and/or urogenital disorders, as well as possible long-term complications, including osteoporosis and a higher risk of cardiovascular disease [1].
HFs constitute an early menopausal symptom, with prevalence rates ranging from 55% to 85%, and usually lasting for several years [2]. HFs present with sufficient intensity to require medical attention in up to 60% of cases [2,3] and have been associated with a marked reduction in the health-related quality of life (HRQoL) of menopausal women [4]. Interestingly, HRQoL is a subjective and multidimensional concept, involving different physical and psychological dimensions [5]. Apart from the menopausal area, HFs have been suggested to significantly impact all these HRQoL dimensions during perimenopause and postmenopause [3,6].
Menopausal hormonal therapy (MHT) has been the gold therapeutic standard to manage the symptoms of estrogen deficiency, at the minimum sufficient dose [7]. However, the uncertain risk of thromboembolism and breast cancer, together with the associated contraindications, has led patients and healthcare professionals to seek treatment alternatives for managing VMS [8,9].
Phytoestrogen-based therapies are among the most common alternatives to MHT [8]. Genistein-containing soy isoflavones have shown efficacy in treating HFs [10,11,12]; they represent the best-studied phytotherapy to date. The clinical benefits of these preparations are generally noticed from week 12 [12,13], this waiting period being the main drawback of the therapy. Isoflavones containing over 18.8 mg of genistein have been shown to double the effectiveness in reducing HFs compared to extracts of lower genistein content [12]. Strikingly, treatment with isoflavones has not only been associated with a reduction in HFs; several studies might suggest an improvement in the HRQoL of postmenopausal women [14,15]. Hops (Humulus lupulus L.), specifically its active molecule 8-prenylnaringenin (8-PN), has been classified as one of the most potent phytoestrogens [16,17,18]. Use of hops extracts enriched in 8-PN have been recently introduced, showing efficacy in alleviating menopausal discomfort in clinical studies [16,17,18]. Furthermore, the European Medicines Agency (EMA) [19] has recognized hops preparations’ sedative effects.
Altogether, there is little available literature on the benefits of phytoestrogens in HRQoL during menopause. There is also a need for further evidence on the clinical value of combined formulations. The main aim of the present study was to evaluate the effects of a food supplement containing soy isoflavones (including genistein), 8-PN and melatonin (among other nutrients), on the HRQoL of postmenopausal women presenting moderate-to-severe HFs.
MATERIALS AND METHODS
Study design and participants
This 12-week prospective, multicenter, open-label, exploratory clinical study (FLAVIE study) was conducted in the gynecological service of 7 centers in Spain from September 2020 to May 2021. Healthy postmenopausal women (45–65 years old) experiencing ≥ 5 daily or ≥ 35 weekly moderate-to-severe HFs, according to Food and Drug Administration (FDA) [20] and the National Institutes of Health (NIH) [21] scales, were included. FDA and NIH similarly define mild HFs as the sensation of heat without sweating, moderate HFs as the sensation of heat with sweating, but being able to continue activity and severe HFs as the sensation of heat with sweating, causing cessation of activity [20,21].
Exclusion criteria were contraindication of MHT, previous use of MHT (within 3 months) or or any other VMS treatment (last month), recent use of gonadotropin agonist drugs (6 months), glucocorticoids (3 months) or selective serotonin/noradrenalin reuptake inhibitors (last month). Vegan and soy-enriched diets, and smoking (> 10 cigarettes/day) were also prohibited. Additional exclusion criteria included body mass index (BMI) > 40 kg/m2, presence of clinical conditions including present/past breast, endometrial or other hormone-dependent cancers, current disorders of the genitourinary tract (bleeding, myomas, endometriosis, abnormal Papanicolau test) and thrombotic comorbidities, hepatic and biliary vesicle disorders, pregnancy and allergy to any product component. The study was conducted in accordance with Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki. Ethics approval was granted by the reference institutional review board of the Puerta del Hierro University Hospital, and all participants provided written informed consent.
Participants received one daily oral soft capsule (before bedtime) of a food supplement containing 54.4 mg standardized soy isoflavones (including 24.5 mg genistein and 16.3 mg daidzein), 100 µg 8-PN and 1 mg melatonin, among other nutrients, for 12 weeks (Flavia Nocta®; Italfarmaco Group). Study visits were scheduled at baseline, 4 weeks and 12 weeks.
Study outcomes
Primary outcome-health-related quality of life
The primary outcome was the analysis of HRQoL following 12 weeks of treatment, compared to baseline. We employed the Menopause-Specific Quality of Life Questionnaire (MENQoL) and the Cervantes Scales, both interviewer administered questionnaires, validated for patient-report of menopause experience, for self-assessment of the week prior to the visit. The MENQoL [22] is a 29-item questionnaire with four domains (vasomotor, psychosocial, physical, and sexual). Items are rated on a 7-point scale ranging from 0 (not at all bothered) to 6 (extremely bothered). Scores in each item were converted to an analysis score with a range of 1 (no symptoms) to 8 (extremely bothered). Domain scores are calculated as the mean of its included item scores. A higher score indicates lower HRQoL, and a reduction therefore reflects improvement.
Likewise, the Cervantes Scale [23] consists of 31 items (score 0 to 5 each) grouped into 4 domains: menopause and health (sub-dimensions: VMS, health, and aging), psychic, sexuality, and couple relationship. Domain scores are calculated considering scores of their individual items. The global score ranges from 0 to 155, with a higher score indicating lower HRQoL, as described for MENQoL.
Evaluation of HRQoL following 4 weeks of intervention and between 4–12 weeks were secondary outcomes.
Secondary outcomes
Hot flashes: The number of mild, moderate, severe and total weekly HFs (in the week previous to assessment) following 4 weeks and 12 weeks of product administration were assessed, comparing reduction with baseline or between timepoints. Presence and intensity of HFs were recorded by patient’ self-assesment by a hot flash daily diary, according to the NIH guidelines [21] and reviewed during scheduled study visits. Total HFs were calculated as the sum of any mild, moderate and severe HFs recorded.
Adherence to treatment: Participants were instructed on proper administration. Leftover treatments were collected at 4 weeks and 12 weeks to assess treatment adherence. Therapeutic compliance with the food supplement was established by leftover pill count and it was considered as poor (< 50%), moderate (50%–75%), good (75%–90%), or very good (≥ 90%).
Acceptability: Treatment acceptability was assessed as the degree of satisfaction with the product perceived by both the patient and the researcher at the end of the study (12 weeks), categorizing it as excellent, very good, good, fair, poor, and not rated.
Safety: Any adverse event (AE) was recorded and evaluated to assess severity and product relationship from the moment the informed consent was signed.
Tolerability: Patient and researcher perception of tolerability of possible AEs observed during the study was assessed in the final visit, categorizing it as excellent (absence of AEs), very good, good, fair, poor (AEs leading to withdrawal), and not rated.
Statistical analysis
Sample size was calculated to comply with the guidelines of the International Council for Harmonisation [24]. Based on the Cervantes Scale score [23], 34 patients provided a mean ± SD decrease in the global score of 8 [25] points after 12 weeks of the study product, with 80% power and 95% confidence interval (CI). Assuming a dropout rate of 15%, 40 patients would be needed. It was planned to be 44 people in consideration of the safety population. Calculations were performed with the PASS package, 2011 version (NCSS LLC.). Of note, from a clinical perspective, as it is established in the analysis by the Spanish Association for the Study of Menopause (AEEM) [26], a change of 6.7 points in the global Cervantes Scale score involved a significant improvement in accomplishing working/daily activities, a reduction in healthcare needs and alleviation of the economic burden resulting from menopausal symptoms and lost productivity [26].
Demographics and baseline characteristics were analyzed in the intention-to-treat (ITT) population (study subjects with at least one dose and any follow-up). Efficacy analyses are presented in the valid population for analysis (VPA, ITT individuals with basal and 12-week QoL data), as we observed no differences with the analyses of the ITT or per-protocol (VPA subjects with no protocol deviations) populations. Safety and tolerability were analyzed in the safety population, including any study subject.
Summary statistics are presented as number (frequency,%) for categorical data and median (interquartile range, IQR, or 95% CI) and mean ± SD for continuous data. We calculated both absolute and relative differences (percentage change) between given timepoints as the mean of individual subject change. Paired data (between visits) were analyzed with the paired Student’s t test or the Wilcoxon sign-ranked test, according to normal distribution analyzed by means of the Shapiro-Wilk test. Statistical analyses were performed using SAS software (version 9.4 or higher). A P < 0.05 was considered to be statistically significant.
RESULTS
Disposition and baseline characteristics
Of the 44 women enrolled (safety population), 40 and 38 were included in the ITT and VPA populations, respectively. The most common reasons for discontinuation (n = 6) were lost to follow-up (n = 2), treatment interruption (n = 1) or others (n = 3, coronavirus disease 2019 [COVID-19] infection and unmet inclusion criteria). Participants’ baseline characteristics are summarized in Table 1. The mean ± SD age of participants was 53.4 ± 5.1 years. A total of 92.5% of these underwent natural menopause, while 5.0% were subjected to surgical menopause. Mean time since menopause onset was was 55.1 ± 41.2 months. Baseline HRQoL scores and HFs are summarized in Tables 2 and 3, respectively.
Table 1. Baseline characteristics of the participants (ITT population).
| Characteristics | n = 40 | |
|---|---|---|
| Age (y) | 53.4 ± 5.1 | |
| Weight (kg) | 66.8 ± 9.0 | |
| Height (cm) | 162.6 ± 5.4 | |
| BMI (kg/m2) | 25.3 ± 3.5 | |
| Time since menopause (months)a | 55.1 ± 41.2 | |
| Reason for menopause | ||
| Natural process | 37 (92.5) | |
| Oophorectomy | 2 (5.0) | |
| Otherb | 1 (2.5) | |
| Medication in the previous month | 0 (0.0) | |
| Previous clinically relevant comorbidities and/or surgery | 8 (20.0) | |
Data are presented as mean ± SD or number (%).
BMI: body mass index, ITT: intention-to-treat population.
aMonths elapsed between the date of the last menstrual period and the date of informed consent. bSpecification of other reasons for menopause (n = 1; early menopause).
Table 2. MENQoL and Cervantes Scales scores (global and by domains) throughout the study Data are presented as mean ± SD.
| Scales and domains | Baseline | 4 wk | 12 wk | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Score | Score | Reduction (%) | P valuea | Score | Reduction (%) | P valueb | P valuec | ||
| MENQoL | |||||||||
| Vasomotor | 7.2 ± 1.0 | 5.8 ± 0.9 | –19.0 | < 0.001 | 4.2 ± 1.0 | –41.2 | < 0.001 | < 0.001 | |
| Psychosocial | 3.8 ± 1.9 | 3.2 ± 1.5 | –14.5 | < 0.001 | 2.8 ± 1.5 | –26.3 | < 0.001 | < 0.001 | |
| Physical | 4.9 ± 1.6 | 4.3 ± 1.3 | –11.9 | < 0.001 | 3.7 ± 1.4 | –25.0 | < 0.001 | < 0.001 | |
| Sexual | 5.3 ± 1.8 | 4.9 ± 1.5 | –0.3 | 0.0125 | 4.6 ± 1.7 | –7.1 | < 0.001 | < 0.001 | |
| Cervantes scale | |||||||||
| Global | 77.2 ± 26.0 | 63.1 ± 23.5 | –18.6 | < 0.001 | 51.6 ± 26.3 | –35.4 | < 0.001 | < 0.001 | |
| M&H | 46.8 ± 12.9 | 37.5 ± 11.8 | –20.5 | 0.001 | 29.4 ± 14.9 | –39.3 | < 0.001 | < 0.001 | |
| Vasomotor | 14.1 ± 1.6 | 11.0 ± 1.7 | –22.0 | < 0.001 | 7.3 ± 3.1 | –47.3 | < 0.001 | < 0.001 | |
| Health | 15.0 ± 6.0 | 11.4 ± 4.8 | –22.9 | < 0.001 | 9.1 ± 5.3 | –39.3 | < 0.001 | < 0.001 | |
| Aging | 17.7 ± 7.3 | 15.1 ± 7.5 | –16.7 | < 0.001 | 13.0 ± 8.1 | –30.1 | < 0.001 | < 0.001 | |
| Psychic | 16.4 ± 10.0 | 12.6 ± 8.1 | –20.2 | < 0.001 | 9.4 ± 7.7 | –51.7 | < 0.001 | < 0.001 | |
| Sexuality | 11.0 ± 4.9 | 10.9 ± 4.9 | –1.0 | 0.279 | 10.6 ± 4.8 | –4.0 | 0.203 | 0.616 | |
| Couple relationship | 2.9 ± 4.1 | 2.2 ± 3.8 | –24.6 | 0.0009 | 2.3 (4.0) | –15.1 | 0.012 | 0.951 | |
Reduction (%) reflects relative difference vs. basal at a given timepoint.
MENQoL: Menopause-Specific QoL Questionnaire, M&H: menopause & health.
Student’s t test or Wilcoxon sign-ranked test: aweek 4 vs. baseline; bweek 12 vs. baseline; cweek 12 vs. week 4.
Table 3. Total and by-intensity weekly hot flashes throughout the study.
| Type of HFs | Baseline | 4 wk | 12 wk | |||||
|---|---|---|---|---|---|---|---|---|
| Number | Number | Reduction (%) | P valuea | Number | Reduction (%) | P valueb | P valuec | |
| Total | 65.3 ± 19.9 | 43.2 ± 29.7 | –32.7 | < 0.001 | 25.7 ± 27.2 | –60.0 | < 0.001 | < 0.001 |
| Mild | 9.7 ± 13.6 | 10.6 ± 13.4 | +33.4 | 0.817 | 12.3 ± 16.7 | +26.0 | 0.761 | 0.557 |
| Moderate | 31.7 ± 16.5 | 19.7 ± 14.2 | –38.3 | < 0.001 | 8.0 ± 7.8 | –72.9 | < 0.001 | < 0.001 |
| Severe | 23.9 ± 10.1 | 12.9 ± 10.7 | –38.0 | < 0.001 | 5.5 ± 9.6 | –73.8 | < 0.001 | < 0.001 |
| Moderate + severe | 55.6 ± 15.5 | 32.7 ± 21.1 | –41.7 | < 0.001 | 13.5 ± 15.8 | –76.5 | < 0.001 | < 0.001 |
Data are presented as mean ± SD.
Mean reduction (%) reflects relative difference vs. basal at a given timepoint.
HFs: hot flashes.
Student’s t test or Wilcoxon sign-ranked test: aweek 4 vs. baseline; bweek 12 vs. baseline; cweek 12 vs. week 4.
Health-related quality of life
Compared with baseline, significant decreases in all MENQoL domains were found at 4 weeks and 12 weeks since supplementation (Fig. 1A, Table 2), reflecting improved HRQoL. The strongest effect was evidenced in the vasomotor domain, whose score was reduced from a mean ± SD of 7.2 ± 1.0 at baseline to 5.8 ± 0.9 (19.0% improvement) and 4.2 ± 1.0 (41.2% improvement) at 4 weeks and 12 weeks, respectively (both P < 0.001 vs. baseline) (Fig. 1A, Table 2). Benefits in the vasomotor area were followed by those in the psychosocial sphere (from 3.8 ± 1.9 to 2.8 ± 1.5 at 12 weeks, 26.3% improvement, P < 0.001) and the physical sphere (from a basal score of 4.9 ± 1.6 to 3.7 ± 1.4 at 12 weeks, 25.0% improvement, P < 0.001) (Fig. 1A, Table 2). Once again, these effects in the MENQoL domains, already observed at 4 weeks, were further improved over time (Table 3). The sexual domain exhibited a slight modification.
Fig. 1. Reduction (%) in score in (A) Menopause-Specific Quality of Life Questionnaire (MENQoL) domains by timepoint (vs. baseline) and (B) Cervantes Scale domains by timepoint (vs. baseline). #P < 0.05 vs. baseline; ***P < 0.001 (12 weeks vs. 4 weeks). Reduction in these scores reflects QoL improvement.
Women reported a mean ± SD of 77.2 ± 26.0 points in the global score of the Cervantes Scale at baseline, 63.1 ± 23.5 at 4 weeks (18.6% decrease, P < 0.001) and 51.6 ± 26.3 at 12 weeks (35.4% reduction, P < 0.001), thus reflecting a positive effect upon HRQoL (Fig. 1B, Table 2). Benefits were clearly evidenced in the menopause and health domain (M&H), from a basal mean score ± SD of 46.8 ± 12.9 at baseline to 37.5 ± 11.8 (20.5% improvement) and 29.4 ± 14.9 (39.3% improvement) at 4 weeks and 12 weeks, respectively (both P < 0.001 vs. baseline) (Fig. 1B, Table 2). All subdomains from the M&H area were markedly improved, including vasomotor , health and aging (all P < 0.001, both timepoints). The psychic domain presented a remarkable improvement (from basal score of 16.4 ± 10.0 to 9.4 ± 7.7 at 12 weeks, 51.7% improvement, P < 0.001) (Fig. 1B, Table 2). Again, the effects on these Cervantes Scale areas and subdomains, already observed at 4 weeks, displayed further improvements over time (Fig. 1B, Table 2). The couple relationship domain only presented a slight change as from 4 weeks, and we detected no effect of the food supplement upon sexuality (Fig. 1B, Table 2).
Hot flashes
Women experienced a mean ± SD of 65.3 ± 19.9 total weekly HFs at baseline, 43.2 ± 29.7 at 4 weeks (32.7% reduction, P < 0.001) and 25.7 ± 27.2 at 12 weeks (60.0% reduction, P < 0.001) (Fig. 2A, Table 3), in line with the improvement observed in HRQoL scores. A markedly significant decrease in moderate-to-severe episodes was found both at 4 weeks (55.6 ± 15.5 at baseline to 32.7 ± 21.1, 41.7% reduction) and 12 weeks of treatment (13.5 ± 15.8, 76.5% reduction) (Table 3). Total and moderate-to-severe HFs were further reduced between both study timepoints (all P < 0.001) (Fig. 2, Table 3). Of note, 15.8% and 39.4% of women reported no severe HFs following 4 weeks and 12 weeks of treatment, respectively (in contrast with 2.6% at baseline). Likewise, 13.1% of women did not experience any moderate or moderate-to-severe HFs at the end of the study. While at baseline mild HFs represented a minor fraction of all episodes (14.9%), the remaining HFs after 12 weeks of treatment were mostly mild (47.9%) (Fig. 2B, Table 3).
Fig. 2. Mean number ± SE of total (A) and by-intensity (B) weekly hot flashes by timepoint. #P < 0.001 vs. baseline; ***P < 0.001 (12 weeks vs. 4 weeks).
Treatment adherence and satisfaction
The mean ± SD of therapeutic compliance was 95.4% ± 10.0 in the VPA population, which was classified as very good (≥ 90%) by 84.2% of the patients during the whole study and for 89.5% when studied per visits. In general terms, 76.3% of the patients reported a very good-to-excellent degree of satisfaction following 12 weeks of treatment, in accordance with the researchers’ perception (78.9% also evaluated acceptability as very good-to-excellent).
Safety and tolerability
A total of 5 AEs was reported in a total of 5 patients. None of these were related to the study medication. No deaths were reported during the study, and only 1 serious AE (COVID-19 infection), unrelated to the study medication, was reported. In this regard, 86.9% of the patients as well as the researchers in the VPA population reported a perception of very good-to-excellent tolerability after 12 weeks of treatment.
DISCUSSION
Despite the fact that they are often ignored, HFs continue to constitute a serious symptom during menopause, affecting over 80% of peri- and post-menopausal women [2]. Notably, the cessation of menstrual periods is also associated with various other prevalent and unpleasant symptoms, including insomnia, anxiety, fatigue and depression; consequently, this causes a strong impact on the HRQoL of menopausal women at the physical and the psychosocial levels [27]. In this sense, appropriate management is needed to alleviate VMS, particularly moderate/severe HFs [2], but also to tackle overall menopausal symptomatology and the impact thereof on HRQoL [28], thus eventually influencing the substantial healthcare use, work productivity and economic burden associated with the menopausal stage [27].
The FLAVIE study demonstrated that the isoflavone-based food supplement examined herein is a safe and effective alternative for improving menopause-related HFs and impact on HRQoL at the vasomotor, physical, psychological, and social levels in menopausal women.
Appropriately validated menopause-specific tools, such as the international MENQoL questionnaire are important for treatment evaluation [29], as they provide a multidimensional evaluation of the different parameters influencing the HRQoL. In particular, the FLAVIE study revealed a significant reduction in the vasomotor, psychosocial and physical domains of the MENQoL scale, both at 4 weeks and 12 weeks since treatment was initiated. Likewise, the Cervantes Scale is the most frequently used validated menopause-QoL scale in the Spanish population and in Spanish-speaking countries [26,30]. Strikingly, an overall significant decrease in the global score of this scale, exceeding 25 points, was found at 12 weeks (35.4% improvement); it was noteworthy in the M&H domain (mean 17.4 points), and specifically in the reduction of VMS subdomain (–47.3%, mean 6.8 points). All this fulfils the study objectives. In the analysis by the AEEM [26], a change of 6.7 points in the global Cervantes Scale score involved a significant improvement in accomplishing working/daily activities, a reduction in healthcare needs and alleviation of the economic burden resulting from menopausal symptoms and lost productivity. According to this analysis, the effectiveness of this food supplement containing soy isoflavones, 8-PN and melatonin would appear to be of notable clinical relevance, as the benefits in the vasomotor subdomain alone reached that score.
As previously stated, the impact of this intervention on HRQoL appeared to be fundamentally associated with an improvement in the vasomotor area; nonetheless, the supplement unequivocally improved other physical and mental HRQoL outcomes in these women, as previously reported in MHT studies [31]. The combination of melatonin [32] and 8-PN [33] with soy isoflavones is proposed as a reason for these benefits, particularly those associated with improvements in sleep disturbances, fatigue, or nervousness [34], as such effects were not observed with other combinations of different isoflavones [35]. Notably, the greater improvements in HRQoL observed in this study are likely associated with the baseline bothersome symptomatology required for recruited subjects [28].
Regarding the specific effect upon HFs, the supplement significantly reduced both the frequency and intensity of HFs, with marked decreases in moderate-to-severe episodes. The mean number of daily HFs at baseline was 9.3, which significantly dropped to 6.2 at 4 weeks and 3.6 at 12 weeks. Considering that the FDA [20] estimates a reduction of at least two episodes of HFs per day necessary for a difference to be considered as clinically significant, the effectiveness data analyzed herein could be deemed as highly relevant.
Of note, the treatment proved to be particularly effective in the higher-intensity HFs, with the remaining mild HFs representing the major fraction at the end of the study (47.9%). It could be speculated that the moderate-to-severe episodes became less intense, falling into the mild category, which would explain the lack of significant difference in this group and would support the effect of this combined phytoestrogen in managing both frequency and intensity of HFs.
As for the controversial side effects of MHT, in the last few years there has been a pressing need to find non-hormonal alternatives for managing menopausal symptomatology. The safety profile of isoflavones combined with the benefits they provide for overall health makes them a compelling treatment option for postmenopausal women unwilling or unable to receive MHT [36]. The results of our study corroborated these previous considerations, since the combiantion of soy isoflavones, 8-PN and melatonin displayed an excellent safety and tolerability profile, with no product-related AEs; this fact is further supported by unequivocal adherence and acceptability.
Interestingly, the results of our study provided evidence of a significant benefit after 4 weeks of intervention. Following the above-mentioned AEEM analysis of the clinical relevance of Cervantes Scale changes [26,30], the improvement observed at this early stage (mean reduction in global score, 14.1 points) goes far beyond what is considered to constitute a clinically meaningful change (6.7 points). This prompt benefit may provide noteworthy advantages, since effects are generally expected as from week 12 of the treatment [13,37]. The combination of soy isoflavones with a potent phytoestrogen such as 8-PN may account for this early onset of clinical benefit. Furthermore, the effectiveness in HFs and QoL was progressive, showing gradual improvements over time (12 weeks), which supports the relevance of continued treatment. In this regard, not only adherence, but also the effect of the dose in these supplements should be taken into account, as shown by a Cochrane systematic review, which indicates that soy extracts alone, with high levels of genistein, reduced VMS frequency [38].
By way of an important strength, the FLAVIE study analyzed clinical evolution from different perspectives, providing vital information to assist clinicians in the management of menopausal women. The study design followed EMA guidelines [39] for clinical evaluation of VMS-directed strategies in postmenopausal women, including inclusion criteria referring to frequency/intensity of HFs, evaluation outcomes (moderate-to-severe HFs), use of validated scales (FDA, 2003) and intervention time (at least 12 weeks) [40]. The compatibility of the data obtained from the two HRQoL questionnaires employed reinforces the validity of these scales as well as the results of our study.
As a limitation, this is an open-label single-arm study with possible associated biases. The results could be susceptible to the placebo effect reported in some studies on menopausal women [41]. In this regard, some studies found a benefit compared with placebo [11,12,13,14,16,42,43,44,45,46], and others did not [47,48,49].The wide range in the amount of total isoflavones and the other active compounds, dose-schedule, time of follow-up and the severity of symptoms in these studies may explain these differences. Notably, in our study the reduction observed in overall and moderate-to-severe HFs seemed to surpass the estimated placebo effect described in other studies [38], similar to what has been described in the study of Nahas et al. [13]. In this study, Nahas et al. [13] found that isoflavones were significantly superior to placebo in reducing the frequency and severity of HFs (P < 0.001) in postmenopausal women with a symptom profile similar to that reported in our study and a 10-month follow-up period.
Regarding the impact of treatment on the HRQoL, the placebo-controlled study carried out by López-Ríos et al. [45] corroborated our findings and found a significant improvement in the Global HRQoL score (38% vs. 18.8%; P = 0.04) on the Cervantes Scale in subjects treated with a botanical extract containing isoflavones compared to subjects in the placebo group and, specifically, in the menopause and health domain (40.7% vs. 13.6%; P = 0.05). Of note, in line with our results, Basaria et al. [15] reported a significant improvement in all 4 QoL subscales of the MENQoL (vasomotor, psychosexual, physical, and sexual) among the women taking high-dose isoflavones, while no changes were seen in the placebo group.
Moreover, intraindividual comparison regarding the baseline status offers a comprehensive overview of real-life clinical settings. The sociodemographic characteristics of the population selected would likely prevent the data from being extrapolated to the general population. Indeed, the use of a combined formulation prevents to analyze the effects of each component individually. Despite the limited size, however, the positive results are in line with previous literature supporting the effect of phytoestrogen-based supplements as an efficient and safe alternative to MHT for relieving VMS and improving HRQoL. Controlled studies of bigger sample size would further corroborate the validity and generalizability of the results found in our study.
Data from the FLAVIE study showed that administration of a food supplement containing 54.4 mg standarized soy isoflavones (including 24.5 mg genistein and 16.3 mg daidzein), 100 µg 8-PN and 1 mg melatonin for 12 weeks to women suffering from moderateto-severe HFs significantly alleviated this vasomotor symptomatology and improved the overall menopause-related HRQoL, thus providing a prompt and sustained benefit. Together with the positive effect of a decrease in HFs upon the HRQoL of menopausal women, the supplement notably improved other physical and mental HRQoL outcomes, which are crucial for the general well-being of women during menopause. In conclusion, this combination of soy isoflavones, 8-PN and melatonin emerges as a safe, effective and comprehensive alternative to MHT for managing menopause-related symptomatology and overcoming the negative impact thereof on the daily activities of menopausal women.
ACKNOWLEDGEMENTS
We wish to thank Adknoma S.L. for their clinical study support and monitoring, including drafting of the study protocol and performance of the statistical analysis. We are gateful to Eva Garcia-Aguilar, Francisca Pereira and Vanesa Garrido for their support in the clinical management of the study. The authors are grateful to Ana Isabel Ortega and Cormac de Brun who provided medical writing assistance and English editing, respectively.
Footnotes
FUNDING: The study was supported by Italfarmaco Research Pharma SLU.
CONFLICT OF INTEREST: PS-L and CNM are employees of ITalfarmaco Research Pharma SLU. NM-LG receives honoraria or consulting fees from Italfarmaco Research Pharma SLU, Astellas or Shionogi.
The rest of authors have no conflicts of interest to declare.
References
- 1.Rees M, Abernethy K, Bachmann G, Bretz S, Ceausu I, Durmusoglu F, et al. The essential menopause curriculum for healthcare professionals: a European Menopause and Andropause Society (EMAS) position statement. Maturitas. 2022;158:70–77. doi: 10.1016/j.maturitas.2021.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Bansal R, Aggarwal N. Menopausal hot flashes: a concise review. J Midlife Health. 2019;10:6–13. doi: 10.4103/jmh.JMH_7_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caan B, LaCroix AZ, Joffe H, Guthrie KA, Larson JC, Carpenter JS, et al. Effects of estrogen and venlafaxine on menopause-related quality of life in healthy postmenopausal women with hot flashes: a placebo-controlled randomized trial. Menopause. 2015;22:607–615. doi: 10.1097/GME.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delamater L, Santoro N. Management of the perimenopause. Clin Obstet Gynecol. 2018;61:419–432. doi: 10.1097/GRF.0000000000000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenabi E, Shobeiri F, Hazavehei SM, Roshanaei G. Assessment of questionnaire measuring quality of life in menopausal women: a systematic review. Oman Med J. 2015;30:151–156. doi: 10.5001/omj.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolge SC, Balkrishnan R, Kannan H, Seal B, Drake CL. Burden associated with chronic sleep maintenance insomnia characterized by nighttime awakenings among women with menopausal symptoms. Menopause. 2010;17:80–86. doi: 10.1097/gme.0b013e3181b4c286. [DOI] [PubMed] [Google Scholar]
- 7.“The 2022 Hormone Therapy Position Statement of The North American Menopause Society” Advisory Panel. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767–794. doi: 10.1097/GME.0000000000002028. [DOI] [PubMed] [Google Scholar]
- 8.Hill DA, Crider M, Hill SR. Hormone therapy and other treatments for symptoms of menopause. Am Fam Physician. 2016;94:884–889. [PubMed] [Google Scholar]
- 9.Parish SJ, Gillespie JA. The evolving role of oral hormonal therapies and review of conjugated estrogens/bazedoxifene for the management of menopausal symptoms. Postgrad Med. 2017;129:340–351. doi: 10.1080/00325481.2017.1281083. [DOI] [PubMed] [Google Scholar]
- 10.North American Menopause Society. The role of soy isoflavones in menopausal health: report of The North American Menopause Society/Wulf H. Utian Translational Science Symposium in Chicago, IL (October 2010) Menopause. 2011;18:732–753. doi: 10.1097/gme.0b013e31821fc8e0. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Lv Y, Xu L, Zheng Q. Quantitative efficacy of soy isoflavones on menopausal hot flashes. Br J Clin Pharmacol. 2015;79:593–604. doi: 10.1111/bcp.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taku K, Melby MK, Kronenberg F, Kurzer MS, Messina M. Extracted or synthesized soybean isoflavones reduce menopausal hot flash frequency and severity: systematic review and meta-analysis of randomized controlled trials. Menopause. 2012;19:776–790. doi: 10.1097/gme.0b013e3182410159. [DOI] [PubMed] [Google Scholar]
- 13.Nahas EA, Nahas-Neto J, Orsatti FL, Carvalho EP, Oliveira ML, Dias R. Efficacy and safety of a soy isoflavone extract in postmenopausal women: a randomized, double-blind, and placebocontrolled study. Maturitas. 2007;58:249–258. doi: 10.1016/j.maturitas.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Riesco E, Choquette S, Audet M, Tessier D, Dionne IJ. Effect of exercise combined with phytoestrogens on quality of life in postmenopausal women. Climacteric. 2011;14:573–580. doi: 10.3109/13697137.2011.566652. [DOI] [PubMed] [Google Scholar]
- 15.Basaria S, Wisniewski A, Dupree K, Bruno T, Song MY, Yao F, et al. Effect of high-dose isoflavones on cognition, quality of life, androgens, and lipoprotein in post-menopausal women. J Endocrinol Invest. 2009;32:150–155. doi: 10.1007/BF03345705. [DOI] [PubMed] [Google Scholar]
- 16.Heyerick A, Vervarcke S, Depypere H, Bracke M, De Keukeleire D. A first prospective, randomized, double-blind, placebo-controlled study on the use of a standardized hop extract to alleviate menopausal discomforts. Maturitas. 2006;54:164–175. doi: 10.1016/j.maturitas.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Sehmisch S, Hammer F, Christoffel J, Seidlova-Wuttke D, Tezval M, Wuttke W, et al. Comparison of the phytohormones genistein, resveratrol and 8-prenylnaringenin as agents for preventing osteoporosis. Planta Med. 2008;74:794–801. doi: 10.1055/s-2008-1074550. [DOI] [PubMed] [Google Scholar]
- 18.Štulíková K, Karabín M, Nešpor J, Dostálek P. Therapeutic perspectives of 8-prenylnaringenin, a potent phytoestrogen from hops. Molecules. 2018;23:660. doi: 10.3390/molecules23030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Medicines Agency. Assessment report on Humulus lupulus L., flos. Amsterdam: Committee on Herbal Medicinal Products (HMPC); 2014. [cited 2022 May 26]. Available from: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-humulus-lupulus-l-flos-revision-1_en.pdf . [Google Scholar]
- 20.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Estrogen and estrogen/progestin drug products to treat vasomotor symptoms and vulvar and vaginal atrophy symptoms - recommendations for clinical evaluation. Rockville: U.S. Food and Drug Administration; 2003. [cited 2022 May 26]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/estrogen-and-estrogenprogestin-drug-products-treat-vasomotor-symptoms-and-vulvar-and-vaginal-atrophy . [Google Scholar]
- 21.National Institutes of Health (NIH) Assessing and improving measures of hot flashes: summary of an NIH workshop. Bethesda: NIH; 2004. [cited 2022 May 28]. Available from: https://files.nccih.nih.gov/s3fs-public/hotflashessumm.pdf . [Google Scholar]
- 22.Hilditch JR, Lewis J, Peter A, van Maris B, Ross A, Franssen E, et al. A menopause-specific quality of life questionnaire: development and psychometric properties. Maturitas. 1996;24:161–175. doi: 10.1016/s0378-5122(96)82006-8. Erratum in: Maturitas 1996; 25: 231. [DOI] [PubMed] [Google Scholar]
- 23.Castelo-Branco C, Palacios S, Ferrer-Barriendos J, Parrilla JJ, Manubens M, Alberich X, et al. Understanding how personality factors may influence quality of life: development and validation of the Cervantes Personality Scale. Menopause. 2008;15:914–918. doi: 10.1097/gme.0b013e318167b916. [DOI] [PubMed] [Google Scholar]
- 24.Therapeutic Goods Administration. International scientific guideline: ICH topic E 9 - note for guidance on statistical principles for clinical trials. Woden ACT: Commonwealth of Australia; 1999. [cited 2022 Dec 6]. Available from: https://www.tga.gov.au/resources/resource/international-scientific-guidelines/international-scientific-guideline-ich-topic-e-9-note-guidance-statistical-principles-clinical-trials . [Google Scholar]
- 25.D'Anna R, Cannata ML, Atteritano M, Cancellieri F, Corrado F, Baviera G, et al. Effects of the phytoestrogen genistein on hot flushes, endometrium, and vaginal epithelium in postmenopausal women: a 1-year randomized, double-blind, placebo-controlled study. Menopause. 2007;14:648–655. doi: 10.1097/01.gme.0000248708.60698.98. [DOI] [PubMed] [Google Scholar]
- 26.Coronado PJ, Monroy M, Fasero M, Baquedano L, Mendoza N, Llaneza P, et al. Predictive and criterion validity of the Cervantes-SF menopause quality of life questionnaire. Menopause. 2021;28:935–942. doi: 10.1097/GME.0000000000001790. [DOI] [PubMed] [Google Scholar]
- 27.Whiteley J, DiBonaventura Md, Wagner JS, Alvir J, Shah S. The impact of menopausal symptoms on quality of life, productivity, and economic outcomes. J Womens Health (Larchmt) 2013;22:983–990. doi: 10.1089/jwh.2012.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coronado PJ, Monroy M, Fasero M, Sánchez-Borrego R, Palacios S, Rejas J, et al. Population-based norms for the Cervantes-SF short-form questionnaire assessing health-related quality of life in menopause. Maturitas. 2021;146:34–41. doi: 10.1016/j.maturitas.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Utian WH, Woods NF. Impact of hormone therapy on quality of life after menopause. Menopause. 2013;20:1098–1105. doi: 10.1097/GME.0b013e318298debe. [DOI] [PubMed] [Google Scholar]
- 30.Coronado PJ, Borrego RS, Palacios S, Ruiz MA, Rejas J. Structural validity of a 16-item abridged version of the Cervantes Health-Related Quality of Life scale for menopause: the Cervantes Short-Form Scale. Menopause. 2015;22:325–336. doi: 10.1097/GME.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 31.Savolainen-Peltonen H, Hautamäki H, Tuomikoski P, Ylikorkala O, Mikkola TS. Health-related quality of life in women with or without hot flashes: a randomized placebo-controlled trial with hormone therapy. Menopause. 2014;21:732–739. doi: 10.1097/GME.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 32.Toffol E, Kalleinen N, Haukka J, Vakkuri O, Partonen T, Polo-Kantola P. Melatonin in perimenopausal and postmenopausal women: associations with mood, sleep, climacteric symptoms, and quality of life. Menopause. 2014;21:493–500. doi: 10.1097/GME.0b013e3182a6c8f3. [DOI] [PubMed] [Google Scholar]
- 33.Benkherouf AY, Eerola K, Soini SL, Uusi-Oukari M. Humulone modulation of GABAA receptors and its role in hops sleep-promoting activity. Front Neurosci. 2020;14:594708. doi: 10.3389/fnins.2020.594708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shahmohammadi A, Ramezanpour N, Mahdavi Siuki M, Dizavandi F, Ghazanfarpour M, Rahmani Y, et al. The efficacy of herbal medicines on anxiety and depression in peri- and postmenopausal women: a systematic review and meta-analysis. Post Reprod Health. 2019;25:131–141. doi: 10.1177/2053369119841166. [DOI] [PubMed] [Google Scholar]
- 35.Amato P, Young RL, Steinberg FM, Murray MJ, Lewis RD, Cramer MA, et al. Effect of soy isoflavone supplementation on menopausal quality of life. Menopause. 2013;20:443–447. doi: 10.1097/gme.0b013e318275025e. [DOI] [PubMed] [Google Scholar]
- 36.Gómez-Zorita S, González-Arceo M, Fernández-Quintela A, Eseberri I, Trepiana J, Portillo MP. Scientific evidence supporting the beneficial effects of isoflavones on human health. Nutrients. 2020;12:3853. doi: 10.3390/nu12123853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evans M, Elliott JG, Sharma P, Berman R, Guthrie N. The effect of synthetic genistein on menopause symptom management in healthy postmenopausal women: a multi-center, randomized, placebo-controlled study. Maturitas. 2011;68:189–196. doi: 10.1016/j.maturitas.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Lethaby A, Marjoribanks J, Kronenberg F, Roberts H, Eden J, Brown J. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev. 2013;2013:CD001395. doi: 10.1002/14651858.CD001395.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.European Medicines Agency. Guideline on clinical investigation of medicinal products for Hormone replacement therapy of oestrogen deficiency symptoms in Post-menopausal women. London: European Medicines Agency; 2005. [cited 2022 Dec 5]. Available from: https://www.ema.europa.eu/en/clinical-investigation-medicinal-products-hormone-replacement-therapy-oestrogen-deficiency-symptoms . [Google Scholar]
- 40.Cuadros JL, Fernández-Alonso AM, Cuadros-Celorrio AM, Fernández-Luzón N, Guadix-Peinado MJ, del Cid-Martín N, et al. Perceived stress, insomnia and related factors in women around the menopause. Maturitas. 2012;72:367–372. doi: 10.1016/j.maturitas.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Newton KM, Carpenter JS, Guthrie KA, Anderson GL, Caan B, Cohen LS, et al. Methods for the design of vasomotor symptom trials: the menopausal strategies: finding lasting answers to symptoms and health network. Menopause. 2014;21:45–58. doi: 10.1097/GME.0b013e31829337a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen MN, Lin CC, Liu CF. Efficacy of phytoestrogens for menopausal symptoms: a meta-analysis and systematic review. Climacteric. 2015;18:260–269. doi: 10.3109/13697137.2014.966241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen LR, Ko NY, Chen KH. Isoflavone supplements for menopausal women: a systematic review. Nutrients. 2019;11:2649. doi: 10.3390/nu11112649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas AJ, Ismail R, Taylor-Swanson L, Cray L, Schnall JG, Mitchell ES, et al. Effects of isoflavones and amino acid therapies for hot flashes and co-occurring symptoms during the menopausal transition and early postmenopause: a systematic review. Maturitas. 2014;78:263–276. doi: 10.1016/j.maturitas.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.López-Ríos L, Barber MA, Wiebe J, Machín RP, Vega-Morales T, Chirino R. Influence of a new botanical combination on quality of life in menopausal Spanish women: results of a randomized, placebo-controlled pilot study. PLoS One. 2021;16:e0255015. doi: 10.1371/journal.pone.0255015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rattanatantikul T, Maiprasert M, Sugkraroek P, Bumrungpert A. Efficacy and safety of nutraceutical on menopausal symptoms in post-menopausal women: a randomized, double-blind, placebo-controlled clinical trial. J Diet Suppl. 2022;19:168–183. doi: 10.1080/19390211.2020.1853648. [DOI] [PubMed] [Google Scholar]
- 47.Knight DC, Howes JB, Eden JA, Howes LG. Effects on menopausal symptoms and acceptability of isoflavone-containing soy powder dietary supplementation. Climacteric. 2001;4:13–18. [PubMed] [Google Scholar]
- 48.Baber RJ, Templeman C, Morton T, Kelly GE, West L. Randomized placebo-controlled trial of an isoflavone supplement and menopausal symptoms in women. Climacteric. 1999;2:85–92. doi: 10.3109/13697139909025571. [DOI] [PubMed] [Google Scholar]
- 49.Kang I, Rim CH, Yang HS, Choe JS, Kim JY, Lee M. Effect of isoflavone supplementation on menopausal symptoms: a systematic review and meta-analysis of randomized controlled trials. Nutr Res Pract. 2022;16(Suppl 1):S147–S159. doi: 10.4162/nrp.2022.16.S1.S147. [DOI] [PMC free article] [PubMed] [Google Scholar]