Abstract
Introduction
Patients with germline variants in CDH1 who undergo prophylactic total gastrectomy (TG) are at risk of altered nutrient and drug absorption due to modified gastrointestinal anatomy. Bone mineral density loss and micronutrient deficiencies have not been described previously in this patient population.
Methods
In this study we included 94 patients with germline CDH1 variants who underwent prophylactic TG between October 2017 and February 2022. We examined pre- and post-gastrectomy bone mineral density (BMD); serum biomarkers including calcium, phosphorus, alkaline phosphatase, and 25 (OH)-vitamin D; and postoperative adherence to calcium and multivitamin supplementation.
Results
Almost all patients (92/94, 98%) lost a substantial amount of weight post-TG, with an average weight loss of 26.5% at 12 months post-surgery. Serum biomarkers of mineral metabolism, namely calcium and phosphorus, did not change significantly after TG. However, average BMD was decreased in all patients at 12 months post-TG. Nonadherence to calcium supplementation was associated with a decrease in BMD. Nonadherence to multivitamin supplementation was associated with greater percent BMD loss in the femoral neck and total hip.
Conclusions
Appropriate micronutrient supplementation and nutritional counseling pre- and postoperatively in patients undergoing prophylactic TG are important to mitigate the long-term effects of gastrectomy on bone health.
Keywords: bone health, prophylactic total gastrectomy, micronutrient supplementation
Prophylactic total gastrectomy (TG) is a major life-altering operation recommended to individuals with germline variants in the CDH1 tumor suppressor gene (1). The rationale for complete removal of the stomach is the elevated lifetime gastric cancer risk of 37% to 42% among men and 25% to 33% among women (2, 3). TG reconfigures normal gastrointestinal anatomy, leading to altered nutrient and drug absorption (4, 5). TG is associated with micronutrient deficiencies, unintentional weight loss, and dumping syndrome and can also increase the likelihood of nephrolithiasis, cholelithiasis, and osteopenia/osteoporosis (6).
Micronutrient deficiencies, including calcium and vitamin D, are well documented following Roux-en-Y gastric bypass (RYGB) for obesity (7) and gastrectomy for gastric cancer (8). Calcium and vitamin D malabsorption after TG is of particular concern due to the potential loss of bone mineral density (BMD), which can lead to osteopenia, osteoporosis, and fractures (9). BMD is also known to predict risk of fractures (10). Effects of bone density following RYGB and gastrectomy for treatment of gastric cancer are chronic and can develop months to years after gastrectomy (7, 8). These effects are multifactorial, related to malnutrition secondary to gastric cancer, decreased oral intake, and impaired absorption (8, 9, 11-13).
The effects of micronutrient malabsorption on bone health have not been described among individuals undergoing prophylactic TG, most of whom are healthy adults at baseline. Bone health in this population is critical because these individuals have a close to normal life expectancy. In this study, we sought to describe the effects of prophylactic TG on serum biomarkers of mineral metabolism and BMD. Furthermore, we aimed to examine the effects of adherence to micronutrient supplementation on serum biomarkers of mineral metabolism and BMD after TG.
Methods
Study Population
Patients enrolled in a natural history study of hereditary gastric cancers (clinicaltrials.gov, NCT03030404) at the National Institutes of Health Clinical Center were analyzed. All patients were carriers of germline CDH1 pathogenic or likely pathogenic variants, which were confirmed at study enrollment. A retrospective analysis was performed using data collected between October 2017 and February 2022. All patients underwent TG in the same manner by the same surgeon and received postoperative follow-up care at our institution. Patients included in the analysis received a baseline bone densitometry scan [dual-energy x-ray absorptiometry (DXA] immediately prior to TG and at 1 and 2 years postoperatively. All patients received counseling on diet modification, vitamin supplementation, and medication adherence prior to surgery and during routine follow-up with the clinical care team. Patients with insufficient follow-up were excluded from analysis. Patients who received DXA scans at an outside institution were excluded from analysis due to lack of formal cross-calibration between individual DXA machines.
Clinical variables for analysis included age, sex, race, height, baseline weight, body mass index (BMI), and past medical history, which included heart disease, hyperlipidemia, hypertension, diabetes mellitus, lung disease, thyroid disease, anxiety/depression, osteopenia/osteoporosis, and preoperative vitamin supplementation. Heart disease encompassed cardiomyopathy, coronary artery disease, and atrial fibrillation. Lung disease included asthma and chronic obstructive pulmonary disease.
Laboratory Measurements
Peripheral blood evaluations included serum calcium (mmol/L), serum phosphorus (mg/dL), alkaline phosphatase (U/L), and 25 (OH)-vitamin D (ng/mL), which were measured at baseline and at 3, 6, 12, and 24 months after TG. 25 (OH)-vitamin D levels were analyzed via the chemiluminescent immunoassay technology using the Diasorin LIAISON® XL Analyzer (DiaSorin S.p.A. Saluggia, VC, Italy). Total vitamin D reference ranges were based on institutional ranges of <10 ng/mL (severe deficiency), 10-32 ng/mL (mild to moderate deficiency), 33-100 ng/mL (optimum levels), and >100 ng/mL (toxicity possible) (14). Serum calcium and phosphorus levels were analyzed using a clinical chemistry analyzer, Architect c8000 (Abbott, Abbott Park, IL, USA). Alkaline phosphatase was analyzed using the Para-nitrophenyl Phosphate assay (New England BioLabs, Inc., Ipswich, MA, USA).
Measurement of BMD
BMD (g/cm3) was measured at baseline, 12 months, and 24 months after TG and included measurements of the lumbar spine (L1-L4), left femoral neck, and total hip by DXA [Dephi W, Hologic Inc., Bedford, MA, USA (vs using Hologic QDR4500SL bone densitometer, Hologic, Waltham, MA, USA)]. The in vivo coefficient of variation was 1.0% for the lumbar spine and 1.65% for the femoral neck.
Supplementation Information and Adherence Classification
All patients received uniform nutrition counseling by a registered dietitian prior to operation, during inpatient admission, and postoperatively at 3-, 6-, 12-, and 24-month evaluations. All patients were educated on the postgastrectomy diet and need for micronutrient supplementation (Table 1). Calcium citrate supplementation and ProCare multivitamin (MVI) capsule or chewable form (ProCare Health, Lake St. Louis, MO, USA, Supplemental Fig. 1) (15) adherence was recorded at each clinical evaluation time point. Adherence to calcium citrate supplementation was defined as an average of 1200 to 1500 mg/day in divided doses. Calcium citrate was the treatment of choice for calcium supplementation as it is a soluble calcium salt and is likely to be better absorbed than insoluble calcium carbonate in the setting of reduced gastric acidity from TG. Adherence to MVI supplementation was defined as intake of the MVI at least 6 days per week. Any deviation from these definitions was considered nonadherence.
Table 1.
Post-gastrectomy diet education
| Must educate about post-gastrectomy diet modifications to promote diet tolerance and adequate absorption |
| Must discuss need for lifelong micronutrient supplementation with a bariatric-formulated multivitamin and calcium citrate, including instructions on American Society for Metabolic & Bariatric Surgery guidelines for dosing of 1200-1500 mg oral calcium citrate daily in divided doses at least 2 hours apart from iron-containing supplements |
| Must discuss the risks related to decreased bone mineral density after total gastrectomy |
| Must discuss the benefits of weight-bearing and weight-resistance exercise |
| Must discuss risks related to unintentional weight loss |
Statistical Analysis
Statistical analysis was performed using SPSS Version 25 (IBM, Armonk, NY, USA) and GraphPad Prism Version 9.3.1. All categorical and continuous variables between adherence groups were compared using the Square test, independent (or paired), pooled t-test, Wilcoxon signed rank test, and 2-way analyses of variance as appropriate. A linear regression model was used to evaluate for an association between weight loss and bone density loss. Variables with a P-value <.05 were considered statistically significant. Biochemical markers and BMD data were presented as a mean with SD.
Results
A total of 94 patients with germline CDH1 variants underwent TG over a 3-year period and were included in this analysis. All patients underwent prophylactic TG, thus no patient received perioperative chemotherapy. Each patient was followed for a minimum of 2 years postgastrectomy. The median age at time of TG was 41 years (range 20-71). Most patients were White (96%, n = 91) and female (74%, n = 70). The median (baseline) BMI was 29 (range 18-51), with the majority of patients (78%, n = 73) classified as overweight (BMI 25-29.9 kg/m2) or obese (BMI >30 kg/m2). Five patients (5%) met criteria for osteoporosis at baseline with a median age of 55 years (range 23-67), and 30% (n = 29) reported taking vitamin supplements prior to operation (Table 2). Serum biochemical markers of mineral metabolism were within normal ranges prior to TG in the majority of patients. Patients exhibited normal serum calcium (93%, n = 88), serum phosphorus (93%, n = 88), and serum alkaline phosphatase (97%, n = 92) in our study. Thirty-six (39%) of 92 patients had normal levels of serum 25 (OH)-vitamin D (normal 33-100 ng/mL) prior to TG.
Table 2.
Baseline characteristics of CDH1 variant carriers who underwent prophylactic total gastrectomy
| Variable | Overall (n = 94) |
|---|---|
| Age, median (range), y | 41 (20-71) |
| Sex, female, n (%) | 70 (74.5) |
| Race, white, n (%) | 91 (96.8) |
| Height, median (range), cm | 170 (149-194.9) |
| Body weight, median (range), kg | 86 (50.1-141.9) |
| Body mass index, median (range), kg/m2 | 29 (18.4-51.4) |
| Comorbidities, n (%) | 54 (57.4) |
| Heart disease, n (%) | 8 (8.5) |
| Hyperlipidemia, n(%) | 18 (19.1) |
| Hypertension, n (%) | 20 (21.3) |
| Diabetes, n (%) | 7 (7.4) |
| Lung disease, n (%) | 7 (7.4) |
| Thyroid disease, n (%) | 14 (14.9) |
| Anxiety/depression, n (%) | 28 (29.8) |
| Osteoporosis, n (%) | 5 (5.3) |
| Pre-op vitamin supplementation, n (%) | 29 (30.8) |
Postgastrectomy Weight Loss
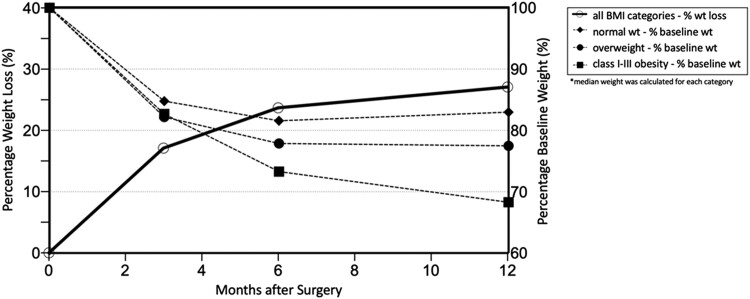
Nearly all patients (92 of 94, 98%) lost weight following gastrectomy, with the most rapid weight loss occurring within the first 3 months following surgery (Fig. 1). Of the 92 patients who lost weight, the average total percent weight loss was 17.3% at 3 months (range 7.3-29%), 23.2% (2.0-35.8%) at 6 months, and 26.5% (0-45.3%) at 12 months post-TG. Obese patients lost the most weight after TG at 1 year (33% average percent weight loss) compared to overweight and normal weight patients (22% and 15% average percent weight loss at 1 year, respectively).
Figure 1.
Percent weight loss and percent baseline weight following prophylactic total gastrectomy.
Bone Density Changes After Total Gastrectomy
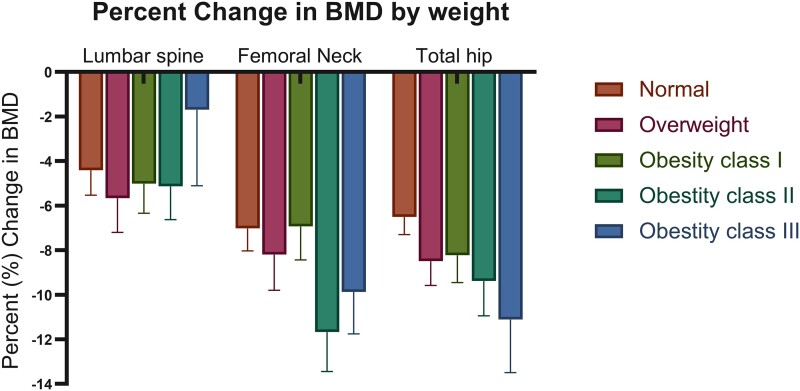
Seventy-one of 94 patients (76%) had baseline and post-TG DXA scan data available for analysis. A decrease in average BMD and T-score was observed in all patients at 12 months post-TG (P < .001). At 12 months post-TG, 31 patients (44%) met criteria for osteopenia and 12 patients (17%) met criteria for osteoporosis based on T-score. There was no significant difference in percent BMD change from baseline to 1-year post-TG when stratified by baseline weight class (Fig. 2). There was a positive correlation between percent average BMD loss and average percent weight loss between baseline and 12-months post-TG. Furthermore, patients who lost more weight also lost more BMD (B = 0.122, P = .008, R2 = 0.97).
Figure 2.
Percent change in bone mineral density from baseline to 12 months post-total gastrectomy by baseline patient weight class. Number of patients varied in each weight class: patients with normal BMI (18.5-24.9 kg/m2) n = 17, overweight BMI (25-29.9 kg/m2) n = 22, obesity class I (30-34.9 kg/m2) n = 17, obesity class II (35-39.9 kg/m2), n = 11 and obesity class III (≥40 kg/m2) n = 6. BMD values are calculated as mean ± SE. Abbreviations: BMD, bone mineral density. BMI, body mass index.
Bone density data were available in 55 of 94 patients (59%) at 24 months after TG. We compared BMD and T-score results between baseline to 24 months and between 12 months to 24 months post-TG. Overall, the rate of decrease in BMD was lower after 12 months post-TG. The median percent decrease in BMD between baseline to 24 months post-TG was −8.4% (range −37.5 to −1.59%) among all sites (lumbar spine, femoral neck, and total hip). We found a −7.6% (range −18.4 to −3.07%) decrease in BMD from baseline to 12 months post-TG and −0.3% (−30.4 to −7.47%) from 12 months to 24 months post-TG.
Serum Biomarkers and Micronutrient Supplement Adherence
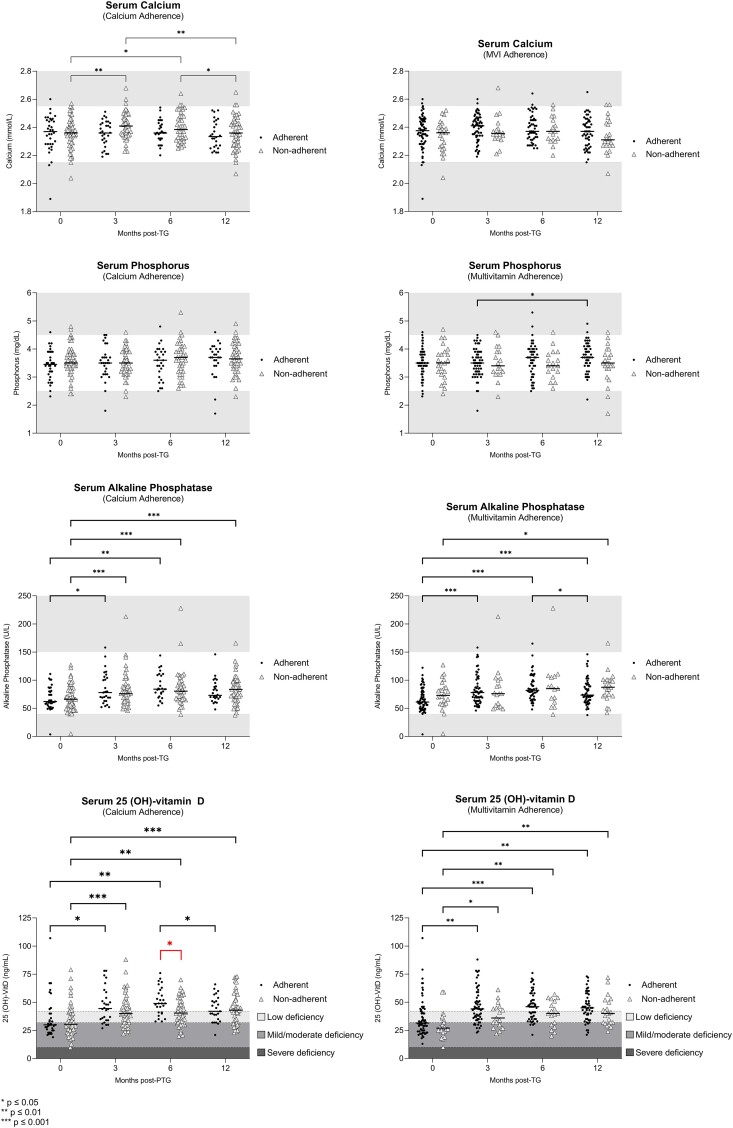
In order to better understand the impact of TG on mineral metabolism, available serum biochemical markers were compared at baseline to 12 months post-TG. We found that calcium and phosphorus levels demonstrated no significant change (P = .978 and .145, respectively) from baseline to 12 months post-TG, whereas alkaline phosphatase and 25 (OH)-vitamin D both increased (P < .001 for both) (Table 3). Next, we assessed changes in serum markers at baseline, 3, 6, and 12 months post-TG (Fig. 3). Serum calcium levels increased from baseline to 3 and 6 months post-TG, then decreased at 12 months in the calcium-nonadherent group. Within the MVI-adherent group, serum phosphorus levels increased from 3 to 12 months. Serum alkaline phosphatase levels also increased in both MVI- and calcium-adherent groups from baseline to 3 and 6 months. However, alkaline phosphatase levels also increased in the calcium-nonadherent group from baseline to 3, 6, and 12 months. In the calcium- and MVI-adherent and nonadherent groups, serum vitamin D levels increased from baseline to 3 and 6 months post-TG (Fig. 3). There were no significant changes between serum calcium levels in MVI-adherent groups and serum phosphorus levels in calcium-adherent groups between baseline, 3, 6, and 12 months post-TG.
Table 3.
Markers of mineral metabolism and bone mineral density findings at baseline, 12 months, and 24 months post-prophylactic gastrectomy
| Baseline | 12-months post-TG | P-valuea | 24-months post-TG | P-valueb | P-valuec | |
|---|---|---|---|---|---|---|
| Serum calcium, mean ± SD, mmol/L | 2.35 ± 0.12 | 2.36 ± 0.11 | .978 | — | — | |
| Serum phosphorus, mean ± SD, mg/dL | 3.52 ± 0.53 | 3.64 ± 0.57 | .145 | — | — | |
| Serum alkaline phosphatase, ± SD, U/L | 68.06 ± 22.0 | 82.3 ± 23.94 | <.001 | — | — | |
| Serum 25 (OH)-vitamin D, mean ± SD, ng/mL | 34.0 ± 15.54 | 44.22 ± 12.75 | <.001 | — | — | |
| Lumbar spine BMD, mean ± SD, g/cm2 | 1.02 ± 0.14 | 0.98 ± 0.16 | <.001 | 0.99 ± 0.15 | .83 | <.001 |
| Femoral neck BMD, mean ± SD, g/cm2 | 0.83 ± 0.14 | 0.77 ± 0.12 | <.001 | 0.75 ± 0.12 | .002 | <.001 |
| Total hip BMD, mean ± SD, g/cm2 | 0.98 ± 0.17 | 0.88 ± 0.12 | <.001 | 0.85 ± 0.16 | .11 | <.001 |
| Lumbar spine T-score, mean ± SD | −0.31 ± 1.29 | −0.84 ± 1.42 | <.001 | −0.62 ± 1.37 | .50 | <.001 |
| Femoral neck T-score, mean ± SD | −0.31 ± 1.15 | −0.90 ± 1.03 | <.001 | −1.05 ± 1.04 | .02 | <.001 |
| Total hip T-score, mean ± SD | 0.04 ± 1.06 | −0.60 ± 0.99 | <.001 | −0.74 ± 0.97 | .008 | <.001 |
Abbreviations: BMD, bone mineral density; TG, total gastrectomy.
paired t-test between baseline and 12 months post-TG.
paired t-test between 12 months and 24 months post-TG.
paired t-test between baseline and 24 months post-TG.
Figure 3.
Micronutrient serum levels for each patient at baseline, 3, 6, and 12 months following total gastrectomy according to supplementation adherence. Horizontal bars indicate median values. Grey areas represent values outside of normal range. Number of patients varied by serum biomarker and time point due to incomplete data. For serum calcium levels: patient samples were n = 92 at baseline, n = 79 at 3 months, n = 68 at 6 months, and n = 73 at 12 months. For serum phosphorus levels: patient samples were n = 92 at baseline, n = 79 at 3 months, n = 67 at 6 months, and n = 73 at 12 months. For serum alkaline phosphatase levels: patient samples were n = 92 at baseline, n = 77 at 3 months, n = 65 at 6 months, and n = 73 at 12 months. For serum 25 (OH)-vitamin D levels: patient samples were n = 92 at baseline, n = 78 at 3 months, n = 70 at 6 months, and n = 72 at 12 months.
Interestingly, patient adherence to calcium supplementation at 1 year post-TG was 40% (n = 36) and to MVI use was 73% (n = 69). After 2 years, only 33% (n = 23) of patients were consistently adherent to calcium supplementation and 70% (n = 47) to MVI supplementation. At 6 months post-TG, serum vitamin D levels were significant higher in the calcium-adherent compared to nonadherent group (P = .01) (Fig. 3). There were no significant differences in serum calcium, phosphorus, and alkaline phosphatase between adherent and nonadherent groups (Fig. 3). Moreover, there was no difference in mean BMD and T-score between the adherent and nonadherent groups at 12 months post-TG (Table 4). Patients deemed adherent and nonadherent were not significantly different according to demographics, height, baseline weight and BMI, comorbidities, baseline serum markers, or baseline BMD measurements. While higher cost of calcium citrate may have contributed to the observed nonadherence in this cohort, we did not investigate cost as a potential factor for nonadherence to prescribed supplementation.
Table 4.
Markers of mineral metabolism and bone mineral density findings 12-month post-prophylactic gastrectomy findings in relation to calcium and multivitamin adherence
| Calcium adherence 12 months post-TG | Multivitamin adherence 12 months post-TG | |||||
|---|---|---|---|---|---|---|
| Adherent (n = 36) | Nonadherent (n = 58) | P-value | Adherent (n = 69) | Non-adherent (n = 25) | P-value | |
| Body weight, median (range), kg | 65 (22.5-97.8) | 60 (41.2-106.5) | .412 | 61 (22.5-106.5) | 61 (43.4-89.9) | .971 |
| Body mass index, median (range), kg/m2 | 22 (7.5-33.1) | 21 (15.8-31.2) | .528 | 21 (7.5-33.1) | 22 (17.0-28.4) | .515 |
| Serum calcium, mean ± SD, mmol/L | 2.3 ± 0.11 | 2.3 ± 0.11 | .415 | 2.3 ± 0.10 | 2.3 ± 0.14 | .784 |
| Serum phosphorus, mean ± SD, mg/dL | 3.6 ± 0.59 | 3.6 ± 0.57 | .835 | 3.6 ± 0.58 | 3.60 ± 0.55 | .703 |
| Serum alkaline phosphatase, ± SD, U/L | 84.5 ± 23.7 | 80.9 ± 24.2 | .534 | 83.6 ± 25.06 | 79.0 ± 21.1 | .459 |
| Serum 25 (OH)-vitamin D, mean ± SD, ng/mL | 45.8 ± 13.3 | 43.2 ± 12.4 | .405 | 44.3 ± 13.28 | 43.9 ± 11.6 | .909 |
| Lumbar spine BMD, mean ± SD, g/cm2 | 1.0 ± 0.17 | 0.96 ± 0.14 | .218 | 0.97 ± 0.15 | 0.98 ± 0.16 | .912 |
| Femoral neck BMD, mean ± SD, g/cm2 | 0.75 ± 0.13 | 0.77 ± 0.11 | .471 | 0.76 ± 0.12 | 0.76 ± 0.11 | .317 |
| Total hip BMD, mean ± SD, g/cm2 | 0.87 ± 0.14 | 0.89 ± 0.11 | .435 | 0.88 ± 0.12 | 0.90 ± 0.12 | .479 |
| Lumbar spine t-score, mean ± SD | −0.40 ± 1.30 | −0.76 ± 1.42 | .340 | −0.51 ± 1.20 | −1.04 ± 1.94 | .262 |
| Femoral neck t-score, mean ± SD | −0.91 ± 1.08 | −1.14 ± 1.02 | .433 | −0.97 ± 0.96 | −1.35 ± 1.32 | .275 |
| Total hip t-score, mean ± SD | −0.60 ± 0.99 | −0.83 ± 0.96 | .385 | −0.67 ± 0.92 | −1.02 ± 1.17 | .287 |
Abbreviations: BMD, bone mineral density; TG, total gastrectomy.
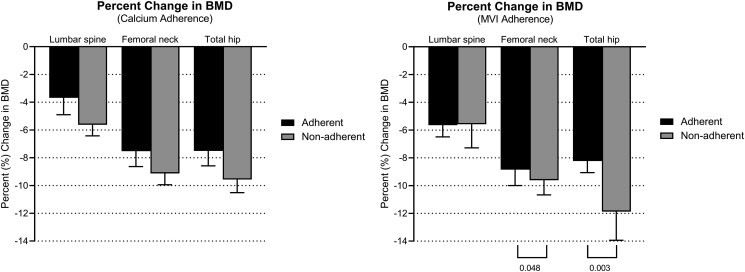
Overall, percentage change in BMD was greater in patients who were nonadherent (Fig. 4). Patients deemed nonadherent with MVI supplementation had greater percent BMD loss in femoral neck (mean Δ9.7%) and total hip (mean Δ11.9%) than adherent patients (femoral neck mean Δ8.4%, total hip mean Δ8.1%) (P = .048, 95% CI, 0.19-5.6 and P = .003, 95% CI, 1.57-7.59, respectively) (Fig. 4). Sixteen patients had ≥10% decrease in BMD in 2 or more measurements, and the majority of these patients (75%, 12/16) were not adherent to calcium supplementation, 43% (7/16) were not adherent to MVI supplementation, and 31% (5/16) were not adherent to either.
Figure 4.
Percent changes in bone mineral density from baseline to 12 months post-total gastrectomy (n = 71). BMD values are calculated as mean ± SE. Abbreviations: BMD, bone mineral density.
In our study, we included 5 patients who had osteoporosis prior to prophylactic TG. All 5 were adherent to MVI supplementation postoperatively, and 2 were adherent to calcium supplementation. Those who were adherent with both calcium and MVI supplements had <7% decrease in BMD compared to those who were only MVI adherent and had a 10-16% decrease in BMD.
Discussion
TG results in unintentional weight loss due to the restrictive and malabsorptive nature of the operation to remove the entire stomach and restore gastrointestinal continuity. In this study, we demonstrated that patients who lost more weight also lost more BMD. Furthermore, nonadherence to calcium supplementation was associated with increased BMD loss. We demonstrated that although serum calcium and phosphorus levels were stable in most patients following TG, BMD decreased in almost all patients at 1-year postgastrectomy.
Although there was no association between serum biomarkers of mineral metabolism and BMD in the postoperative period, nonadherence to MVI supplementation was associated with greater percent BMD loss in the femoral neck and total hip. Additionally, nonadherence with calcium supplementation was associated with a >10% loss in BMD in 16 patients. Two of the 94 patients reported fractures after falls at 1 year and 2 years post-TG, respectively. Furthermore, these data have important implications in the management of this young and healthy group of patients undergoing prophylactic TG who normally would not be considered for bone density screening. Micronutrient malabsorption after TG can be exacerbated by the decrease in caloric intake due to early satiety (lack of reservoir) and loss of hunger cues. Furthermore, this emphasizes the need for (1) appropriate preoperative and postoperative nutrition counseling regarding the necessary micronutrient supplementation after TG to minimize bone density loss and (2) establishing guidelines to intervene if or when patients show signs of declining BMD.
Readily available serum biomarkers of mineral metabolism, namely calcium and phosphorus, did not change in patients after prophylactic TG. These results are consistent with existing data in patients post-RYGB and sleeve gastrectomy and may be explained by compensatory bone resorption by parathyroid hormone (PTH) (11). Mean serum 25(OH)-vitamin D level was 34 ± 15.5 ng/mL at baseline and 44.2 ± 12.8 ng/mL at 12 months post-TG in our patient cohort, which appears to be optimal based on conventional practice but likely reflects the need for higher target levels following gastrectomy due to altered gastrointestinal anatomy and consequent malabsorption. While serum PTH levels on these patients are not available, increased alkaline phosphatase noted in these patients can suggest increased bone turnover (limited by lack of data regarding liver vs bone isoenzyme fractions).
We demonstrate that BMD decreases significantly at 1 year after TG. These findings are consistent with data in bariatric surgery and patients undergoing RYGB (11) and partial gastrectomy for gastric cancer (16). Patients who were nonadherent to their prescribed supplementation had a greater reduction in BMD. These findings are particularly important in the prophylactic TG population given their relatively young age and long life expectancy. Our data emphasizes the importance of calcium and MVI supplementation, nutrition counseling, and standardized postoperative nutritional support and long-term follow-up.
In this study, the majority of patients were adherent to a daily MVI while fewer than half were able to adhere to the rigorous calcium supplementation schedule. Although calcium and MVI supplementation did not significantly impact most serum biochemical markers, average BMD, or T-score at 12 months post-TG, there was a correlation between adherence and percent change BMD. This underscores the importance of calcium and MVI supplementation and the need for standardization of postoperative supplement recommendations as well as the necessity for nutritional education and support following gastrectomy.
Our study is strengthened by its size in an understudied population of patients with germline CDH1 variants undergoing TG. All patients received standardized long-term follow-up and formalized nutritional support. However, limitations of this study include lack of availability of serum bone turnover markers including PTH, bone-specific alkaline phosphatase, C-telopeptide, and amino terminal of type 1 pro-collagen. Lack of patient diversity and clinical practices may also not be representative of other clinical programs Most patients in this analysis were female, which could bias the findings. Additionally, while we accounted for calcium citrate intake, we did not assess for dietary intake of calcium. However, this likely would not have made much of a difference because of the known suboptimal absorption due to both the lack of gastric acid necessary for initial metabolism and bypass of the duodenum, which is the predominant site of active calcium absorption (11, 17). Other factors that can potentially contribute to BMD loss such as change in body composition, hormonal expressions (sex steroids, insulin-like growth factor 1, gut-secreted hormones), gut microbiome, and mechanical unloading was not investigated in this study. Our findings may also reflect selection bias as we excluded patients lost to follow-up, suggesting that the real-world experience may demonstrate a higher degree or frequency of BMD loss.
Conclusions
The intent of prophylactic TG is to reduce gastric cancer risk; however, it also results in significant weight loss and micronutrient malabsorption that adversely impacts bone health. Our findings indicate that although all patients experience a loss of BMD in the first year after TG, the magnitude of BMD loss is higher in patients who are nonadherent with their prescribed supplementation regimen. These findings highlight the critical importance of pre- and postoperative nutrition counseling on dietary supplementation and long-term health risks after TG. These findings also emphasize the essential role of preventative strategies to minimize loss of BMD and the subsequent adverse consequences related to osteopenia and osteoporosis. Furthermore, it is paramount to screen post-TG patients for bone density loss, to monitor micronutrient levels, and to provide dedicated nutrition counseling by an experienced registered dietitian as part of the multidisciplinary team.
Acknowledgments
The authors wish to thank the patient volunteers enrolled in our study of hereditary gastric cancer syndromes. We also acknowledge Karen Chelcun Schreiber for her efforts as a patient advocate and for her fervent support of gastric cancer research.
Contributor Information
Lauren A Gamble, Surgical Oncology Program, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA.
Rachael Lopez, Clinical Center Nutrition Department, National Institutes of Health, Bethesda, MD 20892, USA; US Public Health Service, Washington, DC 20245, USA.
Suraj Rajasimhan, Pharmacy Department, National Institutes of Health Clinical Center, Bethesda, MD 20892, USA.
Sarah G Samaranayake, Surgical Oncology Program, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA.
Cassidy Bowden, Surgical Oncology Program, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA.
Amber L Famiglietti, Surgical Oncology Program, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA.
Andrew M Blakely, Surgical Oncology Program, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA.
Smita Jha, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health Clinical Center, Bethesda, MD 20892, USA.
Mark A Ahlman, Radiology and Imaging Sciences, National Institutes of Health Clinical Center, Bethesda, MD 20892, USA.
Jeremy L Davis, Surgical Oncology Program, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA.
Funding
This study was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute and National Institute of Diabetes and Digestive and Kidney Diseases.
Clinical Trial Registration
Registration number: NCT03030404 (clinicaltrials.gov).
Author Contributions
All authors contributed substantially to the production of this manuscript.
Disclosures
R.L. is a Commissioned Officer in the US Public Health Service. The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the Pubic Health Service, Uniformed Service University of the Health Sciences, or the US Department of Defense. The authors each can claim no conflicts of interest.
Data Availability
Data generated in this study are available upon reasonable request to the corresponding author. Unlinked genomic data will be deposited in public genomic databases such dbGap in compliance with the National Institutes of Health Genomic Data Sharing Policy.
References
- 1. Blair VR, McLeod M, Carneiro F, et al. Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol. 2020;21(8):e386‐e397. Doi: 10.1016/S1470-2045(20)30219-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xicola RM, Li S, Rodriguez N, et al. Clinical features and cancer risk in families with pathogenic CDH1 variants irrespective of clinical criteria. J Med Genet. 2019;56(12):838‐843. Doi: 10.1136/jmedgenet-2019-105991 [DOI] [PubMed] [Google Scholar]
- 3. Roberts ME, Ranola JMO, Marshall ML, et al. Comparison of CDH1 penetrance estimates in clinically ascertained families vs families ascertained for multiple Gastric cancers. JAMA Oncol. 2019;5(9):1325‐1331. Doi: 10.1001/jamaoncol.2019.1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haverkamp L, Weijs TJ, van der Sluis PC, van der Tweel I, Ruurda JP, van Hillegersberg R. Laparoscopic total gastrectomy versus open total gastrectomy for cancer: a systematic review and meta-analysis. Surg Endosc. 2013;27(5):1509‐1520. Doi: 10.1007/s00464-012-2661-1 [DOI] [PubMed] [Google Scholar]
- 5. Brocks DR, Ben-Eltriki M, Gabr RQ, Padwal RS. The effects of gastric bypass surgery on drug absorption and pharmacokinetics. Expert Opin Drug Metab Toxicol. 2012;8(12):1505‐1519. Doi: 10.1517/17425255.2012.722757 [DOI] [PubMed] [Google Scholar]
- 6. Nakazono M, Aoyama T, Hayashi T, et al. Comparison of the dietary intake loss between total and distal gastrectomy for Gastric cancer. In Vivo. 2021;35(4):2369‐2377. Doi: 10.21873/invivo.12514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mechanick JI, Apovian C, Brethauer S, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures—2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, the Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists—executive summary. Endocr Pract. 2019;25(12):1346‐1359. Doi: 10.4158/GL-2019-0406 [DOI] [PubMed] [Google Scholar]
- 8. Hsu P-I, Chuah S-K, Lin J-T, et al. Taiwan nutritional consensus on the nutrition management for gastric cancer patients receiving gastrectomy. J Formos Med Assoc. 2021;120(1):25‐33. Doi: 10.1016/j.jfma.2019.11.014 [DOI] [PubMed] [Google Scholar]
- 9. Oh HJ, Yoon BH, Ha YC, et al. The change of bone mineral density and bone metabolism after gastrectomy for gastric cancer: a meta-analysis. Osteoporos Int. 2020;31(2):267‐275. Doi: 10.1007/s00198-019-05220-2 [DOI] [PubMed] [Google Scholar]
- 10. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312(7041):1254‐1259. Doi: 10.1136/bmj.312.7041.1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schafer AL. Vitamin D and intestinal calcium transport after bariatric surgery. J Steroid Biochem Mol Biol. 2017;173:202‐210. Doi: 10.1016/j.jsbmb.2016.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Climent M, Pera M, Aymar I, Ramon JM, Grande L, Nogues X. Bone health in long-term gastric cancer survivors: a prospective study of high-dose vitamin D supplementation using an easy administration scheme. J Bone Miner Metab. 2018;36(4):462‐469. Doi: 10.1007/s00774-017-0856-1 [DOI] [PubMed] [Google Scholar]
- 13. Rino Y, Aoyama T, Atsumi Y, Yamada T, Yukawa N. Metabolic bone disorders after gastrectomy: inevitable or preventable? Surg Today. 2022;52(2):182‐188. Doi: 10.1007/s00595-021-02253-1 [DOI] [PubMed] [Google Scholar]
- 14. Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18(2):153‐165. Doi: 10.1007/s11154-017-9424-1 [DOI] [PubMed] [Google Scholar]
- 15. ProCare Health . ProCare Health Once Daily Bariatric Multivitamin Capsule. 2023. https://procarenow.com/procare-health-once-daily-bariatric-multivitamin-capsule/ [Google Scholar]
- 16. Noh HM, Yoo JH, Jeong JY, Park YS. Bone mineral density after treatment for gastric cancer: endoscopic treatment versus gastrectomy. Medicine (Baltimore). 2018;97(1):e9582. Doi: 10.1097/MD.0000000000009582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stein EM, Silverberg SJ. Bone loss after bariatric surgery: causes, consequences, and management. Lancet Diabetes Endocrinol. 2014;2(2):165‐174. Doi: 10.1016/S2213-8587(13)70183-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated in this study are available upon reasonable request to the corresponding author. Unlinked genomic data will be deposited in public genomic databases such dbGap in compliance with the National Institutes of Health Genomic Data Sharing Policy.