Abstract
Context
Parathyroid cancer (PC) is a rare endocrine neoplasm with high mortality. While surgery is the treatment for patients with the disease, recurrence rates are high, and patients usually succumb to severe hypercalcemia. There is no effective systemic therapy for the disease.
Objective
To investigate for novel genes causing parathyroid cancer.
Methods
We analyzed the germline DNA of 17 patients with “sporadic” PC and 3 with atypical parathyroid tumors (APTs) who did not have germline CDC73 or MEN1 pathogenic variants. Sequencing of available tumor tissue from 14 patients with PC and 2 with APT was also performed (including 2 patients with no available germline DNA). In addition, sporadic parathyroid adenomas from 74 patients were analyzed for FLCN variants.
Results
We identified germline FLCN variants in 3 unrelated patients with PC. The 2 frameshift variants have been described in patients with Birt-Hogg-Dubé (BHD) syndrome, while the pathogenicity of the missense variant c.124G > C (p.G42R) has not been definitively established. Functional analysis of the missense variant showed a potential effect on posttranslational modification. All 3 patients with germline FLCN variants were noted to have renal cysts and 2 had lung cysts, features associated with BHD syndrome. Somatic FLCN variants were identified in tumors from 2 (1 APT) of 16 patients with PC/APT and in none of the 74 sporadic parathyroid adenomas. No second hits in FLCN were noted on sequencing; however, loss of heterozygosity at the locus was demonstrated in 2 of 3 patients with the identified germline FLCN variant.
Conclusion
The finding of FLCN variants associated with PC may provide the foundation for the development of therapy for this malignancy.
Keywords: parathyroid cancer, CDC73, Birt-Hogg-Dubé syndrome, primary hyperparathyroidism, renal cancer, tumor suppressor
Parathyroid cancer (PC) is a rare endocrine cancer with a 10-year mortality of 33% and a recurrence rate of 50% to 100% (1). It is often challenging to differentiate PC from benign disease preoperatively as there are no existing biomarkers for definitive diagnosis. Fine-needle aspiration biopsy of suspected lesions is not recommended because of the risk of tumor seeding and the limitations of cytology in differentiating an adenoma from carcinoma. Surgical resection is the mainstay of treatment. However, reoccurrences are common and may occur in the regional operative field increasing the risk of reoperation, particularly from vocal cord paralysis, or present as distant metastases. Most patients with PC succumb to severe intractable hypercalcemia. There is currently no approved systemic therapy for PC. Thus, there is a need to develop genetic and molecular markers that can aid in diagnosis and to identify potential therapeutic targets.
CDC73 variants are the most frequently identified genetic variation in PC. Germline variants are found in hyperparathyroidism–jaw tumor (HPT-JT) syndrome, in which 15% to 20% of patients develop PC, while somatic CDC73 variants are found in 40% to 75% of patients with sporadic PC (2). PC may rarely be associated with other familial hyperparathyroidism syndromes, including multiple endocrine neoplasia types 1 and 2 (3).
Germline variants in the tumor suppressor gene FLCN are associated with the autosomal dominant disorder Birt-Hogg-Dubé (BHD) syndrome, which is associated with cutaneous fibrofolliculomas, lung cysts, spontaneous pneumothorax, and/or kidney tumors (4, 5). The FLCN gene encodes folliculin (FLCN), a ubiquitously expressed protein involved in multiple cellular processes, including cell signaling, apoptosis, and autophagy. FLCN functions through its interaction with 2 proteins, folliculin-interacting proteins 1 and 2 (FNIP1, FNIP2) (6, 7). Kidney-specific Flcn inactivation in mice leads to activation of mechanistic target of rapamycin complex 1 (mTORC1) (8). While rare cases of parathyroid adenomas have been reported in BHD patients (9-11), BHD-associated PC has not been described. In the present study, we report the presence of germline FLCN variants in 3 patients with PC and features of BHD, as well as somatic FLCN variants in tumors from 1 patient with PC and a second, with an atypical parathyroid tumor (APT). In addition, we report no somatic FLCN variants in sporadic parathyroid adenomas from 74 patients indicating that these may be seen preferentially with PC/APT vs adenomas.
Materials and Methods
Study Participants and Clinical Characterization
Written informed consent was obtained from all patients under a National Institutes of Health (NIH) Institutional Review Board–approved natural history study of parathyroid disorders (NCT04969926). We included patients with the diagnosis of PC/APT for this study. Sporadic PC was defined as the histological diagnosis of PC in patients with no family history of parathyroid disorders and absence of germline variants in CDC73 or MEN1. We identified 41 patients with PC/APT seen at our institute—15 of 35 patients with PC and 2 of 6 with APT had germline CDC73 variants. Of the remaining, germline CDC73-negative 20 patients with sporadic PC and 4 with APT, patient-1245, our most recent patient who presented with disease recurrence, underwent tumor-normal whole-exome sequencing (WES) comparing germline blood DNA to the metastatic PC. Subsequently, germline FLCN sequencing was performed in 17 of 20 (including patient-1245) unrelated patients with sporadic PC and 3 of 4 with APT (Invitae Corporation) with available germline DNA. We found germline FLCN variants in 3 patients (patients-1245, -1344, and -103; Table 1) with PC. We also performed FLCN sequencing of tumor tissue in 14 of 20 patients with PC tissue (including 3 with germline FLCN variants) and 2 of 4 with APT and found somatic FLCN variants in one germline-FLCN–negative patient with PC (patient-1036) and another with APT (patient-1050). Case summaries of patients with positive findings are described later.
Table 1.
Clinical features of patients with parathyroid cancer and germline FLCN variants
| Patient | Sex | Presenting manifestation | Parathyroid | Renal cysts | Skin findings | Lung findings |
|---|---|---|---|---|---|---|
| 1245 | F | Primary hyperparathyroidism; age 40 y | Parathyroid cancer with distant metastases | Bilateral cysts (largest 1.2 cm) | Negative for fibrofolliculomas | Multiple bilateral lung bullae with emphysematous changes; pneumothorax |
| 1344 | F | Primary hyperparathyroidism; age 34 y | Parathyroid cancer with distant metastases | Unilateral cyst (1.5 cm) | NA | Small air-filled cyst |
| 103 | F | Primary hyperparathyroidism; age 17 y | Parathyroid cancer | S/p unilateral nephrectomy for pyelonephritis, nephrolithiasis Unilateral cyst (9 mm) in remaining kidney |
Negative for fibrofolliculomas | None |
Abbreviations: F, female; FLCN, folliculin; NA, not available.
Patients With Germline FLCN Variants
Patient-1245
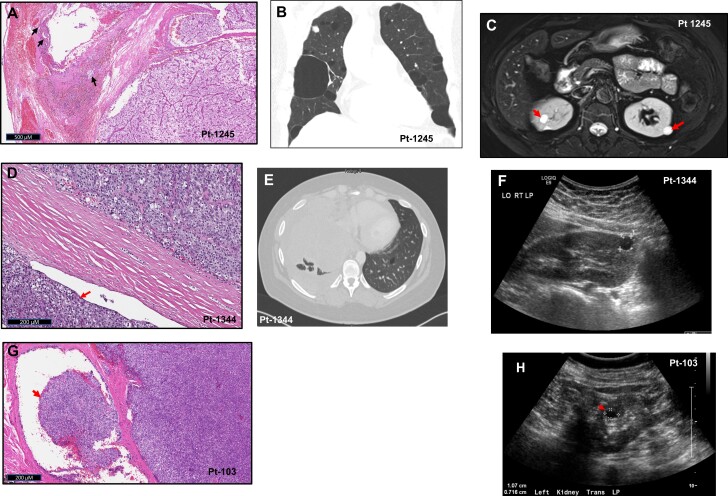
A 40-year-old Asian woman with primary hyperparathyroidism (initial ionized calcium 1.29 mmol/L, parathyroid hormone [PTH]: 178 pg/mL) underwent bilateral neck exploration with excision of a right-sided tumor that was histologically consistent with APT. She had persistent hyperparathyroidism but declined surgery until 5 years later, at which time a tumor histologically consistent with PC (Fig. 1A) was resected. She presented to our hospital at this time with persistent hyperparathyroidism and underwent 2 additional neck operations that were unsuccessful and was started on cinacalcet. Eighteen months later the patient reported dyspnea and dysphagia prompting a computed tomography (CT) scan, which revealed a right paratracheal mass compressing the trachea and bilateral lung blebs (Fig. 1B). She underwent right thoracotomy with resection of a 4.5 × 3 × 2.5-cm mediastinal mass with a prolonged postoperative course complicated by pneumothorax from a ruptured pulmonary bleb. Within 12 months, she had a recurrence necessitating reinitiation of cinacalcet along with denosumab, intravenous fluids, and sorafenib. Five years later, she developed compressive symptoms and underwent resection of an anterior neck mass histologically consistent with metastatic PC. Six months later, the patient continues to have hyperparathyroidism with redevelopment of a new anterior neck mass. She is a nonsmoker and has multiple bilateral cystic kidney tumors (Fig. 1C). No fibrofolliculomas are noted on examination.
Figure 1.
Birt-Hogg-Dubé syndrome phenotype in patients with germline FLCN variants. A, Hematoxylin and eosin (H&E) staining of resected secondary parathyroid cancer from the most recent surgery for patient-1245 demonstrating vascular invasion (arrows). B, Chest computed tomography (CT) (coronal view) demonstrating multiple bilateral lung bullae in patient-1245. C, Abdominal magnetic resonance imaging scan of patient-1245 showing multiple predominantly cystic kidney nodules (arrows). D, H&E staining of resected primary parathyroid cancer from patient-1344 showing extension of tumor beyond the capsule (arrow). E, Chest CT (axial view) showing a large mass (pulmonary metastasis) compressing the right lung and pericardium and one of the multiple lung blebs on the left (arrowhead) in patient-1344. F, Kidney ultrasonogram showing unilateral 1.5-cm kidney cyst in patient-1344. G, H&E staining of resected parathyroid cancer recurrence from patient-103 showing extension of tumor through the capsule (arrow). H, Ultrasonogram of the left kidney showing unilateral 1.07-cm cyst in patient-103 (asterisk).
Patient-1344
A 34-year-old African American woman with incidentally diagnosed primary hyperparathyroidism underwent resection of the left inferior parathyroid, which was histologically consistent with PC (Fig. 1D), followed by subsequent surgical resection of the left thyroid lobe, central lymph nodes, and ipsilateral strap muscles and postoperative neck irradiation. Three years later, imaging studies revealed multiple liver and bilateral lung masses, the largest being a 3.4 × 1.6-cm lung mass with considerable mechanical lung compression. CT-guided biopsies of the largest lung and a liver mass were consistent with metastatic PC. She presented to our center at this time. Biochemical workup revealed a corrected serum calcium of 10 mg/dL (reference rage, 7.9-9.7 mg/dL), ionized calcium of 1.21 mmol/L (reference rage, 1.12-1.32 mmol/L), and PTH of 21.5 pg/mL (reference rage, 15-65 pg/mL). Six months later she presented with 30-pound (13.6 kg) weight loss, night sweats, and intermittent fevers. CT of the chest showed rapid growth of the lung tumors (largest measuring 11 cm), which were compressing the heart, liver, and major blood vessels. She died 2 years later at age 39. Her imaging revealed multiple small air-filled lung cysts (Fig. 1E) and a 1 to 1.5-cm right kidney cyst (Fig. 1F). No fibrofolliculomas or family history of kidney tumors were noted.
Patient-103
A 17-year-old White woman presented with primary hyperparathyroidism associated with a fragility fracture and kidney stones and underwent right inferior parathyroidectomy. She had a recurrence 3 years later and had a 3-gland parathyroidectomy that failed, requiring reoperation for resection of a right inferior parathyroid mass. Pathology on these surgeries were all benign. Two decades later, she presented to our center with recurrent hyperparathyroidism (corrected serum calcium: 12.4 mg/dL, ionized calcium: 1.7-1.8 mmol/L, PTH: 446 pg/mL). Imaging studies demonstrated a 2-cm mass in the right tracheoesophageal groove. A dense and hard mass adherent to the trachea and esophagus was resected with histology consistent with PC (Fig. 1G), with remission of hyperparathyroidism. Subsequently, the patient had another recurrence 4 years later with symptomatic hypercalcemia necessitating resection of a right level IV lymph nodes with histology consistent with PC. The patient is currently aged 66 years with an incidental finding of a 9-mm renal cyst (Fig. 1H). She does not have any lung cysts or fibrofolliculomas or family history of PC or BHD.
Patients With Somatic FLCN Variants
Patient-1036
A 37-year-old African American woman was diagnosed with primary hyperparathyroidism during the third trimester of her pregnancy on presentation with severe symptoms of nausea, vomiting, confusion, polyuria, and polydipsia accompanied by hypercalcemia (serum calcium: 17 mg/dL). A few weeks after delivery, she underwent resection of a 6.8-g, 3.5 × 2.2 × 1.5-cm hypercellular parathyroid gland on the right lacking an atrophic rim of normal parathyroid gland. She remained eucalcemic for about 15 years when she developed confusion and was diagnosed with recurrent hyperparathyroidism (serum calcium: 11.6-12.3 mg/dL, PTH: 233 pg/mL). Localization studies revealed a 2-cm pleural-based mass and a second 6-cm right lower lobe superior segmental mass. She underwent thoracoscopic resection of the pleural-based mass and wedge resection of the superior segmental mass leading to remission of hyperparathyroidism. Pathology was consistent with metastatic parathyroid carcinoma. She is currently aged 68 years and remains eucalcemic. She has no kidney masses or lung blebs on imaging.
Patient-1050
A 49-year-old African American man presented with right flank pain due to nephrolithiasis and was diagnosed with primary hyperparathyroidism (serum calcium 3.32 mmol/L [normal 2.15-2.65 mmol/L]; PTH 1400 pg/mL [normal 13-76 pg/mL]). CT scan of the neck and mediastinum revealed a 9.6 × 7 × 4.5-cm partially cystic right middle mediastinal/paratracheal mass extending from the level of the azygos arch to the root of neck, displacing the trachea to the left. The mass had an enhancing rim on sestamibi scan. The patient underwent bilateral neck exploration and anterior thoracotomy for resection of the mass, which was located posterior to the recurrent laryngeal nerve and therefore assumed to be the right superior parathyroid gland. Histologically, the mass was read as being consistent with cystic atypical parathyroid tumor with large areas of gross necrosis, although there was one area of partial capsular invasion. Postoperatively, his hyperparathyroidism went into remission although further follow-up on the disease course is not available. There were no pulmonary blebs or kidney tumors identified on imaging.
Further tests and experiments were performed as described later.
Whole-Exome Sequencing
WES of Patient-1245's blood (genomic) and tumor DNA was performed at the Laboratory of Pathology, National Cancer Institute. Genomic DNA was extracted from peripheral blood using a PSS DNA Reagent Kit with a magLEAD 5bL automated nucleic acid extraction instrument (Precision System Science Co Ltd). Similarly, tumor DNA was extracted from formalin-fixed paraffin-embedded (FFPE) tissue sections mounted on slides after microdissection to enrich for viable tumor content using the AllPrep DNA/RNA FFPE Kit or QIAmp DNA FFPE Tissue Kit/RNeasy FFPE kit (Qiagen). A total of 100 ng of high-quality, double-stranded genomic DNA extracted from peripheral blood and FFPE tumor tissue was used for library preparation by Nextera Flex for Enrichment assay with the Exome Panel (Illumina Inc). Genomic DNA was tagmented onto the Bead-Linked Transposomes (eBLT) and amplified. The resulting amplified library was hybridized with probes (Coding Exome Oligo Panel, Illumina Inc), and the hybridized probes were captured. The enriched library was amplified, and library quality control was performed. Libraries were then pooled and loaded for target coverage of 50× for normal blood DNA and 150× for tumor DNA and sequenced on the NextSeq 550Dx and/or Novaseq 6000 (Illumina Inc)
The COMPASS Exome Germline Sequencing test is a next-generation sequencing assay that detects germline mutations in the whole exome of the human genome based on the Nextera Exome Kit. The panel captures coding exons and covers 45 Mb of exonic content (≥ 98% of RefSeq, CCDS, and Ensembl coding content). The sequences were compared to the current human reference genome hg19 using internally developed COMPASS somatic Bioinformatics Pipeline V4.0 and mutations reported according to HGVS nomenclature. The assay can detect germline single-nucleotide variations in coding regions (exons ± 2 bp neighboring intronic regions) and small insertion/deletion (indel) events up to 25 bp based on matched tumor/normal DNA sequencing. Classification of pathogenicity and actionability of variants was performed by independent algorithms developed by QCI (QCI Interpret) that are based on integration of data from multiple clinical and population-based bioinformatics databases (eg, COSMIC, OMIM, HGMD, ClinVar, gnomAD), protein function tools (eg, CADD, SIFT, PolyPhen-2), and from experimental or clinical data reported in the biomedical literature. Final review of variant classification and interpretation was performed by a board-certified pathologist.
WES of tumor DNA from an additional 8 patients with parathyroid cancer who were negative for germline FLCN variants was performed at the Frederick National Laboratory, National Cancer Institute. Tumor samples were processed using the Agilent Sure Select Human All Exon V7 capture kit, and resulting libraries were sequenced on an Illumina NovaSeq 6000 SP flowcell. Raw sequencing data was processed, and tumor-only somatic variant calling was performed using the DRAGEN v3.9.5 workflow. Resulting variants were then annotated using VEP v104 for downstream filtering and analysis (12). Raw variants were then filtered using the following criteria: depth greater than 15×, tumor allele fraction greater than 0.01, tumor allele coverage greater than 2, and population frequency less than 0.001.
Germline FLCN Sequencing
Germline DNA was isolated from whole-blood samples using the iPrep Purification Instrument (Invitrogen, Thermo Fisher Scientific). Germline DNA genetic testing for FLCN was performed at Invitae by WES followed by mutation analysis of FLCN.
Loss of Heterozygosity in Parathyroid Tumors
To look for loss of heterozygosity (LOH) in parathyroid cancer tumor samples, germline and tumor DNA was used for polymerase chain reaction (PCR) to amplify the region of the respective FLCN or TP53 mutation sites. The PCR primers included the T3 or T7 sequence in the forward or reverse primer, respectively, for sequencing. PCR was performed using 5 to 10 ng of DNA, 0.2-µM primers, 5% dimethyl sulfoxide, and the AmpliTaq Gold DNA Polymerase mix (Thermo Fisher Scientific) with the following conditions: 94 °C for 10 minutes; and 35 cycles of 94 °C for 30 seconds, 55 °C for 30 seconds, and 72 °C for 90 seconds. PCR products were purified using MinElute PCR Purification Kit (Qiagen), and Sanger sequencing was performed with the T3 or T7 primer at Quintarabio. DNA sequence traces were visually inspected for the presence or absence of LOH. Primer sequences are available on request.
Functional Analysis of Missense Variant p.G42R
Plasmids expressing Flag-tagged or HA-tagged FLCN, FNIP1, or FNIP2 were purchased from Addgene. The plasmid expressing Flag-tagged-FLCN was used to engineer FLCN missense variation p.G42R with a site-directed mutagenesis kit (Agilent) and confirmed by Sanger sequencing. HEK293 (ATCC) or Cos7 cells (ATCC) were transfected with plasmids using Lipofecatmine 2000, and whole-cell extracts (WCEs) were prepared 24 hours post transfection in radioimmunoprecipitation assay buffer (Cell Signaling). Transfected proteins were detected by immunoblot analysis or immunofluorescence (rabbit-Anti-FLCN, Abcam No. ab124885 or Cell Signaling Technology No. 3697, RRID:AB_2231646; mouse-Anti-Flag-tag, Sigma No. F1804, RRID:AB_262044; rabbit-Anti-HA-tag, Abcam No. ab9110, RRID:AB_307019; mouse-Anti-HA-tag, Cell Signaling Technology No. 2367, RRID:AB_10691331; mouse-Anti-p84, GeneTex No. GTX70220, RRID:AB_372637; Secondary anti-rabbit, JacksonImmuno No. 711035152, RRID:AB_10015282; Secondary anti-mouse, JacksonImmuno No. 715035151, RRID:AB_2340771). To analyze protein-protein interaction between HA-tagged-FNIP1/FNIP2 and Flag-tagged-FLCN, immunoprecipitation with the rabbit-Anti-HA-tag was performed as described previously (13), followed by immunoblot analysis to detect co-immunoprecipitated proteins and input proteins. Images of immunoblots were captured on the G-BOX imaging system (Syngene). For detecting nuclear cytoplasmic localization of TFE3, Cos7 cells were transfected in 4-chamber slides with plasmids expressing Flag-tagged-FLCN or Flag-tagged-FLCN (p.G42R). The transfected FLCN and endogenous TFE3 proteins were detected 24 hours post transfection by immunofluorescence with mouse-Anti-Flag and rabbit-Anti-TFE3 (Sigma No. HPA023881, RRID ID: AB_1857931), respectively. The secondary antibodies used were mouse-Alexa-Fluor-488 (Thermo Fisher No. A-11029, RRID ID: AB_2534088) to detect Flag-tagged-FLCN and rabbit-Alexa-Fluor-594 (Thermo Fisher No. A-11037, RRID ID: AB_2534095) to detect endogenous TFE3. Images were captured on an epifluorescence microscope with appropriate filters (Carl Zeiss). Anti-TFE3 was validated by immunoblot analysis of WCE from HEK293 cells transfected with a plasmid expressing TFE3.
Sanger Sequencing for Somatic FLCN Variants
Tumor DNA was isolated from unstained sections of FFPE tissue specimens using the QIAamp DNA FFPE tissue kit (Qiagen). DNA concentration was measured with the NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific). For somatic genetic testing and LOH analysis, tumor DNA was used to screen the coding exons of FLCN with in-house primers or previously published primers (4) with the T3 or T7 sequence in the forward or reverse primer, respectively. PCR was performed using 5 to 10 ng of DNA, 0.2 µM primers, 5% dimethyl sulfoxide, and the AmpliTaq Gold DNA Polymerase mix (Thermo Fisher Scientific) with the following conditions: 94 °C for 10 minutes; and 35 cycles of 94 °C for 30 seconds, 55 °C for 30 seconds, and 72 °C for 90 seconds. PCR products were purified using MinElute PCR Purification Kit (Qiagen), and Sanger sequencing was performed with the T3 or T7 primer at Quintarabio. DNA sequences were analyzed for variants with Mutation Surveyor software (SoftGenetics). FFPE tissue sections of FLCN variant-positive tumors were subsequently processed for independent DNA isolation, exon-specific PCR amplification, and Sanger sequencing. Primer sequences used in the study are available on request.
TSO500 Oncology Panel Testing for Somatic Variants
Tumor DNA was extracted as described under “Whole Exome Sequencing.” Libraries were prepared per kit instructions (TruSight Oncology 500 [TSO500, Illumina]), a capture-based next-generation sequencing assay that analyzes 523 cancer-relevant genes and assesses multiple variant types, including single-nucleotide variations, indels, copy number variations, and immunotherapy biomarkers that rely on analysis of multiple genomic loci, such as tumor mutational burden (and microsatellite instability. Sequencing was performed on a Nextseq 550Dx system (Illumina). Proprietary TruSight Oncology 500 v2.1 local applications (Illumina) was used for alignment, variant calling, gene fusion detection, and for determination of tumor mutational burden and microsatellite instability. All alignments were performed against Illumina designed browser extensible data (BED) files that are based on the human genome reference GRCh37/hg19 assembly. The resultant vcf file was uploaded to QIAGEN Clinical Insight (QCI) for variant filtering, annotation, classification, interpretation, and reporting. Variant classification was as described under “Whole Exome Sequencing.”
Polymerase Chain Reaction–based FLCN Sequencing on Tumor DNA From Sporadic Parathyroid Adenomas
FLCN variant analysis in DNA from a random selection of 74 sporadic parathyroid adenomas that were previously described (14) was performed by multiplex PCR products sequencing at the NCI Genomics core facility.
FLCN and GPNMB Expression in Tumor Tissue
FFPE parathyroid tumor specimens processed for immunohistochemistry (Vitro Vivo) revealed no clear loss of FLCN staining or positive GPNMB staining in any of the processed samples with either germline or somatic FLCN variants (rabbit-Anti-FLCN; goat-Anti-GPNMB, R&D Systems No. AF2550, RRID:AB_416615; Secondary Anti-goat, Santa Cruz Biotechnology No. sc-2056, RRID:AB_631730).
Results
Germline FLCN Variants in Patients With Parathyroid Cancer
Among the 17 patients with sporadic PC and 3 with APTs who had available germline DNA, patient-1245 underwent WES of the resected metastatic parathyroid tumor and germline blood DNA for comparison analysis. WES of blood DNA from the patient revealed a pathogenic heterozygous FLCN variant (c.1285insC) that was confirmed by Sanger sequencing (Fig. 2A) of tumor DNA (tumor content 80%). The FLCN c.1285insC variant causes a frameshift, resulting in a shorter protein that is unstable and with loss of tumor suppressor activity (15), and is frequently reported in the germline of individuals affected with BHD syndrome (4, 16). The patient also has a history of pneumothorax due to rupture of a lung bleb and kidney cysts, consistent with the diagnosis of BHD syndrome (Fig. 1A-1C). Tumor WES also identified a variant in TP53 (variant allele frequency [VAF]: 68%), RUFY2 (VAF: 70%), and SECISBP2L (VAF: 68%). The TP53 variant c.742C > T (p.R248W) found in the PC sample has been described in Li-Fraumeni syndrome, a cancer predisposition syndrome caused by germline-inactivating variants in TP53. However, functional characterization in other tumors shows TP53 R248W to be a gain-of-function, rather than loss-of-function, variant (17).
Figure 2.
Analysis of germline FLCN variant in patients with parathyroid cancer and Birt-Hogg-Dubé syndrome phenotype. A to C, Germline DNA and loss-of-heterozygosity (LOH) analysis for FLCN in parathyroid cancer tissue of A, patient-1245; B, patient-1344; and C, patient-103. Each panel represents sequence of unaffected blood DNA (normal), patient blood DNA (germline, heterozygous), and tumor DNA (LOH analysis). Arrows shows the location of the variants. Brackets in panel B mark codon 42 (c.124G > C, GGT/CGT).
Subsequently, we identified 2 additional PC patients with germline FLCN variants. Patient-1344 was found to have the variant c.584delG (p.G195fs*28) (Fig. 2B), which has been previously reported in BHD patients (18, 19), while patient-103 was found to have a variant of uncertain significance (c.124G > C; p.G42R) (Fig. 2C). Patient-1344 was noted to have a 1.5-cm kidney cyst and multiple small lung blebs (Fig. 1D-1F), while patient-103 had no lung blebs but had a 1-cm kidney cyst in her solitary kidney (Fig. 1G and 1H). Sequencing of tumor tissue from 2 patients with no available germline DNA did not identify any pathogenic FLCN variants, suggesting absence of a germline FLCN variant in these patients.
Loss of Heterozygosity in Tumor Tissue From Patients With Germline FLCN Variants
Tumorigenesis secondary to inheritance of a mutated tumor-suppressor gene requires a somatic “second hit” on the unaffected allele (deletion resulting in LOH, or variant) (20). LOH analysis showed evidence for deletion of the unaffected allele in tumors from patients-1245 (Figs. 2A and 3A) and 1344 (Fig. 2B), but not from patient-103 (Fig. 2C). LOH was consistently observed in PC specimens removed from patient-1245 in 3 separate surgeries (Fig. 3A). Thus, biallelic disruption of FLCN was observed in PC tissue from 2 of the 3 patients with germline-heterozygous FLCN variants. However, LOH for the somatic TP53 variant was also detected in metastases from patient-1245 (Fig. 3B).
Figure 3.
FLCN and TP53 variants in primary tumor and metastases in patient-1245. A and B, Loss of heterozygosity of A, germline FLCN variant, and B, somatic TP53 variant in parathyroid cancer specimens obtained from various surgeries from 2012 to 2021 from patient-1245.
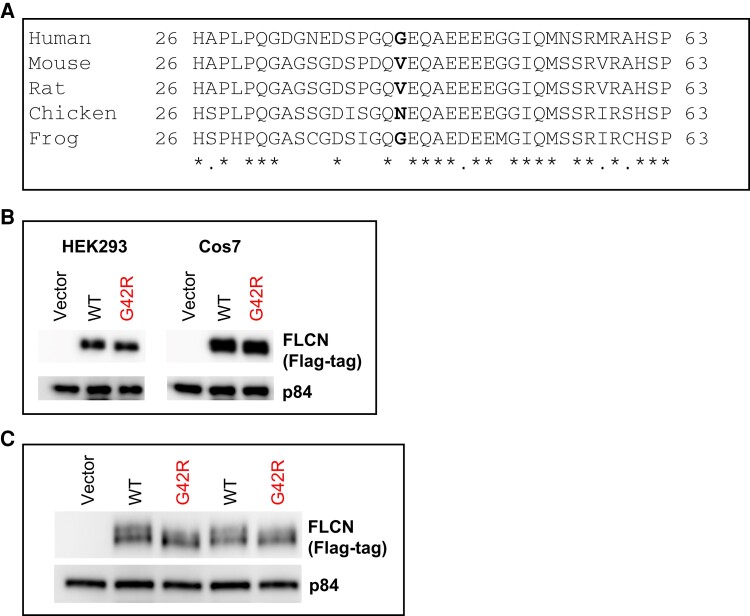
Germline FLCN Variant p.G42R Shows a Structural or Posttranslational Defect
The G42 amino acid residue in the FLCN protein sequence is not highly conserved among vertebrate species (Fig. 4A). Immunoblotting of WCEs of HEK293 or Cos7 cells transfected with wild-type (WT) or G42R FLCN showed comparable levels of FLCN expression (Fig. 4B). Further immunoblot analysis of HEK293 WCEs with longer separation times showed that FLCN G42R did not display the top band of the doublet (likely phosphorylated form) seen in the WT FLCN protein (Fig. 4C) (6), indicating that FLCN G42R may be missing a posttranslational modification or may have a structural defect. However, coimmunoprecipitation experiments showed that the interaction of FLCN G42R with FNIP1 or FNIP2 was unaffected, indicating proper protein folding of the G42R variant (Fig. 5A-5C). Additionally, expression of both FLCN G42R protein or WT FLCN in Cos7 cells resulted in cytoplasmic sequestration of the transcription factor TFE3, while TFE3 remained localized to the nucleus in untransfected cells (Fig. 5A, 5C, and 5D). These results suggest a structural defect or a missing posttranslational modification in FLCN G42R that does not affect its functional interactions in the 2 assays analyzed. However, the variant may be defective in other yet unknown specific function(s) of FLCN in parathyroid cells.
Figure 4.
Expression of transfected FLCN variant p.G42R in cell culture. FLCN protein sequence conservation at position G42. FLCN protein sequences were retrieved from NCBI HomoloGene. Multiple sequence alignment of the region near human amino acid residue G42 (residues 26-63) was performed using ClustalW (MacVector software). A, The accession numbers of the proteins aligned are NP_659434.2 (human), NP_001258285.1 (mouse), NP_955422.1 (rat), XP_414807.2 (chicken), and NP_001011353.1 (frog). Asterisk (*) indicates fully conserved residue; dot (.) indicates conservation of residues with similar properties. The human amino acid residue G42 (bold) is not conserved among the selected vertebrate FLCN protein sequences. B, Immunoblot of whole cell extracts of HEK293 or Cos7 cells transfected with Flag-tagged empty vector or the indicated Flag-tagged FLCN plasmids. FLCN proteins were detected with Anti-Flag, and p84 was used as the loading control. C, Immunoblot showing protein migration in 10% polyacrylamide gel electrophoresis gel from whole-cell extracts of HEK293 cells transfected with Flag-tagged empty vector or the indicated Flag-tagged FLCN plasmids. The blot was first probed with Anti-Flag, and then with p84 as the loading control. The blot was probed with the mouse secondary antibody to detect both p84 and Flag-tagged FLCN proteins simultaneously.
Figure 5.
Functional analysis of FLCN variant p.G42R. A, Diagram showing interaction of FLCN (folliculin) with FNIP1/FNIP2 in the cytoplasm, and nuclear or cytoplasmic localization of TFE. B, Coimmunoprecipitation (co-IP) assay showing interaction of Flag-tagged FLCN (wild-type [WT] or p.G42R variant) with HA-tagged FNIP1/FNIP2. Whole-cell extracts (WCEs) of HEK293 cells expressing Flag-tagged FLCN protein (WT or p.G42R) and HA-tagged FNIP protein (FNIP1 or FNIP2) underwent immunoprecipitation using an anti–HA-tag antibody. Input WCE and co-IP proteins underwent immunoblotting with the indicated antibodies. Anti-p84 was used as the loading control for input WCE. C, Immunofluorescence analysis of Cos7 cells transfected with Flag-tagged FLCN (WT or p.G42R variant) and the localization of endogenous TFE3. Top panels: The subcellular localization of p.G42R is similar to WT (in the nucleus and cytoplasm). Middle and bottom panels: In the presence of high expression of WT or p.G42R, endogenous TFE3 translocates to the cytoplasm (red dot). D, Anti-TFE3 was validated by immunoblot analysis of protein WCEs of HEK293 cells transfected with empty vector or a plasmid expressing TFE3 (Sino Biological). Anti-p84 was used as the loading control. In the Anti-p84 blot, note that protein loaded on the gel was more for the vector transfected to observe endogenous protein level.
Immunohistochemical staining of tumor samples was attempted to evaluate expression level of FLCN and glycoprotein nonmetastatic B (GPNMB) shown to have an inverse expression pattern with GPNMB overexpression and loss of intact form of FLCN in renal tumors from patients with BHD syndrome (21). No difference in staining pattern was noted between the positive control (germline FLCN and LOH-positive sample, patient-1245) and negative control (germline and somatic FLCN-variant negative sample), indicating a failed attempt (Fig. 6). This may be because the folliculin antibody has not been well characterized or folliculin loss in parathyroid tumors may not act through GPNMB.
Figure 6.
Immunohistochemical staining of tumor tissue for FLCN from A, a patient with no germline or somatic FLCN variant (negative control), and B, patient-1245 with a pathogenic germline FLCN variant (positive control) for FLCN and glycoprotein nonmetastatic B (GPNMB) (negative control: C; patient-1245: D).
Somatic FLCN Variants in Sporadic Parathyroid Cancer
The presence of somatic FLCN variants was examined in available PC/APT specimens from germline FLCN-negative patients by Sanger sequencing, WES, multiplex PCR, or 500-Gene Oncology Panel (Table 2). Multiple sequencing modalities were used because of poor FLCN coverage noted on initial WES of tumor samples. Sanger sequencing was successful in amplifying FLCN in tumor samples from 3 FLCN-germline negative patients due to poor quality DNA from the archived FFPE samples and detected a recurrent FLCN c.1285insC variant in tumors from 2 patients (patient-1036 with PC and patient-1050 with a large APT) (Fig. 7A-7C). This is the same variant identified in germline DNA from patient-1245. Clear LOH was observed in the tumor DNA from patient-1036 but not from patient-1050, possibly due to normal DNA contamination (see Fig. 7A). Neither of the 2 patients with somatic FLCN variants (patients-1036 and 1050) had any kidney cysts or nodules. No lung cysts were noted on chest x-ray performed on either of the 2 patients (patients with germline FLCN variant had CT scan of lung available). Exome sequencing of tumor DNA from both samples did not detect CDC73, MEN1, or TP53 variants. Multiplex PCR analysis of tumor DNA from 5 patients did not detect any FLCN variants. WES and 500-Gene Oncology Panel testing of tumor samples failed to identify FLCN variants in any of the samples, including the 2 samples in which variants were identified by Sanger sequencing. Furthermore, germline FLCN variants were missed on tumor sequencing of fresh samples multiple times (WES and 500-Gene Oncology Panel) for patient-1245 and on archived FFPE specimens from patient-1344 (WES). This lack of sensitivity may be driven by poor coverage of FLCN by exome sequencing of DNA isolated from archived FFPE tumor tissue (Fig. 8).
Table 2.
Tumor sequencing modality used for identification of somatic FLCN variants (N = 16)
| Whole-exome sequencing (n = 9) | 500-Gene Oncology Panel (n = 6) | Sanger sequencing (n = 6) | Multiplex PCR (n = 5) | |
|---|---|---|---|---|
| Patient-1245 | Germline variant missed; no FLCN second hit noted (performed on fresh tumor sample); no pathogenic variants in CDC73 or MEN1; pathogenic variant in TP53 noted | Germline FLCN variant missed on tumors from 2 successive surgeries; no second hit in FLCN noted; pathogenic variant in TP53 noted | LOH noted in addition to germline variant | No FLCN second hit noted; germline variant identified, LOH suggested (variant allele frequency 87.18%) |
| Patient-1344 | Germline FLCN variant missed; no FLCN second hit noted; no pathogenic variants in CDC73, MEN1, or TP53 | Not done | LOH noted in addition to germline variant | Not done |
| Patient-103 | Not done | Germline FLCN variant noted—no second hit identified; no pathogenic variants in CDC73, MEN1, or TP53 | Germline variant noted—no LOH | Not done |
| Patient-1036 | No FLCN variant noted; no pathogenic variants in CDC73, MEN1, or TP53 | Not done | LOH noted in addition to somatic variant c.1285insC | Not done |
| Patient-1050 | No FLCN variant noted; no pathogenic variants in CDC73, MEN1, or TP53 | Not done | somatic variant c.1285insC No LOH |
Not done |
Abbreviations: FLCN, folliculin; LOH, loss of heterozygosity; PCR, polymerase chain reaction.
Figure 7.
Somatic FLCN variants and tumor histology of parathyroid cancer tissues. A, Somatic FLCN hot spot variant in FLCN-germline–negative patients with sporadic parathyroid cancer. “Hot spot” FLCN variant c.1285insC in exon 11 is identified in 2 of 3 sporadic parathyroid tumor DNA samples. The variant was validated in independent DNA preps. Arrow shows the location of the variant. B, Pulmonary metastasis of patient-1036 composed of chief cells. C, Primary tumor from patient-1050 showing capsular invasion.
Figure 8.
Depth and coverage of the FLCN locus on exome sequencing of FFPE tissues. Cumulative coverage distribution from all targeted FLCN exons (blue) vs all other targeted exons (red) illustrating the clear coverage deficit for FLCN relative to the majority of other targeted regions from the tumor exome sequencing.
FLCN Variants are Rare in Sporadic Parathyroid Adenomas
Fresh-frozen parathyroid adenoma samples from 74 patients without family history of parathyroid disease or germline MEN1 variants (previously described in (14)) were analyzed for somatic FLCN variants by multiplex PCR. The median age of initial presentation with primary hyperparathyroidism for the cohort was 47 years (age range, 31-54 years), and 19% had recurrent disease. The significant proportion of recurrent disease in the cohort is because our institution serves as a referral base for patients with potential underlying heritable hyperparathyroidism as indicated by the lower quartile of 31 years for median age of initial presentation. Variants were filtered for those affecting protein sequence, greater than or equal to 10× depth, greater than 2× coverage on the mutant allele, and less than 0.01 frequency in the general population. Variants were then manually confirmed for sequencing errors. No FLCN variants were identified in the samples sequenced, indicating that these variants are rare in sporadic parathyroid adenomas.
Parathyroid Cancer in Published Literature and the Surveillance, Epidemiology, and End Results Database
Eight publications with sequencing of parathyroid tumors from 2012 to 2021 were identified and access to exome sequencing data requested where necessary (22-29). No germline or somatic variants in FLCN were identified on querying the available exome sequencing data from prior publications of sporadic parathyroid tumors (23, 25-28). However, raw sequencing data could be accessed from only 3 publications (23, 25, 28). In addition, the Surveillance, Epidemiology, and End Results (SEER) Program was queried for co-occurrence of parathyroid and renal cancer as a proxy for patients with underlying BHD syndrome. This identified 12 of 790 patients with co-occurring PC and renal cancer (histological characteristics of renal tumor not available). Of note, only approximately 30% of patients with BHD syndrome develop renal tumors, primarily hybrid and chromophobe tumors (5).
Discussion
In this study we report germline (3/20) and somatic (2/13 tumors from germline FLCN-negative patients) FLCN variants associated with PC/APT, which appear to be rare in parathyroid adenomas. Patients-1245 and 1344 met the criteria for the diagnosis of BHD and therefore, we present the first 2 cases of BHD associated with PC. This finding expands the BHD phenotype to include PC and has implications for the diagnosis and surveillance of these patients. Two of 3 patients with PC and germline FLCN variants developed distant metastases, which is seen in about 25% of patients with PC and is associated with decreased overall survival (2). None of the patients in whom we identified germline FLCN variants were suspected to have or diagnosed with BHD before this workup. It is noteworthy that both HPT-JT and BHD syndromes manifest parathyroid and kidney tumors.
A frequently observed mechanism for tumor growth in hereditary syndromes is inactivation of tumor suppressor genes as described by Knudson's 2-hit model of tumorigenesis (20). We demonstrate LOH of FLCN in tumors from patients-1245 and 1344, although neither LOH nor a second-hit FLCN variant could be identified in tumor tissue from patient-103. LOH on the short arm of chromosome 17 has been described in 17% and somatic “second hit” mutations in about 53% of BHD-associated renal tumors (30). Nevertheless, 30% of BHD-associated renal tumors and the majority of BHD-associated cutaneous lesions and lung cysts show no LOH or somatic mutations and it is thought that gene silencing by hypermethylation, variants in the regulatory region of FLCN, or insufficiency of only one WT allele for a normal phenotype may explain this observation (31). It is also possible that a microRNA can act as an oncomir, blocking gene expression through an epigenetic mechanism and thereby resulting in a “second hit” in parathyroid tissue. c.1285insC is a “hot spot” for variants in BHD and appears to also be a “hot spot” for PC. The discovery of FLCN variants in PC is consistent with disruption of the PI3K/AKT/mTOR pathway, which has been implicated in PC, with gene alterations in the pathway reported in approximately 10% to 30% of samples (PIK3CA, PTEN, AKT1, mTOR, TSC1, and NF1) (2).
Variants in TP53 have been reported in 10% to 30% of PC (32). However, it does not appear to be a driver of oncogenesis (23, 33-35) and PC has not been reported in patients with Li-Fraumeni syndrome who have germline TP53 variants. Both TP53 and FLCN are located on chromosome 17p, and chromosomal regions of LOH can often be large so the LOH of both the TP53 and FLCN genes observed in patient-1245 may result from a single large deletion on chromosome 17. However, no variant in TP53 was identified in tumor samples from patient-1344 and the TP53 R248W appears to be a gain-of-function, rather than loss-of-function, variant (17). Nevertheless, a synergistic effect of variants in both genes as a cause of PC in patient-1245 cannot be definitively excluded (36). Germline variant in CDC73 has been reported in a patient with BHD (37), but both germline or somatic variants in CDC73 were ruled out in the patients with germline or somatic FLCN variants described in this report.
The FLCN variant c.124G > C (p.G42R) found in patient-103 is rare in the gnomAD population database and has been reported in ClinVar in the germline of 2 individuals with multiple fibrofolliculomas and suspected hereditary cancer-predisposing syndrome. The mutant arginine is bulkier and more hydrophilic than WT glycine and is positively charged whereas glycine has a neutral charge. Change of the glycine to arginine may result in an abnormal protein conformation that may affect optimal FLCN function. Our biochemical analysis of this variant suggests a defect in its posttranslational modification; however, its pathogenicity cannot be definitively ascertained with the experimental data available currently. Lack of in vitro models and parathyroid cell line and failure of immunohistochemical staining for GPNMB and FLCN to assess expression level severely limits our ability to perform functional characterization of this variant of uncertain significance.
PC is an ultrarare disorder, which makes research in the field challenging because of the rarity of tissue specimens. The published raw sequencing data for PC accessed for this study include data from sequencing performed on archived FFPE samples from 48 patients, a relatively small cohort (23, 25). We found that the exonic coverage for FLCN was lower in comparison to other genes on WES (see Fig. 8), which may explain our inability to detect any FLCN variants in the accessed published sequencing data. Furthermore, the germline status of patients is not described in either of the publications (23, 25). Our reported frequency of germline or somatic FLCN variants in patients with PC/APT may be higher because we routinely exclude germline CDC73 variants in patients with PC. Thus, the initially identified cohort of 41 patients with PC/APT may serve as a better denominator to assess the frequency of FLCN variants in patients with PC/APT. However, our institute serves as a referral base for patients suspected of heritable forms of primary hyperparathyroidism, which can bias the findings. Of note, there was racial heterogeneity among the 3 patients with germline FLCN variants.
It should be noted that a pathogenic FLCN variant was also seen in an APT, but not in any of the 74 parathyroid adenomas. While the molecular characteristics of APT suggest that these are not precursor lesions of PC, it is not uncommon for a patient originally diagnosed with APT to be diagnosed with PC, as with patient-1245. This reflects the challenge in establishing the diagnosis of PC, particularly its distinction from APT. Similarly, although histology for patient-1050 was thought to be more consistent with APT, one small area of partial capsular invasion was noted. Unfortunately, we do not have long-term follow-up information on this patient to further describe his disease course.
While parathyroid adenomas have been reported in patients with BHD (9-11), we report no variants in FLCN in tumor DNA from 74 patients with sporadic parathyroid adenomas. More samples will need to be analyzed to draw firm conclusions; the initial results presented in this study (Fig. 9) suggest that the presence of FLCN variants has the potential to be useful as a biomarker for PC. Our finding of germline and somatic FLCN variants in patients with PC/APT is important as this gene has not previously been reported to have a role in the pathogenesis of PC/APT. These findings may provide the foundations for improved diagnosis and identification of a potential therapeutic target for PC.
Figure 9.
Summary of study findings (created with BioRender.com).
Acknowledgments
The authors thank Della Cox and Craig Cochran for coordinating patient visits and tissue procurement and David Sun and Xiaolin Wu for performing multiplex PCR-based variant analysis. We wish to thank the trainees of the NIH Inter-Institute Endocrine Training Program and the nurses and staff at the NIH Clinical Center for providing care to the study participants. We are especially grateful to the patients for their support of our research. We thank Nancy Perrier, MD, for access to dbGAP phs001765.v1.p1.
Abbreviations
- APT
atypical parathyroid tumor
- BHD
Birt-Hogg-Dubé
- CT
computed tomography
- FFPE
formalin-fixed paraffin-embedded
- FLCN
folliculin
- FNIP1
folliculin-interacting protein 1
- FNIP2
folliculin-interacting protein 2
- HPT-JT
hyperparathyroidism–jaw tumor
- LOH
loss of heterozygosity
- NIH
National Institutes of Health
- PC
parathyroid cancer
- PCR
polymerase chain reaction
- PTH
parathyroid hormone
- VAF
variant allele frequency
- WCE
whole-cell extract
- WES
whole-exome sequencing
- WT
wild-type
Contributor Information
Smita Jha, Metabolic Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892, USA.
James Welch, Metabolic Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892, USA.
Rana Tora, Metabolic Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892, USA.
Justin Lack, NIAID Collaborative Bioinformatics Resource, National Institute for Allergy and Infectious Diseases, Bethesda, MD 20892, USA.
Andrew Warner, Frederick National Laboratory, National Institutes of Health, Bethesda, MD 21701, USA.
Jaydira del Rivero, Developmental Therapeutics Branch National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA.
Samira M Sadowski, Endocrine Surgery Section, Surgical Oncology Program, Bethesda, MD 20892, USA.
Naris Nilubol, Endocrine Surgery Section, Surgical Oncology Program, Bethesda, MD 20892, USA.
Laura S Schmidt, Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA; Frederick National Laboratory for Cancer Research, Basic Science Program, Frederick, MD 21701, USA.
W Marston Linehan, Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA.
Lee S Weinstein, Metabolic Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892, USA.
William F Simonds, Metabolic Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892, USA.
Sunita K Agarwal, Metabolic Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892, USA.
Funding
This work was supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases (ZIA DK043006-46), National Institute for Allergy and Infectious Diseases, the National Cancer Institute, the National Institutes of Health (NIH), and was funded in part with federal funds from the Frederick National Laboratory for Cancer Research. Part of the research reported in this publication was supported by the NIH (under award No. T32CA009599-24), the Baylor College of Medicine Comprehensive Cancer Training Program (award No. RP160283), and an MD Anderson Cancer Center Parathyroid Cancer Research Grant.
Author Contributions
S.J. contributed to study design, enrolling patients, data acquisition, and writing the paper; J.W. contributed to data acquisition and enrolled patients; R.T. contributed to data acquisition; J.L. performed genetic data analyses and helped in writing the paper; A.W., J.D.R., S.M.S., and N.N. contributed to data acquisition; L.S.S. and W.M.L. contributed to data acquisition and writing the manuscript; L.S.W. contributed to enrolling patients and writing the paper; W.F.S. contributed to enrolling patients and data acquisition; and S.K.A. contributed to study design, data acquisition, and writing the paper. All coauthors reviewed and revised the manuscript.
Disclosures
The authors have nothing to disclose.
Data Availability
Data generated in this paper is not publicly available but available from corresponding author on reasonable request.
References
- 1. Fingeret AL. Contemporary evaluation and management of parathyroid carcinoma. JCO Oncol Pract. 2021;17(1):17‐21. [DOI] [PubMed] [Google Scholar]
- 2. Perrier ND, Arnold A, Costa-Guda J, et al. Hereditary endocrine tumours: current state-of-the-art and research opportunities: new and future perspectives for parathyroid carcinoma. Endocr Relat Cancer. 2020;27(8):T53‐T63. [DOI] [PubMed] [Google Scholar]
- 3. Cinque L, Sparaneo A, Salcuni AS, et al. MEN1 gene mutation with parathyroid carcinoma: first report of a familial case. Endocr Connect. 2017;6(8):886‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nickerson ML, Warren MB, Toro JR, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome. Cancer Cell. 2002;2(2):157‐164. [DOI] [PubMed] [Google Scholar]
- 5. Schmidt LS, Linehan WM. FLCN: the causative gene for Birt-Hogg-Dubé syndrome. Gene. 2018;640:28‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baba M, Hong SB, Sharma N, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci U S A. 2006;103(42):15552‐15557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hasumi H, Baba M, Hong SB, et al. Identification and characterization of a novel folliculin-interacting protein FNIP2. Gene. 2008;415(1-2):60‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hasumi Y, Baba M, Ajima R, et al. Homozygous loss of BHD causes early embryonic lethality and kidney tumor development with activation of mTORC1 and mTORC2. Proc Natl Acad Sci U S A. 2009;106(44):18722‐18727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chung JY, Ramos-Caro FA, Beers B, Ford MJ, Flowers F. Multiple lipomas, angiolipomas, and parathyroid adenomas in a patient with Birt-Hogg-Dubé syndrome. Int J Dermatol. 1996;35(5):365‐367. [DOI] [PubMed] [Google Scholar]
- 10. Mikesell KV, Kulaylat AN, Donaldson KJ, Saunders BD, Crist HS. A rare soft tissue tumor masquerading as a parathyroid adenoma in a patient with Birt-Hogg-Dubé syndrome and multiple cervical endocrinopathies. Case Rep Pathol. 2014;2014:753694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vinit J, Friedel J, Bielefeld P, Muller G, Goudet P, Besancenot JF. Birt-Hogg-Dubé syndrome and multiple recurrent tumors [article in French]. Rev Med Interne. 2011;32(3):e40‐e42. [DOI] [PubMed] [Google Scholar]
- 12. McLaren W, Gil L, Hunt SE, et al. The Ensembl Variant Effect Predictor. Genome Biol. 2016;17(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clausen L, Stein A, Grønbæk-Thygesen M, et al. Folliculin variants linked to Birt-Hogg-Dubé syndrome are targeted for proteasomal degradation. PLoS Genet. 2020;16(11):e1009187. (In Eng). [DOI] [PMC free article] [PubMed]
- 14. Guan B, Welch JM, Sapp JC, et al. GCM2-activating mutations in familial isolated hyperparathyroidism. Am J Hum Genet. 2016;99(5):1034‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nahorski MS, Reiman A, Lim DHK, et al. Birt Hogg-Dubé syndrome-associated FLCN mutations disrupt protein stability. Hum Mutat. 2011;32(8):921‐929. [DOI] [PubMed] [Google Scholar]
- 16. Sattler EC, Steinlein OK. Delayed diagnosis of Birt-Hogg-Dubé syndrome due to marked intrafamilial clinical variability: a case report. BMC Med Genet. 2018;19(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klemke L, Fehlau CF, Winkler N, et al. The gain-of-function p53 R248W mutant promotes migration by STAT3 deregulation in human pancreatic cancer cells. Front Oncol. 2021;11:642603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmidt LS, Nickerson ML, Warren MB, et al. Germline BHD-mutation spectrum and phenotype analysis of a large cohort of families with Birt-Hogg-Dubé syndrome. Am J Hum Genet. 2005;76(6):1023‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Toro JR, Wei MH, Glenn GM, et al. BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dubé syndrome: a new series of 50 families and a review of published reports. J Med Genet. 2008;45(6):321‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knudson AG. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68(4):820‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Furuya M, Hong SB, Tanaka R, et al. Distinctive expression patterns of glycoprotein non-metastatic B and folliculin in renal tumors in patients with Birt-Hogg-Dubé syndrome. Cancer Sci. 2015;106(3):315‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu Y, Zhang X, Wang O, et al. Integrated whole-exome and transcriptome sequencing of sporadic parathyroid adenoma. Front Endocrinol (Lausanne). 2021;12:631680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clarke CN, Katsonis P, Hsu TK, et al. Comprehensive genomic characterization of parathyroid cancer identifies novel candidate driver mutations and core pathways. J Endocr Soc. 2019;3(3):544‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei Z, Sun B, Wang ZP, et al. Whole-exome sequencing identifies novel recurrent somatic mutations in sporadic parathyroid adenomas. Endocrinology. 2018;159(8):3061‐3068. [DOI] [PubMed] [Google Scholar]
- 25. Pandya C, Uzilov AV, Bellizzi J, et al. Genomic profiling reveals mutational landscape in parathyroid carcinomas. JCI Insight. 2017;2(6):e92061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu W, McPherson JR, Stevenson M, et al. Whole-exome sequencing studies of parathyroid carcinomas reveal novel PRUNE2 mutations, distinctive mutational spectra related to APOBEC-catalyzed DNA mutagenesis and mutational enrichment in kinases associated with cell migration and invasion. J Clin Endocrinol Metab. 2015;100(2):E360‐E364. [DOI] [PubMed] [Google Scholar]
- 27. Newey PJ, Nesbit MA, Rimmer AJ, et al. Whole-exome sequencing studies of nonhereditary (sporadic) parathyroid adenomas. J Clin Endocrinol Metab. 2012;97(10):E1995‐E2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cromer MK, Starker LF, Choi M, et al. Identification of somatic mutations in parathyroid tumors using whole-exome sequencing. J Clin Endocrinol Metab. 2012;97(9):E1774‐E1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Soong CP, Arnold A. Recurrent ZFX mutations in human sporadic parathyroid adenomas. Oncoscience. 2014;1(5):360‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vocke CD, Yang Y, Pavlovich CP, et al. High frequency of somatic frameshift BHD gene mutations in Birt-Hogg-Dubé-associated renal tumors. J Natl Cancer Inst. 2005;97(12):931‐935. [DOI] [PubMed] [Google Scholar]
- 31. Tong Y, Schneider JA, Coda AB, Hata TR, Cohen PR. Birt-Hogg-Dubé syndrome: a review of dermatological manifestations and other symptoms. Am J Clin Dermatol. 2018;19(1):87‐101. [DOI] [PubMed] [Google Scholar]
- 32. Kang H, Pettinga D, Schubert AD, et al. Genomic profiling of parathyroid carcinoma reveals genomic alterations suggesting benefit from therapy. Oncologist. 2019;24(6):791‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hakim JP, Levine MA. Absence of p53 point mutations in parathyroid adenoma and carcinoma. J Clin Endocrinol Metab. 1994;78(1):103‐106. [DOI] [PubMed] [Google Scholar]
- 34. Kishikawa S, Shan L, Ogihara K, et al. Overexpression and genetic abnormality of p53 in parathyroid adenomas. Pathol Int. 1999;49(10):853‐857. [DOI] [PubMed] [Google Scholar]
- 35. Kutahyalioglu M, Nguyen HT, Kwatampora L, et al. Genetic profiling as a clinical tool in advanced parathyroid carcinoma. J Cancer Res Clin Oncol. 2019;145(8):1977‐1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cryns VL, Rubio MP, Thor AD, Louis DN, Arnold A. P53 abnormalities in human parathyroid carcinoma. J Clin Endocrinol Metab. 1994;78(6):1320‐1324. [DOI] [PubMed] [Google Scholar]
- 37. Mahtani K, Park D, Abbott J, Selvam PP, Atwal PS. Importance of family history in the era of exome analysis: a report of a family with multiple concurrent genetic diseases. Hum Hered. 2021;86(1-4):28‐33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated in this paper is not publicly available but available from corresponding author on reasonable request.