Abstract
Context
Management of sporadic medullary thyroid microcarcinoma smaller than 1 cm (micro-MTC) is controversial because of conflicting reports of prognosis. As these cancers are often diagnosed incidentally, they pose a management challenge when deciding on further treatment and follow-up.
Objective
We report the outcomes of surgically managed sporadic micro-MTC in a specialist endocrine surgery and endocrinology unit and identify associations for recurrence and disease-specific survival in this population.
Methods
Micro-MTCs were identified from a prospectively maintained surgery database, and slides were reviewed to determine pathological grade. The primary end points were recurrence, time to recurrence and disease-specific survival. Prognostic factors assessed included size, grade, lymph node metastasis (LNM), and postoperative calcitonin.
Results
From 1995 to 2022, 64 patients were diagnosed with micro-MTC with 22 excluded because of hereditary disease. The included patients had a median age of 60 years, tumor size of 4 mm, and 28 (67%) were female. The diagnosis was incidental in 36 (86%) with 4 (10%) being high grade, 5 (12%) having LNM and 9 (21%) having elevated postoperative calcitonin. Over a 6.6-year median follow-up, 5 (12%) developed recurrence and 3 (7%) died of MTC. High grade and LNM were associated with 10-year survival estimates of 75% vs 100% for low grade and no LNM (hazard ratio = 831; P < .01). High grade, LNM, and increased calcitonin were associated with recurrence (P < .01). Tumor size and type of surgery were not statistically significantly associated with recurrence or survival. No patients with low grade micro-MTC and normal postoperative calcitonin developed recurrence.
Conclusion
Most sporadic micro-MTCs are detected incidentally and are generally associated with good outcomes. Size is not significantly associated with outcomes. Using grade, LNM, and postoperative calcitonin allows for the identification of patients at risk of recurrence to personalize management.
Keywords: medullary thyroid microcarcinoma, surgery, medullary thyroid cancer, tumor grade, outcomes
Medullary thyroid carcinoma (MTC) is a rare and aggressive neuroendocrine tumor derived from the parafollicular cells of the thyroid gland, with around a quarter of patients having cancers associated with hereditary syndromes and the remaining 3 quarters thought to occur in a sporadic fashion. MTC constitutes less than 5% of thyroid cancers, with 5-year survival rates of 83% to 89%, falling to 36% to 51% in metastatic disease (1). Medullary thyroid microcarcinoma (micro-MTC) can be defined as MTC with a maximum diameter of 1 cm or less. There is some debate regarding this definition, with prior studies arguing that this should be redefined to tumors smaller than 0.5 cm based on favorable clinical outcomes, while other research suggests that micro-MTC does not have a biology different from larger cancers (2). In addition, many prior series included both sporadic and hereditary cancers, limiting their clinical application. Further complicating the natural history of micro-MTC is the observation that they have been found incidentally in 0.14% to 0.42% of patients at autopsy (3).
In the absence of effective treatment with radioactive iodine, chemotherapy, or radiotherapy, curative management of MTC is surgical, with targeted RET inhibitors such as selpercatinib and pralsetinib demonstrating efficacy for metastatic and advanced disease (4, 5). Current guidelines recommend total thyroidectomy with dissection of lymph nodes of the central compartment, with lateral nodal dissection indicated in cases of clinical nodal involvement and can be considered when patients have known increased serum calcitonin levels at diagnosis (6). Owing to conflicting results regarding the biology of micro-MTC, its management is less uniform, with Machens and Dralle (7) reporting that unifocal tumors smaller than 0.5 cm found incidentally on hemithyroidectomy do not require completion thyroidectomy or lymph node dissection . This is in contrast to tumors of size 0.5 to 1 cm having been reported as having the same metastatic potential as larger MTC (2).
Established prognostic factors for MTC include age at diagnosis, and tumor stage and size. Until recently there had been no widely accepted grading scale for MTC. However, now a consensus has developed that MTCs can be graded on the basis of the combination of proliferative activity (defined by mitotic count per 2 mm (2) and Ki67 proliferative index) and tumor necrosis (8, 9). In 2022 an international consortium agreed on consensus cut-offs for mitotic rate and Ki67 proliferative index, and then validated this approach in a multicenter study (10). This grading scale has been endorsed by the World Health Organization/International Agency for Research on Cancer (WHO/IARC), and it is now recommended that all MTCs be graded using this system. Under this WHO/IARC grading scale, high-grade MTCs are defined as tumors with at least 1 of the following 3 features: mitotic index 5 or greater per 2 mm2, Ki67 proliferative index greater than or equal to 5%, or tumor necrosis, whereas MTCs lacking all 3 features are considered low grade (10).
The natural history and clinical significance of sporadic micro-MTC is controversial. Considering this and the uncommon yet significant risk of morbidity associated with surgical management (11), there is a lack of consensus regarding the necessary extent of surgery in cases of micro-MTC. Considering the low prevalence of micro-MTC and variability in reported results, it is unclear which patients require further treatment, including completion thyroidectomy or further lymph node dissection, and what extent of follow-up is necessary. We therefore sought to report the outcomes of surgically managed sporadic micro-MTC in a specialist endocrine surgery and endocrinology unit and to identify associations for recurrence and disease-specific survival (DSS) in this population.
Materials and Methods
This study analyzed data from a prospectively maintained endocrine surgery database at an Australian tertiary referral center. Consecutive patients treated surgically for MTC between 1995 and 2022 were included if the maximum dimension of the largest MTC was less than or equal to 10 mm. Patients were excluded if they had hereditary disease due to multiple endocrine neoplasia (MEN) syndromes. Ethics approval was obtained (approval No. 2019/ETH11857) from the Northern Sydney Local Health District Human Research Ethics Committee.
The information collected from the database included demographics, operative details, tumor pathology, follow-up clinical details, biochemistry, and imaging. Any missing data were collected from the medical records. Patient ethnicity was not available in the database.
As this is a historical series, management of MTC has changed over time and management was undertaken using a multidisciplinary approach. The general management approach was as follows. Preoperative diagnosis of MTC was made by fine-needle aspiration biopsy (FNAB). All patients with a known preoperative diagnosis of MTC underwent assessment of lymph nodes using clinical and ultrasound assessment, along with measurement of preoperative calcitonin. Patients with known MTC were managed with total thyroidectomy and bilateral central lymph node dissection. Lateral lymph node dissection was performed if suspicious lymph nodes were seen on preoperative assessment or intraoperatively during the central lymph node dissection or if the calcitonin was elevated beyond 200 pg/mL (6).
For patients who had an incidental finding of MTC following thyroid surgery, postoperative calcitonin measurements were performed, and pathology, calcitonin, and imaging were reviewed at a multidisciplinary team meeting to discuss further management. Patients who had an incidental finding who had been treated with a hemithyroidectomy were generally recommended for completion thyroidectomy with or without prophylactic lymph node dissection following multidisciplinary review. These treatment recommendations were discussed with patients, and not all elected to proceed with further surgery.
Following surgery, all patients with a diagnosis of MTC were referred for genetic counseling and germline genetic testing. If found to have a hereditary cause of MTC, these patients were excluded from this study. Diagnosis of hereditary disease was made on clinical grounds via family history and/or germline genetic testing, which has evolved over the study period (12). Somatic genetic or molecular testing of the resected cancers were not performed for patients in this study.
A specialist endocrine pathologist confirmed the diagnosis and provided a structured report on all tumor specimens using standard definitions. At the time of this study all pathology slides were again reviewed to confirm the diagnosis by a specialist pathologist (T.F.) and to apply the 2-tiered WHO/IARC grading scale to tumors as high-grade based on the presence of any of the following: mitotic index of 5 or greater per 2 mm (2), Ki67 proliferative index of 5% or greater, or tumor necrosis (10) (Fig. 1). This study included a subset of patients whose pathology had previously being reported in the initial description of the grade and were included in this study, which was expanded to include a wider time range and identification of additional cases. For this study, eligible patients were identified from a separate surgical database and notes and outcomes reviewed independently from the prior report (10).
Figure 1.
Representative image of low-grade medullary thyroid microcarcinoma, A, serial hematoxylin and eosin (H&E), and B, Ki67 immunohistochemistry H&E-stained section from a 2-mm tumor. The tumor has a mitotic count of less than 1 per 2 mm2, a Ki67 proliferative index of less than 1%, and lacks tumor necrosis. Therefore, under the grading system the tumor is low grade (original magnification 200×).
Prognostic factors assessed included primary tumor size—both as a continuous variable and divided into smaller than 5 mm and 5 to 10 mm groups, tumor grade, presence of nodal disease, and elevated postoperative calcitonin. Statistical analysis was performed using GraphPad Prism 8 (GraphPad Software). The primary end points were structural recurrence, time to recurrence (TTR), and DSS. Recurrence was diagnosed by detection of structural disease on imaging with radiologically guided biopsy confirmation. TTR was calculated from the date of diagnosis to diagnosis of structural recurrence.
Survival analysis was performed using the log-rank test and variables were compared using the Mann-Whitney U test and Fisher exact test, depending on variable type, with P less than .05 indicating statistical significance. Postoperative calcitonin was considered to be increased at 5 pg/mL or greater, representing biochemical persistent disease. Recurrence was defined as structural recurrence after completion of initial treatment, identified during follow-up by imaging and histologically confirmed.
Results
Demographic and Clinical Characteristics
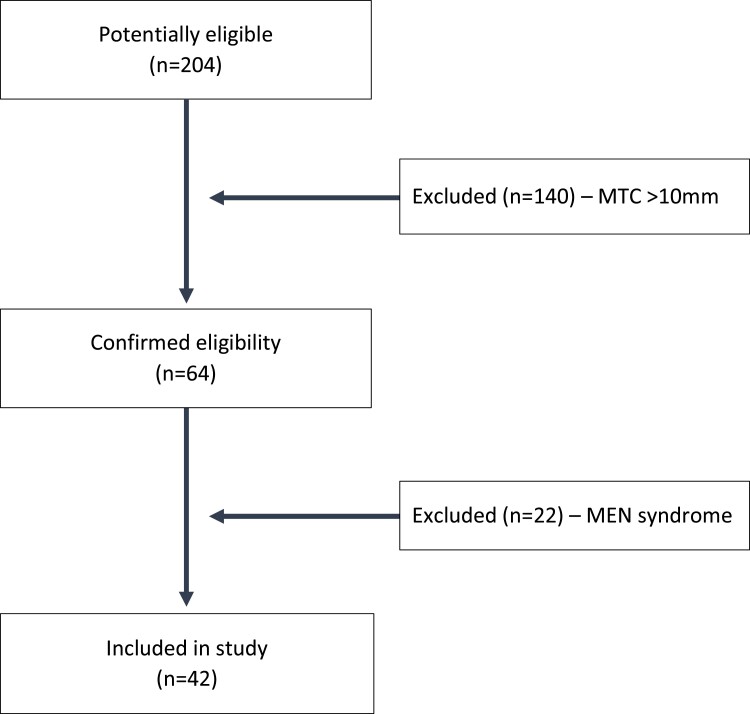
From 1995 to 2022, a total of 204 patients underwent surgery and had a diagnosis of MTC; 64 of these had a maximum tumor size of 10 mm or less (Fig. 2). Twenty-two patients had a diagnosis of hereditary disease due to MEN syndromes and were excluded, leaving 42 patients for analysis. Of these 42 patients, 28 (67%) were female, and the median age was 60 years (range, 25-93 years; Table 1). The diagnosis of micro-MTC was mostly incidental with 36 (86%) patients having a diagnosis following surgery for other indications, the most common being multinodular goiter for 12 patients (29%), other thyroid malignancies for 12 (29%) patients, and thyrotoxicosis for 8 (19%) patients. Four (10%) patients presented with hyperparathyroidism and also underwent thyroid surgery leading to an incidental diagnosis (Table 2).
Figure 2.
Patient flowchart. MEN, multiple endocrine neoplasia; MTC, medullary thyroid carcinoma.
Table 1.
Demographics and tumor characteristics of patients with medullary thyroid microcarcinoma treated by thyroidectomy
| Total population (n = 42) | |
|---|---|
| Sex (female), n (%) | 28 (67%) |
| Age at operation, y, median (interquartile range) | 60 (47-70) |
| Follow-up time, y, median (interquartile range) | 6.6 (3-10) |
| Tumor size, mm, median (interquartile range) | 4 (2-7) |
| Tumor size category, mm, n (%) | |
| < 5 | 25 (60%) |
| 5-10 | 17 (41%) |
| Tumor staging, n (%) | |
| pT1 | 41 (98%) |
| pT2 | 0 (0%) |
| pT3 | 0 (0%) |
| pT4 | 1 (2%) |
| Node staging, n (%) | |
| NX | 15 (36%) |
| N0 | 22 (52%) |
| N1a | 4 (10%) |
| N1b | 1 (2%) |
| Tumor grade, n (%) | |
| Low | 37 (90%) |
| High | 4 (10%) |
| TNM stage, n (%) | |
| I | 37 (88%) |
| II | 0 |
| III | 3 (7%) |
| IV | 2 (5%) |
| Lymphovascular invasion, n (%) | 3 (7%) |
| Multifocal MTC n (%) | 2 (5%) |
| Multifocal thyroid cancer (%) | 14 (33%) |
| Postoperative calcitonin, pg/mL, median (range)a | 5 (< 1-99) |
| Elevated postoperative calcitonin, n (%)a | 8 (20%) |
Abbreviation: MTC, medullary thyroid carcinoma.
n = 40 with postoperative calcitonin result available.
Table 2.
Presentation and management
| Total population (n = 42, %) | |
|---|---|
| Initial presentation | |
| Nodule suspicious for malignancy/confirmed PTC | 12 (29%) |
| Confirmed MTC | 6 (14%) |
| Thyrotoxicosis | 8 (19%) |
| Multinodular goiter | 12 (29%) |
| Hyperparathyroidism | 4 (10%) |
| Surgical approach | |
| Hemithyroidectomy | 4 (10%) |
| Hemithyroidectomy followed by completion | 7 (17%) |
| Central lymph node dissection | 5 (12%) |
| Lateral lymph node dissection | 1 (2%) |
| Total thyroidectomy | 31 (74%) |
| Central lymph node dissection | 11 (26%) |
| Lateral lymph node dissection | 3 (7%) |
Abbreviations: MTC, medullary thyroid carcinoma; PTC, papillary thyroid cancer.
Eleven patients (27%) initially underwent a hemithyroidectomy, with 7 (10%) subsequently having a completion thyroidectomy; 1 patient had a multifocal micro-MTC with 2 2-mm cancers in either lobe. Five patients also underwent central and/or lateral lymph node dissection at the time of completion that did not show any metastatic lymph node disease (see Table 2). The remaining 31 patients (74%) had a total thyroidectomy. Eleven patients (38%) had a central lymph node dissection at the time of initial operation, with 4 of these also having a lateral lymph node dissection (see Table 2). Overall, 25% of lymph node dissections were performed with therapeutic intent and the remainder were performed prophylactically.
Pathological Features
Ten (10%) patients presented with an atypical or suspicious thyroid nodule and underwent surgery for diagnosis and 2 patients (5%) presented with an FNAB Bethesda category VI papillary thyroid cancer (PTC) and subsequently were found to have a second micro-MTC. For patients with a nonincidental diagnosis, all 6 (14%) patients had a diagnosis of MTC before surgery with 5 by confirmation of diagnosis of a suspicious nodule by FNAB and 1 patient following an excisional lymph node biopsy of a level V lymph node that showed MTC and subsequently underwent thyroidectomy.
The median micro-MTC size was 4 mm (range, 0.9-10 mm) and 5 (12%) patients had lymph node metastasis at initial surgery with a mean of 3 metastatic MTC lymph nodes (range, 1-20). No patients had distant metastatic disease at the time of presentation. Of note, 14 patients had multifocal cancers, most of whom (n = 12) had simultaneous PTCs, while 2 had multifocal micro-MTCs. The PTCs were larger than MTC in 7 patients, with the largest cancer being a 16-mm PTC. For 25 patients (60%), the greatest dimension of the largest micro-MTC was smaller than 5 mm, which, compared to 5- to 10-mm tumors, were less likely to have nodal involvement (4% n = 1, vs 24% n = 4; P = .14) or be high grade (0% vs 24% n = 4; P = .02).
Grade was able to be assessed for 41 patients, with 1 patient not having slides available for review, and showed 37 patients (90%) had low-grade cancers and 4 (10%) had high-grade cancers. A high grade was associated with larger cancers (median 9 vs 3 mm), increased calcitonin, and the presence of lymph node metastasis (Table 3). Lymph node metastasis was present in 5 (12%) patients and was associated with a high grade and increased calcitonin (see Table 3). Postoperatively, 40 patients also had at least 1 calcitonin result available for review, of which 8 (20%) were raised, with a median postoperative calcitonin of 5 ng/mL (range, < 1-99 ng/mL).
Table 3.
Association of factors with lymph node metastasis and grade
| LNM (n = 5) | No LNM (n = 37) | P | High grade (n = 4) | Low grade (n = 37) | P | |
|---|---|---|---|---|---|---|
| Sex (female) | 3 (60%) | 25 (68%) | ≥ .999 | 3 (75%) | 24 (65%) | ≥ .999 |
| Age, y | 55 | 61 | .3 | 60 | 58 | .8 |
| Median size (10) | 4.0 | 3.0 | ≥ .999 | 9.0 | 3.0 | .008 |
| Raised calcitonin (n = 8) | 5 | 3 | < .01 | 3 | 5 | .02 |
| High grade (n = 4) | 3 (60%) | 1 (3%) | .004 | — | — | |
| LNM (n = 5) | — | — | — | 3 | 2 | .004 |
Age shown as median. Bold indicates P < 0.05.
Abbreviation: LNM, lymph node metastasis.
Outcomes and Survival
Over a median follow-up of 6.6 years (range, 0.1 to 23 years; interquartile range, 2.7-10.1), 5 patients (12%) developed recurrence at a median of 4 years. For 2 patients (5%), recurrence was first detected in the lateral cervical lymph nodes. The remaining patients were diagnosed with distant recurrence, with 2 patients developing recurrence in the mediastinal lymph nodes and lung, and 1 patient developing distant metastasis with liver, lung, and bone metastases.
During follow-up 11 patients died, of which 3 deaths were due to MTC. For patients who died of MTC, none presented with distant metastasis but developed multiorgan distant metastasis to the lung, mediastinal nodes, and/or bone and liver at 4 to 8 years following initial diagnosis. In total, 5 patients developed recurrence, 3 had initial recurrence with local lymph node disease in the neck, 1 with distant disease and 1 with both local and distant disease. Demographic, clinical, and pathologic details of these patients are presented in Supplementary Table 1 (13). The patients with local disease underwent further lymph node dissection with the aim of achieving local control in the neck, and one patient went on to have tyrosine kinase inhibition as part of a clinical trial during the study period.
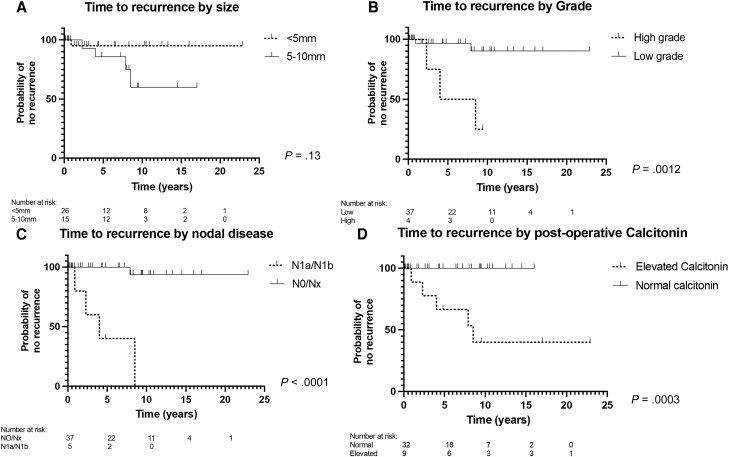
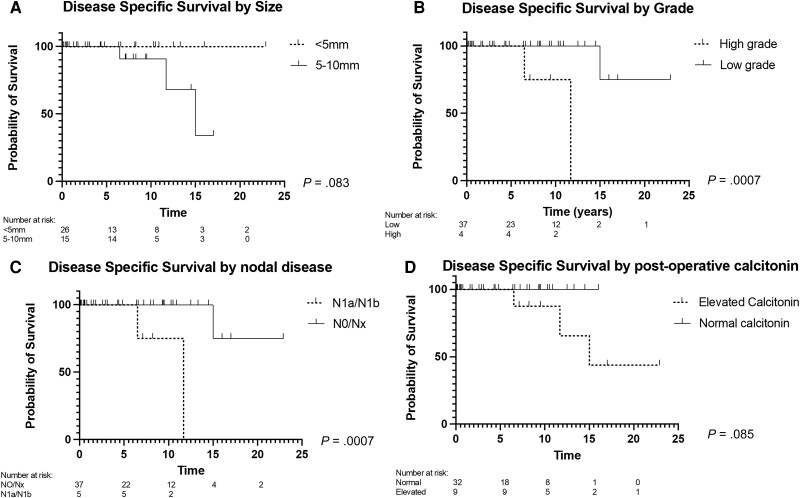
The 5-year estimate of having been recurrence free was 90.5% (95% CI, 73.3%-96.9%), 5-year DSS was 100%, and 5-year overall survival (OS) was 84.3% (95% CI, 66.2%-93.9%). There was no statistical difference in rates of recurrence, by analysis of smaller than 5 mm compared to 5- to 10-mm micro-MTCs (P = .13; Fig. 3A; Table 4) or DSS (Fig. 4A; see Table 4).
Figure 3.
Kaplan-Meier curves comparing patients with medullary thyroid microcarcinoma on the basis of A, time to recurrence (TTR) for tumor size (< 5 mm/5-10 mm); B, TTR for tumor grade (low/high); C, TTR for lymph node involvement (negative/positive); and D, TTR for postoperative calcitonin (normal/increased).
Table 4.
Relationships between prognostic factors
| Recurrence | 5-y estimatea(95% CI) | Hazard ratio (95% CI) | P (log-rank) |
|---|---|---|---|
| Tumor size, mm | |||
| < 5 | 95.0% (69.5%-99.3%) | 3.98 (0.68-23.24) | .12 |
| 5-10 | 85.7% (53.9%-96.2%) | ||
| Grade | |||
| Low | 99.6% (78.6%-99.6%) | 81.51 (5.68-1170) | .001 |
| High | 50% (5.8%-84.5%) | ||
| Nodal involvement | |||
| N0/Nx | 100% | 2118 (116.0-38 682) | < .0001 |
| N1a/N1b | 40% (5.2%-75.3%) | ||
| Postoperative calcitonin | |||
| Normal | 100% | 56.94 (5.87-552.3) | .0005 |
| Raised | 62.5% (22.7%-86.0%) | ||
| Initial surgical management | |||
| Total thyroidectomy | 100% | 3.62 (0.43-30.19) | .23 |
| Hemithyroidectomy | 87.7% (66.0%-95.9%) | ||
| No dissection | 100% | 2.40 (0.40-14.34) | .34 |
| Nodal dissection | 84.9% (60.0%-95.0%) | ||
| Disease-specific survival | 10-y estimate (95% CI) | Hazard ratio (95% CI) | P (log-rank) |
|---|---|---|---|
| Tumor size, mm | |||
| < 5 | 100% | 7.39 (0.77-71.04) | .083 |
| 5-10 | 90.9% (50.8%-98.7%) | ||
| Grade | |||
| Low | 100% | 830.9 (16.9-40 901) | .0007 |
| High | 75% (12.8%-96.1%) | ||
| Nodal involvement | |||
| N0/Nx | 100% | 830.9 (16.9-40 901) | .0007 |
| N1a/N1b | 75% (12.8%-96.1%) | ||
| Postoperative calcitonin | |||
| Normal | 100% | 15.08 (0.84-269.8) | .065 |
| Raised | 85.7% (33.5%-97.7%) | ||
| Initial surgical management | |||
| Total thyroidectomy | 95% (69.4%-99.2%) | 3.14 (0.049-200.1) | .29 |
| Hemithyroidectomy | 100% | ||
| No dissection | 100% | 2.58 (0.24-28.04) | .44 |
| Nodal dissection | 92.9% (59.0%-98.9%) | ||
Estimates for recurrence show no risk of recurrence.
Figure 4.
Kaplan-Meier curve comparing patients with medullary thyroid microcarcinoma on the basis of A, disease-specific survival (DSS) for tumor size (< 5 mm/5 to 10 mm); B, DSS for tumor grade (low/high); C, DSS for lymph node involvement (negative/positive); and D, DSS for postoperative calcitonin (normal/increased).
For grade, 5-year estimates for TTR rates were significantly different at 100% for low-grade and 50% for high-grade (P = .0012; Fig. 3B; see Table 4). The median TTR for high-grade cancers was 6.25 years. DSS rates were also significantly different for high-grade cancers, having worse survival (P = .0007; Fig. 4B; see Table 4) with a median survival of 11.7 years.
For patients with nodal involvement, recurrence was more common, and survival was poorer compared to those with no known nodal involvement (Figs. 3C and 4C; see Table 4). Patients with LNM had a median time to recurrence of 4 years and survival of 11.7 years.
For patients with biochemical persistent disease, represented by increased postoperative calcitonin (n = 8), structural recurrence was significantly more common than for those with a normal postoperative calcitonin (n = 33; Fig. 3D; see Table 4), of which 23 were detectable at 5 pg/mL or less and 10 were undetectable. No significant association was found between elevated postoperative calcitonin and DSS (Fig. 4D; see Table 4). Patients with an increased calcitonin level had a median follow-up of 9 years with a median TTR of 8.5 years.
For the 8 patients with an increased calcitonin, serial results were available for review for 7 patients. Four patients had calcitonin return to levels of less than 5 pg/mL during follow-up, which for 2 patients returned to normal within 12 months of surgery without further treatment. For 2 patients, calcitonin returned to normal 1 and 3 years from the time of initial surgery after being diagnosed with recurrence in the lateral lymph nodes during follow-up and undergoing lateral lymph node dissection. Two patients had persistently high calcitonin with a high-grade cancer and were later diagnosed with distant metastatic disease. For the remaining patient, also with a high-grade cancer, calcitonin returned to normal 12 months after surgery and during follow-up rose after they developed recurrence with distant metastatic disease.
With regard to surgical approach, there was no statistical difference in recurrence for patients treated by hemithyroidectomy compared to total thyroidectomy (0% vs 16%; P ≥ .999) or completion thyroidectomy compared to total thyroidectomy (0% vs 16%; P ≥ .999). For lymph node dissection, excluding therapeutic dissections there was no statistical difference in recurrence for patients who underwent a prophylactic lymph node dissection vs those who did not undergo a dissection (8.3% vs 3.9%; P = .54). Compared to patients with an incidental diagnosis, patients who presented with MTC diagnosed preoperatively were less likely to have stage 1 disease (50% vs 94%) and had higher rates of lymph node metastasis (50% vs 6%) and higher rates of recurrence and poorer survival (Supplementary Table 2; Supplementary Fig. 1) (13).
Discussion
The results of this study demonstrate that patients with sporadic micro-MTC generally have good outcomes with most cancers being detected incidentally and that, if low grade with a normal postoperative calcitonin, these are not associated with structural recurrence. In this study, a high-grade cancer, lymph node metastasis, and an increased postoperative calcitonin were associated with structural recurrence. As grade is available at the time of pathological review, the use of this system offers the opportunity to tailor follow-up and further treatment to higher-risk patients. In this series no patient with a low-grade cancer and a normal postoperative calcitonin developed structural recurrence, suggesting that such patients may not require further treatment or extensive follow-up after a diagnosis of micro-MTC if these findings can be confirmed in other series.
This series demonstrates that most patients presented with an incidental diagnosis of micro-MTC, with presentations that range from thyrotoxicosis to multinodular goiter. The management of patients following an incidental postoperative diagnosis of micro-MTC is varied, with previous management being guided by the postoperative calcitonin level, genetic testing to exclude hereditary causes of MTC, and patient preference. A number of patients treated in the earlier period of the series underwent completion thyroidectomy and/or further surgery with prophylactic central and lateral lymph node dissection following diagnosis with clear lymph nodes. All of these patients' cancers would, if being treated in the current period, be classified as low grade.
The existing literature regarding micro-MTC is limited and variable, resulting in controversy regarding the appropriate extent of surgical treatment especially regarding a size threshold for cancers, with larger than 5 mm suggested as a cutoff for clinically relevant cancers (2). Reported rates of nodal involvement in sporadic micro-MTC have varied greatly, from 5% to 31% (14). Recent studies have demonstrated lower, but not insignificant, rates of nodal involvement in patients with tumors smaller than 5 mm. A patient in this series with a cancer of 4 mm later developed lateral lymph node recurrence, suggesting that this size cut-off has limitations when applied to practice (15).
A strength of this series is the detailed clinical information offered by a specialist center database, which complements population studies such as those using the Surveillance, Epidemiology, and End Results (SEER) registry, which provide population-level analyses including information regarding extent of disease and survival. Such studies have described 10-year OS of 96% for localized disease and 50% for patients with distant metastasis (16). However, many studies, including those using the SEER registry, do not separate sporadic and hereditary MTC, despite substantial differences in age and stage at diagnosis (7).
This study demonstrates the utility of tumor grade in predicting outcomes. Using patient cohorts of any size MTC, this grade has previously demonstrated significance in predicting OS, DSS, and disease-free survival (10, 17). Given its proven validity in MTC, its association with nodal involvement and recurrence, as well as its postoperative availability, the use of grading in micro-MTC warrants further research as it represents a potential tool to assist in determining the appropriate extent of surgery and follow-up following the identification of sporadic micro-MTC. The ability to effectively distinguish patients at risk of recurrence and a worse DSS using results available from the time of surgery has the potential to facilitate clinical decision-making to minimize morbidity associated with MTC and surgical management itself. In addition, for these patients there is the potential benefit of allowing earlier identification of those at risk for closer follow-up and consideration of further therapies and clinical trials.
While this study is retrospective, it brings together a substantial number of patients with a rare presentation and a median follow-up time of more than 6 and a half years with follow-up of some patients over 20 years. The results of this series must be assessed acknowledging the small number of events with recurrence (n = 5) that limit analysis. This low number of events meant that further exploration of the relationships of these associations using Cox regression was not possible. One patient (who died of unrelated causes) had missing grade information. Imputing this patient's grade as high or low had a minimal effect on the results.
In addition, TTR as determined in this study is dependent on the timing of imaging studies to diagnose structural recurrence and so may be affected by follow-up practices and the clinicians and patients' concerns for recurrence, which could lead to differences in the TTR diagnosis. While this is a valid drawback of this series, we would maintain the recurrence rate of patients in this series shows that recurrence is a rare event for small MTCs. Despite these acknowledged limitations, this series brings together one of the larger series of these patients and challenges previous reports with regard to size and outcome (2).
For these results to have a wider effect it is important the grade be reproduced, which has recently been assessed as being reproducible within a specialist pathology unit (18). Following a diagnosis of micro-MTC, current practice includes referral for genetic counseling and testing with follow-up and further treatment dependent on the patient's clinical presentation, which has included completion thyroidectomy or extensive follow-up. If the results from this study can be replicated, it would support discharge from follow-up for patients with sporadic disease, low-grade cancers, and normal postoperative calcitonin. In addition, for patients with a low-grade cancer diagnosed following hemithyroidectomy with a normal contralateral lobe, these results would support conservative management without completion thyroidectomy.
For this study, the pathologist assigning grade (T.F.) was blinded to the history and outcomes of the patients but aware of the diagnosis of micro-MTC, and patients received a high grade because of the presence of necrosis. Further research in applying how grading can apply to micro-MTCs from hereditary disease will be a future area of research, and efforts to assess the application of this grading system outside specialist pathologists with thyroid cancer expertise will need to be assessed (17).
Conclusions
Sporadic micro-MTC has a low, but not insubstantial, rate of recurrence. High-grade cancers, lymph node metastasis, and increased postoperative calcitonin are associated with structural recurrence, and high grade and lymph node metastasis are associated with reduced DSS. No patients with a low-grade cancer and normal postoperative calcitonin developed structural recurrence. Use of tumor grade, nodal involvement, and postoperative calcitonin has the potential to allow individualized management following the identification of sporadic micro-MTC, allowing clinicians and patients to balance treatment decisions when faced with a likely unexpected diagnosis.
Acknowledgment
An earlier version of this paper was presented at the 2022 Royal Australasian College of Surgeons Annual Scientific Congress.
Abbreviations
- DSS
disease-specific survival
- FNAB
fine-needle aspiration biopsy
- LNM
lymph node metastasis
- MEN
multiple endocrine neoplasia
- micro-MTC
medullary thyroid microcarcinoma
- MTC
medullary thyroid carcinoma
- OS
overall survival
- PTC
papillary thyroid cancer
- SEER
Surveillance, Epidemiology, and End Results
- TTR
time to recurrence
- WHO/IARC
World Health Organization/International Agency for Research on Cancer
Contributor Information
Nicholas Kesby, Endocrine Surgery Unit, Royal North Shore Hospital, Northern Sydney Local Health District, St Leonards, NSW 2065, Australia; The Kinghorn Cancer Centre, Garvan Institute of Medical Research, St Vincent's Clinical School, Faculty of Medicine, University of New South Wales, Darlinghurst, NSW 2010, Australia.
Robert Mechera, Endocrine Surgery Unit, Royal North Shore Hospital, Northern Sydney Local Health District, St Leonards, NSW 2065, Australia; Clarunis, University Hospital Basel, Basel, Basel-Stadt 4031, Switzerland.
Talia Fuchs, NSW Health Pathology, Department of Anatomical Pathology, Royal North Shore Hospital, St Leonards, NSW 2065, Australia.
Alexander Papachristos, Endocrine Surgery Unit, Royal North Shore Hospital, Northern Sydney Local Health District, St Leonards, NSW 2065, Australia; Northern Clinical School, Sydney Medical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW 2065, Australia.
Matti Gild, Northern Clinical School, Sydney Medical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW 2065, Australia; Department of Endocrinology, Royal North Shore Hospital, Northern Sydney Local Health District, St Leonards, NSW 2065, Australia.
Venessa Tsang, Northern Clinical School, Sydney Medical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW 2065, Australia; Department of Endocrinology, Royal North Shore Hospital, Northern Sydney Local Health District, St Leonards, NSW 2065, Australia.
Roderick Clifton-Bligh, Northern Clinical School, Sydney Medical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW 2065, Australia; Department of Endocrinology, Royal North Shore Hospital, Northern Sydney Local Health District, St Leonards, NSW 2065, Australia.
Bruce Robinson, Northern Clinical School, Sydney Medical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW 2065, Australia; Department of Endocrinology, Royal North Shore Hospital, Northern Sydney Local Health District, St Leonards, NSW 2065, Australia.
Mark Sywak, Endocrine Surgery Unit, Royal North Shore Hospital, Northern Sydney Local Health District, St Leonards, NSW 2065, Australia; Northern Clinical School, Sydney Medical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW 2065, Australia.
Stan Sidhu, Endocrine Surgery Unit, Royal North Shore Hospital, Northern Sydney Local Health District, St Leonards, NSW 2065, Australia; Northern Clinical School, Sydney Medical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW 2065, Australia.
Angela Chou, NSW Health Pathology, Department of Anatomical Pathology, Royal North Shore Hospital, St Leonards, NSW 2065, Australia; Northern Clinical School, Sydney Medical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW 2065, Australia.
Anthony J Gill, NSW Health Pathology, Department of Anatomical Pathology, Royal North Shore Hospital, St Leonards, NSW 2065, Australia; Northern Clinical School, Sydney Medical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW 2065, Australia.
Anthony Glover, Endocrine Surgery Unit, Royal North Shore Hospital, Northern Sydney Local Health District, St Leonards, NSW 2065, Australia; The Kinghorn Cancer Centre, Garvan Institute of Medical Research, St Vincent's Clinical School, Faculty of Medicine, University of New South Wales, Darlinghurst, NSW 2010, Australia; Northern Clinical School, Sydney Medical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW 2065, Australia.
Funding
A.G. is supported by a Cancer Institute NSW Early Career Fellowship (2019/ECF1081) for this project.
Author Contributions
N.K.: methodology, data curation, formal analysis, writing—original draft, writing—reviewing and editing; R.M.: supervision, writing—reviewing and editing; T.F.: data curation, formal analysis, writing—reviewing and editing; A.P.: writing—reviewing and editing. M.G.: writing—reviewing and editing; V.T.: writing—reviewing and editing; R.C.B.: writing—reviewing and editing; B.R.: writing—reviewing and editing; M.S.: writing—reviewing and editing; S.S.: writing—reviewing and editing; A.C.: writing—reviewing and editing; A.G.: writing—reviewing and editing; A.G.: supervision, conceptualization, methodology, writing—reviewing and editing. All authors were responsible for the final approval of the manuscript.
Disclosures
The authors have nothing to disclose.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Randle RW, Balentine CJ, Leverson GE, et al. Trends in the presentation, treatment, and survival of patients with medullary thyroid cancer over the past 30 years. Surgery. 2017;161(1):137‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pillarisetty VG, Katz SC, Ghossein RA, Tuttle RM, Shaha AR. Micromedullary thyroid cancer: how micro is truly micro? Ann Surg Oncol. 2009;16(10):2875‐2881. [DOI] [PubMed] [Google Scholar]
- 3. Valle LA, Kloos RT. The prevalence of occult medullary thyroid carcinoma at autopsy. J Clin Endocrinol Metab. 2011;96(1):E109‐E113. [DOI] [PubMed] [Google Scholar]
- 4. Wirth LJ, Sherman E, Robinson B, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. 2020;383(9):825‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jozaghi Y, Zafereo M, Williams MD, et al. Neoadjuvant selpercatinib for advanced medullary thyroid cancer. Head Neck. 2021;43(1):E7‐E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wells SA Jr, Asa SL, Dralle H, et al. ; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma . Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Machens A, Dralle H. Biological relevance of medullary thyroid microcarcinoma. J Clin Endocrinol Metab. 2012;97(5):1547‐1553. [DOI] [PubMed] [Google Scholar]
- 8. Fuchs TL, Nassour AJ, Glover A, et al. A proposed grading scheme for medullary thyroid carcinoma based on proliferative activity (Ki-67 and mitotic count) and coagulative necrosis. Am J Surg Pathol. 2020;44(10):1419‐1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alzumaili B, Xu B, Spanheimer PM, et al. Grading of medullary thyroid carcinoma on the basis of tumor necrosis and high mitotic rate is an independent predictor of poor outcome. Mod Pathol. 2020;33(9):1690‐1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu B, Fuchs TL, Ahmadi S, et al. International medullary thyroid carcinoma grading system: a validated grading system for medullary thyroid carcinoma. J Clin Oncol. 2022;40(1):96‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hayward NJ, Grodski S, Yeung M, Johnson WR, Serpell J. Recurrent laryngeal nerve injury in thyroid surgery: a review. ANZ J Surg. 2013;83(1-2):15‐21. [DOI] [PubMed] [Google Scholar]
- 12. Learoyd DL, Marsh DJ, Richardson AL, Twigg SM, Delbridge LW, Robinson BG. Genetic testing for familial cancer. Consequences of RET proto-oncogene mutation analysis in multiple endocrine neoplasia, type 2. Arch Surg. 1997;132(9):1022‐1025. [DOI] [PubMed] [Google Scholar]
- 13. Kesby N, Mechera R, Fuchs TL, et al. Supplementary material for “Natural history and predictive factors of outcome in medullary thyroid microcarcinoma.” Sydney eScholarship Repository: University of Sydney; Submitted 29/4/2023. [DOI] [PMC free article] [PubMed]
- 14. Kim JH, Pyo JS, Cho WJ. Clinicopathological significance and prognosis of medullary thyroid microcarcinoma: a meta-analysis. World J Surg. 2017;41(10):2551‐2558. [DOI] [PubMed] [Google Scholar]
- 15. Saltiki K, Rentziou G, Stamatelopoulos K, et al. Small medullary thyroid carcinoma: post-operative calcitonin rather than tumour size predicts disease persistence and progression. Eur J Endocrinol. 2014;171(1):117‐126. [DOI] [PubMed] [Google Scholar]
- 16. Kazaure HS, Roman SA, Sosa JA. Medullary thyroid microcarcinoma. Cancer. 2012;118(3):620‐627. [DOI] [PubMed] [Google Scholar]
- 17. Endo M, Huang EC, Roth MY. Newly developed pathologic grading system predicts clinical outcomes in medullary thyroid carcinoma. Clin Thyroidol. 2022;34(2):89‐91. [Google Scholar]
- 18. Williams JF, Zhao M, Najdawi F, et al. Grading of medullary thyroid carcinoma: an interobserver reproducibility study. Endocr Pathol. 2022;33(3):371‐377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.