Abstract
Context
Congenital combined pituitary hormone deficiency (cCPHD) is the loss of ≥2 pituitary hormones caused by congenital factors.
Objective
We aimed to estimate the national incidence of cCPHD diagnosed before age 18 years and in subgroups.
Methods
Patients with cCPHD were identified in the Danish National Patient Registry and Danish hospital registries in the period 1996-2020. Hospital files were reviewed and incidences calculated using background population data. Incidence was the main outcome measure.
Results
We identified 128 patients with cCPHD; 88 (68.8%) were males. The median (range) age at diagnosis was 6.2 (0.01-19.0) years. The median (25th;75th percentile) number of hormone deficiencies at diagnosis was 3 (3; 4) at <1 year vs 2 (2; 2) at 1-17 years, P < .0001. Abnormal pituitary magnetic resonance imaging findings were seen in 70.3% (83/118). For those born in Denmark aged <18 years at diagnosis (n = 116/128) the estimated national incidence (95% CI) of cCPHD was 10.34 (7.79-13.72) per 100 000 births, with an annual incidence rate of 5.74 (4.33-7.62) per million. In subgroup analysis (diagnosis <1 vs 1-17 years), the incidence was highest in the 1-17 years subgroup, 7.97 (5.77-11.00) vs 1.98 (1.39-2.84) per 100 000 births, whereas the annual incidence rate was highest at <1 year, 19.8 (13.9-28.4) vs 4.69 (3.39-6.47) per million births.
Conclusion
cCPHD had the highest incidence rate and the most hormone deficiencies in those diagnosed at <1 year. The incidence was highest in the 1-17 years age group, underscoring the need for multiple pituitary hormone investigations throughout childhood and adolescence in children with only 1 hormone deficiency.
Keywords: congenital hypopituitarism, congenital combined pituitary hormone deficiency, cCPHD, cMPHD, diagnosis of hypopituitarism, incidence
Pituitary hormone deficiency (PHD) may be isolated with only 1 hormone deficiency or combined (CPHD) with ≥2 hormone deficiencies (1, 2). CPHD may be acquired or congenital (cCPHD), the latter defined as partial or complete loss of ≥2 hormones secreted from the pituitary gland caused by genetic factors or malformation (2–4). CPHD is considered congenital in the absence of identified acquired causes such as cerebral tumors, surgery, infection, radiotherapy, hemorrhage, infarction, and infiltrative or granulomatous disease in the hypothalamic–pituitary region (5, 6).
cCPHD may be caused by genetic variants of genes involved in the embryological development of the midbrain and pituitary, including HESX1, LHX3, LHX4, POU1F1, PROP1, SIX6, OTX2, PITX2, GLI2, and SOX3. However, despite increasing knowledge, most patients remain genetically unexplained when screened for genetic mutations (2, 7, 8). Disruption of early-acting transcription factors of pituitary organogenesis may lead to extrapituitary abnormalities such as septo-optic dysplasia, holoprosencephaly, or aplasia/hypoplasia of other brain structures, especially in the midline. In contrast, disruption of later-acting transcription factors leads to a more pituitary-specific phenotype with hormone deficiencies alone (9, 10).
cCPHD has a broad clinical spectrum from potentially life-threatening multiple hormone deficiencies in the neonate to a late-onset gradual presentation in childhood or adolescence (1, 4, 6, 11, 12). A wide variation occurs in both age of onset, presence of cerebral malformations, and composition of hormone deficiencies, even within the same family (3, 13).
Neonates with clinical overt cCPHD may present with micropenis and other features of hypovirilization (boys), hypoglycemia, electrolyte abnormalities, prolonged jaundice, failure to thrive, and severe hypernatremic dehydration due to central diabetes insipidus (3, 6). Onset of cCPHD in later infancy or childhood may occur as a gradual process with the hormone deficiencies developing consecutively with symptoms and signs of growth retardation, constipation, fatigue, developmental delay, polyuria, intense thirst, and/or delayed puberty (13, 14).
A recent Finnish study from 1 tertiary hospital estimated the incidence of cCPHD to 1 in 16 000 live-born children (6). In Denmark, all patients are registered in the Danish National Patient Registry (DNPR) with information on their ICD-10 diagnoses. Treatment of cCPHD in children is only approved in 4 tertiary hospitals. Given these unique possibilities, we performed a nationwide registry and hospital file study, aiming to estimate the national incidence of cCPHD diagnosed before age 18 years and in subgroups, including hormone deficiency characteristics and brain magnetic resonance imaging (MRI) abnormalities in the patients.
Materials and Methods
Study Population
The study included all patients with cCPHD in Denmark born between January 1, 1996, and December 31, 2020, identified through ICD-10 diagnosis code searches in the DNPR and locally at the 4 Danish tertiary hospitals.
The DNPR contains all inpatients in Denmark since 1977 and all outpatients since 1995, with ICD-10 diagnosis codes since 1994. Whenever a patient is in contact with a Danish hospital, 1 primary and an optional number of secondary ICD-10 diagnosis codes are registered as the reason for the contact, along with codes identifying the reporting hospital and department. The DNPR identifies patients based on a central person registrations number, which is a unique personal identification number, including information on date of birth, given to all Danish citizens since 1968. The validity and completeness of the DNPR ICD-10 diagnosis code registration allow for national incidence estimations according to a systematic review (15).
The DNPR search identified all Danish patients with relevant diagnoses before age 15 years from January 1, 1996, to December 31, 2020. As cCPHD does not have a specific ICD-10 diagnosis code, we had to search the DNPR for broader pituitary-related ICD-10 diagnosis codes E23.0-E23.9 (hypofunction and other disorders of pituitary) and Q89.2G (congenital malformation of pituitary), thus including both isolated PHD and acquired CPHD. A number of additional ICD-10 diagnosis codes indicating an acquired etiology of PHD were used as exclusion criteria, as detailed in Table 1. Furthermore, identified patients who were not treated at a tertiary hospital were excluded. To detect failure of referral of patients with cCPHD to a tertiary hospital, we reviewed the hospital files of all patients registered at 1 secondary hospital in the DNPR data set as a spot sample (Table 2). This was done to examine the frequency of referral failure and, through that, the validity of the method used to identify patients with cCPHD.
Table 1.
Search strategy for cCPHD by ICD-10 diagnosis codes
| ICD-10 code | Diagnoses |
|---|---|
| Inclusion criteria diagnosis codes | |
| E23.0-E23.9 | Hypofunction and other disorders of pituitary |
| Q89.2G | Congenital malformation of pituitary |
| Q04.0-Q04.9a | Other congenital malformations of brain |
| Exclusion criteria diagnosis codes | |
| C69.0-C69.9 | Malignant neoplasm of eye and adnexa |
| C70.0-C70.9 | Malignant neoplasm of meninges |
| C71.0-C71.9 | Malignant neoplasm of brain |
| C72.0-C72.9 | Malignant neoplasm of spinal cord, cranial nerves, and other parts of central nervous system |
| C75.1-C75.3 | Malignant neoplasm of pituitary gland, craniopharyngeal duct, and pineal gland |
| D33.0-D33.9 | Benign neoplasm of brain and other parts of central nervous system |
| D35.2-D35.4 | Benign neoplasm of pituitary gland, craniopharyngeal duct, and pineal gland |
| A80.0-A89.9 | Viral infections of the central nervous system |
| I60.0-I60.9b | Subarachnoid hemorrhage |
| I61.0-I61.9b | Intracerebral hemorrhage |
| I62.0-I62.9b | Other nontraumatic intracranial hemorrhage |
| I63.0-I63.9b | Cerebral infarction |
| I64a | Stroke, not specified as hemorrhage or infarction |
A search on these ICD-10 diagnosis codes was performed at each the 4 tertiary hospitals as a spot sample (n = 80).
These ICD-10 diagnosis codes were only used as an exclusion criterion if the registration occurred prior to registration of E23.0-E23.9 and/or Q89.2G ICD-10 codes.
Table 2.
Hospitals included in the tertiary hospital registry search and the secondary hospital spot sample
| Hospitals | Search period |
|---|---|
| Tertiary hospitals | |
| Department of Pediatrics, Aalborg University Hospital | January 1, 1996-December 31, 2020 |
| Department of Pediatrics and Adolescent Medicine, Aarhus University Hospital | January 1, 2000-December 31, 2020 |
| Hans Christian Andersen Children's Hospital, Odense University Hospital | January 1, 2016-December 31, 2020 |
| Department of Growth and Reproduction, Rigshospitalet | November 6, 2016-December 31, 2020 |
| Secondary hospital, spot sample | |
| Department of Pediatrics, Kolding Hospital | January 1, 1996-December 31, 2020 |
For validation, supplementary ICD-10 diagnosis code searches on E23.0-E23.9, Q89.2G, and Q04.0-Q04.9 (other congenital malformations of brain) were performed locally at the 4 tertiary hospitals for variable periods according to the possibilities of the individual hospital registries (Table 2).
The hospital registry searches, which included patients with an above-mentioned diagnosis code given at the age of 0-17 years, were followed by hospital file examinations to exclude patients born outside the study period 1996-2020 and patients with ICD-10 diagnosis codes indicating acquired PHD. Next, the DNPR and hospital registry searches were merged by central person registration numbers to disallow patient duplets, followed by a retrospective review of all available hospital files of the remaining patients to validate the cCPHD diagnosis and exclude patients with fewer than 2 documented secondary/tertiary hormone deficiencies or acquired CPHD.
Data Collection
Hospital file data were collected from March 1, 2021, to June 30, 2022. For the finally included patients, the following data were included: date of birth, sex as indicated by the presence of male/female genitals (male/female), born in Denmark (yes/no), date and results of blood samples and stimulation tests of interest (low/normal/inadequately high/high), date and result of urine osmolality measurement (low/normal/high), abnormalities of the adenohypophysis, neurohypophysis, pituitary stalk, midline, and cerebrum by the latest MRI (aplasia/hypoplasia/ectopic/normal or yes/no), and clinical signs of hormone deficiencies (yes/no).
Validation of Hormone Deficiencies
All hormone deficiencies were retrospectively validated using the criteria in Table 3. The criteria were defined by 5 experienced pediatric endocrinologists, including a representative from each of the tertiary hospitals and were based on clinical practice and national as well as international literature and guidelines (1, 3, 16–20). We chose to also include validated congenital deficiency of thyrotropin-releasing hormone and gonadotropin-releasing hormone. Blood sample and stimulation test results were categorized as low/normal/inadequately high/high based on the clinical assessment in the hospital file and the existing reference values at the given time and hospital.
Table 3.
Criteria used for validation of secondary and tertiary hormone deficiency
| Pituitary hormone | Criteria for validation of secondary and tertiary hormone deficiency |
|---|---|
| Growth hormone (GH) | Two pathological GH stimulation tests and concomitantly relevant clinical features. |
| One pathological GH stimulation test, low/normal levels of IGF-1, low/normal levels of IGF-BP3 if measured, concomitantly relevant clinical features, and validated secondary/tertiary deficiency of ≥1 hormone (this criterion was solely considered valid if only 1 GH stimulation test was performed). | |
| Low/normal levels of IGF-1, low/normal levels of IGF-BP3 if measured, concomitantly relevant clinical features, and validated secondary/tertiary deficiency of ≥1 hormone (this criterion was solely considered valid if no GH stimulation tests were performed). | |
| Thyrotropin (TSH) | Low levels of thyroxine/free thyroxine, concomitantly low/normal/inadequately high levels of TSH, and concomitantly relevant clinical features. |
| Follicle-stimulating hormone/luteinizing hormone (FSH/LH) | One pathological relevant stimulation test, low levels of estradiol/testosterone, concomitantly low/normal levels of LH and FSHa, and concomitantly relevant clinical features. |
| Low levels of estradiol/testosterone, concomitantly low/normal levels of LH and FSHa, concomitantly relevant clinical features, and validated secondary/tertiary deficiency of ≥1 hormone (this criterion was solely considered valid if no stimulation test was performed). | |
| Relevant clinical features, initiation of sex hormone replacement treatment, and validated secondary/tertiary deficiency of ≥1 hormone (this criterion was solely considered valid if no stimulation test or blood samples were performed). | |
| Persistently low levels of testosterone/estradiol, concomitantly low/normal levels of FSH and LH, 1 GnRH stimulation test showing normal pituitary functiona, no indication of peripheral hormone deficiency, and ≥1 validated secondary/tertiary hormone deficiency. | |
| Adrenocorticotropin (ACTH) | One pathological relevant stimulation test and concomitantly relevant clinical features. |
| Antidiuretic hormone (ADH) | One pathological relevant stimulations test and relevant clinical features. |
| High serum osmolality/high serum sodium, low urine osmolality, concomitantly relevant clinical features, and validated secondary/tertiary deficiency of ≥1 hormone (this criterion was solely considered valid if no stimulation test was performed). |
Abbreviations: GnRH, gonadotropin-releasing hormone; IGF-1, insulin-like growth factor-1; IGF-BP3, insulin-like growth factor binding protein 3.
Stimulation tests and blood samples must be performed at an appropriate age: age 0 to 6 months (minipuberty), age >11 years (girls)/>12 years (boys) if secondary/tertiary deficiency of ≥1 other hormone has been validated, or age >9 years (girls)/>10 years (boys), if secondary/tertiary deficiency of ≥2 others has been validated.
Transient hormone deficiencies were excluded. We included children diagnosed with childhood growth hormone deficiency (GHD) without requiring retesting in the transition phase for adult GHD, where lower GH cut-offs are used. Patients with birth asphyxia or infant brain hemorrhage were considered having cCPHD only if structural pituitary abnormalities were described by MRI. In cases of central hypothyroidism where hormone replacement treatment had not been initiated, thyroid stimulating hormone (TSH) deficiency was retrospectively judged present when at least 2 consecutive and latest blood samples met our defined criteria. To distinguish central hypogonadism from constitutional delay in puberty, we additionally reviewed the patient files for inadequate treatment of TSH or GH deficiency, which can cause a delay in puberty, and for mentions of familial predisposition to constitutional delay in puberty. If any of these conditions were detected, the patients were not considered to have central hypogonadism after validation.
Every validated hormone deficiency was assigned a date of diagnosis, which we defined as the date of the blood sample or stimulation test that led to the diagnosis by clinicians. If the date of the blood sample/stimulation test was unavailable, we instead used the date of the hospital file note commenting on the result. The age at diagnosis of cCPHD was defined as the age at diagnosis of the second hormone deficiency.
Data Analysis
All validated cases of cCPHD made up the national cCPHD cohort. The national cCPHD incidence cohort was formed by excluding patients born outside Denmark and patients diagnosed with cCPHD after age 17 years. The incidence was calculated using the birth year of patients with cCPHD, dividing the number of patients with cCPHD born in a period with the number of live births in Denmark in the same period. Data on the annual birth counts were obtained from Statistics Denmark. Subgroup incidences were calculated by dividing patients into 2 groups according to their age at diagnosis: <1 year and 1-17 years.
Incidence rates were calculated by dividing the number of patients with cCPHD born in a period with the corresponding person-time of the birth cohorts. The person-time was determined by multiplying the birth counts with a factor of 1 (subgroup <1 year); 17 (subgroup 1-17 years); and 18 (the national incidence cohort).
The incidence calculations were subjected to right truncation from 2003 to 2020, as children born in this period did not reach age 18 years in the observation period. To eliminate the influence of right truncation, we calculated the overall incidence in the period 1996-2002. For the subgroup diagnosed before age 1 year, the incidence was calculated in the period 1996-2019. For completeness, we also calculated the incidence of cCPHD diagnosed at all ages (0-19 years) and the incidence of cCPHD diagnosed before age 18 years for the whole period 1996-2020.
The SE and the 95% CI were calculated using the formulas 1/, 2/, and 3/. In the formulas, “a” represents the number of clinical events, “IR” the incidence rate, and “e” the exponential constant.
Statistical calculations were performed using Stata/IC 16.1, Microsoft Excel version 16.57, and Maple 2020.2. Pearson's chi-square goodness-of-fit test was used to analyze differences in the sex distribution. The background population sex distribution from 1996 to 2020 served as reference (Statistics Denmark). Fisher’s exact test was performed to explore associations between the number of hormone deficiencies and MRI abnormalities and age at diagnosis. P < .05 was considered to be statistically significant.
Ethics
This study was approved by the region of Southern Denmark (j.no. 20/58158). To ensure compliance with the General Data Protection Regulation and the Danish Data Protection Act, data were collected and managed in collaboration with the Open Patient data Explorative Network (OPEN) using Microsoft SharePoint and REDCap (Research Electronic Data Capture) (21, 22).
Results
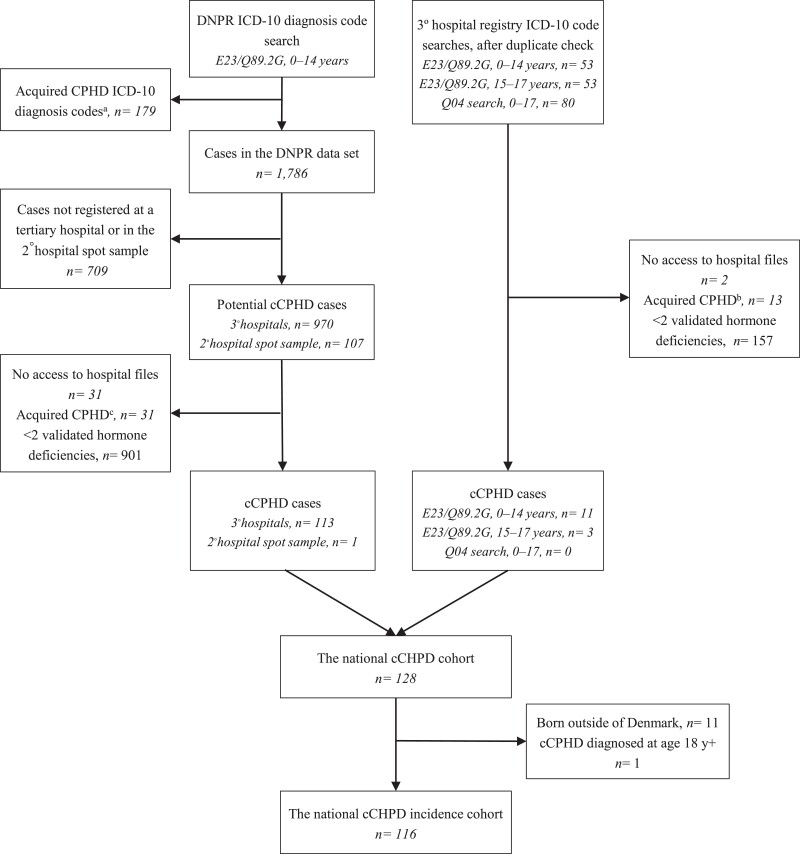
Through the ICD-10 diagnosis code searches and the subsequent hospital file reviews, we identified 114 patients through the DNPR search and 14 patients through the tertiary hospital registries, resulting in a national cCPHD cohort of 128 patients (Table S1 (23)). Of these, 116 were born in Denmark and diagnosed before age 18 years, representing the national incidence cohort of cCPHD diagnosed <18 years (Fig. 1).
Figure 1.
Flow chart of the identification of the national congenital combined pituitary hormone deficiency (cCPHD) cohort and the national cCPHD incidence cohort. Patients at risk of cCPHD were identified through searches on ICD-10 diagnosis codes E23.0-E23.9 (hypofunction and other disorders of pituitary), Q89.2G (congenital malformation of pituitary), and Q04.0-Q04.9 (other congenital malformations of brain). All potential cCPHD cases were examined through retrospective hospital file reviews to validate the cCPHD diagnosis from uniform criteria. DNPR, Danish National Registry; CPHD, combined pituitary hormone deficiency. aC69.0-C72.9; n = 69, C75.1-C75.3; n = 4, D33.0-D33.9; n = 53, D35.2-D35.4; n = 41, A80.0-A89.9; n = 8, I60.0-I64.9; n = 4. bInfections/autoimmunity; n = 1, brain neoplasm and treatment hereof; n = 8, leukemia/lymphoma and treatment hereof; n = 2, severe eating disorder; n = 1, birth asphyxia; n = 1. cInfections/autoimmunity; n = 3, Langerhans cell histiocytosis; n = 6, transfusion-dependent thalassemia; n = 3, brain neoplasm and treatment hereof; n = 3, leukemia/lymphoma and treatment hereof; n = 6, other; n = 10.
Clinical Data
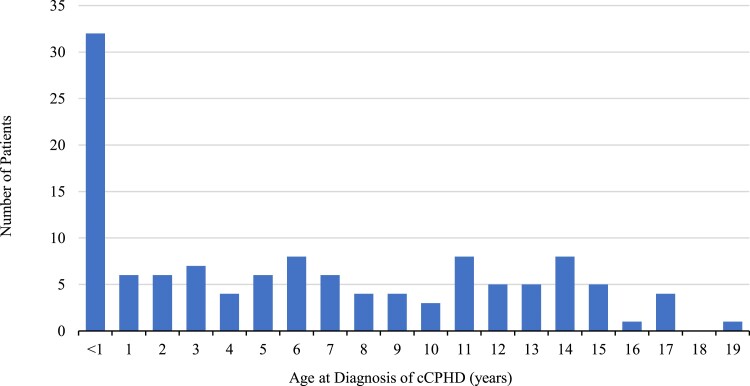
Males were overrepresented in the national cCPHD cohort (88/128; 68.8%), and in both subgroups (Table 4); however, they were only statistically significant in the subgroup of patients diagnosed aged 1-17 years (chi-square P < .0001). The median age (range and 25th;75th percentile) at cCPHD diagnosis was 6.2 (0.01-19.0 and 0.6; 11.9) years (Fig. 2 and Table 4). Age at diagnosis was not available in 5 patients, however diagnosed with cCPHD at some point before age 18 years. Of the remainer, thirty-two patients (26.2%) were diagnosed aged <1 year and 90 patients (73.8%) between 1 and 17 years of age. The median (range) patient age at last follow-up was 15.6 (1.0-24.8) years, and 51 patients (39.8%) had reached 18 years of age at last follow-up.
Table 4.
Magnetic resonance imaging (MRI) and hormone deficiency characteristics of the 128 patients in the national cCPHD cohort
| Diagnosis at age <1 year | Diagnosis at age 1-17 years | Total | |
|---|---|---|---|
| Number | 32 (26.2) | 90 (73.8) | 128a (100) |
| Sex | |||
| Male | 20 (62.5) | 65 (72.2)d | 88 (68.8)d |
| Female | 12 (37.5) | 25 (27.8) | 40 (31.2) |
| Age at observation period end | 11.4 (5.9; 17.8) | 16.5 (12.3; 20.1) | 15.6 (10.7; 20.4) |
| Age at diagnosis of | |||
| First hormone deficiency | 0.06 (0.03; 0.2) | 4.8 (3.0; 7.5) | 3.6 (0.5; 6.5) |
| Second hormone deficiency | 0.1 (0.04; 0.2) | 8.3 (4.2; 13.1) | 6.2 (0.6; 11.9) |
| Third hormone deficiency | 0.08 (0.05; 0.2) | 8.2 (6.0; 12.6) | 3.1 (0.1; 10.0) |
| Fourth hormone deficiency | 0.4 (0.08; 0.9) | 13.0 (12.0; 14.0) | 10.8 (0.4; 13.0) |
| Fifth hormone deficiency | 12.2 (12.2; 12.2) | 11.8 (11.8; 11.8) | 12.0 (11.8; 12.2) |
| Number of hormone deficiencies | |||
| At cCPHD diagnosis | 3 (3; 4)e | 2 (2; 2) | 2 (2; 3) |
| At latest follow-up | 4 (3; 4)e | 2 (2; 3) | 2 (2; 4) |
| Type of deficiencies at last follow-up | |||
| Adrenocorticotropin (ACTH) | 27 (24.1) | 32 (13.9) | 61 (16.9) |
| Growth hormone (GH) | 30 (26.8) | 83 (35.9) | 119 (33.0) |
| Thyrotropin (TSH) | 31 (27.7) | 74 (32.0) | 110 (30.5) |
| Follicle-stimulating/luteinizing hormone (FSH/LH) | 19 (17.0) | 40 (17.3) | 63 (17.5) |
| Antidiuretic hormone (ADH) | 5 (4.5) | 2 (0.9) | 8 (2.2) |
| Brain MRI findingsb | |||
| Abnormal brain MRI | 29 (96.7) | 70 (85.4) | 105 (89.0) |
| Abnormal pituitary gland or stalk | 25 (83.3) | 52 (63.4) | 83 (70.3) |
| Adenohypophysis hypoplastic or absent | 18 (60.0) | 42 (51.2) | 66 (55.6) |
| Neurohypophysis hypoplastic, absent or ectopic | 21 (70.0) | 44 (53.7) | 71 (60.2) |
| Pituitary stalk hypoplastic or absent | 17 (56.7)g | 28 (34.1) | 48 (40.7) |
| Additional midline or other cerebral abnormalities | 18 (60.0) | 37 (45.1) | 57 (48.3) |
| Cerebral midline malformationsc | 11 (36.7) | 16 (19.5) | 27 (22.9) |
| Other cerebral abnormalities | 15 (50.0) | 33 (40.2) | 50 (42.4) |
| Normal brain MRI | 1 (3.3) | 12 (14.6) | 13 (11.0) |
| Missing brain MRI | 2 (6.2) | 8 (8.9) | 10 (7.8) |
| Combined brain MRI abnormalitiesb | |||
| Pituitary abnormality only | 11 (36.7) | 33 (40.2) | 48 (40.7) |
| Adeno- and neurohypophysis only | 1 (3.3) | 7 (8.5) | 10 (8.5) |
| Pituitary stalk, adeno- and neurohypophysis | 4 (13.3) | 14 (17.1) | 20 (16.9) |
| Pituitary and midline abnormalities only | 3 (10.0) | 2 (2.4) | 5 (4.2) |
| Pituitary and other cerebral abnormalities only | 6 (20.0) | 11 (13.4) | 19 (16.1) |
| Pituitary, midline, and other cerebral abnormalities | 5 (16.7) | 6 (7.3) | 11 (9.3) |
Data are presented as median (25th;75th percentile) or n (%).
All hormone deficiencies diagnosed within 6 months of the second hormone deficiency was considered present at the time of cCPHD diagnosis.
Age at diagnosis was not available in 5 patients and 1 patient was diagnosed after age 17 years.
The percentage distribution was calculated from the number of available brain MRI scan.
Including Rathke’s pouch cysts, incomplete Rathke’s pouch closure, malformations of the optic chiasm, hypothalamus, third ventricle, corpus callosum and septum pellucidum.
Fisher's P < .0001 vs female.
Fisher’s P < .0001 vs subgroup 1-17 years.
Fisher’s P = .049 vs subgroup 1-17 years.
Figure 2.
The age distribution at diagnosis of congenital combined pituitary hormone deficiency (cCPHD). The age at cCPHD diagnosis was defined as the age on the date of the second hormone deficiency diagnosis (n = 123).
The number of recorded hormone deficiencies was 2 in 66 patients (51.6%), 3 in 21 patients (16.4%), 4 in 39 patients (30.5%), and 5 in 2 patients (1.6%). GHD was the most common deficiency, succeeded by deficiency of TSH, follicle-stimulating hormone (FSH)/luteinizing hormone (LH), adrenocorticotropic hormone, and antidiuretic hormone. However, deficiency of TSH was more common than GHD in patients diagnosed <1 year of age. Patients with cCPHD diagnosed at <1 year of age had the highest number of hormone deficiencies, both at diagnosis and at last follow-up (Fisher’s P < .0001 vs subgroup 1-17 years). In the 1-17 years subgroup, 58 (64.4%) patients were diagnosed with only 2 hormone deficiencies. The most prevalent combination was GHD and TSH deficiency (n = 37), followed by GHD and FSH/LH deficiency (n = 11).
Abnormal pituitary gland or stalk MRI findings was seen in 83.3% (n = 25/30) for those diagnosed at <1 year of age, decreasing to 63.4% (n = 53/82) in the 1-17 years subgroup (missing data, n = 10). Hypoplastic, absent, or ectopic neurohypophysis was the most common solitary abnormality, followed by hypoplastic or absent adenohypophysis, the category “other cerebral abnormality,” pituitary stalk abnormality, and midline malformation. Fifty-seven patients (48.3%) had additional midline or other cerebral abnormalities. As the only significant difference between the age subgroups, hypoplastic or absent pituitary stalk was more frequent <1 vs 1-17 years (17/30 vs 28/82, P = .049).
The National Incidence of cCPHD
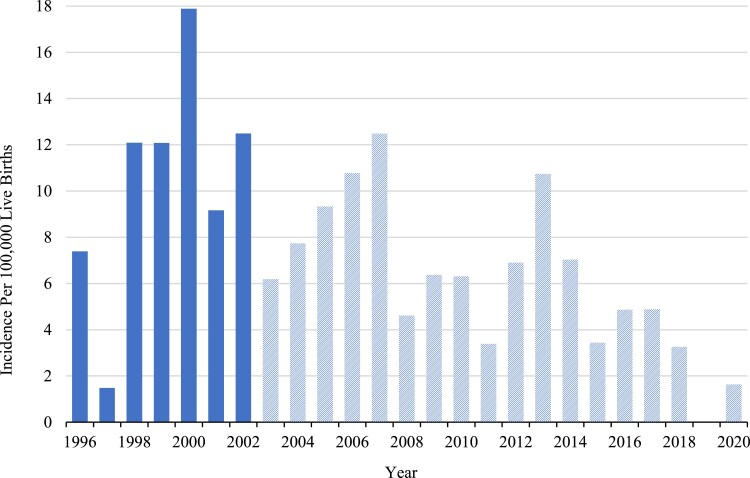
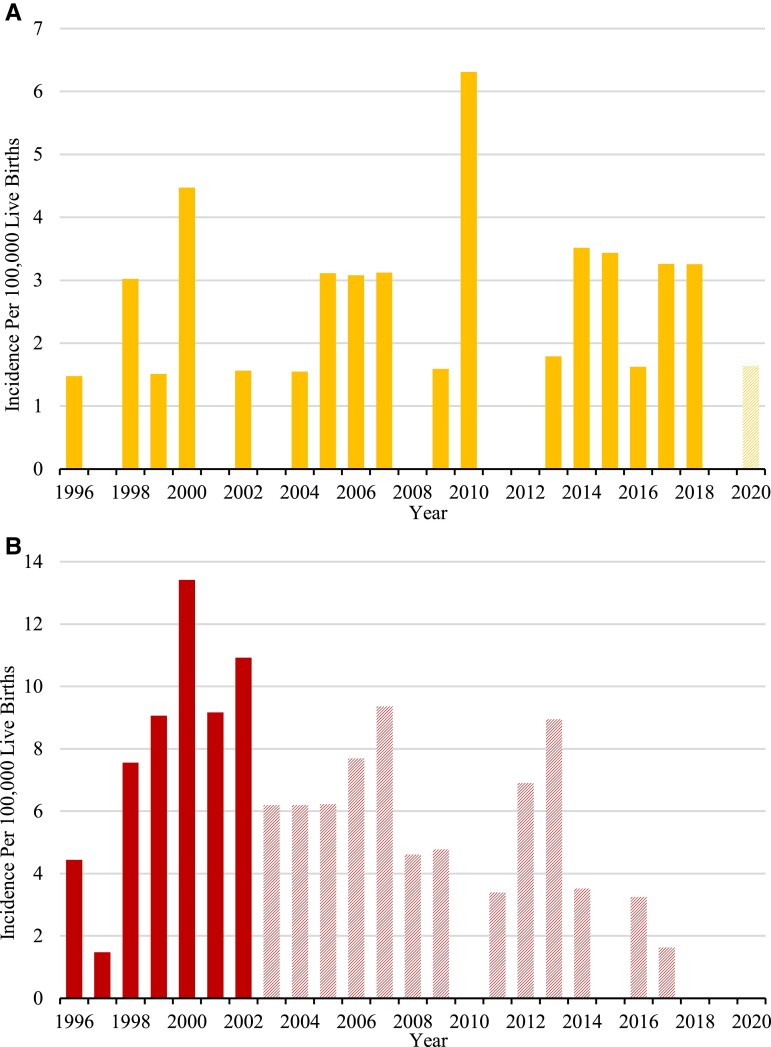
The national incidence (95% CI) of cCPHD diagnosed before age 18 was calculated to be 10.34 (7.79-13.72) per 100 000 live births in the period 1996-2002, giving an incidence rate of 5.74 (4.33-7.62) per million live births per year. No trend in the incidence through this observation period was observed (Figs. 3 and 4). The incidence estimate including all patients diagnosed in the whole observation period 1996-2020 was somewhat, yet nonsignificantly lower, probably owing to right truncation (Table 5). The incidence and annual incidence rate were by far highest for patients diagnosed before age 1 year, 19.8 (13.9-28.4) per million live births per year, compared with the later ages. Incidences split by age 1-8 years and 9-17 years are shown elsewhere (Table S2 (23)).
Figure 3.
The incidence of congenital combined pituitary hormone deficiency (cCPHD) in Denmark diagnosed before age 18 years. The figure only includes patients with cCPHD who were born in Denmark and diagnosed before age 18 years (n = 116). Solid columns represent the incidence estimations that are not influenced by right truncation, as all patients born in the period 1996-2002 have turned 18 years old by the end of the observation period. Hatched columns represent incidence estimations that may be influenced by right truncation.
Figure 4.
The incidence of congenital combined pituitary hormone deficiency (cCPHD) in Denmark diagnosed before age 1 year (A), and at age 1-17 years (B). Solid columns represent the incidence estimations that are not influenced by right truncation, as all patients born in these years had turned 1 year (A), or 18 years (B) by the end of the observation period. Hatched columns represent incidence estimations that may be influenced by right truncation.
Table 5.
National incidences and incidence rates of congenital combined pituitary hormone deficiency from 1996 to 2020
| Age at cCPHD diagnosis | Perioda | Number of patients | Incidence per 100 000 live births | Incidence rates per million live births per year |
|---|---|---|---|---|
| 0-17 years | 1996-2002 | 48 | 10.34 (7.79-13.72) | 5.74 (4.33-7.62) |
| 0-17 years | 1996-2020b | 116 | 7.38 (6.15-8.85) | 4.10 (3.42-4.92) |
| 0-19 years | 1996-2000 | 35 | 10.46 (7.51-14.56) | 5.23 (3.76-7.28) |
| Subgroups | ||||
| <1 year | 1996-2019 | 30 | 1.98 (1.39-2.84) | 19.8 (13.9-28.4) |
| 1-17 years | 1996-2002 | 37 | 7.97 (5.77-11.00) | 4.69 (3.39-6.47) |
Incidences and incidence rates with 95% CI in brackets.
To eliminate the influence of right truncation, incidences were calculated in various periods according to the included age ranges. This was done to ensure all patients reached 1/18/20 years of age in the observation period.
For completeness, we calculated the incidence of cCPHD diagnosed before age 18 years for the whole period 1996-2020.
Discussion
In this first of its kind national study, we identified 128 patients with cCPHD, of which nearly 50% were diagnosed before age 6 years. Above half of the patients had 2 hormone deficiencies, whereas deficiency of all 5 hormones were only seen in 2 patients. Cerebral abnormalities by MRI were seen in almost 90% of the patients.
The incidence of cCPHD diagnosed before age 18 years was calculated to be 10.34 (7.79-13.72) per 100 000 live births with an incidence rate of 5.74 (4.33-7.62) per million live births per year. A by far higher incidence (∼1:50 000) was seen in patients diagnosed before age 1 year, who also presented with the highest incidence rates and highest number of hormone deficiencies.
Published data on the incidence of hypopituitarism are scarce. The incidence rate of all-cause adult-onset hypopituitarism in northwestern Spain was estimated to be 4.21 per 100 000 inhabitants per year (19), whereas another study suggested that the incidence of all-cause CPHD during childhood is less than 3 per million per year (24). We identified a higher incidence rate, even when including only cCPHD and not all-cause CPHD. We primarily ascribe this to the unique access to national and hospital registries in Denmark and detailed hospital file validation. The tertiary hospital registry searches and the secondary hospital spot sample identified 12 additional patients with cCPHD diagnosed before age 15 years, and 3 additional patients aged 15-19 years at diagnosis, indicating incomplete case identification from the national registry search strategy. On the contrary, the identified national cohort was regarded as complete or almost complete, providing high validity to the incidence estimates. The denominator, the annual birth counts in Denmark, was moreover accurate owing to Statistics Denmark, whose calculations were based on data from both the central person registrations and the DNPR registry. Recently, a Finnish study from 1 tertiary hospital estimated the incidence of cCPHD to be 1:16 000 (6.25:100 000) based on 26 identified patients in the period 2000-2018 (6). The lower incidence than our study may be attributed to right censoring, as a follow-up time of 18 years was not ensured. In our study, the importance of avoidance of right censoring was underscored, as the incidence was highest in 1-17 years at diagnosis. Furthermore, while the definition of cCPHD resembles ours, it is, however, not clear whether children diagnosed up to and including age 17 years were included in the Finnish study.
Our study revealed an overrepresentation of males with cCPHD, in agreement with some (6, 7, 9), but not all, studies (25). X-linked genetic factors have been suggested as an explanation of male predominance, but the sex disparity may also be attributed to ascertainment bias, as short stature in boys tends to become a greater cause for concern and more often conveys hormonal investigations than in girls (9, 12). Furthermore, boys show early apparent clinical signs of hypovirilization, which could account for the male predominance, especially among patients diagnosed before 1 year of age.
We found GHD to be the most abundant hormone deficiency, followed by TSH deficiency. These findings are supported by several previous studies (7, 26). Conversely, other studies report FSH/LH deficiencies as the second most frequent (3, 9, 25). The discrepancy may be explained by differences in study design, hormone deficiency criteria, and follow-up period. Additionally, many studies are based on patients with GHD and 1 or more additional hormone deficiencies. Therefore, it was interesting to observe that deficiency of TSH was the most frequent deficiency in the patients diagnosed at age <1 year. Additionally, we identified 9 patients with cCPHD who did not have GHD. Such patients will be overlooked in studies based on the diagnosis of GHD, which fail to examine the full spectrum of cCPHD.
A diagnosis peak was seen at age <1 year, with smaller peaks at age 6, 11, and 14 years. This highlights the broad age spectrum of clinical onset of cCPHD. The early peak <1 year represented the diagnosis of the most severe cCPHD cases, as the median number of hormone deficiencies at diagnosis and at last follow-up was significantly higher in this subgroup. The highest incidence of cCPHD was seen in the 1-17 years subgroup, which underscores the need of testing all pituitary hormone axes through childhood and adolescence when 1 or more axes are deficient.
In clinical practice and in the literature, the definition of PHD in childhood may be subject to variations. To minimize the risk of misclassification of late puberty as hypogonadotropic hypogonadism, we chose to define sex-specific age ranges, within which biochemical analyses of the hypothalamic–pituitary–gonadal axis should be performed. The risk of misclassification of hypogonadotropic hypogonadism increases with decreasing age of diagnosis, but with an increasing number of hormone deficiencies clinicians tended to investigate the gonadal hormone axes at an earlier age. The hormone deficiency criteria, therefore, had to reflect the clinical setting.
Furthermore, we chose to include patients who developed hypothyroidism during GH therapy even though evidence suggests that GH therapy may affect thyroid hormone levels per se. Some studies suggest that the changes in thyroid hormone levels during GH therapy are caused by increased peripheral thyroxine to triiodothyronine conversion (27, 28), whereas others suggest unmasking of latent central hypothyroidism (29–31). We included patients with GHD and TSH deficiency, as 2 recent studies on children with nonacquired isolated GHD support the latter mechanism (29, 30), and as our included patients had repeating levels of free thyroxine/thyroxine below the normal reference range.
The strengths of our study include the use of national registries with unique high completeness regarding patient diagnoses and birth counts, our supplementary tertiary hospital diagnosis code search, the method validation, the broadness of our diagnosis code searches for all-cause pituitary deficiency and pituitary malformation, and our hospital file validation of the cCPHD diagnosis by uniform criteria.
Our study also had limitations. A few children with cCPHD may have been overlooked due to incorrect diagnoses, lack of correct reporting to the DNPR, or lack of referral to a tertiary hospital. On the other hand, acquired causes of CPHD may have been undiagnosed in a few children.
The definition and validation of the hormonal deficiencies could be questioned, including inclusion of children with partly missing information and the potential of incorrect reference values for the hormone assays used in clinical practice. Despite our validation efforts, we had to rely on the diagnostic practice to some extent in this retrospective study. We were likewise unable to evaluate the extent of delay from disease onset to diagnosis.
The diagnosis of cCPHD may be challenging, especially in those with normal MRI findings. However, other studies have shown that normal anatomy of the pituitary gland does not exclude abnormal function and MRI findings are not included in the Danish or international diagnosis criteria guidelines (1, 3, 6, 16–20). A few patients did not have MRI performed, which could lead to minor underestimation or overestimation of the rate of pituitary, midline, and other cerebral abnormalities. Furthermore, as not all patients had reached 18 years’ age at last follow-up, not all late-onset hormonal deficiencies may have been recorded. Lastly, the external validity of our incidence estimates may be limited due to differences in population characteristics such as ethnicity and the frequency of consanguinity and founder mutations (2, 9).
Conclusion
In this national registry study, the incidence of cCPHD diagnosed before age 18 years was calculated to be 10.34 (7.79-13.72) per 100 000 live births with an incidence rate of 5.74 (4.33-7.62) per million live births per year. cCPHD diagnosed before age 1 year had by far the highest incidence rate, with the highest number of hormonal deficiencies and with 96.7% having abnormal brain MRI findings. On the other hand, cCPHD diagnosed at age 1-17 years had the highest incidence, emphasizing the need for investigations of cCPHD throughout childhood and adolescence in children with isolated PHD. The data presented provide information that can be used to shape future screening programs for children at risk of developing cCPHD. Further research should be undertaken to investigate, for instance, the incidence of cCPHD in other populations, the genetics of cCPHD, genotype–phenotype relations, the management of cCPHD, and the correlation between the clinical signs and symptoms at onset and the disease course on follow-up.
Acknowledgments
The management teams at Hans Christian Andersen Children's Hospital, Odense University Hospital, Department of Growth and Reproduction, Rigshospitalet, Department of Pediatrics, Aalborg University Hospital, and Department of Pediatrics and Adolescent Medicine, Aarhus University Hospital, are thanked for providing access to the department's hospital files and ICD-10 diagnosis code registries. The secretaries at the aforementioned departments are acknowledged for their assistance with conducting the ICD-10 diagnosis code searches. The management team at Department of Pediatrics, Kolding Hospital, are also thanked for giving access to the department's hospital files. Pernille Thomsen, the mother of a 3-year-old child with cCPHD, is thanked for providing valuable insights into cCPHD from a parent's perspective.
Abbreviations
- cCPHD
congenital combined pituitary hormone deficiency
- DNPR
Danish National Patient Registry
- FSH
follicle-stimulating hormone
- GH
growth hormone
- GHD
growth hormone deficiency
- LH
luteinizing hormone
- MRI
magnetic resonance imaging
- PHD
pituitary hormone deficiency
- TSH
thyroid-stimulating hormone
Contributor Information
Louise Kjersgaard Jakobsen, Hans Christian Andersen Children’s Hospital, Odense University Hospital, 5000 Odense, Denmark; OPEN, Open Patient data Explorative Network, Odense University Hospital, 5000 Odense, Denmark.
Rikke Beck Jensen, Department of Growth and Reproduction, Copenhagen University Hospital—Rigshospitalet, 2100 Copenhagen, Denmark; Department of Clinical Medicine, University of Copenhagen, 2200 Copenhagen, Denmark.
Niels Holtum Birkebæk, Department of Pediatrics and Adolescent Medicine and Steno Diabetes Center Aarhus, Aarhus University Hospital, 8200 Aarhus, Denmark; Department of Clinical Medicine, Aarhus University, 8200 Aarhus, Denmark.
Dorte Hansen, Hans Christian Andersen Children’s Hospital, Odense University Hospital, 5000 Odense, Denmark; Department of Clinical Research, University of Southern Denmark, Odense, Denmark.
Ann-Margrethe Rønholt Christensen, Department of Pediatrics, Aalborg University Hospital, 9000 Aalborg, Denmark.
Maja Carsting Bjerrum, Hans Christian Andersen Children’s Hospital, Odense University Hospital, 5000 Odense, Denmark.
Henrik Thybo Christesen, Hans Christian Andersen Children’s Hospital, Odense University Hospital, 5000 Odense, Denmark; OPEN, Open Patient data Explorative Network, Odense University Hospital, 5000 Odense, Denmark; Department of Clinical Research, University of Southern Denmark, Odense, Denmark.
Funding
This work was funded by Odense University Hospital Fund (j.no. A4599) and Dagmar Marshalls Fund (29/04/2021). This work was supported by Odense University Hospital, Denmark, the University of Southern Denmark, Denmark, the Danish National Patient Registry, Statistics Denmark, the Management Secretariat and the Regional Secretariat and Law of The Region of Southern Denmark (j.no. 20/58158), Odense University Hospital Fund (j.no. A4599), and Dagmar Marshalls Fund (29/04/2021). OPEN, Open Patient data Explorative Network, Odense University Hospital, Region of Southern Denmark are thanked for supporting this work through the facilities OPEN Project Coordination & Administration and OPEN IT & Data Management.
Author contributions
H.T.C. conceived the original idea and supervised the project. L.K.J. and H.T.C. performed the literature search and acquired the project funding. L.K.J. collected the data from the Danish National Patient Registry, Statistics Denmark, and the hospital files at the 4 tertiary hospitals. M.C.B. and L.K.J. collected the data from the hospital files at Kolding Hospital. L.K.J., H.T.C., R.B.J., D.H., N.B.H., and A.R.C. contributed to the study design and to the analysis and interpretation of data. L.K.J. and HTC both directly accessed and verified the underlying data. L.K.J. led the statistical analyses. L.K.J. and H.T.C. drafted the original manuscript. All authors contributed to the writing and revision of the final manuscript.
Disclosures
Authors have no financial or other conflicts of interest to declare. The Funders had no role in the design of this study, in the writing of this report, or in the decision to submit the manuscript for publication.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided. The de-identified patient data that underlie this study are available to researchers upon approval by the Region of Southern Denmark. The full study protocol is available at https://portal.findresearcher.sdu.dk/en/publications/the-incidence-of-congenital-combined-pituitary-hormone-deficiency and the Stata do-files are available to anyone with a request. Proposals can be submitted up to 3 years following article publication and should be directed to henrik.christesen@rsyd.dk.
Clinical Trials Information
ClinicalTrials.gov ID:NCT05334563 (registered April 19, 2022).
References
- 1. Romero CJ, Nesi-França S, Radovick S. The molecular basis of hypopituitarism. Trends Endocrinol Metab. 2009;20(10):506‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Romero CJ, Pine-Twaddell E, Radovick S. Novel mutations associated with combined pituitary hormone deficiency. J Mol Endocrinol. 2011;46(3):R93‐R102. [DOI] [PubMed] [Google Scholar]
- 3. Haim-Pinhas H, Kauli R, Lilos P, Laron Z. Growth, development, puberty and adult height of patients with congenital multiple pituitary hormone deficiencies. Growth Horm IGF Res. 2016;27:46‐52. [DOI] [PubMed] [Google Scholar]
- 4. Cerbone M, Dattani MT. Progression from isolated growth hormone deficiency to combined pituitary hormone deficiency. Growth Horm IGF Res. 2017;37:19‐25. [DOI] [PubMed] [Google Scholar]
- 5. Di Iorgi N, Morana G, Allegri AE, et al. Classical and non-classical causes of GH deficiency in the paediatric age. Best Pract Res Clin Endocrinol Metab. 2016;30(6):705‐736. [DOI] [PubMed] [Google Scholar]
- 6. Hietamäki J, Kärkinen J, Iivonen AP, et al. Presentation and diagnosis of childhood-onset combined pituitary hormone deficiency: a single center experience from over 30 years. EClinicalMedicine. 2022;51:101556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Graaff LC, Argente J, Veenma DC, Drent ML, Uitterlinden AG, Hokken-Koelega AC. PROP1, HESX1, POU1F1, LHX3 and LHX4 mutation and deletion screening and GH1 P89L and IVS3 +1/+2 mutation screening in a Dutch nationwide cohort of patients with combined pituitary hormone deficiency. Horm Res Paediatr. 2010;73(5):363‐371. [DOI] [PubMed] [Google Scholar]
- 8. Alatzoglou KS, Gregory LC, Dattani MT. Development of the pituitary gland. Compr Physiol. 2020;10(2):389‐413. [DOI] [PubMed] [Google Scholar]
- 9. De Rienzo F, Mellone S, Bellone S, et al. Frequency of genetic defects in combined pituitary hormone deficiency: a systematic review and analysis of a multicentre Italian cohort. Clin Endocrinol (Oxf). 2015;83(6):849‐860. [DOI] [PubMed] [Google Scholar]
- 10. Kelberman D, Rizzoti K, Lovell-Badge R, Robinson IC, Dattani MT. Genetic regulation of pituitary gland development in human and mouse. Endocr Rev. 2009;30(7):790‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cameron FJ, Khadilkar VV, Stanhope R. Pituitary dysfunction, morbidity and mortality with congenital midline malformation of the cerebrum. Eur J Pediatr. 1999;158(2):97‐102. [DOI] [PubMed] [Google Scholar]
- 12. Child CJ, Blum WF, Deal C, et al. Development of additional pituitary hormone deficiencies in pediatric patients originally diagnosed with isolated growth hormone deficiency due to organic causes. Eur J Endocrinol. 2016;174(5):669‐679. [DOI] [PubMed] [Google Scholar]
- 13. Ascoli P, Cavagnini F. Hypopituitarism. Pituitary. 2006;9(4):335‐342. [DOI] [PubMed] [Google Scholar]
- 14. Toogood AA, Stewart PM. Hypopituitarism: clinical features, diagnosis, and management. Endocrinol Metab Clin North Am. 2008;37(1):235‐261. [DOI] [PubMed] [Google Scholar]
- 15. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fleseriu M, Hashim IA, Karavitaki N, et al. Hormonal replacement in hypopituitarism in adults: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(11):3888‐3921. [DOI] [PubMed] [Google Scholar]
- 17. Johannsen TH, Main KM, Ljubicic ML, et al. Sex differences in reproductive hormones during mini-puberty in infants with normal and disordered sex development. J Clin Endocrinol Metab. 2018;103(8):3028‐3037. [DOI] [PubMed] [Google Scholar]
- 18. Stochholm K, Gravholt CH, Laursen T, et al. Incidence of GH deficiency—a nationwide study. Eur J Endocrinol. 2006;155(1):61‐71. [DOI] [PubMed] [Google Scholar]
- 19. Regal M, Páramo C, Sierra SM, Garcia-Mayor RV. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol (Oxf). 2001;55(6):735‐740. [DOI] [PubMed] [Google Scholar]
- 20. Birkebaek NH, Patel L, Wright NB, et al. Endocrine status in patients with optic nerve hypoplasia: relationship to midline central nervous system abnormalities and appearance of the hypothalamic-pituitary axis on magnetic resonance imaging. J Clin Endocrinol Metab. 2003;88(11):5281‐5286. [DOI] [PubMed] [Google Scholar]
- 21. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jakobsen LK, Beck Jensen, R., Birkebaek, N. H., et al. Supplementary material to Diagnosis and incidence of congenital combined pituitary hormone deficiency in Denmark—a national observational study. University of Southern Denmark Research Portal. Date of deposit 10 March 2023. 10.21996/y547-kp962023 [DOI] [PMC free article] [PubMed]
- 24. Lamberts SW, de Herder WW, van der Lely AJ. Pituitary insufficiency. Lancet. 1998;352(9145):127‐134. [DOI] [PubMed] [Google Scholar]
- 25. Otto AP, França MM, Correa FA, et al. Frequent development of combined pituitary hormone deficiency in patients initially diagnosed as isolated growth hormone deficiency: a long term follow-up of patients from a single center. Pituitary. 2015;18(4):561‐567. [DOI] [PubMed] [Google Scholar]
- 26. Coya R, Vela A, Pérez de Nanclares G, et al. Panhypopituitarism: genetic versus acquired etiological factors. J Pediatr Endocrinol Metab. 2007;20(1):27‐36. [DOI] [PubMed] [Google Scholar]
- 27. Portes ES, Oliveira JH, MacCagnan P, Abucham J. Changes in serum thyroid hormones levels and their mechanisms during long-term growth hormone (GH) replacement therapy in GH deficient children. Clin Endocrinol (Oxf). 2000;53(2):183‐189. [DOI] [PubMed] [Google Scholar]
- 28. Seminara S, Stagi S, Candura L, et al. Changes of thyroid function during long-term hGH therapy in GHD children. A possible relationship with catch-up growth? Horm Metab Res. 2005;37(12):751‐756. [DOI] [PubMed] [Google Scholar]
- 29. Witkowska-Sędek E, Kucharska AM, Rumińska M, Paluchowska M, Pyrżak B. Decreased thyroxine levels during rhGH therapy in children with growth hormone deficiency. J Clin Med. 2021;10(21):5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Iersel L, van Santen HM, Zandwijken GRJ, Zwaveling-Soonawala N, Hokken-Koelega ACS, van Trotsenburg ASP. Low FT4 concentrations around the start of recombinant human growth hormone treatment: predictor of congenital structural hypothalamic-pituitary abnormalities? Horm Res Paediatr. 2018;89(2):98‐107. [DOI] [PubMed] [Google Scholar]
- 31. Agha A, Walker D, Perry L, et al. Unmasking of central hypothyroidism following growth hormone replacement in adult hypopituitary patients. Clin Endocrinol (Oxf). 2007;66(1):72‐77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided. The de-identified patient data that underlie this study are available to researchers upon approval by the Region of Southern Denmark. The full study protocol is available at https://portal.findresearcher.sdu.dk/en/publications/the-incidence-of-congenital-combined-pituitary-hormone-deficiency and the Stata do-files are available to anyone with a request. Proposals can be submitted up to 3 years following article publication and should be directed to henrik.christesen@rsyd.dk.