Abstract
Context
Long COVID is an emerging syndrome affecting 50% to 70% of COVID-19 survivors that still lacks predicting factors.
Objective
Due to the extraskeletal effects of vitamin D, we retrospectively assessed the association between 25(OH) vitamin D levels and long COVID in COVID-19 survivors 6 months after hospitalization.
Methods
Long COVID was defined according to NICE guidelines. Fifty long COVID and 50 non–long-COVID subjects matched on a 1:1 basis were enrolled from an outpatient clinic post-COVID cohort seen from August to November 2020. Therapies/comorbidities affecting calcium/vitamin D/bone metabolism, and/or admission to the intensive care unit during hospitalization were exclusion criteria. 25(OH) Vitamin D was measured at hospital admission and 6 months after discharge.
Results
We observed lower 25(OH) vitamin D levels, evaluated at follow-up, in subjects with long COVID than those without (20.1 vs 23.2 ng/mL, P = .03). Regarding the affected health areas evaluated in the entire cohort, we observed lower 25(OH) vitamin D levels in those with neurocognitive symptoms at follow-up (n = 7) than those without (n = 93) (14.6 vs 20.6 ng/mL, P = .042). In patients presenting vitamin D deficiency (<20 ng/mL), both at admission and at follow-up (n = 42), those affected by long COVID (n = 22) presented lower 25(OH) vitamin D levels at follow-up than those not affected (n = 20) (12.7 vs 15.2 ng/mL, P = .041). In multiple regression analyses, lower 25(OH) vitamin D levels at follow-up were the only variable significantly associated with long COVID in our cohort (P = .008, OR 1.09, CI 1.01-1.16).
Conclusion
COVID-19 survivors with long COVID have lower 25(OH) vitamin D levels than matched patients without long COVID. Our data suggest that vitamin D levels should be evaluated in COVID-19 patients after hospital discharge. The role of vitamin D supplementation as a preventive strategy of COVID-19 sequelae should be tested in randomized controlled trials.
Keywords: COVID-19, vitamin D, Long COVID, SARS-CoV-2, hypovitaminosis D
The negative role of lower vitamin D levels in patients affected by Coronavirus disease-19 (COVID-19) was reported early to be a possible risk factor for worse acute clinical outcomes (1). Several retrospective case–control studies, meta-analyses, including observational and interventional studies, as well as prospective studies consistently revealed inverse associations between serum vitamin D levels and the risk of developing acute severe COVID-19 with an increased risk of mortality, intensive care unit (ICU) admission, length of ICU stay, and need for mechanical ventilation (2-10).
After the first pandemic wave, emerging evidence observed early that patients previously affected by SARS-CoV-2 infection often reported several persistent clinical symptoms and signs, even for long periods after the acute disease, with a marked impairment of their quality of life (11). The concomitant presence of multiple and different persistent symptoms continuing for more than 12 weeks after acute COVID-19 was early denominated as long COVID syndrome (12, 13). This syndrome was described to involve different health systems in patients, including neurocognitive, cardiorespiratory, gastrointestinal, constitutional, and musculoskeletal areas, and taste and smell disorders (11). To date, most of published studies on long COVID suggest that in 50% to 70% of COVID-19 survivors several post-COVID symptoms can be observed up to 3 months after acute disease (11). However, to date, the predisposing factors for this syndrome are still poorly understood. A meta-analysis including 20 articles and involving 13 340 patients highlighted the role of female sex and severity of acute disease as independent prognostic factors for long-term sequelae (14). Similarly, another meta-analysis, including 37 peer-reviewed studies, revealed that female sex and presence of concomitant comorbidities, such as pulmonary disease, diabetes, and obesity, could be identified as potential risk factors for long COVID occurrence (15).
Vitamin D is well-known to regulate the immune response and immunocompetence regarding both innate and adaptive immunity, supporting antimicrobial and antiviral immune responses (16-18). Furthermore, among the consistently reported extraskeletal effects of vitamin D, this hormone is also characterized to, firstly, influence musculoskeletal health and function, improving muscle recovery and cellular turnover and reducing muscle cells atrophy (19-21); secondly, reduce nonspecific musculoskeletal pain, myalgia, and arthralgia (22-24); thirdly, influence neurocognitive functions and disorders (25-27); and, finally, promote respiratory recovery after pneumonia (28, 29). However, despite these findings and the negative influence of hypovitaminosis D on the acute clinical course of patients affected by COVID-19, very little is known regarding the role of vitamin D in recovery from symptoms and signs often present in COVID-19 survivors after the acute disease. To date, only a few studies have investigated the role of vitamin D in risk the of long COVID occurrence (30-34), reporting conflicting results principally due the inclusion of different cohorts of patients (ie, including those with different grades of acute disease severity), different timing of follow-up evaluations (ie, mostly up to only 3 months after the acute infection), and different health areas of symptoms and signs collected (ie, some studies evaluated only muscle and physical performances).
The aim of this study was to evaluate the impact of circulating 25(OH) vitamin D levels on risk of long COVID occurrence in a cohort of patients previously hospitalized for acute COVID-19 and reassessed 6 months after hospital discharge.
Material and Methods
Study Design
This was an observational cross-sectional retrospective study performed at IRCCS Ospedale San Raffaele, a tertiary health care center in Milan, Italy. The study protocol complies with the Declaration of Helsinki and was approved by the hospital ethics committee (protocol ABIO/NC/04 no. 36/2022). This study is a cohort substudy part of monocentric prospective observational research, the COVID-BioB study, conducted in our hospital (35). Signed informed consent was obtained from all patients participating in this study.
Confirmed COVID-19 was defined by a positive real-time reverse transcriptase polymerase chain reaction (RT-PCR) from a nasal and/or throat swab together with signs, symptoms, and/or radiological findings suggestive of COVID-19 pneumonia. Patients admitted to hospital for other reasons and subsequently diagnosed with superimposed SARS-CoV-2 infection were excluded. Remission was defined as 2 negative RT-PCR from a nasal and/or throat swab performed 24 hours apart, and no symptoms of acute infection.
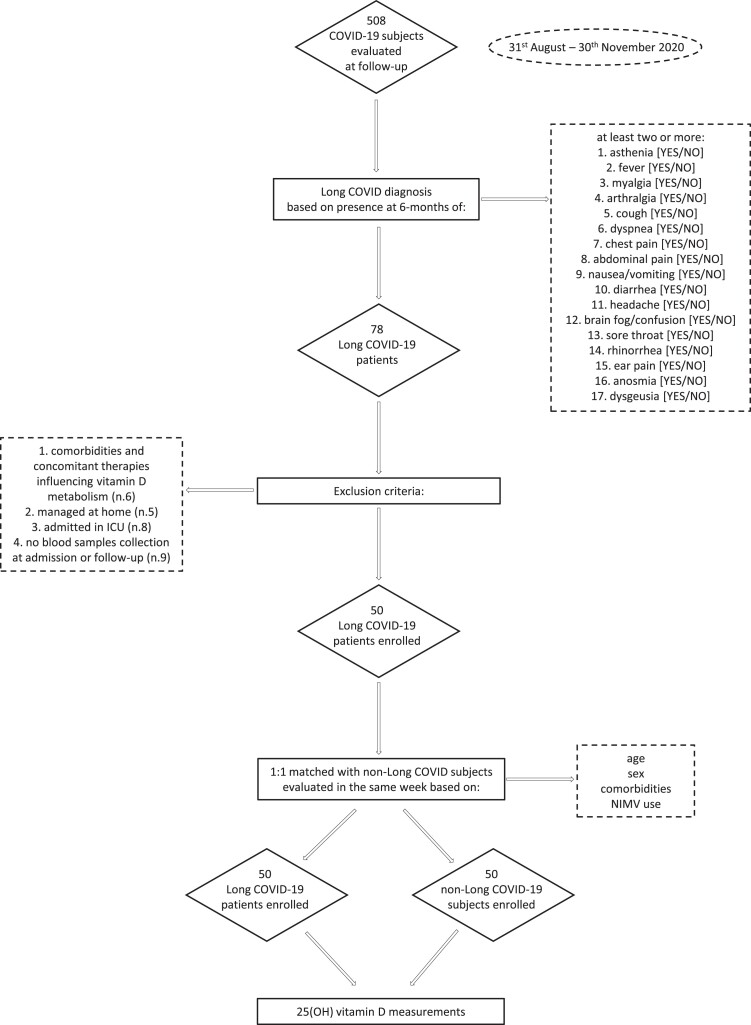
We included only adult (age ≥18 years) patients with a confirmed diagnosis of COVID-19 who had been hospitalized and subsequently discharged from IRCCS Ospedale San Raffaele during the first pandemic wave from March to May 2020, and were re-evaluated 6 months after discharge at the outpatient COVID-19 follow-up clinic in the same institution. Accordingly, 500 patients were consecutively evaluated in our follow-up clinic from August 31 to November 30, 2020, after the first pandemic as being potentially eligible for the study (36). However, only those fulfilling the following inclusion and exclusion criteria were included: (1) patients with the following comorbidities and concomitant active therapies influencing vitamin D metabolism including chronic kidney disease, osteoporosis, chronic glucocorticoid and antiepileptic treatments, vitamin D/calcium supplements, loop/thiazide diuretics, and patients with an estimated glomerular filtration rate of less than or equal to 30 mL/min/1.73 m2 were excluded; (2) in order to minimize the possible bias due to the reported negative influence of severe acute disease on risk of post-COVID symptom persistence (11, 14, 15), we excluded patients who were only managed at home, and those who, during the hospitalization, were admitted to the ICU; (3) only patients with available medical data recorded upon admission and at 6-month follow-up were included; (4) only patients with available blood samples for 25(OH) vitamin D measurements collected upon admission and at 6-month follow-up were included. In our study, based on the National Institute for Health and Care Excellence (NICE) guidelines (12, 13), long COVID was defined by the concomitant presence of at least 2 or more symptoms and signs (complete list reported below under “Data Collection”) observed at the 6-month follow-up visits that were not explained by an alternative medical diagnosis and could only be attributed to the previous COVID-19. As defined by NICE guidelines, none of the symptoms and signs characterizing long COVID were present before the acute disease. After excluding patients who did not fulfil the above-mentioned criteria, 50 patients with long COVID were eligible for study enrolment. From the same cohort attending the COVID follow-up outpatient clinic and based on the same inclusion and exclusion criteria, we also enrolled in the study, on a 1:1 ratio, 50 subjects without long COVID matched for age, sex, concomitant comorbidities, and need for noninvasive mechanical ventilation (NIMV) with long COVID patients included in the study. In order to minimize the possible differences related to the known seasonality effects on the 25(OH) vitamin D measurements (37), the matched non–long COVID subjects were specifically selected from those evaluated at follow-up in the same week of the corresponding enrolled long COVID patients. Therefore, each patient with long COVID and matched subjects without long COVID were evaluated at follow-up and previously admitted in hospital in the same time periods. The study design and the enrolment flow chart are summarized in detail in Fig. 1.
Figure 1.
Study design and enrolment flow chart.
Data Collection
Data were collected directly by patient interview or from medical chart review and entered on a dedicated electronic case record form specifically developed for the study. Before the analysis, data were cross-checked with medical charts and verified by data managers and clinicians for accuracy. For this study the following variables were collected: age, sex, body mass index (BMI) (calculated as the ratio of weight in kilograms divided by height in meters squared. Data from overweight (defined as a BMI >25 kg/m2) and obese (defined as BMI >30 kg/m2) patients were analyzed together. We collected comorbidities (including history of hypertension, diabetes mellitus, cardiovascular disease, active neoplasia, chronic kidney disease), biochemical parameters including lactate dehydrogenase (LDH, U/L), high-sensitivity C-reactive protein (CRP, mg/dL), leukocyte count, albumin levels (g/L), and clinical outcomes occurred during the entire disease course (hospitalization, need for NIMV, and admission to ICU). Data about the initial onset presentation of COVID-19 and the disease course were retrospectively scrutinized from medical records in the presence of the patient during follow-up evaluation and collected.
A comprehensive evaluation of physical, constitutional, neurocognitive, cardiorespiratory, and gastrointestinal health systems was performed by a multidisciplinary team during the follow-up visits (36, 38). The complete physical examination was integrated with detailed patient medical history. Symptoms and signs collected at the 6-month follow-up visits and included in the present study for the analyses were categorized into: constitutional (asthenia [yes/no], fever [yes/no], myalgia [yes/no], arthralgia [yes/no]), cardiorespiratory (cough [yes/no], dyspnea [yes/no], chest pain [yes/no]), gastrointestinal (abdominal pain [yes/no], nausea/vomiting [yes/no], diarrhea [yes/no)], neurocognitive (headache [yes/no], brain fog/confusion [yes/no]), ear nose and throat (sore throat [yes/no], rhinorrhea [yes/no], ear pain [yes/no]), and senses area (anosmia [yes/no] and dysgeusia [yes/no]). At follow-up visits for all patients fasting blood glucose levels using a point of care capillary-based glucometer were also evaluated. Laboratory parameter assessments at follow-up were recommended on the basis of the physician's decisions and were not routinely assessed in all patients. In our center, a COVID-19 biobank was established with the aim of collecting high-quality and well-annotated human biospecimens of patients affected in acute and post-COVID-19 phases (35). In this specific study, after the enrolment of long COVID and non–long COVID patients who fulfilled the requested inclusion and exclusion criteria, 25(OH) vitamin D levels were retrospectively measured using the blood samples collected at hospital admission and at 6-month follow-up visits (using Roche Cobas 8000 WKC/MET/036 electrochemiluminescence immunoassays (ng/mL) (coefficient of variation of 5%)). Vitamin D deficiency was defined as 25(OH) vitamin D levels below 20 ng/mL, according to the cut-off values reported by Sempos et al (37).
Statistical Analysis
Descriptive statistics were obtained for all study variables. Categorical variables were summarized as counts and percentages. The Kolmogorov–Smirnov normality test was performed (P < .05); continuous variables are expressed as medians and interquartile range (IQR) (25th-75th percentile). The Fisher exact test or chi-squared test and the Wilcoxon signed-rank test or the Kruskal–Wallis test were used to determine statistical significance of the differences in proportions and medians, respectively. Correlations were analyzed using the Spearman rank correlation analysis.
Multiple logistic regression analyses were used to estimate the odds ratio (OR) with 95% CI of 25(OH) vitamin D levels for long COVID occurrence risk. All statistical tests were 2-sided. P < .05 was considered to be statistically significant. Statistical analysis was conducted using IBM SPSS Statistics (IBM SPSS Statistics for Windows, Version 23.0; IBM Corp., Armonk, NY) and GraphPad Prism (GraphPad Software for Windows, version 9.0.0, San Diego, CA USA).
Results
Long COVID Features
A total of 100 patients were enrolled in the study in a 1:1 ratio for the presence/absence of long COVID and matched for age, sex, comorbidities and previous COVID-19 severity. The cohort included 50 (50%) patients with and 50 (50%) without long COVID. Demographic characteristics and concomitant comorbidities prevalence of these groups are summarized in Table 1. Median (IQR) age of patients was 61 (51-70) years, and 56 (56%) were male. The most frequent concomitant comorbidity in our cohort was history of hypertension (39%), followed by cardiovascular disease (9%). Twenty-nine patients (29%) required NIMV during hospitalization. Median BMI was 27.5 (24.6-31.7), 32 (32%) patients were obese, and 42 (42%) were overweight. There were no statistical differences regarding demographic characteristics, comorbidities prevalence, NIMV needs, inflammatory parameters at admission and at follow-up, and glucose levels at follow-up between patients with long COVID and those without (Table 1).
Table 1.
Demographic characteristics, concomitant comorbidities prevalence and NIMV requirement in patients with and without long COVID
| Long COVID (n = 50) | Non–long COVID (n = 50) | P value | |
|---|---|---|---|
| Age, years | 61 (51-73) | 61 (53-69) | .85 |
| Male gender, n (%) | 28 (56%) | 28 (56%) | >.99 |
| BMI at follow-up, kg/m2 | 27.5 (25-32) | 27.7 (24-31) | .63 |
| Overweight/obese, n (%) | 37 (74%) | 37 (74%) | >.99 |
| Hypertension, n (%) | 17 (34%) | 22 (44%) | .4 |
| Cardiovascular disease, n (%) | 5 (10%) | 4 (8%) | >.99 |
| Diabetes mellitus, n (%) | 3 (6%) | 5 (10%) | .71 |
| Active neoplasia, n (%) | 2 (4%) | 1 (2%) | >.99 |
| NIMV, n (%) | 14 (28%) | 15 (30%) | >.99 |
| CRP at admission, mg/dL | 73.8 (31-133) | 78.5 (29-119) | .68 |
| LDH at admission, U/L | 456 (315-584) | 411 (288-504) | .71 |
| N/L ratio at admission | 5.1 (3.3-7.2) | 4.8 (3.1-7) | .82 |
| Albumin at admission, g/L | 31 (28-37) | 32.1 (27.5-38) | .56 |
| CRP at follow-up, mg/dL | (n = 41) 4.1 (2.5-8) | (n = 36) 3.9 (2.2-6.8) | .55 |
| LDH at follow-up, U/L | (n = 18) 168 (113-235) | (n = 22) 147 (108-215) | .81 |
| N/L ratio at follow-up | 1.8 (1.4-2.1) | 1.74 (1.2-2.4) | .85 |
| Glucose levels at follow-up, mg/dL | 98.5 (92-106) | 97.5 (89-113) | .71 |
Abbreviations: BMI, body mass index; CRP, C-reactive protein; LDH, lactate dehydrogenase; NIMV, noninvasive mechanical ventilation; N/L neutrophil to lymphocyte.
The most frequent symptoms reported in our long COVID cohort were asthenia (19%), followed by dysgeusia (17%), and dyspnea (17%) (Table 2). The most frequent affected health areas were the constitutional and the cardiorespiratory ones (both 21%), followed by senses (18%) and neurocognitive areas (7%) (Table 2).
Table 2.
Signs, symptoms, and health areas affected in patients with long COVID 6 months after hospital discharge
| Signs and symptoms reported at 6-month follow-up visit (n = 50) | |
| Dysgeusia, n (%) | 17 (34%) |
| Anosmia, n (%) | 12 (24%) |
| Asthenia, n (%) | 19 (38%) |
| Fever, n (%) | 1 (2%) |
| Myalgia, n (%) | 8 (16%) |
| Arthralgia, n (%) | 5 (10%) |
| Cough, n (%) | 3 (6%) |
| Dyspnea, n (%) | 17 (34%) |
| Chest pain, n (%) | 2 (4%) |
| Abdominal pain, n (%) | 2 (4%) |
| Nausea/vomiting, n (%) | 0 (0%) |
| Diarrhea, n (%) | 0 (0%) |
| Headache, n (%) | 4 (8%) |
| Brain fog/confusion, n (%) | 3 (6%) |
| Sore throat, n (%) | 2 (4%) |
| Rhinorrhea, n (%) | 5 (10%) |
| Ear pain, n. (%) | 0 (0%) |
| Health areas affected at 6-month follow-up visit (n = 50) | |
| Senses area, n (%) | 18 (36%) |
| Constitutional, n (%) | 21 (42%) |
| Cardiorespiratory, n (%) | 21 (42%) |
| Gastrointestinal, n (%) | 2 (4%) |
| Neurocognitive, n (%) | 7 (14%) |
| Ear nose and throat, n (%) | 6 (12%) |
25(OH) Vitamin D Levels
In the entire cohort, median 25(OH) vitamin D levels were 14.7 (9.3-21.7) ng/mL and 20.6 (15.2-25.2) ng/mL at hospital admission and at 6-month follow-up, respectively. At admission, vitamin D deficiency was found in 71 patients (71%) and at the 6-month follow-up visit in 46 patients (46%). Although there were no differences in demographic characteristics, comorbidity prevalence, NIMV needs, and inflammatory response between patients with long COVID compared with those without, we observed lower 25(OH) vitamin D levels, evaluated at 6-month follow-up visit, in patients with long COVID than in those without (20.1 [13.6-21.8] ng/mL vs 23.2 [16.7-26.6] ng/mL, P = .03) (Table 3). No differences were observed regarding 25(OH) vitamin D levels at hospitalization and prevalence of vitamin D deficiency at hospital admission and at follow-up visits between those with and without long COVID. In overweight/obese patients, we observed more frequently vitamin D deficiency at admission than those with normal weight (77% vs 54%, P = .025); vitamin D deficiency at admission was also found more frequently in male patients than in females (84% vs 54%, P = .002). No other differences were found regarding demographic characteristics, comorbidities prevalence, NIMV needs, and glucose levels at follow-up between patients with vitamin D deficiency and those without, at admission and at follow-up visits. Consistently, lower 25(OH) vitamin D levels at admission were observed in male patients (12.4 [8.8-28.6] ng/mL vs 18.1 [10.9-30.1] ng/mL; P = .009). A nonsignificant trend toward lower 25(OH) vitamin D levels at admission was observed in patients who required NIMV compared with those without (11.9 [8.2-18.9] ng/mL vs 15.8 [10.8-25.5] ng/mL; P = .077). No other differences were found regarding 25(OH) vitamin D levels, at admission and at follow-up visits, and the other demographic characteristics.
Table 3.
25(OH) Vitamin D levels at admission and at 6-month follow-up visit in the entire study cohort subdivided by presence of long COVID and of specific alterations in each affected clinical area
| 25(OH) vitamin D levels at admission (ng/mL) | P value | ||
|---|---|---|---|
| Long COVID, yes/no | Yes (n = 50): 13 (9-21) | No (n = 50): 15.8 (9.9-25) | .41 |
| Senses area, yes/no | Yes (n = 18): 12.1 (8-17.5) | No (n = 82): 15.5 (9.8-25.5) | .12 |
| Constitutional, yes/no | Yes (n = 21): 13.2 (9.3-36) | No (n = 79): 15.1 (9.2-21) | .57 |
| Cardiorespiratory, yes/no | Yes (n = 21): 14.3 (9.2-21) | No (n = 79): 16.1 (9.8-25.8) | .63 |
| Gastrointestinal, yes/no | Yes (n = 2): 13.8 (11.6-13.8) | No (n = 98): 14.7 (9.1-22) | .82 |
| Neurocognitive, yes/no | Yes (n = 7): 9.1 (7.1-11.7) | No (n = 93): 15.3 (10.3-23.4) | .03 |
| Ear nose and throat, yes/no | Yes (n = 6): 13 (9.1-21.4) | No (n = 94): 19 (16-29.5) | .12 |
| 25(OH) Vitamin D levels at 6-month follow-up (ng/mL) | |||
| Long COVID, yes/no | Yes (n = 50): 20.1 (13.6-22) | No (n = 50): 23.2 (16.7-26.6) | .03 |
| Senses area, yes/no | Yes (n = 18): 18.4 (14-21.5) | No (n = 82): 20.7 (15.1-29) | .15 |
| Constitutional, yes/no | Yes (n = 21): 20 (12-26) | No (n = 79): 20.8 (15.3-25) | .41 |
| Cardiorespiratory, yes/no | Yes (n = 21): 20.4 (15-26) | No (n = 79): 20.6 (14-22) | .68 |
| Gastrointestinal, yes/no | Yes (n = 2): 16.2 (12-16.2) | No (n = 98): 20.6 (15.2-25) | .38 |
| Neurocognitive, yes/no | Yes (n = 7): 14.6 (12.6-21.2) | No (n = 93): 20.6 (15.3-25.6) | .042 |
| Ear nose and throat, yes/no | Yes (n = 6): 20.9 (18-22.1) | No (n = 94): 20.4 (14-25.5) | .81 |
P values in bold are statistically significant.
Subgroup Analysis and Correlations
No significant correlations were observed between 25(OH) vitamin D levels, at hospital admission and follow-up visits, and age, and between 25(OH) vitamin D levels, at hospitalization and follow-up visits, and BMI. We found significant negative correlations between 25(OH) vitamin D levels, at admission and follow-up visits, and glucose levels, evaluated at follow-up visits (admission: P = .008, r = −0.29; follow-up: P = .038, r = −0.22) (Fig. 2).
Figure 2.
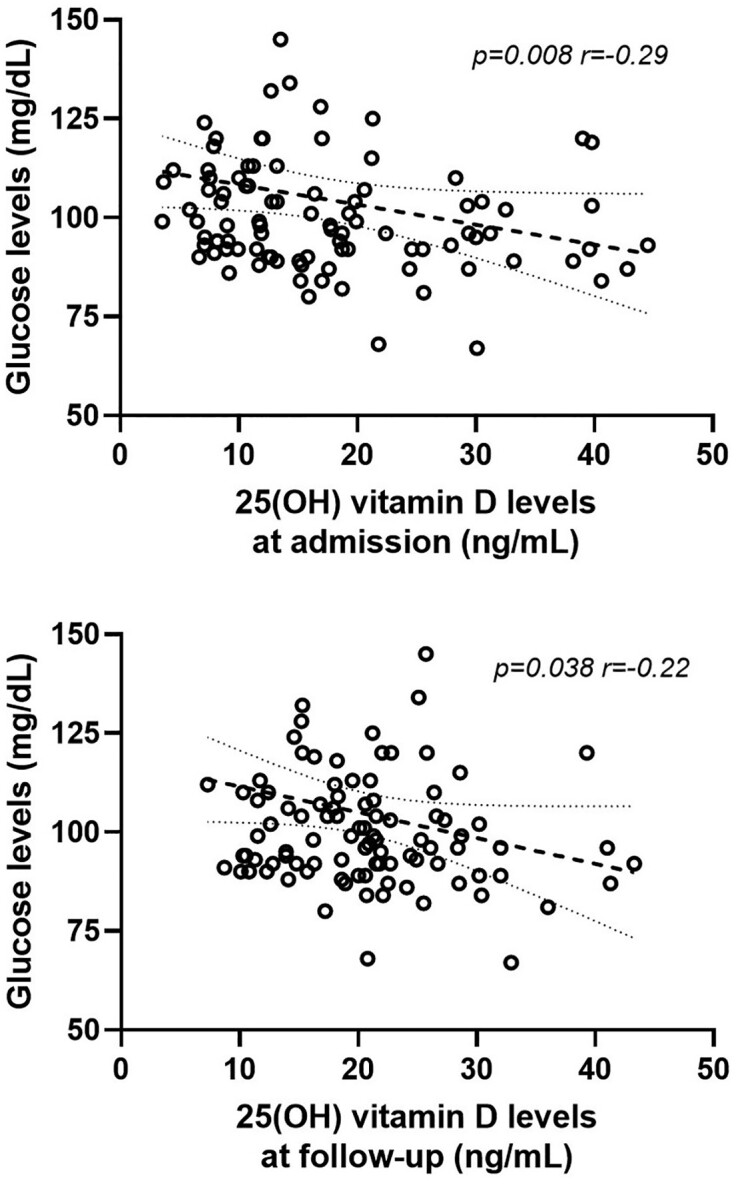
Linear correlations between 25(OH) vitamin D levels, at admission and at follow-up, and glucose levels evaluated at follow-up.
In patients presenting vitamin D deficiency both at hospital admission and at the follow-up visit (n = 42), those affected by long COVID (n = 22) were characterized by lower 25(OH) vitamin D levels, evaluated at follow-up visits, than those not affected (n = 20) (12.7 [11.4-15.1] ng/mL vs 15.2 [12.1-18.2] ng/mL, P = .041).
Regarding the different affected health areas evaluated in the entire cohort, we observed lower 25(OH) vitamin D levels, evaluated at both hospital-admission and follow-up visits, in those with neurocognitive symptoms at 6-month follow-up (n = 7) than in those without (n = 93) (admission: 9.1 [7.1-11.7] ng/mL vs 15.3 [10.3-23.4] ng/mL, P = .03) (follow-up: 14.6 [12.6-21.2] ng/mL vs 20.6 [15.3-25.6] ng/mL, P = .042) (Table 3). No other significant differences were found regarding demographic and disease characteristics between patients with neurocognitive symptoms at 6-month follow-up (n = 7) compared with those without (n = 93) (Table 4). Three patients with neurocognitive symptoms at follow-up who were previously admitted to hospital for acute disease in March were subsequently re-evaluated in the follow-up outpatient clinic in September, 2 admitted in April were then re-evaluated in October, and 2 admitted in May were re-evaluated in November, in line with the seasonality evaluation of the whole cohort. None of these 7 patients had neurocognitive symptoms before the acute disease. A nonsignificant trend toward lower 25(OH) vitamin D levels at hospital admission was observed in patients who presented dysgeusia at 6-month follow-up (n = 17) compared with those without (n = 83) (11.8 [8.1-16.5] ng/mL vs 15.8 [9.9-25.5] ng/mL, P = .072). A significant higher prevalence of vitamin D deficiency at the follow-up visit was observed in those who presented with headache at the 6-month follow-up (n = 4) compared with those without (n = 96) (8.7% vs 0%, P = .042). A significant higher prevalence of vitamin D deficiency at admission was observed in those who presented dysgeusia at the 6-month follow-up (n = 17) compared with those without (n = 83) (22.5% vs 3.4%, P = .02), and a nonsignificant trend toward a higher prevalence of vitamin D deficiency at admission was observed in those who presented an impaired senses area at 6-month follow-up (n = 18) compared with those without (n = 82) (22.5% vs 6.8%, P = .086). No other statistically significant differences were observed regarding 25(OH) vitamin D levels at admission and follow-up visits, prevalence of vitamin D deficiency at admission and at follow-up visits, and symptoms and impaired health areas reported at 6-month follow-up visits.
Table 4.
Demographic characteristics and concomitant comorbidities prevalence in patients with and without neurocognitive symptoms at follow-up visits
| Neurocognitive symptoms—yes (n = 7) | Neurocognitive symptoms—no (n = 93) | P value | |
|---|---|---|---|
| Age, years | 62 (52-71) | 61 (53-70) | .77 |
| Male gender, n (%) | 3 (43%) | 53 (57%) | .69 |
| BMI at follow-up, kg/m2 | 27.3 (24-32) | 27.1 (23-30) | .53 |
| Overweight/obese, n (%) | 5 (70%) | 69 (74%) | >.99 |
| Hypertension, n (%) | 3 (43%) | 36 (39%) | .43 |
| Cardiovascular disease, n (%) | 1 (14%) | 8 (8.6%) | .49 |
| Diabetes mellitus, n (%) | 1 (14%) | 7 (7.5%) | .45 |
| Active neoplasia, n (%) | 0 (0%) | 3 (3.2%) | >.99 |
| NIMV, n. (%) | 1 (14%) | 28 (30%) | .67 |
| CRP at admission, mg/dL | 77.7 (35-151) | 74 (33-139) | .86 |
| LDH at admission, U/L | 426 (305-545) | 444 (311-564) | .81 |
| N/L ratio at admission | 4.9 (3.5-7.1) | 5.2 (3.8-8) | .54 |
| Albumin at admission, g/L | 31.5 (29-37) | 31.8 (28-36) | .85 |
| CRP at follow-up, mg/dL | (n = 5) 4.2 (2.7-7.6) | (n = 72) 4 (2.2-7) | .75 |
| LDH at follow-up, U/L | (n = 3) 158 (131-225) | (n = 37) 145 (112-218) | .38 |
| N/L ratio at follow-up | 1.78 (1.3-2.1) | 1.76 (1.2-2.4) | .82 |
Abbreviations: BMI, body mass index; CRP, C-reactive protein; LDH, lactate dehydrogenase; NIMV, noninvasive mechanical ventilation; N/L neutrophil to lymphocyte.
In patients presenting vitamin D deficiency both at admission and at follow-up visits (n = 42), we observed lower 25(OH) vitamin D levels, evaluated at follow-up visits, in those with constitutional symptoms at the 6-month follow-up (n = 9) compared with those without (n = 33) (11.7 [10.9-13.4] ng/mL vs 15.2 [12.2-17.7] ng/mL, P = .025) and in those with asthenia (n = 8) compared with those without (n = 34) (11.6 [10.7-12.7] ng/mL vs 15.2 [12.3-17.5] ng/mL, P = .009).
Multiple logistic regression analyses including demographic characteristics, comorbidities, and 25(OH) vitamin D levels were performed for long COVID occurrence risk. Lower 25(OH) vitamin D levels, evaluated at follow-up visits, were the only factor significantly and independently associated with the long COVID occurrence (P = .008, OR 1.09, CI 1.01-1.16) in our cohort (Table 5).
Table 5.
Multiple logistic regression analysis of possible risk factors to predict long COVID in a matched cohort for age, sex, concomitant comorbidities and acute disease severity
| OR | 95% CI | P value | |
|---|---|---|---|
| Sex | 1.29 | 0.53-3.14 | .58 |
| BMI | 0.97 | 0.9-1.05 | .44 |
| Age | 0.99 | 0.96-1.03 | .56 |
| Hypertension | 1.69 | 0.65-4.13 | .26 |
| Cardiovascular disease | 0.53 | 0.11-2.7 | .44 |
| Diabetes mellitus | 1.76 | 0.24-12.9 | .57 |
| Active neoplasia | 0.32 | 0.02-4.4 | .39 |
| NIMV requirement | 1.3 | 0.45-3.72 | .63 |
| Glucose levels at follow-up visit | 1.02 | 0.99-1.05 | .11 |
| 25(OH) vitamin D levels at follow-up visit | 1.09 | 1.01-1.16 | .008 |
P values in bold are statistically significant.
Abbreviations: BMI, body mass index; OR, odds ratio.
Discussion
In this study we have observed that COVID-19 survivors who reported persistent signs and symptoms 6 months after hospital discharge consistent with long COVID syndrome were characterized by lower 25(OH) vitamin D levels than those without the syndrome, and lower 25(OH) vitamin D levels were an independent risk factor for long COVID occurrence.
During the last 3 years, increasing evidence has highlighted the potential role of hypovitaminosis D as a modifiable risk factor for SARS-CoV-2 infection and worse acute COVID-19 (1). These findings were initially reported by only retrospective and observational studies conducted during the first pandemic wave, and therefore were unable to adequately clarify the possible reverse causality between acute illness and lower 25(OH) vitamin D levels, which are known to possibly decrease during the acute inflammatory and immune responses (1). Subsequent prospective and interventional studies conducted with better and rigorous study designs were able to consistently confirm the negative role of low vitamin D in these patients not supporting a central role for reverse causality between COVID-19 and hypovitaminosis D observed during the acute illness (1, 7-9, 39). These findings, in addition to the highly reported rates of acute hypocalcemia (40, 41) and skeletal complications (42, 43), have suggested the presence of a distinct and emerging osteo-metabolic COVID-19 phenotype within the endocrine and metabolic comorbidities reported in these patients (44-46). In contrast, the consequences of low vitamin D levels and hypovitaminosis D after the acute phase of COVID-19 are still poorly understood. In fact, COVID-19 survivors are often characterized by the presence of persistent clinically relevant signs and symptoms not explained by other medical conditions and only attributable to the previous SARS-CoV-2 infection, markedly impairing their quality of life and postinfection recovery even several months after the acute phase (11-15). So far, different characteristics of patients including gender, concomitant comorbidities, and grade of acute disease severity have been implicated to be possible underlying factors influencing long COVID occurrence risk (14, 15).
Due to the well-known extraskeletal role of vitamin D in regulating immune response and immunocompetence and the consistently reported positive effects on musculoskeletal, neurocognitive, metabolic, and cardiorespiratory health areas, a possible role of vitamin D in recovery of COVID-19 survivors was also hypothesized.
This hypothesis is firstly supported by the observed lower 25(OH) vitamin D levels reported among 120 not-hospitalized young COVID-19 survivors 3 months after acute disease compared with matched healthy participants. COVID-19 survivors were also characterized by higher inflammatory markers and therefore reverse causality could not be excluded (47). Later studies have subsequently investigated the effect of vitamin D on the risk of the presence of persistent symptoms in these patients, reporting, to date, contrasting results.
Townsend et al investigated the relationship between vitamin D and fatigue and reduced exercise tolerance in a total of 149 patients recruited at a median of 79 days after COVID-19 illness (30). The median vitamin D level was 62 nmol/L (about 24 ng/dL), higher than the levels observed in our cohort in which, differently from the study conducted by Townsend et al, we excluded those with vitamin D supplementation, and fatigue was very common (58%), possibly related to the short-term follow-up visits and to the inclusion in the study also of those who were previously admitted to the ICU. The authors reported no significant relationships between vitamin D status and any of the physical performance outcomes assessed. A later pilot clinical trial aimed at analyzing the effect of vitamin D supplementation on muscle fitness in 30 elderly patients during the recovery phase after SARS-CoV-2 infection has reported that treatment with cholecalciferol (2000 IU/day) vs placebo carried out for 6 weeks was associated with a reduction of serum creatine kinase levels and with slight nonsignificant improvements in physical tests (31). An Egyptian cross-sectional study aimed to evaluate the frequency of vitamin D deficiency in post-COVID-19 patients and its relation to persistent symptoms included 219 patients with confirmed COVID-19 diagnoses in the previous 3 months and who were mostly managed at home during the acute clinical course (32). The authors evaluated different symptom categories, including constitutional, respiratory, neuropsychiatric, and gastrointestinal areas, and reported no differences among vitamin D status and these symptoms, although only 16% of the entire cohort presented with not-deficient vitamin D levels. A recent study conducted in an Italian center aimed to determine the relationship between vitamin D status and physical performance including a sample of recovered patients, both hospitalized and not hospitalized, with a mean age of 53 years and re-evaluated in a post-acute outpatient service about 3 months after COVID-19 diagnosis (33). Vitamin D deficiency was detected in 35.6% of participants, with a higher prevalence in men than in women, similarly to our data. The authors reported that patients with vitamin D deficiency performed worse on the 6-minute walking test, particularly in those older than 65 years. Finally, in a very recent interventional clinical trial including patients receiving a single dose of 200 000 IU of vitamin D3 or placebo at the time of hospital admission for acute COVID-19 and re-evaluated by telephone interviews at 6 months and 1 year after hospital discharge, no significant differences between vitamin D-treated and placebo groups were observed for the different constitutional and respiratory post-COVID-19 symptoms (34). The authors concluded that the use of vitamin D supplementation was not useful for the management of post-COVID-19 symptoms. In this study, no data regarding 25(OH) vitamin D levels after supplementation was reported, both during hospitalization and during follow-up; moreover, at baseline, both vitamin D–treated and placebo groups presented median vitamin D levels around 21 ng/mL, reducing the potential beneficial effect of vitamin D supplementation in a population yet to be characterized by a not-deficient vitamin D status (48); finally, the study did not rule out the possibility that intermediate doses of vitamin D administered during follow-up, and not tested, could modify the clinical course of these patients.
The contrasting results reported in these studies can be, at least in part, explained, as already mentioned, by the different inclusion and exclusion criteria of the patients, the different symptoms and outcomes collected, and the different timepoints evaluated after acute disease.
In our study, in order to minimize the possible biases derived from the influence on long COVID occurrence of patient characteristics, such as gender and age, presence of comorbidities, and the different grade of acute disease severity, we aimed to evaluate the role of vitamin D in post-COVID recovery in the presence of a controlled cohort matched 1:1 for long COVID presence/absence, baseline characteristics, and acute disease severity. Moreover, we excluded patients previously admitted to the ICU and those managed at home in order to include a homogeneous population.
In this controlled clinical situation, we observed that patients affected by long COVID syndrome were characterized by lower 25(OH) vitamin D levels than those without. No differences were observed regarding vitamin D deficiency prevalence. These data could be related, at least in part, to the adequate vitamin D status observed in part of our cohort mostly characterized by non–older patients with a low prevalence of severe comorbidities and re-evaluated with medium-long term follow-up visits after acute disease.
Furthermore, two-thirds of the patients with low vitamin D levels at hospital admission still presented hypovitaminosis D at the 6-month follow-up. This suggests that reverse causality of COVID-19 on vitamin D levels may occur in some patients but also shows that poor vitamin D status may be intrinsic to several COVID-19 patients (1). In fact, reinforcing the possible negative role of hypovitaminosis D in the recovery of COVID-19 survivors, we also observed that considering only patients presenting vitamin D deficiency both at hospital admission and at follow-up visits, thus excluding those with a possible reverse causality effect of the acute disease on hypovitaminosis D occurrence, long COVID patients were characterized by lower 25(OH) vitamin D levels than controls.
Confirming these findings, no differences regarding inflammatory parameters at admission and at follow-up were found between patients with and without long COVID; therefore, these data did not support the influence of disease severity on the differences in 25(OH) vitamin D levels observed in our cohort. Previous studies have hypothesized a possible role of higher inflammation or immunological dysfunction in long COVID risk occurrence (49, 50), particularly in the first months after the acute disease. However, these observations were not confirmed in several later studies evaluating commonly used and available inflammatory biomarkers (51-54). One possible explanation for our findings could be persistent inflammation in patients with long COVID, but not in patients without long COVID, reducing vitamin D binding protein, which in turn could lead to reduced 25(OH) vitamin D levels. Interestingly, we were not able to observe any significant differences in commonly used inflammatory parameters such as CRP, LDH (available only in a part of patients), and neutrophil to lymphocyte ratio between patients with long COVID and patients with non–long COVID. This may be due to the careful selection of study subjects excluding those with more severe acute COVID-19, such as patients requiring ICU admission and including patients with long COVID and patients without long COVID with comparable acute disease severity. Therefore, it appears unlikely that different degrees of inflammation could explain the differences observed in 25(OH) vitamin D levels.
Interestingly, we observed in our entire cohort lower baseline and follow-up 25(OH) vitamin D levels in patients presenting long-term neurocognitive impairment than those without, possibly also confirming the well-known influence of vitamin D in this specific health area, since interfering data such as differences in seasonality of 25(OH) vitamin D measurements and in demographic and comorbidities data were excluded (25-27, 55). Furthermore, for the first time, to our knowledge, we observed strict negative correlations between baseline and follow-up 25(OH) vitamin D levels and follow-up glucose levels, reinforcing the associations between vitamin D and glucose levels previously reported in patients with acute COVID-19 (5, 56, 57) and the well-known relationships between vitamin D and glycemic status (58-60). Moreover, this finding could represent an additional novel mechanism implicated in long COVID occurrence. The risk of occurrence of this syndrome was reported to be increased in patients with diabetes (14, 61-63), and, since low circulating vitamin D levels have been extensively reported to be associated with poor glycemic control in patients with diabetes and with a higher predisposition of developing impaired fasting glucose and diabetes (64-66), hypovitaminosis D could be, firstly, associated with the increased risk of new developing and incident diabetes after acute COVID-19 (67-71) and, secondly, represent, at least in part, the underlying common pathophysiological factor with a bidirectional negative effect associating diabetes and long COVID occurrence.
Finally, we also confirmed the possible negative role of lower 25(OH) vitamin D levels in long COVID occurrence in multivariate logistic regression as an independent risk factor associated with its development.
Our study has limitations. First of all, the number of enrolled patients was relatively limited due to the use of stringent entry criteria, excluding those with factors possibly affecting vitamin D metabolism and including only those with available data and collected blood samples. Moreover, we included only patients who were previously hospitalized, excluding those who required ICU admission and/or those managed at home only. We accurately performed enrolment in a 1:1 ratio matched for age, sex, comorbidities, and acute disease severity on the basis of long COVID presence/absence. Therefore, the highly controlled nature of the study made it a candidate to recognize at best the possible role of lower 25(OH) vitamin D levels in long COVID occurrence. Secondly, its retrospective nature meant we could not evaluate the direct influence of vitamin D on persistence of symptoms and signs. Thirdly, the monocentric cohort, lack of evaluation of other biologically active forms of serum vitamin D as well as vitamin D binding protein, and a complete immunological profile evaluation at follow-up (inflammatory markers at follow-up were also only available in a subgroup of patients) also limit definitive conclusions. However, although a single center experience cannot definitively assign cause and effect, these evaluations should be considered for future larger studies to clarify the underlying mechanisms on the relationship between vitamin D and long COVID syndrome. Fourthly, we cannot exclude the effects of seasonality on the 25(OH) vitamin D levels observed at admission, evaluated with blood samples collected in spring, and at follow-up visits, evaluated with blood samples collected in autumn. Indeed, the improvement of about 6 ng/mL in 25(OH) vitamin D levels observed between these 2 timepoints in our cohort, including only subjects without vitamin D supplementation, should also be related, in addition to the recovery from the acute disease, to different seasonality measurement. Besides this finding, in our study, the seasonality effects cannot explain the differences observed in 25(OH) vitamin D levels between long COVID and non–long COVID patients, since they were individually selected and matched for the time of follow-up visits, and consequentially evaluated 6 months before in the same time periods of hospital admission.
In conclusion, we have reported that patients with long COVID re-assessed 6 months after hospital discharge are characterized by lower 25(OH) vitamin D levels; lower vitamin D were an independent risk factor for the occurrence of this syndrome. Differently from other previous reports, we observed these findings in a controlled study with a homogeneous population; we did not limit our evaluation during follow-up visits to specific health areas but performed a multidisciplinary evaluation, including the collection of a broad spectrum of symptoms and signs reported by patients with long COVID. Furthermore, the medium-long term follow-up used in our study could probably better identify and characterize patients with post-infection recovery impairment, and thus those who needed more intensive follow-up, possibly requiring preventive and therapeutic strategies, including also vitamin D supplementation. This aspect strongly reinforces the clinical usefulness of 25(OH) vitamin D evaluation as a possible modifiable pathophysiological factor underlying this emerging worldwide critical health issue. Finally, our data if confirmed in large, interventional, randomized controlled trials suggests that vitamin D supplementation could represent a possible preventive strategy in reducing the burden of COVID-19 sequelae.
Acknowledgments
The authors wish to thank Abiogen Pharma S.p.A, Pisa, Italy, for supporting this study.
Abbreviations
- BMI
body mass index
- COVID-19
Coronavirus disease-19
- CRP
C-reactive protein
- LDH
lactate dehydrogenase
- NIMV
noninvasive mechanical ventilation
Contributor Information
Luigi di Filippo, Institute of Endocrine and Metabolic Sciences, Università Vita-Salute San Raffaele, IRCCS Ospedale San Raffaele, Milan 20132, Italy.
Stefano Frara, Institute of Endocrine and Metabolic Sciences, Università Vita-Salute San Raffaele, IRCCS Ospedale San Raffaele, Milan 20132, Italy.
Fabrizio Nannipieri, Clinical Research, Abiogen Pharma, Pisa 56121, Italy.
Alice Cotellessa, Laboratory Medicine Service, IRCCS Ospedale San Raffaele, Milan 20132, Italy.
Massimo Locatelli, Laboratory Medicine Service, IRCCS Ospedale San Raffaele, Milan 20132, Italy.
Patrizia Rovere Querini, Division of Immunology, Transplantation & Infectious Diseases, Università Vita-Salute San Raffaele, IRCCS Ospedale San Raffaele, Milan 20132, Italy.
Andrea Giustina, Institute of Endocrine and Metabolic Sciences, Università Vita-Salute San Raffaele, IRCCS Ospedale San Raffaele, Milan 20132, Italy.
Funding
This work was supported by Abiogen Pharma S.p.A, Pisa, Italy.
Author Contributions
All authors contributed equally.
Disclosures
F.N. is an employee of Abiogen Pharma S.p.A. A.G. is a consultant for Abiogen and Takeda and received a research grant to his institution from Takeda. All other authors have no conflicts of interest to declare. The work submitted for publication is original and has not been published in any language or format and has not been submitted elsewhere for print or electronic publication consideration.
Data Availability
All data generated and analyzed during this study are included in this published article.
References
- 1. Bilezikian JP, Binkley N, De Luca HF, et al. Consensus and controversial aspects of vitamin D and COVID-19. J Clin Endocrinol Metab. 2023;108(5):1034–1042. [DOI] [PubMed] [Google Scholar]
- 2. Dissanayake HA, de Silva NL, Sumanatilleke M, et al. Prognostic and therapeutic role of vitamin D in COVID-19: systematic review and meta-analysis. J Clin Endocrinol Metab. 2022;107(5):1484‐1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiodini I, Gatti D, Soranna D, et al. Vitamin D status and SARS-CoV-2 infection and COVID-19 clinical outcomes. Front Public Health. 2021;9:736665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akbar MR, Wibowo A, Pranata R, Setiabudiawan B. Low serum 25-hydroxyvitamin D (vitamin D) level is associated with susceptibility to COVID-19, severity, and mortality: a systematic review and meta-analysis. Front Nutr. 2021;8:660420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. di Filippo L, Allora A, Doga M, et al. Vitamin D levels are associated with blood glucose and BMI in COVID-19 patients, predicting disease severity. J Clin Endocrinol Metab. 2022;107(1):e348‐e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bogliolo L, Cereda E, Klersy C, et al. Vitamin D 25OH deficiency and mortality in moderate to severe COVID-19: a multi-center prospective observational study. Front Nutr. 2022;9:934258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kalichuran S, van Blydenstein SA, Venter M, Omar S. Vitamin D status and COVID-19 severity. S Afr J Infect Dis. 2022;37(1):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parra-Ortega I, Alcara-Ramírez DG, Ronzon-Ronzon AA, et al. 25-Hydroxyvitamin D level is associated with mortality in patients with critical COVID-19: a prospective observational study in Mexico City. Nutr Res Pract. 2021;15(Suppl 1):S32‐S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campi I, Gennari L, Merlotti D, et al. Vitamin D and COVID-19 severity and related mortality: a prospective study in Italy. BMC Infect Dis. 2021;21(1):566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bianconi V, Mannarino MR, Figorilli F, et al. Prevalence of vitamin D deficiency and its prognostic impact on patients hospitalized with COVID-19. Nutrition. 2021;91-92:111408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Cuadrado ML, Florencio LL. Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18(5):2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Institute for Health and Care Excellence (NICE), Royal College of General Practitioners, Healthcare Improvement Scotland SIGN . COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. National Institute for Health and Care Excellence; 2020. Accessed 30 December, 2020. www.nice.org.uk/guidance/ng188 [Google Scholar]
- 13. Sivan M, Taylor S. NICE Guideline on long COVID. BMJ. 2020;371:m4938. [DOI] [PubMed] [Google Scholar]
- 14. Notarte KI, de Oliveira MHS, Peligro PJ, et al. Age, sex and previous comorbidities as risk factors not associated with SARS-CoV-2 infection for long COVID-19: a systematic review and meta-analysis. J Clin Med. 2022;11(24):7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maglietta G, Diodati F, Puntoni M, et al. Prognostic factors for post-COVID-19 syndrome: a systematic review and meta-analysis. J Clin Med. 2022;11(6):1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bouillon R, Marcocci C, Carmeliet G, et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev. 2019;40(4):1109‐1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bilezikian JP, Bikle D, Hewison M, et al. Mechanisms in endocrinology: vitamin D and COVID-19. Eur J Endocrinol. 2020;183(5):R133‐R147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020;12(7):2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garcia M, Seelaender M, Sotiropoulos A, Coletti D, Lancha AH Jr. Vitamin D, muscle recovery, sarcopenia, cachexia, and muscle atrophy. Nutrition. 2019;60:66‐69. [DOI] [PubMed] [Google Scholar]
- 20. Olsson K, Saini A, Strömberg A, et al. Evidence for vitamin D receptor expression and direct effects of 1α, 25(OH)2D3 in human skeletal muscle precursor cells. Endocrinology. 2016;157(1):98‐111. [DOI] [PubMed] [Google Scholar]
- 21. Wagatsuma A, Sakuma K. Vitamin D signaling in myogenesis: potential for treatment of sarcopenia. Biomed Res Int. 2014;2014:121254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78(12):1463‐1470. [DOI] [PubMed] [Google Scholar]
- 23. Makrani AH, Afshari M, Ghajar M, Forooghi Z, Moosazadeh M. Vitamin D and fibromyalgia: a meta-analysis. Korean J Pain. 2017;30(4):250‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ellis SD, Kelly ST, Shurlock JH, Hepburn ALN. The role of vitamin D testing and replacement in fibromyalgia: a systematic literature review. BMC Rheumatol. 2018;2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khairy EY, Attia MM. Protective effects of vitamin D on neurophysiologic alterations in brain aging: role of brain-derived neurotrophic factor (BDNF). Nutr Neurosci. 2021;24(8):650‐659. [DOI] [PubMed] [Google Scholar]
- 26. Nowaczewska M, Wiciński M, Osiński S, Kaźmierczak H. The role of vitamin D in primary headache-from potential mechanism to treatment. Nutrients. 2020;12(1):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gáll Z, Székely O. Role of vitamin D in cognitive dysfunction: new molecular concepts and discrepancies between animal and human findings. Nutrients. 2021;13(11):3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jolliffe DA, Camargo CA Jr, Sluyter JD, et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021;9(5):276‐292. [DOI] [PubMed] [Google Scholar]
- 29. Haugen J, Basnet S, Hardang IM, et al. Vitamin D status is associated with treatment failure and duration of illness in Nepalese children with severe pneumonia. Pediatr Res. 2017;82(6):986‐993. [DOI] [PubMed] [Google Scholar]
- 30. Townsend L, Dyer AH, McCluskey P, et al. Investigating the relationship between vitamin D and persistent symptoms following SARS-CoV-2 infection. Nutrients. 2021;13(7):2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Caballero-García A, Pérez-Valdecantos D, Guallar P, et al. Effect of vitamin D supplementation on muscle status in old patients recovering from COVID-19 infection. Medicina (Kaunas). 2021;57(10):1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mohamed Hussein AAR, Galal I, Amin MT, et al. Prevalence of vitamin D deficiency among patients attending post COVID-19 follow-up clinic: a cross-sectional study. Eur Rev Med Pharmacol Sci. 2022;26(8):3038‐3045. [DOI] [PubMed] [Google Scholar]
- 33. Galluzzo V, Ciciarello F, Tosato M, et al. Association between vitamin D status and physical performance in COVID-19 survivors: results from the Gemelli against COVID-19 post-acute care project. Mech Ageing Dev. 2022;205:111684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fernandes AL, Sales LP, Santos MD, Caparbo VF, Murai IH, Pereira RMR. Persistent or new symptoms 1 year after a single high dose of vitamin D3 in patients with moderate to severe COVID-19. Front Nutr. 2022;9:979667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rovere-Querini P, Tresoldi C, Conte C, et al. Biobanking for COVID-19 research. Panminerva Med. 2020;64(2):244‐252. [DOI] [PubMed] [Google Scholar]
- 36. Rovere Querini P, De Lorenzo R, Conte C, et al. Post-COVID-19 follow-up clinic: depicting chronicity of a new disease. Acta Biomed. 2020;91(9-S):22‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sempos CT, Heijboer AC, Bikle DD, et al. Vitamin D assays and the definition of hypovitaminosis D: results from the first international conference on controversies in vitamin D. Br J Clin Pharmacol. 2018;84(10):2194‐2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Lorenzo R, Conte C, Lanzani C, et al. Residual clinical damage after COVID-19: A retrospective and prospective observational cohort study. PLoS One. 2020;15(10):e0239570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. di Filippo L, Uygur M, Locatelli M, Nannipieri F, Frara S, Giustina A. Low vitamin D levels predict outcomes of COVID-19 in patients with both severe and non-severe disease at hospitalization. Endocrine. Published online March 1, 2023. Doi: 10.1007/s12020-023-03331-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Di Filippo L, Formenti AM, Rovere-Querini P, et al. Hypocalcemia is highly prevalent and predicts hospitalization in patients with COVID-19. Endocrine. 2020;68(3):475‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. di Filippo L, Doga M, Frara S, Giustina A. Hypocalcemia in COVID-19: prevalence, clinical significance and therapeutic implications. Rev Endocr Metab Disord. 2022:23(2):299‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. di Filippo L, Formenti AM, Doga M, Pedone E, Rovere-Querini P, Giustina A. Radiological thoracic vertebral fractures are highly prevalent in COVID-19 and predict disease outcomes. J Clin Endocrinol Metab. 2021;106(2):e602‐e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. di Filippo L, Compagnone N, Frara S, et al. Vertebral fractures at hospitalization predict impaired respiratory function during follow-up of COVID-19 survivors. Endocrine. 2022;77(2):392‐400. [DOI] [PubMed] [Google Scholar]
- 44. di Filippo L, Frara S, Giustina A. The emerging osteo-metabolic phenotype of COVID-19: clinical and pathophysiological aspects. Nat Rev Endocrinol. 2021;17(8):445‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. di Filippo L, Frara S, Doga M, Giustina A. The osteo-metabolic phenotype of COVID-19: an update. Endocrine. 2022;78(2):247‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Giustina A, Bilezikian JP. Revisiting the endocrine and metabolic manifestations of COVID-19 two years into the pandemic. Rev Endocr Metab Disord. 2022;23(2):133‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gameil MA, Marzouk RE, Elsebaie AH, Rozaik SE. Long-term clinical and biochemical residue after COVID-19 recovery. Egypt Liver J. 2021;11(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bilezikian JP, Formenti AM, Adler RA, et al. Vitamin D: dosing, levels, form, and route of administration: does one approach fit all? Rev Endocr Metab Disord. 2021;22(4):1201‐1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Conti V, Corbi G, Sabbatino F, et al. Long COVID: clinical framing, biomarkers, and therapeutic approaches. J Pers Med. 2023;13(2):334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Galán M, Vigón L, Fuertes D, et al. Persistent overactive cytotoxic immune response in a Spanish cohort of individuals with Long-COVID: identification of diagnostic biomarkers. Front Immunol. 2022;13:848886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kedor C, Freitag H, Meyer-Arndt L, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. 2022;13(1):5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. García-Abellán J, Fernández M, Padilla S, et al. Immunologic phenotype of patients with long-COVID syndrome of 1-year duration. Front Immunol. 2022;13:920627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mandal S, Barnett J, Brill SE, et al. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76(4):396‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Clemente I, Sinatti G, Cirella A, Santini SJ, Balsano C. Alteration of inflammatory parameters and psychological post-traumatic syndrome in long-COVID patients. Int J Environ Res Public Health. 2022;19(12):7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Giustina A, Adler RA, Binkley N, et al. Consensus statement from 2nd international conference on controversies in vitamin D. Rev Endocr Metab Disord. 2020;21(1):89‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Puig-Domingo M, Marazuela M, Yildiz BO, Giustina A. COVID-19 and endocrine and metabolic diseases. An updated statement from the European Society of Endocrinology. Endocrine. 2021;72(2):301‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Giustina A. Hypovitaminosis D and the endocrine phenotype of COVID-19. Endocrine. 2021;72(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Giustina A, Bouillon R, Binkley N, et al. Controversies in vitamin D: a statement from the third international conference. JBMR Plus. 2020;4(12):e10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tecilazich F, Formenti AM, Giustina A. Role of vitamin D in diabetic retinopathy: pathophysiological and clinical aspects. Rev Endocr Metab Disord. 2021;22(4):715‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maddaloni E, Cavallari I, Napoli N, Conte C. Vitamin D and diabetes mellitus. Front Horm Res. 2018;50:161‐176. [DOI] [PubMed] [Google Scholar]
- 61. Raveendran AV, Misra A. Post COVID-19 syndrome (“long COVID”) and diabetes: challenges in diagnosis and management. Diabetes Metab Syndr. 2021;15(5):102235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881‐895.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Alkodaymi MS, Omrani OA, Fawzy NA, et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin Microbiol Infect. 2022;28(5):657‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Al Dossari KK, Ahmad G, Aljowair A, et al. Association of vitamin D with glycemic control in Saudi patients with type 2 diabetes: a retrospective chart review study in an emerging university hospital. J Clin Lab Anal. 2020;34(2):e23048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Afzal S, Bojesen SE, Nordestgaard BG. Low 25-hydroxyvitamin D and risk of type 2 diabetes: a prospective cohort study and metaanalysis. Clin Chem. 2013;59(2):381‐391. [DOI] [PubMed] [Google Scholar]
- 66. Tsur A, Feldman BS, Feldhammer I, Hoshen MB, Leibowitz G, Balicer RD. Decreased serum concentrations of 25-hydroxycholecalciferol are associated with increased risk of progression to impaired fasting glucose and diabetes. Diabetes Care. 2013;36(5):1361‐1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10(5):311‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang T, Mei Q, Zhang Z, et al. Risk for newly diagnosed diabetes after COVID-19: a systematic review and meta-analysis. BMC Med. 2022;20(1):444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lai H, Yang M, Sun M, et al. Risk of incident diabetes after COVID-19 infection: a systematic review and meta-analysis. Metabolism. 2022;137:155330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Banerjee M, Pal R, Dutta S. Risk of incident diabetes post-COVID-19: a systematic review and meta-analysis. Prim Care Diabetes. 2022;16(4):591‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shrestha DB, Budhathoki P, Raut S, et al. New-onset diabetes in COVID-19 and clinical outcomes: a systematic review and meta-analysis. World J Virol. 2021;10(5):275‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analyzed during this study are included in this published article.