Graphical abstract
Explicit mechanistic insights of Prosopis juliflora extract in Streptozotocin-induced diabetic rats at the molecular level.
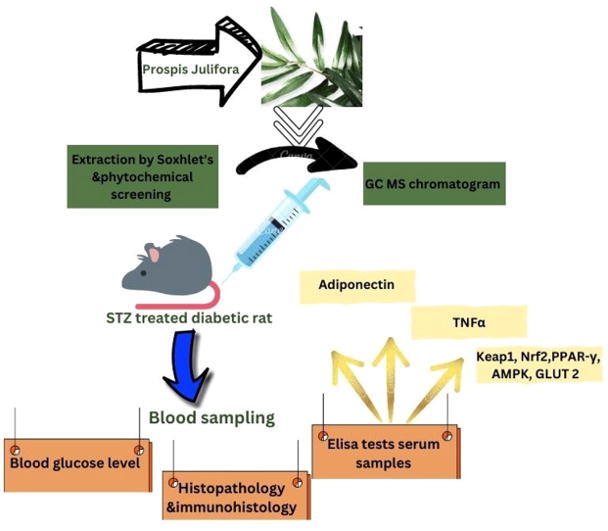
Keywords: P. juliflora, Anti-diabetic, Streptozotocin, Blood glucose, Inflammatory, Pancreatic tissue, Phytochemistry, GC–MS
Abstract
Background
The Ancient system of medicine showed the limelight on the use of herbal remedies and was found to possess minimal side effects and acceptable therapeutic outcomes. In this context, Prosopis juliflora gained importance in managing chronic diseases such as cancer, dermatological diseases, and chronic inflammatory disorders. Hence, P. juliflora was selected for further investigation associated with diabetes and inflammation.
Aim
The present study aimed to evaluate the anti-diabetic activity in chemically induced experimental rats and explore the nature of phytocomponents that may produce this activity.
Methods
Experimentally, diabetes was induced by a single administration of streptozotocin at 50 mg/kg intraperitoneally in Wistar rats. The animals were treated orally with P. juliflora at low and high doses (200 and 400 mg/kg) for 10 days. Blood collected from the retro-orbital plexus was analyzed for parameters like blood glucose levels, insulin, adiponectin, Keap1 and Nrf2. PPAR-γ, AMPK and GLUT 2 levels were analyzed in the pancreatic tissue. Besides, at the end of the experiment, animals were sacrificed, and the pancreatic tissue sections were subjected for histopathological, morphometrical and immune histochemical exploration. The phytochemical composition of the plant was investigated by GC–MS.
Results
The administration of P. juliflora higher dose showed a significant decrease (**p< 0.001) in blood glucose levels with a rise in adiponectin, PPARγ, Keap1, Nrf2, Glut 2, and AMPK significantly (**p< 0.001). The inflammatory cytokine TNFα was also estimated and was found to be lowered significantly (**p< 0.001) in test drug-treated animals. Furthermore, in the pancreatic tissue, the number of Islets, the area, and the number of β-cells were improved significantly with the sub-chronic treatment of P. juliflora extract. The structure and function of β-cells were also revamped.
Conclusion
The study results demonstrated a significant effect of P. juliflora on glycemic status, inflammatory condition, and the architecture of pancreatic tissue. In the identification and isolation process by GC MS, it was noticed that P. juliflora contained few phytochemical constituents from which it might be considered a promising drug for type 2 diabetes mellitus.
1. Introduction
Diabetes mellitus is a metabolic disorder, considered a lifestyle disease, reckons as the most rampant ailment worldwide. Diabetes is also a comorbidity of several inflammatory diseases In 2019, the global age-standardized point prevalence and death rates for type 2 diabetes were 5282.9 and 18.5 per 100 000, an increase of 49% and 10.8%, respectively, since 1990. Moreover, the global age-standardized DALY rate in 2019 was 801.5 per 100 000, an increase of 27.6% since 1990. In diabetes, there is either a derailment in insulin production by β-cells of the pancreas or the development of insulin resistance/deficit in insulin sensitivity, inevitably inclined to disrupt the metabolism of carbohydrates, proteins, and fats. Consumption of increased food, lack of physical activity, and mental stress are the main factors that are remarkable to cause diabetes; other multiple factors are also concerned. Concerning the management of diabetes, several remedies were heeded, considering the adverse effects of long-term therapy and complications of diabetes. Investigations related to the mechanism of action of drugs at the molecular level are mandated, and identification of new bioactive substances must be focused on to get explored better therapy and patient compliance. Prosopis juliflora belonging to Leguminoseae (Fabaseae) is a small shrub native to Mexico, South America, and the Caribbean (Pasiecnik, 2001). Forty-four species are currently used as folk medicine with most medicinal values, such as astringent, anti-rheumatic drugs, and snake bites. Other properties include antibacterial (Ahmad et al., 1986), antifungal (Ahmad et al., 1989, Tapia et al., 2000), haemolytic (Kanthasamy et al., 1989), anti-inflammatory (Ahmad et al., 1989), antihypercholesterolemic (Narasimhacharya A.V.R.L. et al., 2010), antitumor (Batatinha, 1997) and antioxidant (Sirmah et al., 2009). On the other side, the phytochemical constituents responsible for their respective effect might be due to active constituents such as phenols, piperidine alkaloids, flavanol glycosides, and hydroxycinnamic acids juliprosopine and mesquitol (Ahmad et al., 1989, Sirmah et al., 2009). However, there is a deficit in the investigation of the medicinal values of the plant, which is not accomplished accurately; hence a meticulous evaluation is necessary to grasp the use of the mechanism at a molecular level. At the pre-clinical platform, the in-vivo anti-diabetic activity of the plant P. juliflora was evaluated using a suitable animal model termed a sstreptozotocin-induced diabetic model.(See Fig. 1 Table 1, Table 2, Table 3, Table 4, Table 5).
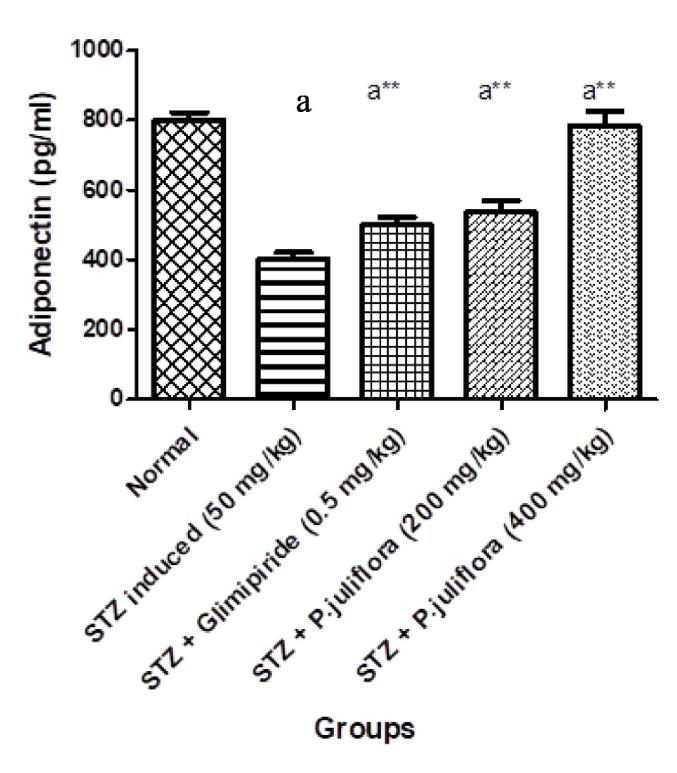
Fig. 1.
Effect of P. juliflora on Adiponectin (pg/ml) in serum samples The data were expressed in Mean ± S.E.M; n = 8 in each group; Values were statistically significant at (a**p< 0.001) when compared to Group II; (ap< 0.001) significant when compared to Group I. Data was analysed using a one-way ANOVA test followed by Tukey’s HSD test.
Table 1.
Effect of P. juliflora on Glucose levels and Insulin in STZ-induced diabetic rats.
| Groups | Treatment and Dose (mg/kg) | Glucose levels (Fasting) mg/dl | Glucose levels (Post-prandial) mg/dl | Insulin (uIU/ml) |
|---|---|---|---|---|
| I | Normal (0.9% NaCl) | 102.75 ± 6.26 | 110.7 ± 8.3 | 13 ± 0.39 |
| II | Streptozotocin (50 mg/kg i.p) | 422 ± 9.6 a | 426 ± 9.8 a | 8.93 ± 0.15 a |
| III | STZ + Glimepiride (0.5 mg/kg, p.o.) | 345.6 ± 8.7 a ** | 180.2 ± 7.6 a ** | 10.16 ± 0.68 a ** |
| IV | STZ + P. juliflora (200 mg/kg, p.o.) | 340.5 ± 12.0 a ** | 108.75 ± 2.49 a ** | 9.93 ± 0.68 a ** |
| V | STZ + P. juliflora (400 mg/kg, p.o.) | 350 ± 7.31 a ** | 81 ± 2.49 a ** | 11.2 ± 0.61 a ** |
Table 2.
Effect of P. juliflora on PPAR-γ, AMPK and GLUT 2 levels in the pancreatic tissue of STZ induced diabetic rats.
| Groups | Treatment and Dose (mg/kg) | PPAR-γ | AMPK | GLUT 2 |
|---|---|---|---|---|
| I | Normal (0.9% NaCl) | 1.1 ± 0.03 | 1.06 ± 0.06 | 1.03 ± 0.03 |
| II | Streptozotocin (50 mg/kg i.p) | 0.2 ± 0.03 a | 0.09 ± 0.01 a | 0.11 ± 0.01 a |
| III | STZ + Glimepiride (0.5 mg/kg, p.o.) | 0.76 ± 0.05 a** | 0.76 ± 0.03 a** | 0.69 ± 0.02 a** |
| IV | STZ + P. juliflora (200 mg/kg, p.o.) | 0.39 ± 0.01 a** | 0.27 ± 0.02 a** | 0.30 ± 0.02 a** |
| V | STZ + P. juliflora (400 mg/kg, p.o.) | 0.88 ± 0.04 a** | 0.78 ± 0.05 a** | 0.75 ± 0.02 a** |
Table 3.
Effect of sub- chronic treatment P. juliflora on islet number and area in pancreatic tissue sections.
| Groups | Treatment and Dose (mg/kg) | Islet number (N/10 mm2) | Islet area (μm2) |
|---|---|---|---|
| I | Normal (0.9% NaCl) | 10 ± 0.97 | 27.36 ± 2.793 |
| II | Streptozotocin (50 mg/kg i.p) | 2.16 ± 0.19 a | 10.968 ± 1.388 a |
| III | STZ + Glimepiride (0.5 mg/kg, p.o.) | 7 ± 0.87 a** | 12.464 ± 1.957 a** |
| IV | STZ + P. juliflora (200 mg/kg, p.o.) | 6 ± 0.53 a** | 22.395 ± 2.035 a** |
| V | STZ + P. juliflora (400 mg/kg, p.o.) | 9 ± 0.98 a** | 25.214 ± 3.769 a** |
The data were expressed in Mean ± S.E.M; n = 8 in each group; Values were considered statistically significant at (a**p< 0.001) when compared to Group II; (ap< 0.001) significant when compared to Group I. Data were analysed using a one-way ANOVA test followed by Tukey’s HSD test.
Table 4.
Effect of P. juliflora on number of β cells in pancreatic tissue sections.
| Groups | Treatment and Dose (mg/kg) | B cell number (N/10 mm2) |
|---|---|---|
| I | Normal (0.9% NaCl) | 143 |
| II | Streptozotocin (50 mg/kg i.p) | 52 |
| III | STZ + Glimepiride (0.5 mg/kg, p.o.) | 98 |
| IV | STZ + P. juliflora (200 mg/kg, p.o.) | 125 |
| V | STZ + P. juliflora (400 mg/kg, p.o.) | 138 |
Table 5.
Phytochemical analysis of Prosopis juliflora having anti-diabetic properties.
| S. No | RT | Compound | MF | MW | Area % | Reference |
|---|---|---|---|---|---|---|
| 1. | 17.32 | 9,12,15-Octadecatrienoic acid, 2-[(trimethylsilyl)oxy]-1-[[(trimethylsilyl)oxy]methyl]ethyl ester, (Z,Z,Z)- | C27H52O4Si2 | 496 | 0.19 | Rao, Anisha et al. 2019 |
| 2. | 64.45 | d-Mannose | C6H12O6 | 194 | 1.15 | Van de Venter et al., 2008, Rahman et al., 2021 |
| 3. | 70.19 | à-D-Glucopyranoside, O-à-D-glucopyranosyl-(1.fwdarw.3)-á-D-fructofuranosyl (steroid) | C18H32O16 | 504 | 0.27 | Kadhim, Al-Rubaye et al. 2017 |
| 4. | 71.56 | n-Hexadecanoic acid (Palmitic acid) | C16H32O2 | 256 | 3.58 | Parker, Moore et al. 2003 |
| 5. | 1.45 | Hexadecanoic acid, ethyl ester | C18H36O2 | 284 | 1.45 | Ahmed, Badawi et al. 2018 |
| 6. | 76.89 | 9,12-Octadecadienoic acid (Z,Z)- (Linoleic acid) | C18H32O2 | 280 | 5.61 | Ahmad, Zamhuri et al. 2012 |
| 7. | 77.38 | 9,12-Octadecadienoic acid, ethyl ester (Linoelaidic acid ethyl ester) | C20H36O2 | 308 | 2.44 | Adeoye-Isijola, Olajuyigbe et al. 2018 |
| 8. | 98.88 | 1,2-Benzenedicarboxylic acid, diisodecyl ester | C28H46O4 | 446 | 4.01 | Devi, Ramprasad et al. 2017 |
| 9. | 106.62 | 1-Monolinoleoylglycerol trimethylsilyl ether | C27H54O4Si2 | 498 | 0.22 | Mani, Sridhar et al. 2018 |
| 10. | 111.81 | Stigmasterol (steroid) | C29H48O | 412 | 0.15 | Tyagi and Agarwal 2017 |
| 11. | 114.24 | b-Sitosterol (steroid) | C29H50O | 414 | 0.12 | Gupta, Sharma et al. 2011 |
Streptozotocin is a nitrosourea compound produced by Streptomyces achromogens, which induced DNA breakage in the β-cells, causing diabetes mellitus (Szkudelski T 2001). This damage leads to insulin deficiency, which increases blood glucose levels. In the pathogenesis of type 2 diabetes, adipocytes participate, which engenders insulin resistance accompanied by low-grade inflammation (Pscherer S et al., 2010). As there is a link between diabetes and obesity, the adipocytes are augmented, altering the expression and secretion of adipokines recommending to a pro-inflammatory condition, dropping the levels of adiponectin whilst enhancing the production of inflammatory cytokines such as TNF-α and IL-6 (Kern PA et al., 2001). As per the studies carried out, there exists a positive correlation between obesity, hyperinsulinemia, and TNF-α (Skurk et al., 2007, El-Shafei et al., 2023). Conversely, adiponectin possesses an anti-diabetic effect with insulin-sensitizing action. (Boyle PJ 2004). Nuclear factor erythroid 2-related factor 2 (NRF2) is a transcription factor that regulates defence systems in the cells and fights against toxins; it interacts with Keap1 (Kelch-like ECH-associated protein 1), which is an adaptor protein. In a normal situation (unstressed condition), Keap 1 represses Nrf2 signalling by processing ubiquitination and degradation through the ubiquitin–proteasome pathway (Furukawa M and Xiong Y 2005). A mechanism exists; whenever Keap1 gets exposed to reactive oxygen species (ROS), a conformational change ensues and influences the hindrance of Nrf2 degradation (Kobayashi A 2004). Correspondingly, Nrf2 eventually participates in several cellular defence systems. This is termed the Keap1-Nrf2 system, which provides a stress response gene regulator and acts as a protective response against free radicals and electrophiles (Ishii T et al., 2000). In the pathophysiology of type 2 diabetes with associated complications, there is a dysfunction in this master antioxidant pathway (Keap1-Nrf2 pathway), and a promising approach has begun to elucidate the possible related mechanisms utilizing both animal and human models (Kobayashi M et al., 2009). This Keap1-Nrf2 pathway is a target, identified in treating the pancreatic destruction, insulin sensitivity, and resistance implicated in oxidative damage (Uruno A and Motohashi H 2011). On top of that, the deficit of Nrf2 may augment both type 1 and type 2 diabetes, a study emphasized (Cnop M et al., 2005). Studies done in animals have shown that in diabetes, the pancreatic β – cells are protected by the Keap1-Nrf2 pathway at physiological and pathological levels (Taguchi K et al., 2010). Thus, the present study explored the determination of Keap1-Nrf2 in streptozotocin-induced diabetic animals treated with a test drug.
In the process of glucose homeostasis, a nuclear receptor, peroxisome proliferator-activated receptor (PPAR)-c was found to be present in certain amounts in diabetic pancreatic cells, though the exact mechanism of PPAR- and its effects on insulin secretion remain speculative (Yamauchi T et al., 2001). However, it was reported that PPAR- agonists exhibit safeguard against apoptosis, thus reinstating the cellular function (Berger et al., 1996, Barroso et al., 1999). Additionally, in diabetic subjects, through the stimulation of GLUT 2 gene expression, PPAR-gets activated, causing remodelling of the glucose-sensing capacity (Tamori et al., 2002, Iwata et al., 2001).
GLUT2 (Glucose transporter 2) is located in the hepatocytes and entailed in the transportation of glucose in two ways, solely depending on the concentration gradient of a substrate molecule (Cheeseman CI 1993). The influx of glucose occurs in the postprandial state, whereas efflux favors the rise in intracellular glucose. Hence, GLUT2 modulates glucose transport incidentally regulated by insulin hormone in accordance with the glucose concentration (Corpe CP et al., 1996). In diabetes, a reduction in muscular glucose influx ensues with escalated glucose efflux in the liver, thus causing loss of glycaemic control, related to a rise in GLUT2 expression (Helliwell PA et al., 2000). As it is aware that type 2 diabetes mellitus is strongly correlated with insulin resistance (IR), few biological pathways have been picked out to overcome the insulin resistance and metabolic dysfunction (Kellett GL and Helliwell PA 2000). Thus, a pathway has been focused on called AMP-activated protein kinase (AMPK), an enzyme in the limelight to regulate metabolism (Koepsell H 2020). AMPK functions as an activator responsible for restoring normal energy levels via ATP generation; it finally improvises insulin sensitivity and balances the glucose homeostasis, which is most required in type 2 diabetes (Xiao B et al., 2007). AMPK operates different skeletal muscle and heart functions, such as elevated glucose uptake and fatty acid oxidation. The decreased insulin secretion is associated with AMPK activation in pancreatic β-cells (Chen L et al., 2012).
Glimepiride, a conventional drug that belongs to the class sulphonyl urea, was used as standard, acts by intensifying the release of insulin and action, thus, lowering the blood glucose levels. Therefore, given the above considerations, any drug used in treating type 2 diabetes necessitates a rigorous interpretation of pharmacodynamic patterns endorsed at the molecular level. Hence in the present study, parameters like glucose, insulin, adiponectin TNFα Keap1, Nrf2, AMPK, PPAR- and GLUT2 were determined in the serum of streptozotocin-induced diabetic rats in the post-treatment of P. julifora plant extract.
2. Materials and methods
2.1. Plant material and preparation of extract
Prosopis juliflora was collected from Western Sudan. Specimen were prepared and subjected to taxonomical identification and authentication at the Medicinal and Aromatic Plants and Traditional Medicine Research Institute, National Centre for Research, Khartoum, Sudan.Extraction was conducted by a slightly modified method previously described by Harborne [31]. Powder of the under shade-dried samples (500 g) was dipped in 2.5 litters of 80% ethanol and petroleum ether separately for 72 h at room temperature with constant shaking. The supernatant was filtered through Whatman filter paper (0.45 µm). This process was repeated twice. The extracts obtained were allowed to dry at room temperature. The extracts were then refrigerated at 4 °C in dark bottles until used.
2.2. Animals
Fifty male Wistar rats (250–350 g) were procured from the animal breeding unit at the National Research Centre -Dokki- Giza – Egypt. Animals were housed in cages with water and food ad libitum, and the animal room temperature was kept at a constant temperature of 20 ± 1 °C on a 12-hour light/12-hour dark cycle. The animals were not in any pain or discomfort because of the procedures adopted. All experiments were done after the approval of Standing Committee for Scientific Research - Jazan University (HAPO-10-Z-001). Approval number (REC-44/4/352).
2.3. Drugs
Streptozotocin was purchased from Sigma, St. Louis – USA. All other chemicals used in the experiment were of the highest available grade.
2.4. Acute oral toxicity study
Toxicity studies were carried out per the OECD guidelines no 423 (OECD 2001). The extract was orally given to various groups of rats at 100 and 200 mg/kg body weight doses, respectively. Readings were noted after 48 h of keen observation to check the expected behavior and examine if there were any issues with nervous problems and any lethality.
2.5. Induction of diabetes
Diabetes was induced by a single intraperitoneal injection of streptozotocin (STZ) at 50 mg/kg, dissolved in cold citrate buffer at a pH of 4.5 (Salama et al. 2022). Twenty-four hours after induction, serum glucose levels were estimated using a relevant method; animals that showed ≥ 300 mg/dl of serum glucose levels were considered diabetic and included in the study. The remaining animals were excluded from the study.
2.6. Experimental protocol
Twenty-four hours after induction of diabetes ethanolic extract of Prosopis juliflora at both doses (200 and 400 mg/kg/p.o.) was administered for 10 consecutive days (Salama et al. 2022). 50 Rats were divided randomly into groups: Group 1: the control group (n = 8), and the STZ-induced diabetic group (n = 40). Of the 40 rats injected with STZ the dead and non-hyperglycaemic rats were excluded, and only 32 hyperglycaemic rats which remained alive were included for the study. These rats were then divided into four groups as follows: Group 2: STZ group (n = 8), Group 3: STZ group (n = 8) treated with Glimepiride (0.5 mg/kg/p.o) (Salama et al. 2022) for 10 days, Group 4: STZ group (n = 8) treated with Prosopis juliflora (200 mg/kg/p.o) for 10 days and Group 5: STZ group (n = 8) treated with Prosopis juliflora (400 mg/kg/p.o) for 10 days. The diabetic rats were divided into groups according to the mean glucose levels (mg/dl) as follows:
| Groups | Glucose level (mg/dl) (24 hrs after STZ injection) |
|---|---|
| STZ (50 mg/kg/i.p) | 422 ± 9.6 |
| STZ + Glimepiride (0.5 mg/kg/p.o) | 345.6 ± 8.76 |
| STZ + Prosopis juliflora (200 mg/kg/p.o) | 340.25 ± 12.05 |
| STZ + Prosopis juliflora (400 mg/kg/p.o) | 350 ± 7.31 |
2.7. Determination of blood glucose level
Blood samples were collected from the rats' tail vein and immediately fixed on the glucose strips, then blood glucose levels were determined using One Touch Sure Step Meter, Life Scan, California, USA.
2.8. Preparation of serum samples
After 12 h of fasting, blood samples were collected from each animal via the retro-orbital plexus using a capillary glass tube at the end of the experiment. Blood samples were centrifuged using a cooling centrifuge (Laborezentrifugen, 2 k15, Sigma, Germany) at 3000 rpm for 15 min. The serum was kept at −20 °C until analysed. Keap1, Insulin, Nrf2, Adiponectin, and TNFα were quantified in the serum samples.
2.9. Enzyme-linked immunosorbent assay (ELISA)
According to the manufacturer's instructions, the ELISA technique used Keap1, Insulin, Nrf2, Adiponectin, and TNFα were estimated in the serum samples. The kits related to Keap1, Insulin, and Nrf2 were procured from SinoGene Clon Biotech Co., Ltd (No.28 Cangxin Road, YuHang District 311112, HangZhou, China). Also, Adiponectin and TNFα were determined by using kits purchased from Elabscience (14780 Memorial Drive, Suite 216, Houston, Texas 77079, United States).
2.10. Preparation of tissue samples for RNA extraction and RT-PCR
Animals were sacrificed by cervical dislocation under high anaesthesia. (Abdelhameed et al., 2021). One part of the tissues of all groups were homogenized (20%), and total RNA was extracted with Direct-zol RNA Mini-Prep Plus (Cat# R2072, ZYMO RESEARCH CORP. USA). Then, the quantity and quality were assessed using Beckman dual spectrophotometer (USA). Reverse transcription of extracted RNA was utilized by Super Script IV One-Step RT-PCR kit (Cat# 12594100, Thermo Fisher Scientific, Waltham, MA USA), followed by PCR. A thermal profile containing a 96-well plate Step One instrument (Applied Biosystem, USA) was used. Reverse transcription was carried out for 10 min at 47 °C, RT inactivation was done for 2 min at 98 °C and 35 cycles of 10 secs each were initiated by initial denaturation at 98 °C, the step of amplification was performed for 10 s at 57 °C and 30 s at 72 °C respectively. The data were expressed in Cycle threshold (Ct) for the target and housekeeping genes. Normalization for variation in the expression of each target gene; PPARα, AMPK &Glut2 was performed, referring to the mean critical threshold (CT) expression value of GAPDH housekeeping gene by the ΔΔCt method. Each target gene's relative quantitation (RQ) is quantified according to the 2-ΔΔCt method calculation.
Primer sequence:
PPARα gene; forward 5′-TGGCGTACGACAAGTGTGAT-3′ and reverse 5′-GTTTGCAAAGCCTGGGATAG-3′ (gene bank accession number is XM_032919101.1).
Glut2 gene; forward 5′-GTTGGTTCATGGTTGCTGAAT-3′ and reverse 5′-GAAGTCCGCAATGTACTGGAA-3′ (gene bank accession number is L28134.1).
AMPK gene; forward 5′-AGCTCGCAGTGGCTTATCAT-3′ and reverse 5′-GGGGCTGTCTGCTATGAGAG-3′ (gene bank accession number is XM_032901222.1); GAPDH housekeeping gene; forward 5′-CCTCGTCTCATAGACAAGATGGT −3′ and reverse 5′- GGGTAGAGTCATACTGGAACATG −3′ (gene bank accession number is NM_001394060.2).
2.11. Histopathology study
Both pancreatic and liver tissues were dissected immediately. The tissues were embedded using 10% buffered formalin. After washing the specimens with tap water for 30 min, they were dehydrated with ascending grades of alcohol, cleared in xylene, and fixed in paraffin. Each block's serial slices of pancreatic and liver tissue (4 m) were cut and stained with haematoxylin and eosin for histological and morphometric examination. Finally, images were examined and photographed under a digital camera (Microscope Digital Camera DP 70, Tokyo, and processed using Adobe Photoshop, Version 8.0).
2.12. Immunohistochemistry study
Xylene and graded ethanol solutions were used to rehydrate paraffinized pancreatic sections. After blocking endogenous peroxidase activity with hydrogen peroxide block for 10–15 min, the slide was washed and incubated in ultra-V block for 5 min to inhibit non-specific binding. Samples were incubated for 30 min at room temperature by primary antibody for insulin Ab-6 (INS04 + INS05); mouse monoclonal antibody 0.5–1 μg/ ml (Lab Vision Corporation-Themo Fisher Scientific, UK). After washing with buffer, the sections were incubated for 10 min at room temperature with a biotinylated goat anti-polyvalent secondary antibody. The sections were rinsed four times before being treated with streptavidin peroxidase for ten minutes at room temperature, as directed by the insulin Ab-6 kit (Lab Vision Corporation-Thermo Fisher Scientific, UK). The response was revealed by diaminobenzidine (DAB) Plus Chromogen and DAB plus substrate (Lab Vision Corporation-Thermo Fisher Scientific, UK) and incubated for 5–15 min, after which the sections were counterstained with Mayer's Hematoxylin and the cover slid for light microscopic analysis.
Image Analysis: The image analysis was performed at Pathology Department, National Research Centre, using Leica Qwin 500 Image analyser (LEICA Imaging System, Ltd, Cambridge, UK), which consisted of Leica DM-LB microscope with a JVC colour video camera attached to a computer.
2.13. Morphometric examination
The pancreatic tissue was morphometrically examined. The slides that were being inspected were put on the microscope's stage. The brightness of the light source was adjusted to the desired level. On the monitor, the successful illumination adjustment was verified. In assessing islets number and islet area %, each group was evaluated for the number of islets, and the number was expressed as N/10 mm2 of the pancreatic parenchyma according to Hebatollah E et al., 2019. Using the system's interactive software, the area of islets glands was determined as an area per field in micrometre square, area fraction, and area percentage. The results have appeared automatically on the monitor in the form of a table with the total, mean, standard deviation, and standard error. Each slide included five fields where the area was measured. An objective lens with a magnification of 20x was used to measure the area. Anti-insulin monoclonal antibody was used to determine the number of B cells/islet utilizing an inter-active measurement procedure at 200x magnification. Data were entered in an Excel sheet and subjected to statistical analysis.
2.14. Statistical analysis
Data are expressed as mean ± S.E.M. and analysed with Graph Pad Prism 4.0 (Graph Pad software, USA). Multiple comparisons were analysed using one-way ANOVA followed by Tukey’s HSD test for multiple comparisons. Statistical significance was considered when probability values (p< 0.001).
3. Results
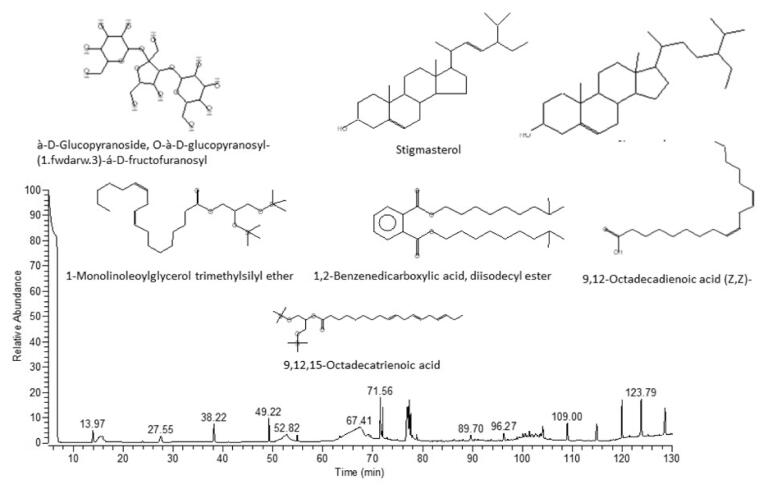
3.1. Identification of isolated compounds in P. juliflora
In the test drug, different compounds were isolated and identified. They were 9,12,15-Octadecatrienoic acid, 2-[(trimethylsilyl)oxy]-1-[[(trimethylsilyl)oxy]methyl]ethyl ester, (Z,Z,Z)-, d-mannose, à-D-glucopyranoside, O-à-D-glucopyranosyl-(1.fwdarw.3)-á-D-fructofuranosyl (steroid), n-hexadecanoic acid (palmitic acid), hexadecanoic acid, ethyl ester, 9,12-octadecadienoic acid (Z,Z)- (linoleic acid), 9,12-octadecadienoic acid, ethyl ester (linoelaidic acid ethyl ester), 1,2-benzenedicarboxylic acid, diisodecyl ester, 1-monolinoleoylglycerol trimethylsilyl ether, stigmasterol and b-Sitosterol.
3.2. Acute oral toxicity study
Acute toxicity tests revealed no toxicity observed, and the P.julifora was found to be safe for the study to be carried out because of no signs of death. Hence, in the current study, rats were administered lower and higher doses of poppy seed oil (200 and 400 mg/kg body weight, p.o).
3.3. Effect of test drug on serum glucose and insulin levels
STZ inebriated diabetic rats set forth a significant (ap< 0.001) rise in blood glucose level in both fasting and post-prandial compared to the control rats. After the treatment with the test extract – P. juliflora at two specified doses- 200 and 400 mg/kg orally, there was a tremendous descend in blood glucose levels with fasting blood glucose as 340.5 ± 12.0 and post-prandial glucose levels as 180.2 ± 7.6 mg/dl respectively, which was found to be significant (a**p< 0.001). Similarly, the fasting insulin levels were also determined in streptozotocin-induced diabetic rats, which declined significantly (ap< 0.001) compared to normal rats. P.juliflora treated animals at a higher dose (400 mg/kg) depicted a significant increase (a**p< 0.001) in insulin levels with 11.2 ± 0.61 uIU/ml as compared to STZ-induced diabetic rats. All the values were comparable with the standard drug-treated animals.
The data were expressed in Mean ± S.E.M; n = 8 in each group; Values were considered statistically significant at (a**p< 0.001) when compared to Group II; (ap< 0.001) significant when compared to Group I. Data were analysed using a one-way ANOVA test followed by Tukey’s HSD test.
3.4. Effect of P. juliflora on adiponectin (pg/ml) levels in serum samples
In diabetes-induced animals, adiponectin levels were determined in serum samples. There was a significant reduction (ap< 0.001) with the value of 403.3 ± 0.33 pg/ml compared to the normal animals. After the oral treatment with P. juliflora at a higher dose (400 mg/kg), there was an escalation in the levels to 784.6 ± 2.6 pg/ml of adiponectin in a significant manner (a**p< 0.001) as compared to other groups.
3.5. Effect of P. juliflora on TNFα (pg/ml) in serum samples
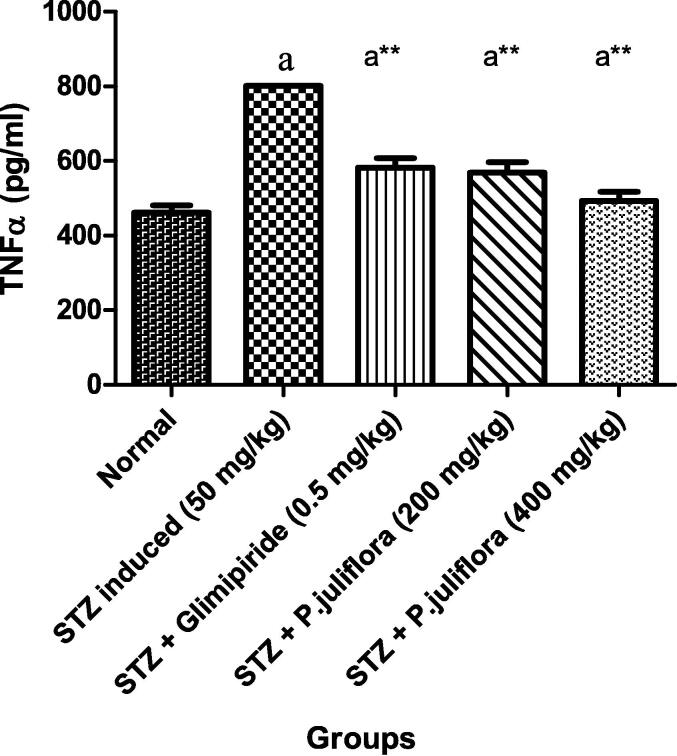
In streptozotocin-induced diabetic rats, there was an elevation in the TNFα with a value of 801.3 ± 0.88 pg/ml significantly (a**p< 0.001). With the completion of treatment with the test extract, P. juliflora at a higher dose (400 mg/kg), there was a significant (ap< 0.001) fall in the TNFα with a value of 493.3 ± 3.01 pg/ml as compared to the control animals as shown in Fig. 2.
Fig. 2.
Effect of P. juliflora on TNFα (pg/ml) in serum samples The data were expressed in Mean ± S.E.M; n = 8 in each group; Values were considered statistically significant at (a**p< 0.001) when compared to Group II; (ap< 0.001) significant when compared to Group I. Data were analysed using a one-way ANOVA test followed by Tukey’s HSD test.
3.6. Effect of P. juliflora on Keap1 (pg/ml) in serum samples
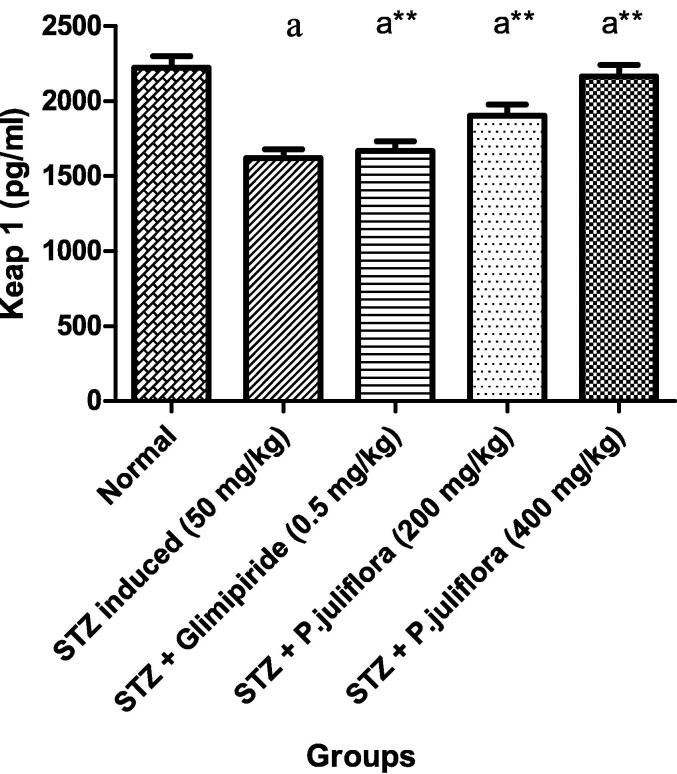
Streptozotocin-induced diabetic rats presented a reduced Keap1 level significantly (ap< 0.001) compared to normal animals. After sub- chronic treatment with the test drug for 10 days, rats that received test extract at a high dose (400 mg/kg) showed an enormous rise in Keap1 values as 2162.6 ± 4.91 pg/ml significantly (a**p< 0.001) as compared to the positive control rats. However, it was noticed that P. juliflora extract at a lower dose (200 mg/kg) had an increase in Keap1 levels significantly, as shown in Fig. 3.
Fig. 3.
Effect of P. juliflora on Keap1 (pg/ml) in serum samples The data were expressed in Mean ± S.E.M; n = 8 in each group; Values were statistically significant at (a**p< 0.001) when compared to Group II; (ap< 0.001) significant when compared to Group I. Data were analysed using one-way ANOVA test followed by Tukey’s HSD test.
3.7. Effect of P. juliflora on Nrf2 (ng/ml) in serum samples
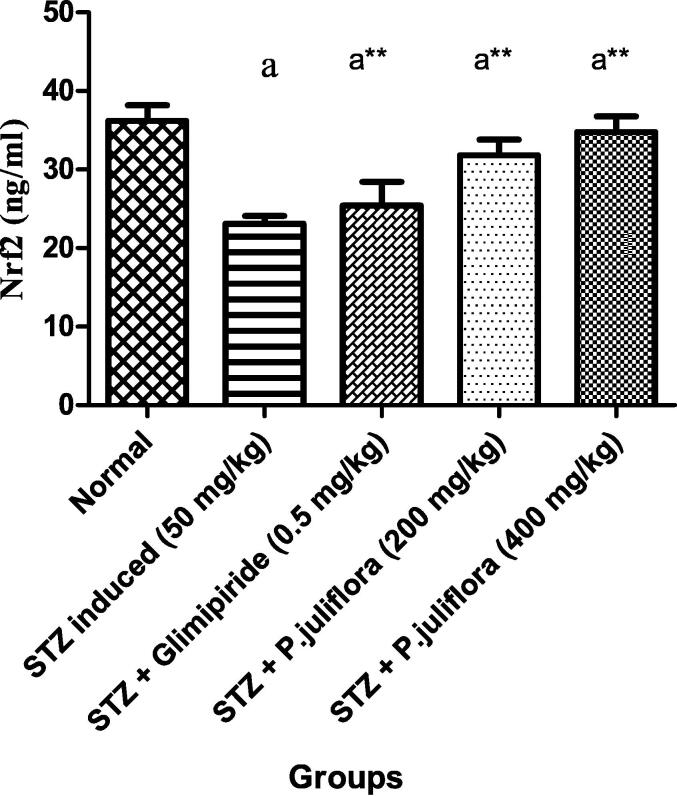
Streptozotocin-induced diabetic rats revealed a reduction in Nrf2 levels significantly (ap< 0.001) compared to normal animals. On treatment with the test drug for 10 days, rats that received test extract at both the lower and higher dose (200 & 400 mg/kg) showed a significant rise (a** p< 0.001) in Nrf2 values as 31.8 ± 0.1 and 34.76 ± 0.03 ng/ml as compared to the positive control rats as shown in Fig. 4.
Fig. 4.
Effect of P. juliflora on Nrf2 (pg/ml) in serum samples The data were expressed in Mean ± S.E.M; n = 8 in each group; Values were statistically significant at (a**p< 0.001) when compared to Group II; (ap< 0.001) significant when compared to Group I. Data were analysed using a one-way ANOVA test followed by Tukey’s HSD test.
3.8. Effect of test drug on PPAR-γ, AMPK and GLUT 2 levels in pancreatic tissue
STZ-induced diabetic rats exhibited a significant (ap< 0.001) fall in PPAR-γ, AMPK, and GLUT 2 levels in pancreatic tissue, which was processed compared to the control rats. After the treatment with the test extract – P. juliflora at two specified doses- 200 and 400 mg/kg orally, there was a tremendous ascend in PPAR-γ levels with 0.39 ± 0.01 and 0.88 ± 0.04, respectively, which was found to be significant (a**p< 0.001). Similarly, AMPK levels were also determined and were observed with a profound increase in a significant manner (a **p< 0.001) in P. juliflora treated animals at lower and higher doses (200 & 400 mg/kg) but with a significant trek in values of 0.78 ± 0.05 as compared to a higher dose of the extract (400 mg/kg). Correspondingly, the same effect was found with the measurement of GLUT 2 levels with intense lowered values with test drug-treated animals at a higher dose (400 mg/kg). All the values were comparable with the standard drug-treated animals.
The data were expressed in Mean ± S.E.M; n = 8 in each group; Values were considered statistically significant at (a**p< 0.001) when compared to Group II; (ap< 0.001) significant when compared to Group I. Data were analysed using a one-way ANOVA test followed by Tukey’s HSD test.
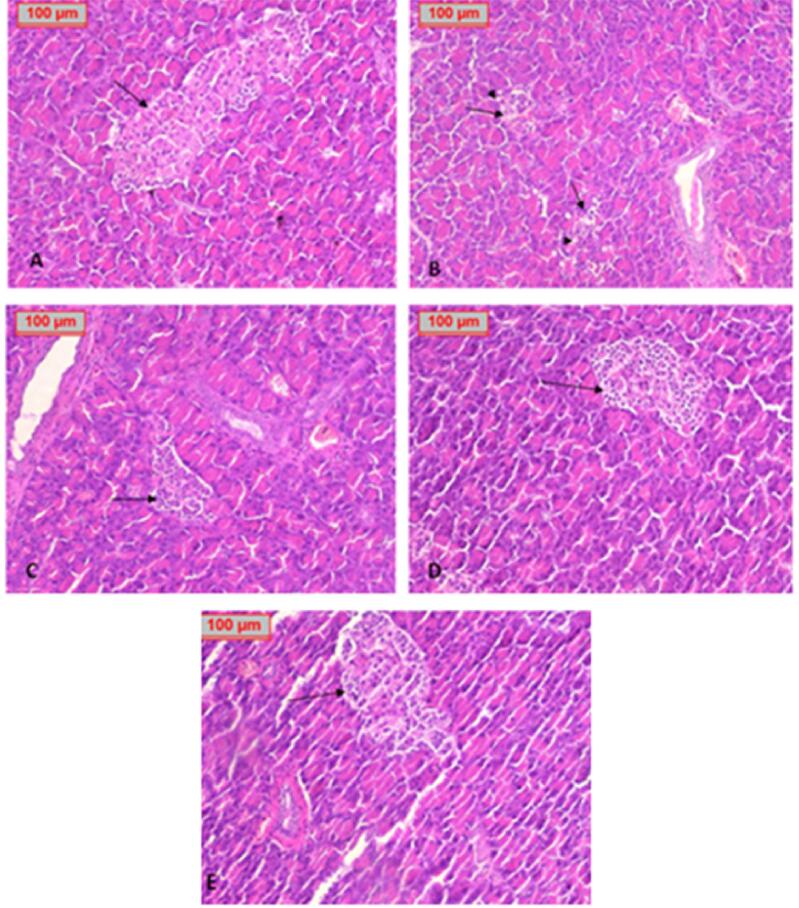
3.9. Effect of sub- chronic treatment of test drugs on the histology of specific tissues
Streptozotocin-induced oxidative damage in the cells was evident by the histopathological observation of sections. Pathologically, the pancreatic histological structure was normal in the healthy control group (Fig. 5A), while in STZ group pancreatic tissue showed marked depletion in islets of Langerhans. There was a decrease in the number of islets with disrupted architecture, shrunken hypocellular islets, and focal vacuolar degeneration (Fig. 5B). After the sub- chronic treatment with standard drug - glimepiride (Fig. 5C) and Prosopis juliflora, either low dose (Fig. 5D) or high dose (Fig. 5E) showed improvement in islet size and histo architecture, especially in the high dose group. Different groups saw no noticeable histological changes in the surrounding pancreatic acini.
Fig. 5.
Histological study of the pancreatic tissue in each group showing A. healthy control group with normal appearance of the islets (arrow). B. positive control group with distorted, shrunken, hypocellular, disintegrated islets with disturbed structure with vacuolar degeneration (arrowhead). C. Glimepiride group enhancement of islet size and structure and cell number. D. Low dose of Prosopis juliflora showed moderate enhancement of islet size, structure, and cell number. E. High dose of P. juliflora showed a marked improvement in islet size and cellularity (H&E x100).
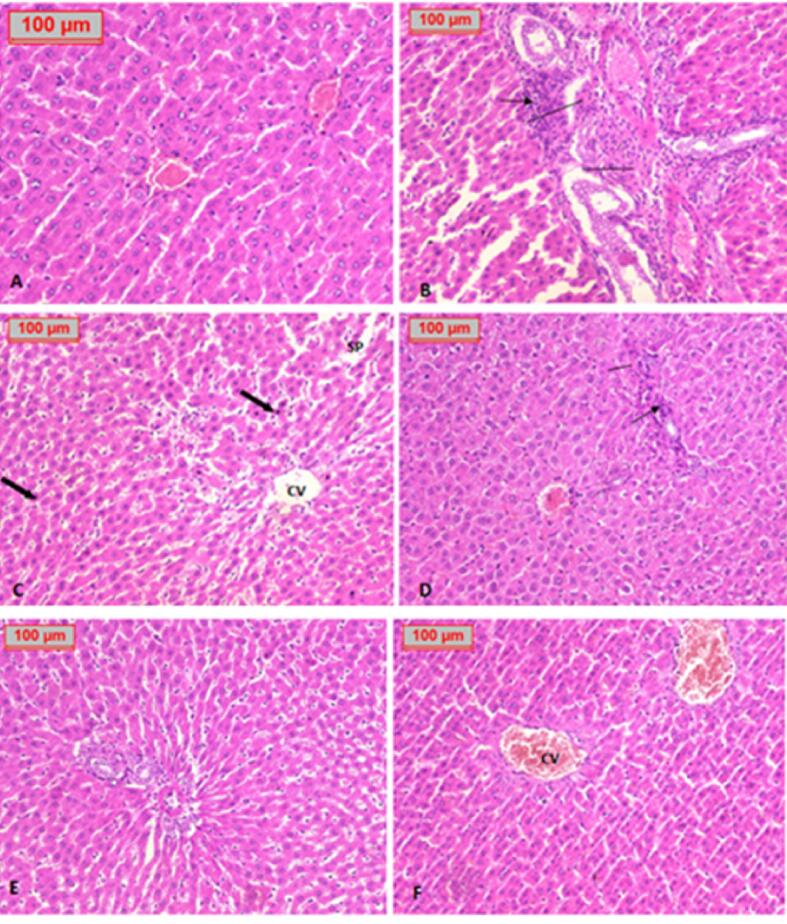
In liver specimens, the hepatic histological structure was normal in the healthy control group (Fig. 6 A). The positive control group showed marked infiltration of the portal tract by inflammatory cells and dilated sinusoidal spaces, hydropic changes, and fatty changes (Fig. 6B, C). Treatment with glimepiride showed enhancement of portal tract and normal appearance of liver cells (Fig. 6D). Treatment with Prosopis juliflora at low dose showed moderate inflammatory infiltration of the portal tract with mild hydropic changes (Fig. 6E). In contrast, high dose administration showed a marked improvement of liver tissue (Fig. 6F).
Fig. 6.
Histological study of the liver tissue in each group shows A. Healthy control group with normal liver tissue and central vein. B, C. Positive control group showed marked infiltration of the portal tract by inflammatory cells (thin arrow), fibrosis (line segment), dilated sinusoidal spaces (SP), scattered pyknotic nuclei (thick arrow), hydropic changes, and fatty changes. D. Glimepiride treated group showed enhancement of portal tract and normal appearance of liver cells. E. Treatment with a low dose of P. juliflora showed moderate inflammatory infiltration of the portal tract with mild hydropic changes. F. Treatment with a high dose of P. juliflora showed a marked improvement in liver tissue. (H&E x100).
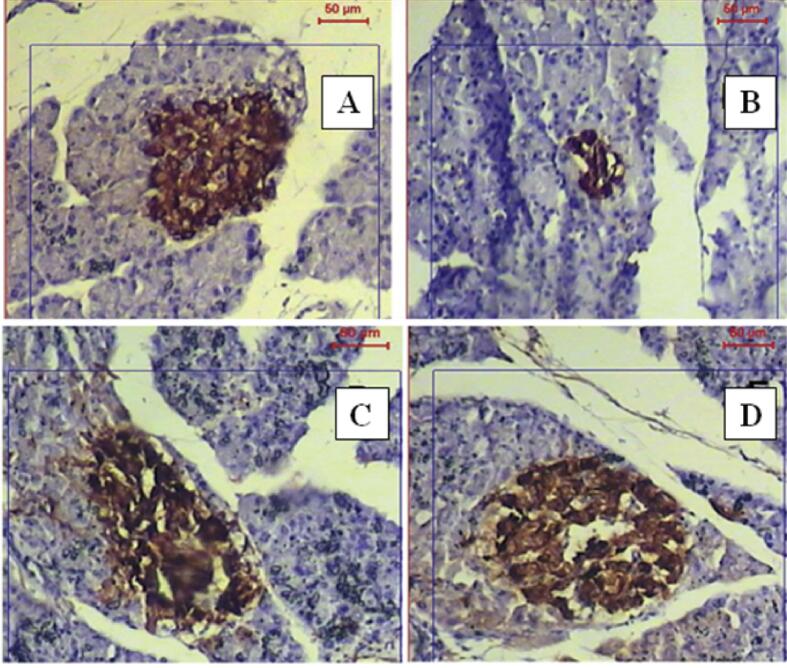
3.10. Immunohistochemical staining after the treatment with test drugs
Immunohistochemical staining of normal rats' pancreatic tissues demonstrated high insulin antigen positivity in the cytoplasm of islet cells. (Fig. 7A), while the STZ group of animals revealed a marked reduction in immune reaction for insulin antibodies inside islet β-cells, as apparent by only a few β-cells displaying minimal insulin immunoreaction (Fig. 7B). Inversely, the treatment with glimepiride induced a significant increase in insulin antigen positivity in the majority of β-cells (Fig. 7C). Additionally, administration of P. juliflora induced a significant increase in β-cells number (Fig. 7D).(See Fig. 8).
Fig. 7.
Immunohistochemical illustrations of the pancreatic tissue denoting β cells of different groups. A. Control group showed β cell count, B. STZ group showed a marked decrease in β cells, C. Glimepiride treated group showed improvement in β cell count. D. P. juliflora treated group showed restored β cells to normalcy.
Fig. 8.
GC MS chromatogram and the structure of some anti-diabetic phytocompounds detected in Prosopis juliflora.
4. Discussion
Type 2 diabetes mellitus is considered a chronic disorder with a derailment of β – cell secretion; people rely on medication persevering. However, the medications possibly produce adverse drug reactions (Rao PS and Mohan GK 2017). A persistent rise in blood glucose levels can lead to the generation of free radicals and lipid peroxidation emerging to complications. Therefore, drugs are used to optimally control the blood glucose levels in the treatment, which favors evolving side effects and adverse drug reactions. In this context, it is always safe to rely on alternative medicine of natural origin like plant sources. From ancient times, medicinal plants have gained importance in herbal remedies for treating chronic illnesses, considering the minimal side effects and therapeutic applications (Ononamadu CJ et al., 2019). Hence the present study explored the therapeutic intimation of consumption of herbal drugs in relation to diabetes mellitus.
Streptozotocin (STZ) is considered a standard animal model for the induction of diabetes. It acts by causing necrosis of pancreatic β-cell by alkylation of DNA, which ultimately has a deleterious effect on the synthesis, production, and release of insulin. The toxic nature of streptozotocin was because of the penetration of STZ into the β-islets through a glucose transporter, thereby generating free radicals. Indeed, when the DNA gets damaged, there would be depletion of NAD + and ATP, causing ATP dephosphorylation, originating the generation of free radicals such as hydrogen peroxide and nitric oxide, thus participating in the impairment of DNA. Subsequently, there is a destruction in the β-cells, which lessens insulin synthesis (Akbarzadeh A et al., 2007). Accordingly, insulin deficiency ensues, giving rise to gluconeogenesis and glycogenolysis (Farhangi A et al., 2014). A perpetual high glucose level is always damaging and causes a diminished insulin secretion/action. With the continuous rise in blood glucose levels, a condition termed hyperinsulinemia emanates, in due course, with a fall or deficit in the release of insulin from β cells of Langerhans. Indeed, from the previous studies, there was a lessened β cell mass for about 40 – 60% of type 2 diabetics (Leibowitz G et al., 2011). In the present study, a hyperglycemic state was observed in the STZ induced diabetic rats with alteration in the other parameters like fasting serum glucose levels, post-prandial serum glucose levels, and insulin levels. In the present study, STZ induced diabetic rats to evaluate the related parameters after the sub- chronic treatment with the test extract P.juliflora.
In the dry and semi-arid zones worldwide, Prosopis juliflora is one of the most economically and environmentally important tree species (Mibrahim et al., 2013). There are many beneficial effects of P.juliflora highlighted in the literature because of phytochemical constituents such as terpenes, alkaloids, flavonoids, and phenols (N. Deepa et al., 2013). Terpenes served as responsible for the activities such as antibacterial, antifungal, anthelmintic, and antimalarial agents (Kanthasamy A et al., 1989). The flavonoid content holds antioxidant and anti-cancer effects. The presence of phenols showed the properties of anti-inflammatory, immune system enhancer, and anti-clotting agents. Regarding the anti-diabetic effect, the terpenes isolated from the plant were found to be responsible for the action on the body's physiological system, thereby controlling the blood glucose levels (Donga JJ et al., 2011).
The results obtained from the identification of phytoconstituents by GC–MS were analyzed and subjected to evaluation. In the different compounds identified, linoleic acid was found to be of great importance in reducing risk for type 2 diabetes mellitus and improving insulin sensitivity (Belury M A et al., 2018). Similarly, identifying the presence of palmitic acid in the test drug was responsible for the glucose uptake in skeletal muscle cells through AMPK/Akt and PI3K/ERK1/2 pathways that usually occur on the cell surface. (Pu J et al., 2011). Further, phytosterols like stigmasterol and sitosterol were also identified in the P.juliflora; both phytoconstituents were probably responsible for restoring glucose levels to normalcy, probably the mechanism might have coincided with rejuvenation of pancreatic β – cells.(Poulose, N et al., 2021). A phytoconstituent named 9,12,15-Octadecatrienoic acid was found in the P.juliflora, was proven to possess to alleviate hyperglycemic condition from the previous study (Yang, JY et al., 2017). Thus, the anti-diabetic potential of the test drug P.juliflora might be because of the presence of the above highlighted phytochemical constituents.
Into the bargain, the plant exhibited therapeutic efficacy as from the previous reports; there is much concern about the out-and-out requirement of investigation of constituents present in the plant to get explored to the pharmacodynamics potential for betterment in terms of therapy in the clinical platform. In the current study, Glimepiride was used as the reference standard, given for 10 days as a part of treatment, noticeably reduced the glucose levels significantly with a rise in the insulin secretions. It belongs to the class sulphonyl ureas and is also found to possess anti-diabetic effect consistent with the previous studies (Basit A et al., 2012). It acts on the potassium channels of the cellular membrane and enhances their closure, thus depolarizing the cell membrane.
On the contrary, this drug activates the voltage-gated calcium channels, thereby increasing the entry of Ca+2 ions inside. Previous studies suggest that Glimepiride improved insulin resistance in streptozotocin-induced diabetic rats (Basit et al., 2012, Trerattanavong and Glimepiride, 2021). In the current study, as a part of treatment, the test drug, P. juliflora leaf extract, was administered for about ten days after the induction of diabetes in animals. The extract exhibited an anti-hyperglycaemic effect by improving insulin levels at a higher dose (400 mg/kg), with lowered glucose levels in post-prandial conditions. This might be due to the presence of phytochemical constituents, which act on the β-cells of the pancreas, causing induction of insulin secretion and release.
The results of the present study illustrated that adiponectin and TNFα were altered in streptozotocin-induced diabetic rats. Disorders such as diabetes, hyperlipidaemia, obesity, and cardiovascular diseases are associated with low adiponectin levels (Margoni A et al., 2011). Studies reported that adipose tissue that secretes adiponectin and TNF – α played a critical role in type 2 diabetes mellitus (Warne JP 2003). In the present investigation, Adiponectin exhibited an inverse relationship with blood glucose levels. The results analysed were consistent with the previous studies, particularly with fasting blood glucose. (Warne JP 2003).
Additionally, the insulin levels in the current study demonstrated an inverse relationship with the adiponectin concentrations. Through the secretions of adipocytokines like leptin and adiponectin, adipose tissue provides storage for triglycerides and free fatty acids, assist in ruling the homeostasis (Havel PJ. 2002). A direct relationship exists between insulin sensitivity and a negative relationship with insulin resistance (Kadowaki T et al., 2006). The present study explored that after the treatment with P. juliflora leaf extract for ten days at the dose of 400 mg/kg orally, there was a significant improvement in the adiponectin levels and control in the blood glucose status. These improved adiponectin levels were modulated by PPAR γ, which accelerated in amelioration of insulin sensitivity, revamp the glucose metabolism and subsequently spurn the inflammation (Yan E et al, 2008).
In the pathogenesis of type 2 diabetes mellitus, inflammation is considered one of the triggering factors contributing to insulin resistance development (Boucher J et al., 2014). In the insulin signalling pathway, the serine/threonine kinases get activated by an inflammatory stimulus, which in turn manifests an inhibitory effect on insulin sensitivity, thereby causing diabetes (Aguirre V et al., 2002). Indeed, TNF-α produces free fatty acids, develops insulin resistance, and hinders adiponectin synthesis (Borst SE 2004). The results of the present study emphasized that the streptozotocin-induced diabetic rats depicted a remarkable rise in the serum TNF-α as comparable to the control group. Oral treatment of animals with a high dose (400 mg/kg) of P. juliflora extract significantly declined the TNF-α levels in diabetic rats. The results are consistent with the property analysed as an anti-inflammatory (Ahmad et al., 1989) from the previous reports.
Diabetes and oxidative stress are associated with each other; cells in diabetic conditions experience a loss due to oxidative damage due to a continual hyperglycaemia state. Nrf2 is a nuclear transcription factor that modulates the antioxidant enzymes inside the cells, inevitably protecting the cellular loss due to oxidative stress (Furukawa M and Xiong Y 2005). Keap1 harbours Nrf2 present in the cells. However, Nrf2 gets activated and dissociated from Keap1 only in oxidative stress, thus triggering the genes responsible for the defence system by cohering to the antioxidant response elements (ARE), accordingly overcoming the stress environment (Kobayashi A et al., 2004). In fact, Nrf2 is responsible for being protective to the organs, promoting its activation in certain chronic diseases such as diabetes, cancer, and cardiovascular diseases. In the present study, Keap1 and Nrf2 were determined in the serum samples of streptozotocin induced diabetic rats. There was a downregulation in both indices, probably the disease-induced animals suffered from oxidative stress (Akbarzadeh A et al., 2007). In diabetes, whenever there is OS (Oxidative stress), a conformation change occurs on Keap 1 due to the production of ROS (Reactive oxygen species), Keap 1 further depletes own potential to have an ally with Nrf2. This uncoupling feature is highlighted and causes upregulation of detoxifying enzymes, providing protection. In the current investigation, the test drug P. juliflora at 200 & 400 mg/kg treated animals showed a significant effect by upregulating the Keap 1 and Nrf2; the underlying mechanism might be the protection thus explained above.
In maintaining homeostasis, a nuclear receptor PPAR-γ plays a climacteric role by revamping insulin sensitivity (Kim HI and Ahn YH 2004). It also ameliorates the peripheral utilization of glucose, thus diminishing the urging for secretion of insulin and glucose production from the liver (Thorens B 2015). A study report on pancreatic islets identified that GLUT 2 acted as a glucose sensor, a test drug treatment that increased the expression of GLUT 2 in rats (Tomaz LM et al., 2016). Also, study emphasized that there might be the involvement of PPAR in glucose regulation. The present study explored a significant effect in the upregulation of PPAR-γ and GLUT 2, ensuing the protection of β-cells of the pancreas by restoration.
The present investigation found that STZ caused damage to Langerhans islets and -cells, as evidenced by the loss of their architecture and insulin antibody positivity, which is consistent with prior findings (EL Baz et al., 2020, Ibrahim et al., 2020). In diabetic rats, both histological and morphometrical evaluations revealed degenerative alterations in pancreatic islets, with a drop in the number of -cells/islet and a decrease in islet area compared to the control group. Treatment with glimepiride improved the histopathological alterations in the pancreas, which in turn improved the overall health of the pancreas, consistent with Bassant et al, 2020.
The photomicrograph of STZ treated hepatic tissue showed inflammatory infiltrate, dilatation of sinusoidal spaces, fibrosis, and hydropic and fatty changes, as suggested by Batarfi et al., 2020. Hepatic histopathological changes were ameliorated by treatment with glimepiride in accordance with studies by Khadre et al., 2011. With the sub- chronic treatment of Prosopis juliflora, there was a disappearance in liver changes as noticeable with administering a large dose. The results were compatible with Hassan et al., 2019. All the possible mechanisms were elaborated in relation to the presence of corresponding phytochemical constituents.
5. Conclusion
In conclusion, the current study emphasized the effects of Prosopis juliflora on blood glucose levels and served to inverse the changes noticed in experimentally induced diabetic animals by elevating the levels of PPARγ, adiponectin, Kaep1, Glut 2, Nrf2 and AMPK respectively, thereby acted as an anti-diabetic agent. The effectiveness of P. juliflora in combating the inflammatory status was proved by diminishing the concentrations of TNFα, which might be a promising drug in managing diabetes mellitus. Additionally, P. juliflora improved the β – cell structure and function after the sub- chronic treatment with the low and high doses, of which higher doses exhibited a significant therapeutic effect. Thus, it can be derived that P. juliflora might be a promising anti-diabetic drug against type 2 diabetes mellitus and its complications. Based on the literature survey, it was concluded that eleven important phytocompounds were identified in the plant extract with anti-diabetic properties.
Funding
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through project number: ISP22-17.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdelhameed M.F., Asaad G.F., Ragab T.I., Ahmed R.F., El Gendy A.E.N.G., El-Rahman A., Elshamy A.I. Oral and topical anti-inflammatory and antipyretic potentialities of Araucaria bidiwillii shoot essential oil and its nanoemulsion in relation to chemical composition. Molecules. 2021;26(19):5833. doi: 10.3390/molecules26195833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeoye-Isijola M.O., et al. Bioactive compounds in ethanol extract of Lentinus squarrosulus Mont-a Nigerian medicinal macrofungus. Afr. J. Tradit. Complement. Altern. Med. 2018;15(2):42–50. [Google Scholar]
- Aguirre V., Werner E.D., Giraud J., Lee Y.H., Shoelson S.E., White M.F. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- Ahmad Z., et al. In vitro anti-diabetic activities and chemical analysis of polypeptide-k and oil isolated from seeds of Momordica charantia (bitter gourd) Molecules. 2012;17(8):9631–9640. doi: 10.3390/molecules17089631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A., Khan K.A., Ahmad V.U., Qazi S. Antibacterial activity of juliflorine isolated from Prosopis juliflora. Planta Med. 1986;4:185. [PubMed] [Google Scholar]
- Ahmad A., Khursheed A.K., Sabiha Q., Viqaruddin A. Antifungal activity of some hydrosoluble Prosopis juliflora alkaloids. Fitoterapia. 1989;60:86–89. [Google Scholar]
- Ahmed B.E., et al. Human anticancer and anti-diabetic activities of the cyanobacterium Fischerella sp. BS1-EG isolated from River Nile, Egypt. Int. J. Curr. Microbiol. Appl. Sci. 2018;7:3473–3485. [Google Scholar]
- Akbarzadeh A., Norouzian D., Mehrabi M.R., Jamshidi S.h., Farhangi A., Verdi A.A., Mofidian S.M., Rad B.L. Induction of diabetes by Streptozotocin in rats. Indian J. Clin. Biochem. 2007 Sep;22(2):60–64. doi: 10.1007/BF02913315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O'Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999 Dec 23-30;402(6764):880–3. [DOI] [PubMed]
- Basit A., Riaz M., Fawwad A. Glimepiride: evidence-based facts, trends, and observations (GIFTS) [corrected] Vasc. Health Risk Manag. 2012;8:463–472. doi: 10.2147/HIV.S33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafisa Batarfi, Rahma Ali and Hawazen Hamed Histological study on the effect of Punica Granatum on the liver of STZ induced diabetic male rats. International Journal of Pharmacutical and Phytopharmacological Research 2020, 101,3, 147–152.
- Batatinha, M.J.M. (1997). Investigations on toxic influences of Prosopis juliflora D.C. (Algarobeira) on cell cultures as well as on ruminal fermentation in cattle (in vitro). Dissertation - Veterinary Medicine University, Hannover. 189.
- Belury M.A., Cole R.M., Snoke D.B., Banh T., Angelotti A. Linoleic acid, glycemic control, and Type 2 diabetes. Prostaglandins Leukot. Essent. Fat. Acids. 2018;132:30–33. doi: 10.1016/j.plefa.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J., Bailey P., Biswas C., Cullinan C.A., Doebber T.W., Hayes N.S., Saperstein R., Smith R.G., Leibowitz M.D. Thiazolidinediones produce a conformational change in peroxisomal proliferator–activated receptor gamma: binding and activation correlate with anti-diabetic actions in db/db mice. Endocrinology. 1996;137:4189–4195. doi: 10.1210/endo.137.10.8828476. [DOI] [PubMed] [Google Scholar]
- Borst SE. The role of TNF-alpha in insulin resistance. Endocrine. 2004 Mar-Apr;23(2-3):177–82. [DOI] [PubMed]
- Boucher J., Kleinridders A., Kahn C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014 Jan 1;6(1) doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P.J. What are the effects of peroxisome proliferator activated receptor agonists on adiponectin, tumor necrosis factor-alpha, and other cytokines in insulin resistance? Clin. Cardiol. 2004;27:IV11-IV16. doi: 10.1002/clc.4960271604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman C.I. GLUT2 is the transporter for fructose across the rat intestinal basolateral membrane. Gastroenterology. 1993 Oct;105(4):1050–1056. doi: 10.1016/0016-5085(93)90948-c. [DOI] [PubMed] [Google Scholar]
- Chen L., Wang J., Zhang Y.Y., Yan S.F., Neumann D., Schlattner U., Wang Z.X., Wu J.W. AMP-activated protein kinase undergoes nucleotide-dependent conformational changes. Nat. Struct. Mol. Biol. 2012 Jun 3;19(7):716–718. doi: 10.1038/nsmb.2319. [DOI] [PubMed] [Google Scholar]
- Cnop M., Welsh N., Jonas J.C., Jörns A., Lenzen S., Eizirik D.L. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Supplement 2):S97–S. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- Corpe C.P., Basaleh M.M., Affleck J., Gould G., Jess T.J., Kellett G.L. The regulation of GLUT5 and GLUT2 activity in the adaptation of intestinal brush-border fructose transport in diabetes. Pflugers Arch. 1996 Jun;432(2):192–201. doi: 10.1007/s004240050124. [DOI] [PubMed] [Google Scholar]
- N. Deepa, S. Chandra Nayaka, K. Girish, M.P. Raghavendra Synergistic effect of Prosopis juliflora extract and chemical fungicides against seed borne toxigenic fungi, International journal of advanced life sciences. 2013; 6(4), 312–317.
- Devi, J. A. I., et al. (2017). “MOLECULAR DOCKING STUDIES ON PHYTOCONSTITUENTS OF PLEIOSPERMIUM ALATUM AGAINST α-AMYLASE ENZYME.”.
- Donga J.J., Surani V.S., Sailor G.U., Chauhan S.P., Seth A.K. A systematic review on natural medicine used for therapy of diabetes mellitus of some Indian medicinal plants. Int. J. Pharm. Sci. 2011;2:36. [Google Scholar]
- EL Baz FK, Salama A, Salama AA. Dunaliella salina attenuates diabetic neuropathy induced by STZ in rats. Involvement of thioredoxin. Biomed Res Int, 2020,1295492. [DOI] [PMC free article] [PubMed]
- El-Shafei R.A., El-Adl M.A., Ali H.S., Nomier Y. Ameliorative effect of Arabic gum Acacia and mori extracts in streptozotocin-induced diabetic rats: implications of Cas-3 and TGF-β. Eur. Rev. Med. Pharmacol. Sci. 2023 Apr;27(7):2845–2857. doi: 10.26355/eurrev_202304_31915. [DOI] [PubMed] [Google Scholar]
- Farhangi A., Norouzian D., Mehrabi M.R., Chiani M., Saffari Z., Farahnak M., Akbarzadeh A. Immunoisolated transplantation of purified langerhans islet cells in testis cortex of male rats for treatment of streptozotocin induced diabetes mellitus. Indian J. Clin. Biochem. 2014 Oct;29(4):406–417. doi: 10.1007/s12291-013-0331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa M., Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the cullin 3-Roc1 ligase. Mol. Cell Biol. 2005;25:162–171. doi: 10.1128/MCB.25.1.162-171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R., et al. Anti-diabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. J. Diabetes. 2011;3(1):29–37. doi: 10.1111/j.1753-0407.2010.00107.x. [DOI] [PubMed] [Google Scholar]
- Havel P.J. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr. Opin. Lipidol. 2002;13:51–59. doi: 10.1097/00041433-200202000-00008. [DOI] [PubMed] [Google Scholar]
- Helliwell PA, Richardson M, Affleck J, Kellett GL. Regulation of GLUT5, GLUT2 and intestinal brush-border fructose absorption by the extracellular signal-regulated kinase, p38 mitogen-activated kinase and phosphatidylinositol 3-kinase intracellular signalling pathways: implications for adaptation to diabetes. Biochem J. 2000 Aug 15;350 Pt 1(Pt 1):163–9. [PMC free article] [PubMed]
- Ibrahim H., Hashem M., Mohamed N. Aliaa Abd EL-Rahman Assessment of ameliorative effects of Zingeber officinale and Nigella sativa on streptozotocin induced diabetic rats. Adv. Anim. Vet. Sci. 2020;8(11):1211. [Google Scholar]
- Ishii T., Itoh K., Takahashi S., Sato H., Yanagawa T., Katoh Y., Bannai S., Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- Iwata M., Haruta T., Usui I., Takata Y., Takano A., Uno T., Kawahara J., Ueno E., Sasaoka T., Ishibashi O., Kobayashi M. Pioglitazone ameliorates tumor necrosis factor-alpha-induced insulin resistance by a mechanism independent of adipogenic activity of peroxisome proliferator–activated receptor-gamma. Diabetes. 2001 May;50(5):1083–1092. doi: 10.2337/diabetes.50.5.1083. [DOI] [PubMed] [Google Scholar]
- Kadhim M.J., et al. Determination of bioactive compounds of methanolic extract of vitis vinifera using GC-MS. Int. J. Toxicol. Pharmacol. Res. 2017;9(2):113–126. [Google Scholar]
- Kadowaki T., Yamauchi T., Kubota N., Hara K., Ueki K., Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 2006 Jul;116(7):1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanthasamy A., Subramanian S., Govindasamy S. Bactericidal and fungicidal effects of Prosopis juliflora Alkaloidal fraction. Ind Drugs. 1989;26:390–394. [Google Scholar]
- Kanthasamy A., William S., Govindasamy S. Hemolytic effects of Prosopis juliflora alkaloids. Curr. Sci. 1989;58:142–144. [Google Scholar]
- Kellett GL, Helliwell PA. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J. 2000 Aug 15;350 Pt 1(Pt 1):155–62. [PMC free article] [PubMed]
- Kern P.A., Ranganathan S., Li C., Wood L., Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Phys. Endocrinol. Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- Kim H.I., Ahn Y.H. Role of peroxisome proliferator-activated receptor-gamma in the glucose-sensing apparatus of liver and beta-cells. Diabetes. 2004 Feb;53(Suppl 1):S60–S65. doi: 10.2337/diabetes.53.2007.s60. [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Kang M.I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Li L., Iwamoto N., Nakajima-Takagi Y., Kaneko H., Nakayama Y., Eguchi M., Wada Y., Kumagai Y., Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol. Cell Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepsell H. Glucose transporters in the small intestine in health and disease. Pflugers Arch. 2020 Sep;472(9):1207–1248. doi: 10.1007/s00424-020-02439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz G., Kaiser N., Cerasi E. β-Cell failure in type 2 diabetes. J. Diabetes Investig. 2011 Apr 7;2(2):82–91. doi: 10.1111/j.2040-1124.2010.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani P., et al. Molecular docking of bioactive compounds from syzygium aqueum against type 2 diabetes susceptibility gene TCF7L2. Int. J. Pharma. Drug Anal. 2018:271–278. [Google Scholar]
- Margoni A, Perrea DN, Vlachos I, Prokopaki G, Pantopoulou A, Fotis L, Kostaki M, Papavassiliou AG. Serum leptin, adiponectin, and tumor necrosis factor-α in hyperlipidemic rats with/without concomitant diabetes mellitus. Mol Med. 2011 Jan-Feb;17(1-2):36–40. [DOI] [PMC free article] [PubMed]
- Mibrahim, K nadir, Aali, V ahmad, Mrasheed, Phytochemical analyses of Prosopis juliflora swartz dc. Pak. J. Bot. 2013; 45(6): 2101- 2104.
- Narasimhacharya A.V.R.L., Nitesh K.V., Tejal N.D. Prosopis juliflora as an antihypercholesterolemic agent. J. Herbal Med. Toxicol. 2010;4(1):31–34. [Google Scholar]
- OECD guidelines for testing chemicals. Acute Oral Toxicity – Acute Toxic Class Method. 423. (2001).
- Ononamadu CJ, Alhassan AJ, Ibrahim A, Imam AA, Ihegboro GO, Owolarafe TA, Sule MS. Methanol-Extract/Fractions of Dacryodes edulis Leaves Ameliorate Hyperglycemia and Associated Oxidative Stress in Streptozotocin-Induced Diabetic Wistar Rats. J Evid Based Integr Med. 2019 Jan–Dec; 24:2515690X19843832. [DOI] [PMC free article] [PubMed]
- Parker S., et al. Palmitate potentiation of glucose-induced insulin release: a study using 2-bromopalmitate. Metabolism. 2003;52(10):1367–1371. doi: 10.1016/s0026-0495(03)00279-8. [DOI] [PubMed] [Google Scholar]
- Pasiecnik N.M., Felker P., Harris P.J.C., Harsh L.N., Cruz G., Tewari J.C., Cadoret K., Maldonado L.J. HDRA, Coventry; UK: 2001. The Prosopis juliflora-Prosopis pallida complex: A monograph; p. 172. [Google Scholar]
- Poulose, N., Sajaya,n A., Ravindran, A., Chandran, A., Priyadharshini, G. B., Selvin, J., & Kiran, G. S. (2021). Anti-diabetic Potential of a Stigmasterol From the Seaweed Gelidium spinosum and Its Application in the Formulation of Nanoemulsion Conjugate for the Development of Functional Biscuits. Frontiers in nutrition, 8, 694362. [DOI] [PMC free article] [PubMed]
- Pscherer S., Heemann U., Frank H. Effect of Renin Angiotensin system blockade on insulin resistance and inflammatory parameters in patients with impaired glucose tolerance. Diabetes Care. 2010;33:914–919. doi: 10.2337/dc09-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu J., Peng G., Li L., Na H., Liu Y., Liu P. Palmitic acid acutely stimulates glucose uptake via activation of Akt and ERK1/2 in skeletal muscle cells. J. Lipid Res. 2011;52(7):1319–1327. doi: 10.1194/jlr.M011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S., et al. Phytochemical screening and anti-diabetic, antihyperlipidemic, and antioxidant effects of Leptopus cordifolius Decne. in diabetic mice. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.643242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M.R.K., et al. Preliminary Phytochemical and Gas Chromatography-Mass Spectrometry study of one medicinal plant Carissa carandas. Drug Invitation Today. 2019;12(7):1629–1630. [Google Scholar]
- Rao P.S., Mohan G.K. In vitro alpha-amylase inhibition and in vivo antioxidant potential of Momordica dioica seeds in streptozotocin-induced oxidative stress in diabetic rats. Saudi J. Biol. Sci. 2017 Sep;24(6):1262–1267. doi: 10.1016/j.sjbs.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama A., Asaad G.F., Shaheen A. Chrysin ameliorates STZ-induced diabetes in rats: possible impact of modulation of TLR4/NF-κβ pathway. Res. Pharma. Sci. 2022;17(1):1. doi: 10.4103/1735-5362.329921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirmah P., Dumarçay S., Masson E., Gerardin P. Unusual amount of (-)- mesquitol from the heartwood of Prosopis. juliflora. Nat. Prod. Res. 2009;23(2):183–189. doi: 10.1080/14786410801940968. [DOI] [PubMed] [Google Scholar]
- Skurk T., Alberti-huber C., Herder C., Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J. Clin. Endocrinol. Metab. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001;50(6):537–546. [PubMed] [Google Scholar]
- Taguchi K., Maher J.M., Suzuki T., Kawatani Y., Motohashi H., Yamamoto M. Genetic analysis of cytoprotective functions supported by graded expression of Keap1. Mol. Cell Biol. 2010;30:3016–3026. doi: 10.1128/MCB.01591-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamori Y., Masugi J., Nishino N., Kasuga M. Role of peroxisome proliferator-activated receptor-gamma in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes. 2002 Jul;51(7):2045–2055. doi: 10.2337/diabetes.51.7.2045. [DOI] [PubMed] [Google Scholar]
- Tapia A., Feresin G.E., Bustos D., Astudillo L., Theoduloz C., Hirschmann G.S. Biologically active alkaloids and a free radical scavenger from Prosopis species. J. Ethnopharmacol. 2000;71(1–2):241–246. doi: 10.1016/s0378-8741(00)00171-9. [DOI] [PubMed] [Google Scholar]
- Thorens B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia. 2015 Feb;58(2):221–232. doi: 10.1007/s00125-014-3451-1. [DOI] [PubMed] [Google Scholar]
- Tomaz L.M., Barbosa M.R., Farahnak Z., Lagoeiro C.G., Magosso N.S., Lavoie J.M., Perez S.E. GLUT2 proteins and PPARγ transcripts levels are increased in liver of ovariectomized rats: reversal effects of resistance training. J. Exerc. Nutr. Biochem. 2016 Jun;20(2):51–57. doi: 10.20463/jenb.2016.06.20.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trerattanavong K, Tadi P. Glimepiride. [Updated 2021 Dec 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-.
- Tyagi T., Agarwal M. Phytochemical screening and GC-MS analysis of bioactive constituents in the ethanolic extract of Pistia stratiotes L. and Eichhornia crassipes (Mart.) solms. J. Pharmacogn. Phytochem. 2017;6(1):195–206. [Google Scholar]
- Uruno A, Motohashi H. 2011. The Keap1-Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide 25:153–160. 11. [DOI] [PubMed]
- Van de Venter M., et al. Anti-diabetic screening and scoring of 11 plants traditionally used in South Africa. J. Ethnopharmacol. 2008;119(1):81–86. doi: 10.1016/j.jep.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Warne J.P. Tumour necrosis factor alpha: a key regulator of adipose tissue mass. J. Endocrinol. 2003;177:351–355. doi: 10.1677/joe.0.1770351. [DOI] [PubMed] [Google Scholar]
- Xiao B., Heath R., Saiu P., Leiper F.C., Leone P., Jing C., Walker P.A., Haire L., Eccleston J.F., Davis C.T., Martin S.R., Carling D., Gamblin S.J. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007 Sep 27;449(7161):496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Waki H., Kamon J., Murakami K., Motojima K., Komeda K., Miki H., Kubota N., Terauchi Y., Tsuchida A., Tsuboyama-Kasaoka N., Yamauchi N., Ide T., Hori W., Kato S., Fukayama M., Akanuma Y., Ezaki O., Itai A., Nagai R., Kimura S., Tobe K., Kagechika H., Shudo K., Kadowaki T. Inhibition of RXR and PPARgamma ameliorates diet-induced obesity and type 2 diabetes. J. Clin. Invest. 2001 Oct;108(7):1001–1013. doi: 10.1172/JCI12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan E., Chen S., Hong K., Kim W.S., Bajpai A., Treyzon L., Gratton L., Elashoff R., Wang H.J., Li Z., Heber D. Insulin, hs-CRP, leptin, and adiponectin. An analysis of their relationship to the metabolic syndrome in an obese population with an elevated waist circumference. Metab. Syndr. Relat. Disord. 2008 Mar;6(1):64–73. doi: 10.1089/met.2007.0027. [DOI] [PubMed] [Google Scholar]
- Yang J.Y., Park J.H., Chung N., et al. Inhibitory potential of constituents from Osmanthus fragrans and structural analogues against advanced glycation end products, α-Amylase, α-Glucosidase, and oxidative stress. Sci. Rep. 2017;7:45746. doi: 10.1038/srep45746. [DOI] [PMC free article] [PubMed] [Google Scholar]