Abstract
The overlapping epidemics of HIV and unplanned pregnancy disproportionately affect adolescent girls and young women (AGYW) in sub-Saharan Africa. Prevailing dynamics driving benefits of any prevention method at the population level depend on: 1) population size, risk profile, and prevalence of method use, 2) method efficacy, and 3) method use-effectiveness. Adding a multi-purpose technology (MPT) to prevent HIV and pregnancy to this three-part equation results in scenarios that may enhance HIV population impact, even with methods that exhibit less than “perfect” method efficacy, by extending protection among existing users and attracting new users, resulting in greater population coverage. However, the interplay of epidemic drivers is complex and the greatest population benefit of such a MPT would be realized among those most at risk for HIV and pregnancy, and could be harmful if successful contraceptive users switch to a method with lower use–effectiveness. While MPTs are highly desired, and may offer considerable individual, population, and system-level public health benefits, there is no “magic bullet”, nor single prevention method–MPT or otherwise–that will end the HIV epidemic nor fully resolve unmet need for family planning. All methods have inherent tradeoffs and women have varied reproductive and HIV prevention needs across their life course. Key programmatic features to maximize the potential of MPTs include offering them among a range of safe and effective methods with comprehensive information about their features allowing women to make a fully-informed method choice. Programmatic follow-up should support consistent and correct use to maximize use-effectiveness, and then monitor for potential untoward effects.
Keywords: multi-purpose technology, HIV prevention, contraception, unplanned pregnancy, use-effectiveness, method efficacy, adolescent girls, sub-Saharan Africa
Introduction
Multipurpose prevention products (MPTs) under development include a range of delivery platforms such as rings, implants, injectables, films, enemas, and vaginal and rectal inserts for HIV, other sexually transmitted infections (STIs), and contraception (1,2). Currently, condoms are the only MPT available for protection against STIs, HIV, and pregnancy. Condom use is challenging, especially for adolescent girls and young women (AGYW) in sub-Saharan Africa (SSA) (3). Men's and women's desire for children, gender inequality, domestic violence, and stigma hinder agency for use. Warfare, corruption, poverty and competing morbidities create unstable backdrops for HIV prevention programming. Product development funding and product cost also present challenges. These prevailing societal norms and realities challenge introduction and use of any new prevention method. The majority of MPTs in development target HIV and pregnancy (1). Women's reproductive health and HIV are related, with HIV exacerbating the maternal mortality epidemic and mother-to-child transmission significantly contributing to the HIV epidemic in SSA (4). A biomedical MPT preventing HIV and pregnancy could have substantial health benefits for AGYW in SSA. The HIV epidemic disproportionately affects AGYW in SSA with 63% of incident infections occurring in females aged 15–24 (5). Further, across 30 SSA countries, the prevalence of unmet need for contraception is 27% among partnered AGYW aged 15–24, and 32% of pregnancies among women in SSA are unplanned (6,7).
A MPT for HIV and pregnancy–even one with less than perfect method efficacy for HIV prevention–holds potential for enhanced public health impact due to the interplay of three drivers of protection: 1) the population's size, risk profile, and method use prevalence; 2) method efficacy and; 3) method use-effectiveness. Adding MPTs for HIV and pregnancy to this three-part equation results in scenarios that may enhance HIV population impact. However, epidemic drivers are complex, and some MPT introduction scenarios for HIV and pregnancy may detract from net population benefit. The greatest potential population benefit and efficiencies for such a MPT would be realized among those most at risk for HIV and pregnancy.
Three drivers of population benefit
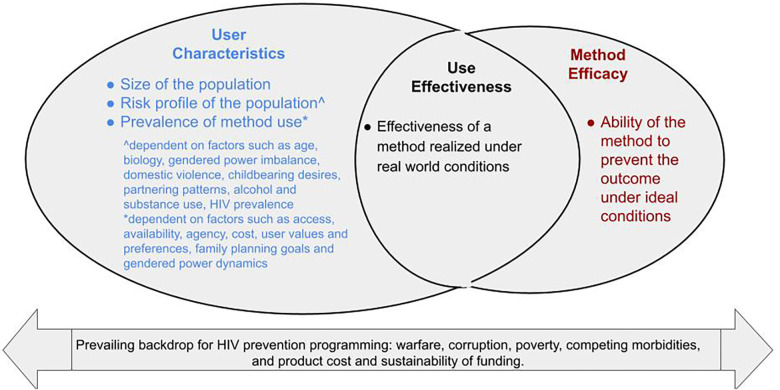
With respect to factors that public health practitioners can influence, drivers of population benefit involve the interplay of three broad elements (Figure 1). First are the population's size, risk profile (whether the group is epidemiologically at high risk for HIV and the total fertility rate), and prevalence of preventive method use in at-risk populations. Second is method efficacy which is the method's ability to prevent the outcome under ideal conditions, such as efficacy observed during pre-clinical challenges or in as-treated analyses in efficacy trials when a method is used consistently and correctly (8). Third, is use-effectiveness which is the effectiveness of a method realized under real-world conditions, where factors such as uptake and consistent and correct use influence the ultimate protection conferred (8).
Figure 1.
Drivers of population benefit.
A highly efficacious method only provides benefit if used consistently and correctly. A widely-used prevention method with only moderate method efficacy may still have population impact as was seen with the use of withdrawal (coitus interruptus) for contraception that contributed to the demographic shift in family size at the end of the 19th century (9). The interplay of method efficacy and user adherence, an element determining use-effectiveness, is demonstrated by the mixed findings of oral PrEP (TDF/FTC) to prevent HIV among women (10). High method efficacy alone is not sufficient to shift an epidemic. The role of the user, their risk profile, their access to methods, and ability to consistently and correctly use a method, are important drivers of protection. Long-acting cabotegravir (CAB-LA) has demonstrated high method efficacy for HIV prevention in clinical trial settings (11). Method efficacy for CAB- LA is derived from select women who met inclusion criteria, received reimbursements for study visits, and were cared for by proactive staff reminding them of injection visits. While many women in SSA state preferences for injectable HIV prevention (2), the use-effectiveness of CAB-LA to protect women from HIV under real-world scale-up is yet to be characterized. Further, at a population level, if new CAB-LA users are those migrating from successful daily oral PrEP use, population impacts on the HIV epidemic may be moderate if one method is simply supplanted for another without a net gain in prevention coverage for those at risk for HIV. The Catalyzing Access to New Prevention Products to Stop HIV (CATALYST) study, will evaluate a multi-product service-delivery platform offering daily oral PrEP, the monthly dapivirine ring, and CAB-LA at public clinics across five countries in southern and eastern Africa and will provide data on use-effectiveness of these single-indication methods and describe the programmatic impact of offering choice (12).
Three drivers + a MPT for HIV and pregnancy
Adding a MPT for HIV and pregnancy to this three-part equation may result in beneficial scenarios by adding new users with unmet need for both indications resulting in a net increase in population coverage, or by expanding protection for a second indication among existing users with unmet need. Increasing uptake for new users holds promise as MPTs are highly desired with 96% of women surveyed in SSA preferring HIV prevention products with multiple indications compared to a single-indication product (2). Preference for multiple over a single indication outweighed preference for type of delivery method (i.e., injectable, pill, implant, etc.) (2). Further, in a worldwide survey of method preference, women were most interested in products offering both HIV and pregnancy prevention (82%) compared with single indications for HIV (76%) or pregnancy only (64%) (13).
In both scenarios above–either attracting new users or expanding coverage to those with unmet need for a second indication–additional benefit is realized so long as the added coverage occurs among women at high risk for the outcomes as suggested by cost effectiveness modeling. Analyses scaling a MPT for HIV and pregnancy found pregnancy prevention would be cost effective if rolled out to women at high risk of HIV (e.g., serodiscordant couples, AGYW or female sex workers) but not to a general population of women (14,15). Notable was that a MPT for HIV and pregnancy was cost effective among populations at high risk for HIV even with HIV method efficacy ranging from 45% to 75% (15). Modeling of the dual prevention pill (DPP) found that scaling the DPP was sensitive to both risk of HIV in the population and adherence (e.g., use-effectiveness), where if adherence was low, the health risks from unintended pregnancy could outweigh the health benefit of HIV prevention (14).
MPT potential benefits and pitfalls for individual users and public health
Expanding protection for the dual outcomes of HIV and unplanned pregnancy would be especially beneficial among AGYW in SSA who are disproportionately at risk for HIV, have unmet contraceptive needs, and face well-established barriers to accessing sexual and reproductive health services (16). The availability of a MPT may ease how AGYW interface with the healthcare system by meeting dual health care needs with only one health encounter which may be less stigmatizing if focused on reproductive health compared with HIV prevention, and by reducing multiple disclosures about HIV and sexual activity (2, 17). Additionally, use-effectiveness for a HIV and pregnancy MPT may be enhanced if women are highly motivated to use the method consistently and correctly in order to avoid unplanned pregnancy as self-perceived risk for HIV is often inaccurate (18,19). However, there are potential pitfalls for a MPT highly dependent on adherence, such as the daily oral DPP. As per DPP cost-effectiveness modeling, expanded population coverage achieved could be outweighed by poor adherence and lowered use-effectiveness, as daily pill taking may be more challenging than using longer-acting methods (14). Since the average probability of conception is higher than HIV transmission, poor adherence to the daily oral DPP could be offset by unplanned pregnancy without necessarily increasing HIV protection.
The introduction of a MPT for HIV and pregnancy, especially among AGYW in SSA, has the potential to offer health dividends not just for the individual user by reducing HIV incidence and unplanned pregnancy in adolescence, but may also improve maternal and child health outcomes. Pregnancy in adolescent girls is associated with disproportionately high maternal mortality, low infant birth weight, severe neonatal outcomes, as well as decreased education and censored socioeconomic potential impacting the welfare of both mother and child (20).
A MPT for HIV and pregnancy may have the additive effect of streamlining and integrating service delivery for over-burdened providers and public health systems, a need which has long been noted (21–23). Most family planning and HIV clinics are overburdened and may not have capacity to provide separate but overlapping services yet several models for integrated care have been proposed (24,25). Integrated service delivery settings are associated with increased method uptake, enhanced client satisfaction, reduced HIV-related stigma, and may facilitate the involvement of men and improve joint decision making around protection (21, 23, 26). Yet, the development of a biomedical MPT for HIV and pregnancy will be challenging, as it requires meeting safety and efficacy thresholds for two indications while regulatory standards for a dual-indication product remain unclear (1, 27).
Discussion
Adolescent girls and young women who bear a disproportionate burden of HIV and unplanned pregnancy have much to gain from a MPT for HIV and pregnancy. While such MPTs are highly desired and have considerable potential for public health impact, like single-indication products, there is no single MPT, regardless of method efficacy level, that alone, will stem the twin epidemics of HIV and unplanned pregnancy. The most effective method is the one that gets used consistently and correctly. While continued efforts should focus on developing MPTs offering high method efficacy for intended indications, a MPT for pregnancy and HIV, even with limited method efficacy, has the potential to have significant epidemic impact if used among those at high risk for HIV. Further, women need a range of prevention methods that can be varied across their lifetime, given that values, preferences, needs and risks vary across the life course. The contraceptive field has shown that offering a choice of methods, even with a range of method efficacy, lessens the burden of unmet need for family planning because values and preferences of users are varied (28). Contraceptive method decision-making is influenced by a variety of factors beyond efficacy including reproductive health events, relationship status, partner approval, childbearing desires, and societal norms which all affect the ultimate ability to adopt and use a method. Key programmatic features that would maximize the potential of MPTs include offering MPT as part of a range of safe and effective contraceptive and HIV prevention methods with full information about their features such as indications addressed (single, dual, multiple), method efficacy, use-effectiveness, mechanism of action, potential side effects, return to fertility, duration, and respectful support in how to use. This would allow women to make a truly informed method choice. Offering an array of both single and dual methods for contraception and HIV and ensuring providers have strong integrated counseling guidelines and training may mitigate against pitfalls, such as inducing women to switch to a less-effective MPT. A method array that includes multiple and single indications, locally-acting and systemic products, as well as on-demand, medium-, and long-acting methods is necessary to address women's complex and evolving needs.
USAID is investing in the development of an array of biomedical HIV prevention products for AGYW, and a few of these products are being developed as MPTs for HIV and pregnancy. Additionally, many USAID service delivery initiatives are underway to streamline and decentralize HIV services in high HIV-burden countries. In the short term, USAID supports moving to integrated care models for sexual and reproductive health, including moving more services into primary care, which is important for sustaining and simplifying service delivery. In the absence of MPTs available for implementation, additional testing of co-delivery models providing existing HIV and pregnancy prevention methods (e.g., the administration of injectable CAB-LA along with contraception) are needed given the promising association that integrated service delivery has on dual method use. In the longer term, sustained investments to make safe, effective, acceptable, and scalable MPTs a reality should continue.
Acknowledgments
The authorship team is grateful to our colleagues at USAID for their review.
Funding Statement
This article was made possible by the support of the American people through the United States Agency for International Development (USAID) under the U.S. President's Emergency Plan for AIDS Relief (PEPFAR). The contents in this article are the sole responsibility of the authors, and do not necessarily reflect the views of USAID, PEPFAR or the United States Government.
Data availability statement
The original contributions are included in the article. Further inquires can be directed to the corresponding author.
Author contributions
ML, and KV contributed to the conception and design of the study. ML wrote the first draft of the manuscript. KV wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version. All author contributed to the article and approved the submitted version.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views and opinions in this article are those of the author and do not necessarily represent the views of USAID, PEPFAR, or the United States Government.
Conflict of interest
Authors KV and AD are employed by Credence LLC, Washington, D.C.; author MG is employed by Public Health Institute, Washington, D.C., all three via the GHTASC award. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Young-Holt B, Turpin JA, Romano J. Multipurpose prevention technologies: opportunities and challenges to ensure advancement of the most promising MPTs. J Front Reprod Health. (2021) 3:704841. 10.3389/frph.2021.704841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Multipurpose Prevention Technologies (MPTs): Technology Landscape and Potential for Low- and Middle-Income Countries. (2021) Available online at: (Accessed April 14, 2023).
- 3.Aventin Á, Gordon S, Laurenzi C, Rabie S, Tomlinson M, Lohan M, et al. Adolescent condom use in Southern Africa: narrative systematic review and conceptual model of multilevel barriers and facilitators. BMC Pub Health. (2021) 21:1228. 10.1186/s12889-021-11306-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdool Karim S, Baxter C, Frohlich J, Abdool Karim Q. The need for multipurpose prevention technologies in sub-saharan Africa. BJOG. (2014) 121(5):27–34. 10.1111/1471-0528.12842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNAIDS. Global HIV & AIDS statistics — Fact Sheet. (2022) Available online at: https://www.unaids.org/en/resources/fact-sheet (Accessed May 2, 2023).
- 6.Ahinkorah BO, Ameyaw EK, Seidu AA. Socio-economic and demographic predictors of unmet need for contraception among young women in sub-saharan Africa: evidence from cross-sectional surveys. Reprod Health. (2020) 17:63. 10.1186/s12978-020-01018-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ameyaw EK, Budu E, Sambah F, Baatiema L, Appiah F, Seidu AA, et al. Prevalence and determinants of unintended pregnancy in sub-saharan Africa: a multi-country analysis of demographic and health surveys. PLoS One. (2019) 14(8):e0220970. 10.1371/journal.pone.0220970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singal AG, Higgins PD, Waljee AK. A primer on effectiveness and efficacy trials. Clin Transl Gastroenterol. (2014) 5(1):e45. 10.1038/ctg.2013.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogow D, Horowitz S. Withdrawal: a review of the literature and an agenda for research. Stud Fam Plann. (1995) 26(3):140–53. 10.2307/21378337570764 [DOI] [Google Scholar]
- 10.Hanscom B, Janes HE, Guarino PD, Huang Y, Brown ER, Chen YQ, et al. Preventing HIV-1 infection in women using oral preexposure prophylaxis: a meta-analysis of current evidence. J Acquir Immune Defic Syndr. (2016) 73(5):606–8. 10.1097/QAI.0000000000001160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delany-Moretlwe S, Hughes JP, Bock P, Ouma SG, Hunidzarira P, Kalonji D, et al. Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. Lancet. (2022) 399(10337):1779–89. 10.1016/S0140-6736(22)00538-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.AVAC. CATALYST: Catalyzing Access to New Prevention Products to Stop HIV. Available online at: https://www.prepwatch.org/catalyst/ (Accessed June 7, 2022).
- 13.Friedland BA, Plagianos M, Savel C, Kallianes V, Martinez C, Begg L, et al. Women want choices: opinions from the share.learn.shape global internet survey about multipurpose prevention technology (MPT) products in development. AIDS Behav. (2023) 27:2190–204. 10.1007/s10461-022-03951-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milali MP, Resar D, Kaftan D, Campbell J, Olowu A, Edwards D, et al. Cost effectiveness of the dual prevention pill for contraception and HIV pre-exposure prophylaxis. J Front Reprod Health. (2023) 5:1144217. 10.3389/frph.2023.1144217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quaife M, Terris-Prestholt F, Eakle R, Cabrera-Escobar MA, Kilbourne-Brook M, Mvundura M, et al. The cost-effectiveness of multi-purpose HIV and pregnancy prevention technologies in South Africa. JAIDS. (2018) 21:e25064. 10.1002/jia2.250641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maharaj NR. Adolescent pregnancy in sub-saharan Africa—a cause for concern. J Front Reprod Health. (2022) 4:984303. 10.3389/frph.2022.984303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minnis AM, Etima J, Musara P, Browne EN, Mutero P, Kemigisha D, et al. Couples’ preferences for “2 in 1” multipurpose prevention technologies to prevent both HIV and pregnancy: results of a discrete choice experiment in Uganda and Zimbabwe. AIDS Behav. (2022) 26(12):3848–61. 10.1007/s10461-022-03713-6. PMID: 35674885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamire V, Magut F, Khagayi S, Kambona C, Muttai H, Nganga L, et al. HIV Risk factors and risk perception among adolescent girls and young women: results from a population-based survey in western Kenya, 2018. J Acquir Immune Defic Syndr. (2022) 91(1):17–25. 10.1097/QAI.0000000000003021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maughan-Brown B, Venkataramani AS. Accuracy and determinants of perceived HIV risk among young women in South Africa. BMC Public Health. (2017) 18(1):42. 10.1186/s12889-017-4593-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The World Bank. The social and educational consequences of adolescent childbearing. (2022) Available online at: https://genderdata.worldbank.org/data-stories/adolescent-fertility (Accessed June 5, 2023).
- 21.Church K, Mayhew SH. Integration of STI and HIV prevention, care, and treatment into family planning services: a review of the literature. Stud Fam Plann. (2009) 40(3):171–86. 10.1111/j.1728-4465.2009.00201.x [DOI] [PubMed] [Google Scholar]
- 22.FHI360. Integrating Family Planning into HIV Programs: Evidence-Based Practices. (2013) Available at: fp-hiv-evidence based practices 2013.pdf (fhi360.org) (Accessed May 5, 2023).
- 23.Grant-Maimont T, Kranzer K, Ferrant RA. The effect of integration of family planning into HIV services on contraceptive use among women accessing HIV services in low and middle-income countries: a systematic review. Front Glob Womens Health. (2022) 3:837358. 10.3389/fgwh.2022.837358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng C, Pacqué-Margolis S, Kotellos K, Brantley S. Integrating family planning and HIV/AIDS services: Health workforce considerations. (2012) An IntraHealth International technical brief. Available at: https://www.intrahealth.org/sites/default/files/files/media/integrating-family-planning-and-hivaids-services-health-workforce-considerations/integrating-family-planning-hiv-aids-services-health-workforce-considerations.pdf (Accessed May 5, 2023).
- 25.Bhavaraju N, Wilcher R, Regeru RN, Mullick S, Mahaka I, Rodrigues J, et al. Integrating oral PrEP into family planning services for women in sub-saharan Africa: findings from a multi-country landscape analysis. J Front Reprod Health. (2021) 3:667823. 10.3389/frph.2021.667823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newmann SJ, Grossman D, Blat C, Onono M, Steinfeld R, Bukusi EA, et al. Does integrating family planning into HIV care and treatment impact intention to use contraception? Patient perspectives from HIV-infected individuals in nyanza province, Kenya. Int J Gynaecol Obstet. (2013) 123:e16–23. 10.1016/j.ijgo.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brady M, Park H. A brief: What regulatory guidance exists for multipurpose prevention technologies (MPTs)? A review of key guidance documents and their applicability to MPTs. Washington, DC: Population Council; (2011). Available online at: https://knowledgecommons.popcouncil.org/departments_sbsr-hiv/12/ (Accessed on April 15, 2023) doi: 10.31899/hiv1.1011. [Google Scholar]
- 28.Yeh PT, Kautsar H, Kennedy CE, Gaffield ME. Values and preferences for contraception: a global systematic review. Contraception. (2022) 111:3–21. 10.1016/j.contraception.2022.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions are included in the article. Further inquires can be directed to the corresponding author.