Abstract
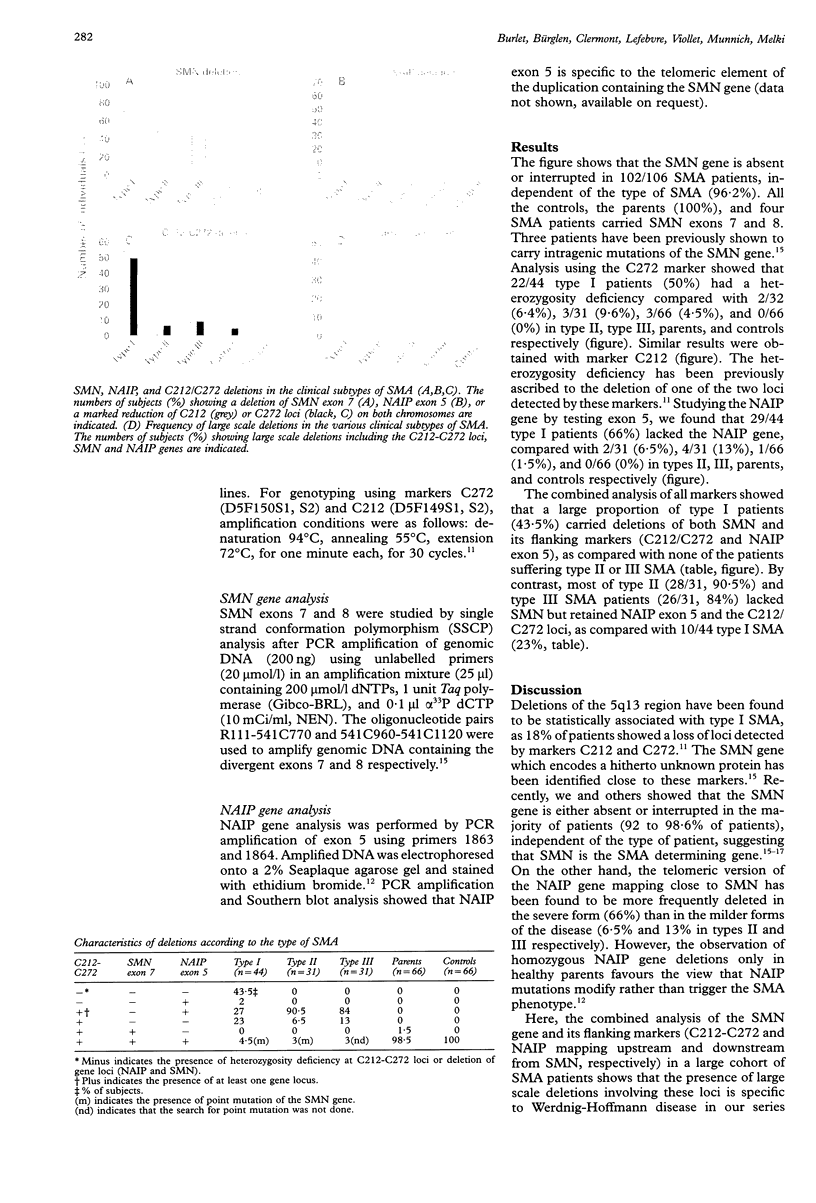
Spinal muscular atrophy (SMA) is characterised by degeneration of anterior horn cells of the spinal cord and represents the second most common, lethal, autosomal recessive disorder after cystic fibrosis. Based on the criteria of the Internatinal SMA Consortium, childhood SMAs are classified into type I (Werdnig-Hoffmann disease), type II (intermediate form), and type III (Kugelberg-Welander disease). Recently, two genes have been found to be associated with SMA. The survival motor neurone gene (SMN) is an SMA determining gene as it is absent in 98.6% of patients. A second gene, XS2G3, or the highly homologous neuronal apoptosis inhibitory protein gene (NAIP) have been found to be more frequently deleted in type I than in the milder forms (types II and III). We investigated the correlation between the clinical phenotype and the genotype at this loci. A total of 106 patients were classified into type I (44), type II (31), and type III (31) and analysed using SMN, markers C212 and C272, and NAIP mapping upstream and downstream from SMN respectively. The combined analysis of all markers showed a large proportion of type I patients (43%) carried deletions of both SMN and its flanking markers (C212/272) and NAIP exon 5), as compared with none of the patients with type II or III SMA. The presence of large scale deletions involving these loci is specific to Werdnig-Hoffman disease (type I) and allows one to predict the severity of the disease in our series.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brzustowicz L. M., Lehner T., Castilla L. H., Penchaszadeh G. K., Wilhelmsen K. C., Daniels R., Davies K. E., Leppert M., Ziter F., Wood D. Genetic mapping of chronic childhood-onset spinal muscular atrophy to chromosome 5q11.2-13.3. Nature. 1990 Apr 5;344(6266):540–541. doi: 10.1038/344540a0. [DOI] [PubMed] [Google Scholar]
- Bussaglia E., Clermont O., Tizzano E., Lefebvre S., Bürglen L., Cruaud C., Urtizberea J. A., Colomer J., Munnich A., Baiget M. A frame-shift deletion in the survival motor neuron gene in Spanish spinal muscular atrophy patients. Nat Genet. 1995 Nov;11(3):335–337. doi: 10.1038/ng1195-335. [DOI] [PubMed] [Google Scholar]
- Cobben J. M., van der Steege G., Grootscholten P., de Visser M., Scheffer H., Buys C. H. Deletions of the survival motor neuron gene in unaffected siblings of patients with spinal muscular atrophy. Am J Hum Genet. 1995 Oct;57(4):805–808. [PMC free article] [PubMed] [Google Scholar]
- Czeizel A., Hamula J. A hungarian study on Werdnig-Hoffmann disease. J Med Genet. 1989 Dec;26(12):761–763. doi: 10.1136/jmg.26.12.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel W. K. Selective and nonselective susceptibility of muscle fiber types. A new approach to human neuromuscular diseases. Arch Neurol. 1970 Feb;22(2):97–117. doi: 10.1001/archneur.1970.00480200003001. [DOI] [PubMed] [Google Scholar]
- Gilliam T. C., Brzustowicz L. M., Castilla L. H., Lehner T., Penchaszadeh G. K., Daniels R. J., Byth B. C., Knowles J., Hislop J. E., Shapira Y. Genetic homogeneity between acute and chronic forms of spinal muscular atrophy. Nature. 1990 Jun 28;345(6278):823–825. doi: 10.1038/345823a0. [DOI] [PubMed] [Google Scholar]
- Hahnen E., Forkert R., Marke C., Rudnik-Schöneborn S., Schönling J., Zerres K., Wirth B. Molecular analysis of candidate genes on chromosome 5q13 in autosomal recessive spinal muscular atrophy: evidence of homozygous deletions of the SMN gene in unaffected individuals. Hum Mol Genet. 1995 Oct;4(10):1927–1933. doi: 10.1093/hmg/4.10.1927. [DOI] [PubMed] [Google Scholar]
- Lefebvre S., Bürglen L., Reboullet S., Clermont O., Burlet P., Viollet L., Benichou B., Cruaud C., Millasseau P., Zeviani M. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995 Jan 13;80(1):155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- Melki J., Abdelhak S., Sheth P., Bachelot M. F., Burlet P., Marcadet A., Aicardi J., Barois A., Carriere J. P., Fardeau M. Gene for chronic proximal spinal muscular atrophies maps to chromosome 5q. Nature. 1990 Apr 19;344(6268):767–768. doi: 10.1038/344767a0. [DOI] [PubMed] [Google Scholar]
- Melki J., Lefebvre S., Burglen L., Burlet P., Clermont O., Millasseau P., Reboullet S., Bénichou B., Zeviani M., Le Paslier D. De novo and inherited deletions of the 5q13 region in spinal muscular atrophies. Science. 1994 Jun 3;264(5164):1474–1477. doi: 10.1126/science.7910982. [DOI] [PubMed] [Google Scholar]
- Melki J., Sheth P., Abdelhak S., Burlet P., Bachelot M. F., Lathrop M. G., Frezal J., Munnich A. Mapping of acute (type I) spinal muscular atrophy to chromosome 5q12-q14. The French Spinal Muscular Atrophy Investigators. Lancet. 1990 Aug 4;336(8710):271–273. doi: 10.1016/0140-6736(90)91803-i. [DOI] [PubMed] [Google Scholar]
- Moosa A., Dubowitz V. Motor nerve conduction velocity in spinal muscular atrophy of childhood. Arch Dis Child. 1976 Dec;51(12):974–977. doi: 10.1136/adc.51.12.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearn J. H. The gene frequency of acute Werdnig-Hoffmann disease (SMA type 1). A total population survey in North-East England. J Med Genet. 1973 Sep;10(3):260–265. doi: 10.1136/jmg.10.3.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearn J. Incidence, prevalence, and gene frequency studies of chronic childhood spinal muscular atrophy. J Med Genet. 1978 Dec;15(6):409–413. doi: 10.1136/jmg.15.6.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues N. R., Owen N., Talbot K., Ignatius J., Dubowitz V., Davies K. E. Deletions in the survival motor neuron gene on 5q13 in autosomal recessive spinal muscular atrophy. Hum Mol Genet. 1995 Apr;4(4):631–634. doi: 10.1093/hmg/4.4.631. [DOI] [PubMed] [Google Scholar]
- Roy N., Mahadevan M. S., McLean M., Shutler G., Yaraghi Z., Farahani R., Baird S., Besner-Johnston A., Lefebvre C., Kang X. The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell. 1995 Jan 13;80(1):167–178. doi: 10.1016/0092-8674(95)90461-1. [DOI] [PubMed] [Google Scholar]
- SOX9 and the switch hitting genes. Nat Genet. 1995 Jan;9(1):1–2. doi: 10.1038/ng0195-1. [DOI] [PubMed] [Google Scholar]
- Thompson T. G., DiDonato C. J., Simard L. R., Ingraham S. E., Burghes A. H., Crawford T. O., Rochette C., Mendell J. R., Wasmuth J. J. A novel cDNA detects homozygous microdeletions in greater than 50% of type I spinal muscular atrophy patients. Nat Genet. 1995 Jan;9(1):56–62. doi: 10.1038/ng0195-56. [DOI] [PubMed] [Google Scholar]
- van der Steege G., Grootscholten P. M., van der Vlies P., Draaijers T. G., Osinga J., Cobben J. M., Scheffer H., Buys C. H. PCR-based DNA test to confirm clinical diagnosis of autosomal recessive spinal muscular atrophy. Lancet. 1995 Apr 15;345(8955):985–986. [PubMed] [Google Scholar]