Summary
Background
Individual doses of dual-dose vaccine-regimens are sequentially administered into the deltoid muscle, but little attention has so far been paid to the immunological effects of choosing the ipsilateral or the contralateral side for the second dose.
Methods
In an observational study, 303 previously naive individuals were recruited, who received the second dose of the COVID-19 vaccine BNT162b2 on either the ipsilateral (n = 147) or the contralateral side (n = 156). Spike-specific IgG, IgG-avidity, and neutralizing antibodies were quantified using ELISA and a surrogate assay 2 weeks after dose 2. A subgroup of 143 individuals (64 ipsilateral, 79 contralateral) was analysed for spike-specific CD4 and CD8 T-cells using flow-cytometry.
Findings
Median spike-specific IgG-levels did not differ after ipsilateral (4590 (IQR 3438) BAU/ml) or contralateral vaccination (4002 (IQR 3524) BAU/ml, p = 0.106). IgG-avidity was also similar (p = 0.056). However, neutralizing activity was significantly lower after contralateral vaccination (p = 0.024). Likewise, median spike-specific CD8 T-cell levels were significantly lower (p = 0.004). Consequently, the percentage of individuals with detectable CD8 T-cells was significantly lower after contralateral than after ipsilateral vaccination (43.0% versus 67.2%, p = 0.004). Spike specific CD4 T-cell levels were similar in both groups, but showed significantly higher CTLA-4 expression after contralateral vaccination (p = 0.011). These effects were vaccine-specific, as polyclonally stimulated T-cell levels did not differ.
Interpretation
Both ipsilateral and contralateral vaccination induce a strong immune response, but secondary boosting is more pronounced when choosing vaccine administration-routes that allows for drainage by the same lymph nodes used for priming. Higher neutralizing antibody activity and higher levels of spike-specific CD8 T-cells may have implications for protection from infection and severe disease and support general preference for ipsilateral vaccination.
Funding
Financial support was provided in part by the State chancellery of the Saarland to M.S.
Keywords: Vaccination, Ipsilateral, Contralateral, Antibodies, T cells, Neutralizing activity, Avidity
Research in context.
Evidence before this study
We searched the Cochrane Central Registry for controlled trials and Medline, without any language restrictions, for studies in humans on immunological effects of sequential vaccinations carried out in different extremities. Terms for systematic search were “(immunization OR immunisation OR vaccination) AND (immune response OR immunogenicity OR antibody response OR T cell) and (contralateral OR ipsilateral OR same arm OR different arm OR consistent arm OR alternating arm OR same limb OR different limb OR consistent limb OR alternating limb OR same leg OR different leg OR consistent leg OR alternating leg)”. Articles resulting from these searches and relevant references cited in those articles were reviewed. There was one randomized trial identified in infants published in 2015, and one study among 46 adults after rabies vaccination published in 1964, which gave conflicting results. Adults received four dosages of a rabies vaccines either in the same arm or the arm was changed for the third and fouth dose (applied at days 0, 7, 14, 21), and antibody response rates were reported before vaccination and after 12 months. Study participants using the same arm had higher antibody response rates (22/24, 92%) compared to individuals who sequentially received two injections in each arm (15/22, 68%). By contrast, results from the infant study were more variable depending on the vaccine and the vaccine dose. The study showed that the alternating limb group had higher geometric mean concentrations of IgG towards a Haemophilus influenzae type b conjugate vaccine (0.61 (0.45–0.82) μg/ml, n = 202) compared to the same limb group (0.41 (0.31–0.54 μg/ml, n = 207, p = 0.0268) at 5 months (1 months after completion of a three dose regimen comparing three doses in the right leg with a regimen of 1 dose in the left leg followed by 2 doses in the right leg), and the effects persisted until month 12, but not after a booster dose. Three doses of the tetanus vaccine in the consistent limb group (0.54 (0.49–0.60) IU/ml, n = 207) did not differ from infants in the alternating limb group (0.56 (0.49–0.63) IU/ml, p = 0.5956) at month 5. This persisted until month 12. A fourth booster dose yielded higher levels in the alternating limb group (2.30 (1.97–2.68) IU/ml) than in the consistent limb group (1.63 (1.40–1.90) IU/ml, p = 0.0008) at month 13, although the administration side for the fourth vaccine was changed in both groups. Finally, the study did not find any effect of the site for three sequential pneumococcal vaccines. Neither the adult nor the infant study analysed the effect on vaccine-induced cellular immunity.
Added value of this study and implications
As published evidence on the role of the vaccine side for sequential vaccinations was controversial, we readdressed this issue using a unique setting where the same type of COVID-19 vaccine was administered in a SARS-CoV-2 naïve population. We performed a detailed analysis of vaccine-induced antibodies and T cells after ipsilateral and contralateral vaccination. We found that spike-specific neutralizing antibody activity was significantly lower after contralateral vaccination, and significantly fewer individuals mounted a sufficient spike-specific CD8 T-cell response.
Implications of all the available evidence
The data support general preference for ipsilateral vaccination. Interindividual variability in specific immune responses was high. However, given that neutralizing antibody activity contributes to protection against SARS-CoV-2 infection, and specific T-cells mediate protection from severe COVID-19 disease, the choice of arm for the second vaccination represents a previously unappreciated factor that may contribute to overall vaccine effectiveness on a population level. Moreover, the findings may have relevance for other vaccines applied as a dual dose regimen. Characterization of the mechanisms for the observed differences needs further study. Documentation of the vaccine sides in future study may help to explore the clinical implications for effectiveness to protect from infection and severe disease.
Introduction
Shortly after the onset of the SARS-CoV-2 pandemic, different vaccines have been developed.1, 2, 3 The mRNA vaccine BNT162b2 was the first vaccine that was given conditional authorisation in the European Union.4 The vaccine has proven to be well tolerated and efficacious in protecting from infection, severe COVID-19 and COVID-19-related death.1,5 Although immunological correlates of protection towards SARS-CoV-2 infection are ill-defined, it is well known that the BNT162b2 vaccine not only induces spike-specific antibodies with neutralizing activity towards the SARS-CoV-2 virus, but also spike-specific CD4 and CD8 T-cells6,7 with important roles in supporting humoral immunity and cytotoxicity, respectively. Originally, a dual-dose vaccination regimen was authorised, with the vaccine recommended to be administered in the deltoid muscle in the upper arm.1,8 As with other vaccine regimens, little attention is generally paid to the choice of the arm used for the first and for the second vaccination. The decision is usually left to the discretion of the vaccinated person and/or vaccinating physician, with most individuals choosing the same non-dominant arm for sequential vaccinations. Knowledge is currently limited, whether the antibody- and T-cell response after a dual-dose vaccination regimen in humans differs depending on whether the second dose is injected into the same ipsilateral or into the contralateral arm. Effects on antibody response rates have been addressed in a small study in the early 1960ies using a four-dose series of rabies vaccine in an adult population,9 and in a cohort of infants after complex series of sequential doses of pneumococcal and Haemophilus influenzae vaccines,10 but the studies were restricted to the analysis of antibodies and have yielded conflicting results. Therefore further studies are needed to address the role of the administration site for sequential vaccines.11 We now used a uniform cohort of SARS-CoV-2 naïve individuals who received a dual-dose BNT162b2 vaccination regimen to study whether the involvement of the ipsilateral or contralateral lymph nodes for the first and the second dose would differentially affect vaccine-induced humoral and cellular immunity.
Methods
Study design and participants
In this prospective observational study, individuals without restriction for sex/gender receiving a dual-dose vaccination with the BNT162b2 vaccine (Comirnaty, BioNtech/Pfizer) were recruited between 1st of March and 10th of September 2021 from either the Saarland University Medical Center (Homburg, Germany) or from the Robert Bosch GmbH (Homburg, Germany) within the respective vaccination programs for employees. Individuals either received both vaccine doses into the same arm or the second vaccine dose was injected into the contralateral arm. The decision for ipsilateral or contralateral vaccination was made randomly assigned based on the day of vaccination (where all study participants of a given day were assigned either the ipsilateral or contralateral side), or at the individual's discretion in a minor fraction of study participants. The number of study participants was given by feasibility of analyses and recruitment. Study participants did not report any history of SARS-CoV-2 infection. A heparinized blood sample was drawn 12–22 days after the second vaccination to analyse nucleocapsid-specific IgG, SARS-CoV-2 spike-specific IgG, spike-specific IgG-avidity, and neutralizing activity. In a subgroup of individuals, differential blood counts, general B-cell and T-cell subpopulations as well as spike-specific CD4 and CD8 T-cell levels were quantified and characterized. In addition, all participants reported vaccine-related adverse events in the first week after the first and the second vaccination, respectively, using a standardized questionnaire. The study was approved by the ethics committee of the Ärztekammer des Saarlandes (reference 76/20 including amendment), and all individuals gave written informed consent.
Quantitation of lymphocyte populations and plasmablasts
100 μl heparinized whole blood was used for quantification of cell populations exactly as described before6 using titered amounts of monoclonal antibodies in saturating concentrations as indicated (see also Table S1). T-cells, B-cells and plasmablasts were quantified using antibodies towards CD3 (clone SK7, final dilution 1:25), CD19 (clone HIB19, 1:40), CD27 (clone L128, 1:200), CD38 (clone HB7, 1:20) and IgD (clone IA6-2, 1:33.3). Expression of CD3 and CD19 was used to identify T and B-cells, respectively, among total lymphocytes. Staining of CD4 (clone SK3, 1:100) and CD8 (clone RPA-T8, 1:100, all antibodies from BD, Heidelberg, Germany) was used to quantify CD4 and CD8 T-cells. Staining of CD38 was used to identify plasmablasts among IgD-CD27+ CD19 positive switched-memory B-cells. Flow-cytometric analyses were performed on a BD FACSLyric instrument and BD FACSuite software v1.4.0.7047. Further data analyses was carried out using FlowJo software 10.6.2 based on a gating strategy as described before.6 Absolute lymphocyte numbers were calculated using differential blood counts.
Quantitation of SARS-CoV-2 specific CD4 and CD8 T-cells
SARS-CoV-2 spike-specific T-cells were measured after a 6 h stimulation of heparinized whole blood with overlapping peptide pools derived from the S1 and S2 domain of the SARS-CoV-2 spike protein (N-terminal receptor binding domain and C-terminal portion including the transmembrane domain, each peptide 2 μg/ml; JPT, Berlin, Germany), and costimulatory antibodies against CD28 and CD49d (clone L293 and clone 9F10, 1 μg/ml each) exactly as previously described.6 Negative control samples were treated with peptide diluent solution (0.64% DSMO), and polyclonal stimulations were carried out with 2.5 μg/ml Staphylococcus aureus Enterotoxin B, both in the presence of antibodies towards CD28 and CD49d. Brefeldin A was added after 2 h to allow intracellular cytokine accumulation. After stimulation, all samples were fixed with BD lysing solution (BD Biosciences) and immunostained using titered amounts of antibodies in saturating concentrations as indicated (see also Table S1). Antibodies included anti-CD4 (clone SK3, 1:33.3), anti-CD8 (clone SK1, 1:12.5), anti-CD69 (clone L78, 1:33.3), anti-IFNγ (clone 4 S.B3, 1:100), anti-IL-2 (clone MQ1-17H12, 1:12.5), anti-TNFα (clone MAb11, 1:20), and anti-CTLA-4 (clone BNI3, 1:50, all antibodies from BD, Heidelberg, Germany). Flow-cytometric analysis was performed on a BD FACS Canto II including BD FACSDiva software 6.1.3 using gating strategies as previously described6,12 Spike-specific CD4 or CD8 T-cells were identified by co-expression of CD69 and IFNγ and further characterized for expression of CTLA-4, and of the cytokines IL-2 and TNFα. Specific CD4 or CD8 T-cell levels ≥0.03% after subtraction of control stimulations were scored as positive as previously established from cohorts of infected and vaccinated individuals.6,12
Quantitation of SARS-CoV-2 specific IgG and neutralizing activity
The amount of SARS-CoV-2 spike-specific IgG antibodies was determined using an ELISA (SARS-CoV-2-QuantiVac, Euroimmun, Lübeck, Germany) at a dilution of 1:64 according to the manufacturer's instructions. Antibody levels were expressed as binding antibody units (BAU/ml) with levels <25.6 BAU/ml scored as negative, levels between ≥25.6 and < 35.2 BAU/ml as intermediate, and levels ≥35.2 BAU/ml as positive. Avidity testing of IgG antibodies was performed as described before13 using ELISA with plasma samples in duplicates at a dilution of 1:64. After incubation with the plasma, the samples were washed three times. One well was incubated with 200 μl PBS and the second well was incubated with 5.5 M urea (in PBS) for 10 min at 37 °C. Thereafter samples were washed and bound antibodies were detected according to the manufacturer's instructions. A relative avidity index (RAI) was calculated by the ratio of detected IgG in the absence and presence of urea. IgG antibodies towards the SARS-CoV-2 nucleocapsid protein (NCAP) was detected using a semiquantitative ELISA (anti-SARS-CoV-2-NCP-ELISA, Euroimmun) at a single serum dilution according to the manufacturer's instruction. The neutralizing activity of the antibodies was measured using a surrogate assay (SARS-CoV-2-NeutraLISA, Euroimmun). The plasma was diluted 1:16 in 2.5% human albumin/PBS buffer as described before,14 and processed further according to manufacturer instructions. Inhibitory activity was quantified as IC50 [%].
Statistical analysis
The Mann–Whitney test (two sided) was used to analyse differences between non-parametric data such as lymphocyte subpopulations, T-cell and antibody levels, and CTLA-4 expression. Data with normal distribution such as cytokine–expression profiles, and age were analysed using a t-test (two-sided). Categorical analyses on vaccine responses were performed using the Fisher's test, sex/gender and adverse events were analysed using Χ2 test. Correlations between the immunological parameters were analysed using a correlation matrix according to Spearman. Analysis was carried out using GraphPad Prism 9.5.1 software (GraphPad, San Diego, CA, USA). Categorical comparisons of the occurrence of adverse events after the first and second vaccination in the ipsilateral and the contralateral group, respectively, was performed using the non-parametrical McNemar test (including two-sided p-values) using IBM SPSS Statistics 26. A p-value <0.05 was considered statistically significant.
Role of funders
The funder did not have any role in study design, data collection, data analyses, interpretation, or writing of report.
Results
Study population
We recruited 303 immunocompetent individuals (39.5 ± 15.1 years) with no known history of SARS-CoV-2 infection who underwent COVID-19 vaccination with two doses of the BNT162b2 vaccine (Comirnaty, BioNTech/Pfizer), which were given 5.8 ± 0.7 weeks apart as per German regulations. Among them, 147 individuals received the second dose on the ipsilateral side, and 156 individuals had the second dose administered on the contralateral side (Table 1). Blood samples were drawn 15 (IQR 2) days after the second vaccination to determine differential blood counts, general lymphocyte subpopulations, NCAP-specific IgG, spike-specific IgG levels, IgG-avidity, and surrogate neutralizing antibody activity towards SARS-CoV-2 spike. Although all individuals did not have any known history of SARS-CoV-2 infection, 2 out of 303 individuals (from the ipsilateral group) had a positive IgG titer towards the nucleocapsid protein and were excluded from further analyses. Among a subgroup of 143 participants (64 ipsilateral and 79 contralateral), we also determined SARS-CoV-2 spike-specific CD4 and CD8 T-cell levels. There was no difference in age and sex between the ipsilateral and the contralateral group (Table 1). Likewise, general leukocyte and lymphocyte numbers and their T- and B-cell subpopulations including plasmablasts did not differ between the two groups (Table 1).
Table 1.
Demographic and clinical characteristics of the study population.
| ipsilateral |
contralateral |
p-value | |
|---|---|---|---|
| n = 145a | n = 156 | ||
| Years of age (mean ± SD) | 38.5 ± 15.0 | 40.4 ± 15.3 | 0.286e |
| Sex/genderb, n (%) | 0.518f | ||
| male | 80 (55.2) | 91 (58.3) | |
| female | 65 (44.8) | 64 (41.0) | |
| transgender | 0 (0.0) | 1 (0.6) | |
| Weeks between 1st and 2nd vaccination, (mean ± SD) | 5.8 ± 0.7 | 5.8 ± 0.8 | |
| Analysis time [days after 2° vaccination], median (IQR) | 15 (2.0) | 14.5 (1) | |
| Differential blood cell counts | n = 143 | n = 153 | |
| Leukocytes (cells/μl), median (IQR) | 6900 (2400) | 7000 (1800) | 0.825g |
| Granulocytes (cells/μl), median (IQR) | 4080 (1711) | 4092 (1539) | 0.674g |
| Monocytes (cells/μl), median (IQR) | 578 (225) | 594 (192) | 0.786g |
| Lymphocytes (cells/μl), median (IQR) | 2356 (926) | 2317 (826) | 0.774g |
| Thrombocytes (cells/μl), median (IQR) | 257,000 (87,000) | 265,000 (86,000) | 0.272g |
| General lymphocyte subpopulations | n = 63c | n = 77c | |
| CD3 T-cells (cells/μl), median (IQR) | 1670 (659) | 1732 (665) | 0.667g |
| CD4 T-cells (cells/μl), median (IQR) | 991c (553) | 1075 (447) | 0.760g |
| CD8 T-cells (cells/μl), median (IQR) | 436d (327) | 482 (264) | 0.139g |
| CD19 B-cells (cells/μl), median (IQR) | 232 (218) | 224 (144) | 0.947g |
| Plasmablasts (cells/μl), median (IQR) | 0.576 (0.807) | 0.726 (0.643) | 0.207g |
Two out of 147 individuals who were included in the ipsilateral group had a positive NCAP-IgG titer (1 male, one female); these two individuals were excluded from further analyses.
Self-declared.
Determined among subgroup of 143 individuals (64 ipsilateral and 79 contralateral) who were tested for both spike-specific antibodies and T-cells.
n = 62.
Unpaired t-test.
Χ2 test.
Mann-Whitney test.
Spike-specific antibodies and T-cells
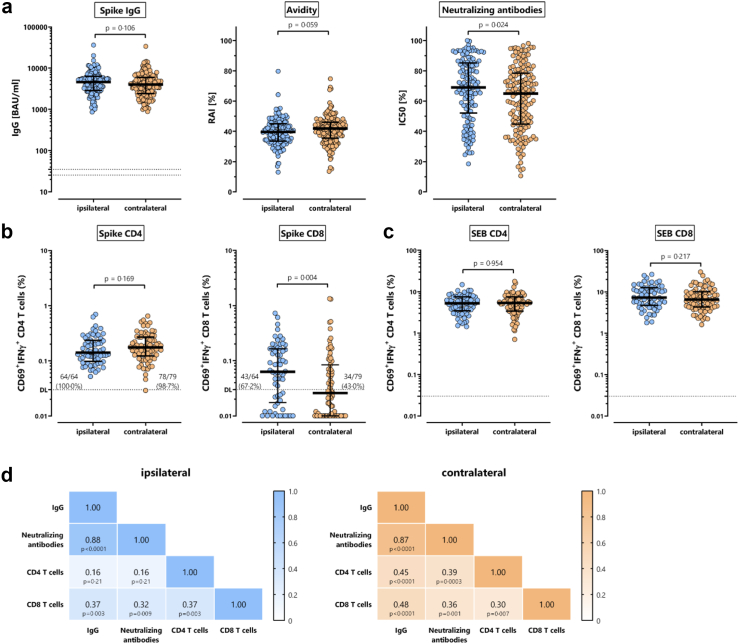
SARS-CoV-2 spike-specific IgG antibodies were strongly induced after the second vaccination, with no significant difference in median antibody levels between the ipsilateral (4590 (IQR 3438) BAU/ml) and the contralateral group (4002 (IQR 3524) BAU/ml, p = 0.106, Fig. 1a). Likewise, IgG-avidity did not differ between the two groups (ipsilateral 39 5% (IQR 11.5%), contralateral 41.9% (IQR 10.5%), p = 0.056, Fig. 1a). However, the median neutralizing activity of the antibodies was significantly lower in the contralateral group (65.0% (IQR 33.8%) versus ipsilateral 69 0% (IQR 33.0%), p = 0.024). SARS-CoV-2 spike-specific T-cell levels were quantified in subgroups of 64 individuals after ipsilateral and 79 after contralateral vaccination, respectively. Specific T-cells were stimulated directly ex vivo from whole blood with overlapping peptide pools spanning the S1 and S2 subunits of the spike protein. Specifically activated T-cells were identified after intracellular staining of IFNγ in CD69 positive CD4 and CD8 T-cells. As shown in Fig. 1b, individuals in the ipsilateral and the contralateral group did not differ in the median percentages of SARS-CoV-2 spike-specific CD4 T-cells (ipsilateral 0.141% (IQR 0.138%), contralateral 0.175% (IQR 0.146%), p = 0.169). In contrast, spike-specific CD8 T-cell levels were significantly lower in the contralateral (0.026% (IQR 0.079%)) than in the ipsilateral group (0.063% (IQR 0.147), p = 0.004). As a result, the percentage of individuals with spike-specific CD8 T-cell levels above the detection limit was significantly lower after contralateral than after ipsilateral vaccination (34/79 (43.0%) versus 43/64 (67.2%), p = 0.004). CD4 and CD8 T-cell levels after polyclonal stimulation with Staphylococcus aureus Enterotoxin B (SEB) did not differ between the two groups (Fig. 1c), which indicates that the effect is vaccine-specific. In both the ipsilateral and the contralateral group, CD8 T-cell levels, IgG levels, and neutralizing activity showed a significant correlation (Fig. 1d). In contrast, significant correlations between CD4 T-cell levels and IgG or between CD4 T-cell levels and neutralizing activity were only found in individuals after contralateral vaccination (Fig. 1d).
Fig. 1.
Spike-specific IgG, neutralizing antibodies, and CD4 and CD8 T-cells after ipsilateral and contralateral administration of the second dose of the BNT162b2 vaccine. (a) Spike-specific IgG levels, IgG-avidity, and neutralizing activity (dilution 1:16) were determined from all individuals (n = 301) after ipsilateral (n = 145) and contralateral (n = 156) vaccination. (b) Spike-specific CD4 and CD8 T-cells as well as (c) SEB-reactive CD4 and CD8 T-cells were quantified in a subset of individuals (ipsilateral n = 64; contralateral n = 79) after stimulation based on expression of CD69 and IFNγ. Stippled lines denote detection limits as defined by the manufacturer (panel a) or as established in previous studies (panel b).6,12 Percentages in panel b refer to individuals with specific CD4 and CD8 T-cell levels above detection limit of 0.03%. Statistical analysis was performed using the non-parametric Mann–Whitney test. Bars represent medians with interquartile ranges. (d) Correlation matrix of spike-specific IgG levels, spike-specific neutralizing antibodies, and spike-specific CD4 and CD8 T-cells. Correlation coefficients (including p-values) were calculated according to two-tailed Spearman and displayed using a color code. SEB, Staphylococcus aureus Enterotoxin B. A separate presentation of the data of this figure for female and male study participants is shown in Figure S1.
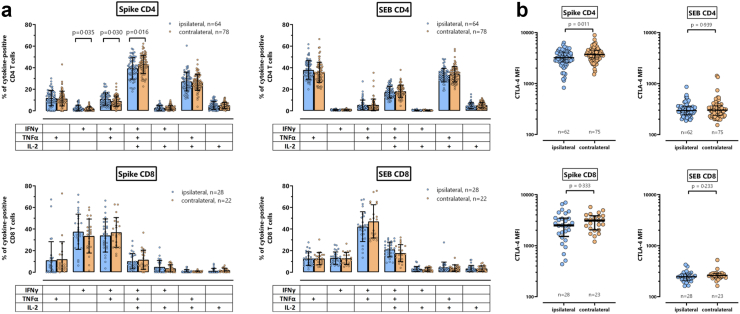
Apart from the cytokine IFNγ, spike-specific T-cells were further characterized for co-expression of IL-2 and TNFα, and for expression of CTLA-4 as phenotypical marker for recent antigen encounter. Although spike-specific CD4 T-cells did not show any quantitative differences between the groups (Fig. 1b), the cytokine profile of spike-specific CD4 T-cells from individuals in the contralateral group showed slightly more multifunctional CD4 T-cells co-expressing IFNγ, IL-2 and TNFα (p = 0.016, Fig. 2a). Likewise, spike-specific CD4 T-cells in the contralateral group had significantly higher CTLA-4 expression levels (p = 0.011, Fig. 2b). The cytokine profile of spike-specific CD8 T-cells or SEB-reactive T-cells was distinct from that of spike-specific CD4 T-cells. However, unlike for spike-specific CD4 T-cells, the ipsilateral and the contralateral group did not show any functional or phenotypical differences for spike-specific CD8 T-cells or for CD4 or CD8 T-cells after polyclonal stimulation with SEB.
Fig. 2.
Cytokine profile and CTLA-4 expression of spike-specific and SEB-reactive CD4 and CD8 T-cells. (b) Spike-specific and SEB-reactive CD4 and CD8 T-cells were analysed for expression of IFNγ, TNFα and IL-2 alone or in combination after Boolean gating. This allowed distinction of seven subpopulations expressing three, two or a single cytokine. All samples were analysed, but to ensure robust statistics, only individuals with at least 30 cytokine-expressing spike-specific CD4 or CD8 T-cells after normalization to the negative control stimulation were considered (with sample size indicated in the figures). Bars represent means and individual values are displayed by circles. Differences between the groups were analysed using the t-test. (b) Spike-specific and SEB-reactive CD4 and CD8 T-cells were analysed for expression of CTLA-4, which is expressed as median fluorescence intensity (MFI). All samples were analysed, but to ensure robust statistics, this analysis was restricted to samples with at least 20 CD69+IFNγ+ CD4 or CD8 T-cells (with sample size indicated in the figures). Bars represent medians with interquartile ranges. Differences between the groups were calculated using the Mann Whitney test.
Adverse events after vaccination
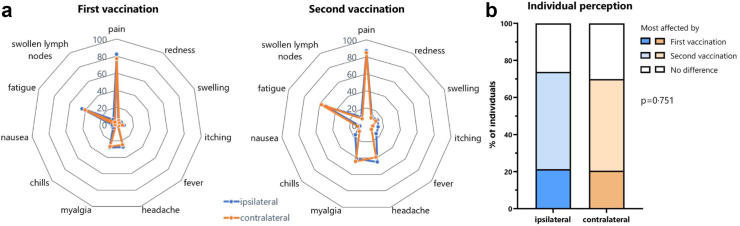
We also analysed vaccine-related adverse events in the first week after the first and the second vaccination based on self-reporting using a questionnaire. Both vaccinations were generally well tolerated with pain at the injection site and fatigue being most frequently reported (Fig. 3a). Local and systemic adverse events after the first dose expectedly did not differ between the two groups, whereas fever was the only adverse event that was more frequently observed in the ipsilateral group after the second vaccination (15.2% versus 7.7%, p = 0.046, Fig. 3a and Table S2). In general, most individuals in both groups felt more affected by the second vaccination (p = 0.751, Fig. 3b). This is also illustrated by a comparison between the first and the second vaccination (Tables S3 and S4, McNemar testing), where the proportion of individuals with local redness and swelling, or systemic adverse events such as headache, myagia and chills showed a significant change from the first to the second vaccination in both the ipsilateral and the contralateral group; a proportional change in fever was only found in the ipsilateral group, whereas proportional changes in local pain, nausea, fatigue and swollen lymph nodes were only observed in the contralateral group.
Fig. 3.
Adverse events after the first and the second vaccination. (a) Local and systemic adverse events in the first seven days after the first and second vaccination, respectively, were self-reported using a questionnaire. Shown is the percentage of individuals who reported a given adverse event in the ipsilateral or the contralateral group. More detailed statistical analyses are shown in Tables S2–S4. (b) Individual perception of relative severity of the two vaccine doses after ipsilateral or contralateral administration is shown based on whether individuals had felt more affected by the first or by the second vaccination or whether both were scored similar. Analyses was performed using Χ2 test.
Discussion
We have studied the role of the ipsilateral and contralateral vaccine side on immunogenicity after secondary vaccination in a unique setting where the same type of COVID-19 vaccine was administered in a SARS-CoV-2 naïve population. Both the ipsilateral and the contralateral vaccination induced a strong humoral and cellular immune response, and were overall similarly well tolerated with adverse events being largely restricted to pain at the injection side and fatigue. As most striking observations, we found that spike-specific neutralizing antibody activity was significantly lower after contralateral vaccination, despite similar quantities of spike-specific IgG and avidity between the groups. Moreover, significantly fewer individuals mounted a sufficient spike-specific CD8 T-cell response (43% versus 67%). While the quantity of spike-specific CD4 T-cells was similar in both groups, their CTLA-4 expression level was significantly higher if induced on the contralateral side. We also noted that interindividual variability in specific immune responses was high. However, considering that neutralizing antibody activity contributes to protection against SARS-CoV-2 infection, and specific T-cells mediate protection from severe COVID-19 disease,15,16 the choice of arm for the second vaccination represents a previously unappreciated factor that may contribute to overall vaccine effectiveness on a population level.
The observed differences in immunogenicity may result from the fact that priming and secondary boosting of the immune response after ipsilateral vaccination occurs in the same draining axillary lymph nodes with limited involvement of the contralateral side. Conceptually, this is supported by 18F-FDG PET/CT studies among BNT162b2-vaccine recipients demonstrating that the ipsilateral lymph nodes on the side where the vaccine had been applied were significantly larger in size and showed higher metabolic activity compared to the contralateral lymph nodes.17 Moreover, studies on fine needle aspirates of lymph nodes after ipsilateral vaccination indicated that follicular helper (Tfh) cells, germinal center (GC) B-cells in general, and spike-specific GC B-cells were induced after the first vaccination and strongly increased after the second.18, 19, 20 As mRNA and spike antigen after ipsilateral vaccination was shown to persist in GC of the axillary draining lymph nodes up to 8 weeks after vaccination,21 this may in part result from antigen persistence on follicular dendritic cells that may facilitate B-cell proliferation and affinity maturation of antibodies upon secondary challenge. It was also found that spike-specific GC B-cells and plasmablasts remained detectable in the draining lymph nodes for at least 12 weeks.20 Interestingly, the vaccine-related induction of these cells after ipsilateral vaccination was restricted to the ipsilateral side, as Tfh cell- and GC B-cell levels in the contralateral lymph node did not increase, and spike-specific GC B-cells were completely absent.18 These data are in line with observations in rhesus macaques vaccinated with an mRNA encoding the H10 hemagglutinin, where priming of influenza-specific CD4 T cells exclusively occurred in the vaccine-draining lymph nodes.22 Similar analyses of lymph nodes after contralateral vaccination have not been performed. However, based on our results, it seems conceivable that administration of the second vaccination on the contralateral side may at least to some extent require de novo induction of specific B and T-cells and germinal center formation in lymph nodes not previously involved in the first vaccination, which overall may result in a less pronounced immune response as compared to ipsilateral vaccination. In this regard, the fact that the correlation between CD4 T-cells and antibodies was primarily found in the contralateral group, and lower levels of CD8 T-cells after contralateral vaccination are reminiscent of immune responses after primary induction.23 In addition, higher levels of CD8 T-cells after ipsilateral vaccination may result from antigen retention on lymphatic endothelial cells followed by capture and cross-presentation of these antigens to migratory dendritic cells upon secondary challenge, which strongly induced specific CD8 T-cells in mice.24,25 While the significance of the slightly altered cytokine profile of spike-specific CD4 T-cells warrants further study, the higher expression levels of CTLA-4 on CD4 T-cells may result in a less pronounced activation and proliferation of B-cells thereby adversely affecting antibody responses. Our data are supported by recent data in mice.26 As with our study, overall IgG levels were similar in mice after secondary influenza vaccination on the ipsilateral and contralateral hock. Consistent with differences in neutralizing activity in our study, the quality of the antibodies also differed, as mice after ipsilateral vaccination had significantly higher numbers of B-cells with high-avidity B-cell receptors.26
Our results on a stronger induction of specific immunity after ipsilateral vaccination are in line with a higher percentage of antibody responders after rabies vaccination in an adult population,9 whereas the differences between ipsilateral and contralateral vaccination in a large study among infants were less clear.10 The reasons for the differences in the results warrant further study, but may result from the fact that repeated vaccinations, in part with longer spacing, were carried out in the infant study which may have offset potential differences seen after the initial doses. Moreover, it may be due to the use of arms in adults (deltoid muscle) and legs in children (vastus lateralis muscle), and attendant anatomical differences between lymphatic vessels draining the upper and lower extremities. The separation of the lymphatic territories of the right and left legs is not strict, since they merge in the region of the lesser pelvis and drain together into the thoracic duct. This contrasts with a lymphatic watershed in the upper extremities, where the right arm has its own drainage via the right lymphatic duct, and the left arm -like the legs-drains via the thoracic duct.27 Together this may indicate that the beneficial effects of ipsilateral vaccination are more pronounced in adults, where the use of the upper extremities is the commonly recommended practice.
Our study is limited by the lack of analyses after the first vaccination to exclude that the ipsilateral group by chance had more high-responders. Moreover, we did not perform any serial analyses after the second vaccination. Therefore, the observed differences between ipsilateral and contralateral administration may in part result from different dynamics in the induction of spike-specific antibodies and T-cells. Although we performed several assays to assess the magnitude and functionality of vaccine-induced antibodies and T cells, our neutralizing activity was performed with a surrogate assay only. However, this assay was shown to correlate well with live viral assays towards the parental SARS-CoV-2 strain based on previous observations.14 Finally, as adverse events may affect immunogenicity,28,29 adverse events after the first vaccination may in principle influence the choice of arm for the second vaccination. However, assignment of the vaccine arm was randomly made based on the day of vaccination in the majority of cases. The minor percentage of study participants where assignment was based on the individual's discretion, adverse events after the first vaccination was not the reasoning for assignment. Particular strengths of our study are the large sample size and the use of COVID-19 as a unique setting where the same vaccine was administered to an immunologically naïve population. Moreover, the rate of infections in our study period was low due to widespread contact restrictions and mask mandates, and all individuals did not report any history of SARS-CoV-2 infection with only 2/303 individuals having a presumed history of infection based on a positive antibody titer towards the NCAP protein. Therefore, infections are considered unlikely as a confounder for the observed side-specific effects on the induction of specific immune responses.
Knowledge on individual factors on how to best induce and improve humoral and cellular immune responses may be relevant for elderly individuals or immunocompromised patients with poor vaccine responses, and for evaluation of effectiveness. During the SARS-CoV-2 pandemic and global introduction of COVID-19 vaccinations, several factors have been identified that influence immunogenicity and effectiveness to varying degrees. Those include the type of vaccine with various delivery principles such as viral vectors, mRNA or proteins. In this respect, vector vaccines generally induced lower antibody levels, and protein-based vaccines were less potent in inducing specific CD8 T-cells.6,30,31 Heterologous combinations of vector- and mRNA-vaccines were found to eliciting stronger antibody and T-cell responses as compared to homologous vector regimens.6,30,31 Moreover, longer time between first and second vaccination resulted in better immunogenicity and effectiveness.32 The impact of vaccine dosage is illustrated by the fact that the mRNA-1273 vaccine elicited slightly higher antibody and T-cell levels as compared to the BNT162b2 vaccine.33 Finally, increasing age or extent of immunodeficiency is associated with lower response rates.23,34 Our study now identified the vaccine side as an additional factor affecting immunogenicity. Despite large interindividual variability in specific antibody- and T-cell responses within each group, the lower neutralizing antibody activity and strikingly lower levels of specific CD8 T-cells after contralateral vaccination may have implications for protection on a population level and support current preference for ipsilateral administration. Moreover, the findings of this study can immediately be translated into clinical practice without additional costs or adverse events, and may have general relevance for other vaccines applied as a dual-dose regimen.
Contributors
L.Z., U.S., B.C.G., and M.S. designed the study; L.Z., V.K., T.S., U.S. and M.S. designed the experiments, L.Z., V.K., and T.S. performed experiments; L.Z., S.S., C.B., J.N., S.L.B., B.C.G., and U.S. contributed to study design, patient recruitment, and clinical data acquisition. L.Z., T.S., U.S. and M.S. performed statistical analysis. L.Z., T.S., U.S., and M.S. supervised all parts of the study, and performed analyses; L.Z. and M.S. have verified all underlying data and wrote the manuscript. All authors approved the final version of the manuscript.
Data sharing statement
Data collected for the study, including individual participant data and a definition of each field in the set, are available to others on a public repository (Zenodo https://doi.org/10.5281/zenodo.8105965) with no end date. Individual participant data that underlie the results reported in this article is provided after de-identification to anyone who wishes to access the data for any purpose.
Declaration of interests
M.S. has received grant support from Astellas and Biotest to the organization Saarland University outside the submitted work, and honoraria and travel support for lectures from Biotest, MSD, Takeda and Novartis, and for advisory boards from Moderna, Biotest, MSD and Takeda. T.S. has received travel support from Biotest for attending a meeting outside the submitted work. S.L.B. has participated in advisory boards with Shionogi and Pfizer outside the submitted work. B.C.G. has received honoraria for lectures from BioNTech, Moderna, Sanofi, CSL Seqirus, and GSK. All other authors of this manuscript have no conflicts of interest to disclose.
Acknowledgements
The authors thank all participants to this study, and the teams of Christina Baum of the occupational health care center at Saarland University Medical Center for their support in enrolling employees, and the team of Dr. Jürgen Neumann from the Robert Bosch GmbH and Patrick Schaaf for their support in enrolling employees to our study. Expert technical assistance by Candida Guckelmus and Rebecca Urschel is acknowledged.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104743.
Appendix A. Supplementary data
References
- 1.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voysey M., Clemens S.A.C., Madhi S.A., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavaleri M., Enzmann H., Straus S., Cooke E. The European Medicines Agency's EU conditional marketing authorisations for COVID-19 vaccines. Lancet. 2021;397(10272):355–357. doi: 10.1016/S0140-6736(21)00085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahin U., Muik A., Vogler I., et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595(7868):572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt T., Klemis V., Schub D., et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med. 2021;27(9):1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh E.E., Frenck R.W., Jr., Falsey A.R., et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Medicines Agency (EMA) 2020. Comirnaty, product information, Annex 1: summary of product characteristics.https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf [Google Scholar]
- 9.Peck F.B., Jr., Kohlstaedt K.C. Pre-exposure rabies prophylaxis problems and procedures. Ind Med Surg. 1964;33:17–21. [PubMed] [Google Scholar]
- 10.Iro M.A., Khatami A., Marshall A.S., et al. Immunological effect of administration of sequential doses of Haemophilus influenzae type b and pneumococcal conjugate vaccines in the same versus alternating limbs in the routine infant immunisation schedule: an open-label randomised controlled trial. Lancet Infect Dis. 2015;15(2):172–180. doi: 10.1016/S1473-3099(14)71057-6. [DOI] [PubMed] [Google Scholar]
- 11.Creech C.B., Edwards K.M. What we don't know might hurt us. Lancet Infect Dis. 2015;15(2):133–135. doi: 10.1016/S1473-3099(14)71083-7. [DOI] [PubMed] [Google Scholar]
- 12.Schub D., Klemis V., Schneitler S., et al. High levels of SARS-CoV-2-specific T cells with restricted functionality in severe courses of COVID-19. JCI Insight. 2020;5(20) doi: 10.1172/jci.insight.142167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz T., Tober-Lau P., Hillus D., et al. Delayed antibody and T-cell response to BNT162b2 vaccination in the elderly, Germany. Emerg Infect Dis. 2021;27(8):2174–2178. doi: 10.3201/eid2708.211145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hielscher F., Schmidt T., Klemis V., et al. NVX-CoV2373-induced cellular and humoral immunity towards parental SARS-CoV-2 and VOCs compared to BNT162b2 and mRNA-1273-regimens. J Clin Virol. 2022;157 doi: 10.1016/j.jcv.2022.105321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 16.Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23(2):186–193. doi: 10.1038/s41590-021-01122-w. [DOI] [PubMed] [Google Scholar]
- 17.Ayati N., Evans S., Zakavi S.R., Gruenewald S.M. Comparison between viral vector and mRNA based COVID-19 vaccination in prevalence and severity of regional immune reactions, and (18)F-FDG PET/CT features. Asia Ocean J Nucl Med Biol. 2023;11(1):4–12. doi: 10.22038/AOJNMB.2022.63110.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lederer K., Bettini E., Parvathaneni K., et al. Germinal center responses to SARS-CoV-2 mRNA vaccines in healthy and immunocompromised individuals. Cell. 2022;185(6):1008–1024.e15. doi: 10.1016/j.cell.2022.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim W., Zhou J.Q., Horvath S.C., et al. Germinal centre-driven maturation of B cell response to mRNA vaccination. Nature. 2022;604(7904):141–145. doi: 10.1038/s41586-022-04527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner J.S., O'Halloran J.A., Kalaidina E., et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596(7870):109–113. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Röltgen K., Nielsen S.C.A., Silva O., et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell. 2022;185(6):1025–1040.e14. doi: 10.1016/j.cell.2022.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang F., Lindgren G., Lin A., et al. Efficient targeting and activation of antigen-presenting cells in vivo after modified mRNA vaccine administration in rhesus macaques. Mol Ther. 2017;25(12):2635–2647. doi: 10.1016/j.ymthe.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt T., Klemis V., Schub D., et al. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am J Transplant. 2021;21(12):3990–4002. doi: 10.1111/ajt.16818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doan T.A., Forward T., Tamburini B.A.J. Trafficking and retention of protein antigens across systems and immune cell types. Cell Mol Life Sci. 2022;79(5):275. doi: 10.1007/s00018-022-04303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kedl R.M., Lindsay R.S., Finlon J.M., Lucas E.D., Friedman R.S., Tamburini B.A.J. Migratory dendritic cells acquire and present lymphatic endothelial cell-archived antigens during lymph node contraction. Nat Commun. 2017;8(1):2034. doi: 10.1038/s41467-017-02247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuraoka M., Yeh C.H., Bajic G., et al. Recall of B cell memory depends on relative locations of prime and boost immunization. Sci Immunol. 2022;7(71) doi: 10.1126/sciimmunol.abn5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pilsl U., Anderhuber F. Anatomy of the lymph system. J Ästhet Chir. 2019;12(1):51–58. [Google Scholar]
- 28.Debes A.K., Xiao S., Colantuoni E., et al. Association of vaccine type and prior SARS-CoV-2 infection with symptoms and antibody measurements following vaccination among health care workers. JAMA Intern Med. 2021;181(12):1660–1662. doi: 10.1001/jamainternmed.2021.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hermann E.A., Lee B., Balte P.P., et al. Association of symptoms after COVID-19 vaccination with anti-SARS-CoV-2 antibody response in the framingham heart study. JAMA Netw Open. 2022;5(10) doi: 10.1001/jamanetworkopen.2022.37908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barros-Martins J., Hammerschmidt S.I., Cossmann A., et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021;27(9):1525–1529. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hillus D., Schwarz T., Tober-Lau P., et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet. 2021;9(11):1255–1265. doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall V.G., Ferreira V.H., Wood H., et al. Delayed-interval BNT162b2 mRNA COVID-19 vaccination enhances humoral immunity and induces robust T cell responses. Nat Immunol. 2022;23(3):380–385. doi: 10.1038/s41590-021-01126-6. [DOI] [PubMed] [Google Scholar]
- 33.Klemis V., Schmidt T., Schub D., et al. Comparative immunogenicity and reactogenicity of heterologous ChAdOx1-nCoV-19-priming and BNT162b2 or mRNA-1273-boosting with homologous COVID-19 vaccine regimens. Nat Commun. 2022;13(1):4710. doi: 10.1038/s41467-022-32321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Müller L., Andree M., Moskorz W., et al. Age-dependent immune response to the biontech/pfizer BNT162b2 coronavirus disease 2019 vaccination. Clin Infect Dis. 2021;73(11):2065–2072. doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.