Summary
Background
Air pollution has been associated with gestational diabetes mellitus (GDM). We aim to investigate susceptible windows of air pollution exposure and factors determining population vulnerability.
Methods
We ascertained GDM status in the prospective Maternal and Developmental Risks from Environmental and Social Stressors (MADRES) pregnancy cohort from Los Angeles, California, USA. We calculated the relative risk of GDM by exposure to ambient particulate matter (PM10; PM2.5), nitrogen dioxide (NO2), and ozone (O3) in each week from 12 weeks before to 24 weeks after conception, adjusting for potential confounders, with distributed lag models to identify susceptible exposure windows. We examined effect modification by prenatal depression, median-split pre-pregnancy BMI (ppBMI) and age.
Findings
Sixty (9.7%) participants were diagnosed with GDM among 617 participants (mean age: 28.2 years, SD: 5.9; 78.6% Hispanic, 11.8% non-Hispanic Black). GDM risk increased with exposure to PM2.5, PM10, and NO2 in a periconceptional window ranging from 5 weeks before to 5 weeks after conception: interquartile-range increases in PM2.5, PM10, and NO2 during this window were associated with increased GDM risk by 5.7% (95% CI: 4.6–6.8), 8.9% (8.1–9.6), and 15.0% (13.9–16.2), respectively. These sensitive windows generally widened, with greater effects, among those with prenatal depression, with age ≥28 years, or with ppBMI ≥27.5 kg/m2, than their counterparts.
Interpretation
Preconception and early-pregnancy are susceptible windows of air pollutants exposure that increased GDM risk. Prenatal depression, higher age, or higher ppBMI may increase one’s vulnerability to air pollution-associated GDM risk.
Funding
National Institutes of Health, Environmental Protection Agency.
Keywords: Air pollution, Gestational diabetes, Sensitive windows, Pre-conception, Susceptibility, Depression
Research in context.
Evidence before this study
We searched PubMed to identify relevant articles published up to June 30, 2022 using (“particulate matter” OR “PM” OR “nitrogen dioxide” OR “NO2” OR “Ozone” OR “O3” OR “air pollution”) AND (“gestational diabetes” OR “GDM”) without restriction on language. Synthesized evidence from meta-analyses indicates increased risk of GDM with exposure to PM2.5, PM10, NO2, or O3. Most studies investigated air pollution exposure in the first trimester, the second trimester, or throughout the whole pregnancy, while only a few studies included exposures before conception. Susceptible exposure windows have not been well defined because little research has examined air pollution at weekly or finer scales. Few studies investigated effect modification by population characteristics.
Added value of this study
Using distributed lag models, we identified a preconceptional to early-pregnancy period (5 weeks before to 5 weeks after conception) as a susceptible window during which exposures to ambient PM2.5, PM10, and NO2 were associated with increased risk of GDM. Importantly, individuals with prenatal depression, higher age, or higher pre-pregnancy BMI had wider periconceptional susceptible window and more pronounced association with GDM risk.
Implications of all the available evidence
Whereas air pollution is an established environmental risk factor, evidence from previous studies and ours emphasizes that air pollution exposure during pregnancy, or even before conception, could increase the risk of developing gestational diabetes. Evidence further suggested individuals with prenatal depression or other risk factors for gestational diabetes maybe particularly vulnerable to air pollution exposure.
Introduction
High blood glucose during pregnancy, or gestational diabetes mellitus (GDM), is one of the most common complications in pregnancy, affecting more than one in twelve pregnancies in the US.1 GDM increases a mother’s lifetime risk of type 2 diabetes and predisposes the offspring to increased risks of macrosomia, obesity, and diabetes.2,3 The past decade witnessed an increasing trend in the prevalence of GDM, possibly due to a high prevalence of obesity and advanced age at pregnancy.4 Recently, GDM risk has also been associated with exposure to environmental pollution, most notably ambient air pollution,5 thus opening novel avenues for GDM prevention.
Various ambient air pollutants have been associated with increased GDM risk, including particulate matter with aerodynamic diameter <2.5 μm (PM2.5),6, 7, 8, 9, 10 and <10 μm (PM10),8 sulfur dioxide (SO2),11,12 and nitrogen dioxide (NO2).8 However, not all studies have found such associations,13 and some have reported decreased GDM risk in association with exposure to PM10 and NO2.14 Such inconsistencies are likely driven by population characteristics that determine baseline GDM risk.5 For example, numerous studies have reported that the association of air pollution exposure with GDM risk is stronger among populations with overweight or obese pre-pregnancy body mass index (ppBMI) compared to normal ppBMI.15, 16, 17 In addition to obesity and other established physiological risk factors of GDM, such as advanced age,3 recent studies also found psychological and mental health factors, such as prenatal depression, are associated with GDM risk, as well as modifying the adverse effect of air pollution.18 A better understanding of how these physiological and psychological characteristics modify the association of air pollution exposure with GDM risk could help identify populations who are more vulnerable to air pollution exposure effect, and thus provide more tailored public health prevention and clinical practices with increased effectiveness.
Timing of exposure during pregnancy is critical because throughout a normal pregnancy, physiological changes, such as decreased insulin sensitivity and pancreatic beta-cell hyperplasia, are precisely regulated to support fetal growth. This dynamic period of metabolic adaptation may increase the mother’s susceptibility to environmental insults,19 especially during specific time windows. Moreover, exposure during a pre-conceptional window may also be relevant to GDM, given the dynamic regulation of metabolism and hormones by ovulation.20 Previous studies have examined ambient air pollution exposure during various time windows in relation to GDM risk and found potentially sensitive exposure windows, including the three months before conception,8,9,12 the first trimester,7,10 the second trimester,6,7,21 or the whole pregnancy period.7 However, because air pollution exposures in adjacent time periods are usually correlated, such identified sensitive windows could be biased by confounding effects from exposure in other time windows.22 In addition, a true sensitive window could be shorter than a month or a trimester.22 Therefore, studies with more finely resolved exposure windows (e.g., in each week or day), which account for the confounding effect of exposure in other periods, are needed to address these questions about timing of exposure. Recent advances in statistics, such as the distributed lag model (DLM),23 has be used to identifying such sensitive exposure windows and thus, could improve our understanding of complex environmental exposure, such as air pollution and effect on GDM risk.
We aim to investigate the susceptible windows of air pollution exposure and factors that determine population vulnerability. We hypothesized that GDM risk could be increased by exposure to air pollution during periconceptional and gestational exposure periods, particularly surrounding conception when hormonal signaling changes dramatically. In addition, we hypothesized a greater vulnerability among pregnant participants with physiological and psychological risk factors, such as higher ppBMI, advanced age, or prenatal depression. We tested these hypotheses using DLMs to identify susceptible exposure windows, and used stratification analysis to identify vulnerable subgroups, in a predominantly low-income, Hispanic prospective pregnancy cohort in Los Angeles, California.
Methods
Study population
Participants were from the Maternal and Developmental Risks from Environmental and Social Stressors (MADRES) study, an ongoing prospective pregnancy cohort study established in 2015.24 We recruited participants from clinical sites that serve low-income populations in Los Angeles, California, if they met the following eligibility criteria: 18 years or older, within 30 weeks of gestation, singleton pregnancy, and speaking English or Spanish fluently. Participants with human immunodeficiency virus, having physical, mental, or cognitive disabilities that would prevent participation, or current incarceration were excluded. All consents were obtained at recruitment. The Institutional Review Board at the University of Southern California approved all aspects of this study. This analysis is reported in accordance with the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guideline.25
A total of 703 MADRES participants had reached delivery and had high-quality pregnancy outcome data abstracted from the electronic medical records (EMR) until September 1st, 2021. Thirty-four participants who had type I or II diabetes diagnosed before pregnancy were excluded. Twelve participants who reported smoking during pregnancy and forty participants with missing information on key covariates, e.g., race/ethnicity were also excluded. No meaningful difference in the prevalence of GDM (the outcome), air pollution levels (the exposure), age, pre-pregnancy BMI, and depression (the effect modifiers) were observed before and after exclusion (Supplementary Table S1). A flow chart describing the final sample size of 617 is provided in Supplementary Fig. S1.
Estimation of ambient air pollution exposure
Details on ambient air pollution exposure estimation have been reported.24,26 Briefly, prior to the third-trimester study visit, participants provided their residential address history starting from 1 year pre-conception, which was further reviewed with study staff to ensure data accuracy. Subsequent residential addresses were prospectively collected at all study timepoints. Addresses were geocoded, and daily residential histories were assembled for each participant with the date of conception estimated based on an ultrasound measurement in the first trimester (<14 gestational weeks, 382/617 [61.9%]) or in the second trimester (14–28 gestational weeks, 159/617 [25.7%]), medical records consensus (71/617 [11.5%]), or last menstrual period (5/617 [0.9%]). We used inverse-distance-squared weighted spatial interpolation to assign daily concentrations of 24-h average PM2.5, PM10, and NO2, and 8-h maximum ground-level ozone (O3), from ambient air quality monitoring data (U.S. EPA Air Quality System), to each participant’s residential location with an average of 4 monitoring stations with nearest monitor within 8–14 km to each residential address (Supplementary Table S2). We calculated one-year pre-conception average levels of air pollution to indicate long-term exposure. We calculated weekly average concentrations from 12 week before to 24 weeks after conception to identify sensitive exposures windows closer to pregnancy, considering 12 weeks before conception as a plausible biologically relevant window of exposure that may affect menstrual cycles and ovulation prior conception.20 We truncated the exposure period at 24 weeks 6 days gestation, because GDM is typically screened between 24 and 28 weeks in the US.27 In addition, we calculated one-year preconception average and weekly temperature using daily temperatures extracted from a gridded surface meteorological dataset with a resolution of 4 km.28
Gestational diabetes mellitus ascertainment
Most GDM cases (55/60 [92%]) were ascertained using physician diagnosis abstracted from maternal EMR. Secondarily, GDM cases (4/60 [7%]) were identified by reviewing EMR results from glucose challenge test (GCT) and oral glucose tolerance test (OGTT), following the American College of Obstetricians and Gynecologists guidelines 229: if the GCT glucose level was ≥200 mg/dL 1 h after drinking a 50 g glucose solution, or if the GCT was 140–200 mg/dL but the following OGTT had two or more high glucose levels, defined as ≥95 mg/dL at baseline, ≥180 mg/dL at 1 h, ≥155 mg/dL at 2 h, or ≥140 mg/dL at 3 h after drinking a standard 100 g glucose solution. One GDM case was self-reported.
Covariates
We a priori selected potential confounders, based on literature review and causal diagram analyses (directed acyclic graph, Supplementary Fig. S2).30 These variables include: year of conception, season of conception, weekly ambient temperature, annual household income, age, ppBMI, ethnicity by nativity (US-born non-Hispanic, US-born Hispanic, foreign-born Hispanic), parity, and enrollment timepoint. Participants self-reported pre-pregnancy weight, race/ethnicity (American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or Other Pacific Islander, more than one race, and White), birth country, marital status (living together, single-never married, divorced or separated, widowed, decline to answer), and annual household income (US dollar) in their preferred language (English or Spanish). Ethnicity and nativity were adjusted as high-level proxy measures of socioeconomic status and lifestyle factors. We recoded enrollment timepoint as early enrollment if the gestational week of initial consent was before 20 weeks and others (20–30 weeks) as late enrollment. Staff measured participants standing height twice by a stadiometer (Perspectives Enterprises Model PE-AIM-101), from which ppBMI (kg/m2) was calculated with self-reported pre-pregnancy weight. We coded marital status to cohabitation status (cohabitate, do not cohabitate, and decline to respond). Parity was determined by the index fetus’ birth order with three categories: first, second, and third or more.
We a priori selected prenatal depression, age, and ppBMI as potential effect modifiers to assess population vulnerability of GDM in association with air pollution exposure.15,17,18 Depressive symptoms were measured in each pregnancy trimester using the Center for Epidemiological Studies Depression (CES-D) Scale, a validated, widely-used instrument to screen depression symptoms.31 Each participant had an average of 2.24 measurements of CES-D over pregnancy. We defined probable prenatal depression if any of the CES-D scores measured over the pregnancy was ≥16, which is the cutoff point representing clinically significant symptoms suggested by the CES-D scale.31 To maximize subgroup sample size, we dichotomized ppBMI (<27.5 vs. ≥27.5 kg/m2) and age (<28 vs. ≥28 years) by their respective medians.
Statistical analysis
We calculated the cumulative incidence of GDM over pregnancy in the overall cohort and in the defined subgroups. We used the Chi-squared test to compare the difference in GDM risk by categorical variables. We described the distribution of continuous variables by GDM status. We first examined the log-linearity of the relationship between air pollution exposure and GDM risk using a 4-df cubic spline of each air pollutant in a generalized additive model. All models showed non-significance for the spline term, suggesting a log-linear dose–response relationship between air pollution exposure and GDM risk. Then we used robust Poisson Regression to analyze the association of each long-term (1-year average) air pollutant and effect modifiers with GDM risk. The use of robust Poisson Regression enabled us to estimate a risk ratio (RR), rather than an odds ratio, since RR is often the preferred metric for interpretation in prospective studies. We used distributed lag models (DLM)23 with robust Poisson Regression to examine the association of weekly exposure to each of the air pollutants (i.e., PM2.5, PM10, NO2, and O3) with GDM risk. Specifically, we fit a model , where is the probability of GDM for subject , is the estimated air pollutant level for week j from 12 weeks before to 24 weeks after pregnancy, and are confounders, including year of conception, season of conception, weekly ambient temperature as a cross-basis function, annual household income, age, ppBMI, ethnicity and nativity, parity, and enrollment timepoint. The DLM uses “cross-basis” to combine a log-linear dose-response function with a nonlinear lag-response function that simultaneously included all weekly levels of each air pollutants from 12 weeks before conception to 24 gestational weeks, assuming their effects are a smooth function, , which was a 5-df (3 evenly placed knots) natural cubic spline to constrain correlated weekly exposures.23 The shape of the spline (number and location of knots) was determined with extensive comparisons, including with various numbers of knots (from 0 to 5 knots, shown in Supplementary Fig. S3), with flexibility of knots location (Supplementary Fig. S4), and further using penalization of the spline (Supplementary Fig. S5). The final spline shape was determined by the minimalization of a quasi-information criterion (QIC).23 The DLM also included weekly temperature of a cross-basis function with a 4-df natural cubic spline for the dose-response function and a 5-df natural cubic spline for the lag-response function. Final estimates were presented as RR for GDM by each interquartile range (IQR) increase in weekly air pollutant concentrations (i.e., 5 μg/m3 for PM2.5,12 μg/m3 for PM10, 11 ppb for NO2, and 15 ppb for O3). Sensitive windows were identified as the weeks during which the RR’s 95% confidence interval did not include 1. We used Bayesian distributed lag interaction models32 to assess the heterogeneity by potential effect modifiers, including ppBMI (<27.5 vs. ≥27.5 kg/m2), age (<28 vs. ≥28 years), and probable prenatal depression status (yes vs. no). Given the presence of heterogeneity (Supplementary Table S7), we conducted stratified analysis. We conducted sensitivity analyses to additionally adjust for respective one-year pre-conception averaged air pollutant levels, year of birth, and gestational week of enrollment, and to remove participants with a history of prior GDM or GDM cases that were not diagnosed by a physician. Because prenatal depression was measured after pre-conceptional exposure, we conducted sensitivity analyses to further adjust for prenatal depression to assess its potential role as a mediator but found little such evidence (Supplementary Fig. S12).
Role of the funding source
Funders, including National Institutes of Health and United States Environmental Protection Agency, had no role in study design, data collection, data analysis, interpretation, or writing of this report.
Results
Among 617 participants (mean age: 28.2 years, SD: 5.9; 78.6% Hispanic, 11.8% non-Hispanic Black), GDM was diagnosed in 60 (9.7%) participants. As shown in Table 1, participants generally were from low-income households, with 276 (45%) reporting annual household income <$30,000, and 343 (55%) having high school education or lower. Over 399 (67%) of participants were overweight or obese prior to pregnancy, and 179 (29%) of participants had probable prenatal depression (CES-D score ≥ 16). Only 19 (3%) of participants reported GDM in a previous pregnancy. Through pregnancy, averaged levels of PM2.5, PM10, NO2, and O3 were 12.05 (SD: 1.16) μg/m3, 29.1 (4.9) μg/m3, 16.69 (2.94) ppb, and 42.4 (3.8) ppb, respectively. Average levels in narrower windows (preconception and trimesters) are shown in Supplementary Table S3. A correlation matrix of air pollutants in various time windows are shown in Supplementary Fig. S6. Ambient air pollutant levels averaged over 1 year pre-conception were comparable between those with and without GDM after adjusting for potential confounders (Supplementary Table S4). In adjusted models (Supplementary Table S4), effect modifiers maternal age (adjusted RR per one additional year of age: 1.06, 95% CI: 1.02–1.11) and ppBMI (≥27.5 vs. <27.5 kg/m2, adjusted RR: 2.56, 95% CI: 1.44–4.55) were both significantly associated with increased risk of GDM, while probable prenatal depression was not (adjusted RR: 0.93, 95% CI: 0.52–1.66). Distribution of covariates, GDM risk, and air pollution by prenatal depression is provided in Supplementary Table S5.
Table 1.
Population characteristics and risk of gestational diabetes mellitus among 617 MADRES participants.
| Population characteristics | N (%) | n of GDM (risk, %) | P value |
|---|---|---|---|
| Overall (denominator) | 617 (100) | 60 (9.7) | – |
| Maternal age, year, mean (SD) | 28.24 (5.9) | 31.1 (5.3) | 0.93 |
| Enrollment timepoint | |||
| Regular entry (<20 weeks) | 463 (75.0) | 47 (10.2) | 0.54 |
| Late entry (20+ weeks) | 154 (25.0) | 13 (8.4) | |
| Language used during interview | |||
| English | 415 (67.3) | 31 (7.5) | 0.02 |
| Spanish | 202 (32.7) | 29 (14.4) | |
| Maternal country origin | |||
| Latin Americaa | 229 (37.1) | 29 (12.7) | 0.13 |
| North Americaa | 298 (48.3) | 22 (7.4) | |
| Others | 90 (14.6) | 9 (10.0) | |
| Maternal race/ethnicity | |||
| Non-hispanic white | 37 (6.0) | 2 (5.4) | 0.88 |
| Non-hispanic black | 73 (11.8) | 7 (9.6) | |
| Hispanic | 485 (78.6) | 49 (10.1) | |
| Non-hispanic multiracial | 6 (1.0) | 0 (0) | |
| Non-hispanic others | 16 (2.6) | 2 (12.5) | |
| Cohabitation status | |||
| Cohabitate with spouse or partner | 392 (63.5) | 42 (10.7) | 0.55 |
| Non-cohabitate | 136 (22.0) | 11 (8.1) | |
| Missing/decline to respond | 89 (14.4) | 7 (7.9) | |
| Annual household income | |||
| <$15,000 | 121 (19.6) | 18 (14.9) | 0.029 |
| $15,000-$29,000 | 155 (25.1) | 16 (10.3) | |
| ≥$30,000 | 143 (23.2) | 16 (11.2) | |
| Unknown | 198 (32.1) | 10 (5.1) | |
| Education | |||
| Below 12th grade | 153 (24.8) | 21 (13.7) | 0.20 |
| Completed 12th grade | 190 (30.8) | 13 (6.8) | |
| Some college | 169 (27.4) | 16 (9.5) | |
| College or above | 105 (17.0) | 10 (9.5) | |
| Pre-pregnancy BMI | |||
| Normal/underweight (<25 kg/m2) | 205 (33.2) | 8 (4.0) | <0.001 |
| Overweight (25-29.9 kg/m2) | 194 (31.4) | 14 (7.2) | |
| Obese (≥30 kg/m2) | 218 (35.3) | 38 (17.4) | |
| GDM history | |||
| No | 591 (95.8) | 48 (8.1) | <0.001 |
| Yes | 19 (3.1) | 11 (57.9) | |
| Missing | 7 (1.1) | 1 (14.3) | |
| Prenatal depression | |||
| No | 385 (62.4) | 40 (10.4) | 0.75 |
| Yes | 179 (29.0) | 15 (8.4) | |
| Missing | 53 (8.6) | 5 (9.4) | |
| Parity | |||
| First born | 200 (32.4) | 15 (7.5) | 0.14 |
| Second born | 178 (28.9) | 15 (8.4) | |
| Third or later born | 169 (27.4) | 24 (14.2) | |
| Missing | 70 (11.4) | 6 (8.6) | |
| Newborn sex | |||
| Female | 316 (51.2) | 26 (8.3) | 0.41 |
| Male | 301 (48.8) | 34 (11.3) |
Note: P value is for a comparison between GDM cases and non-cases.
BMI = body mass index.
Latin America includes participants from Chile, Colombia, El Salvador, Guatemala, Honduras, Medico, Nicaragua, and Venezuela; North America includes US and Canada.
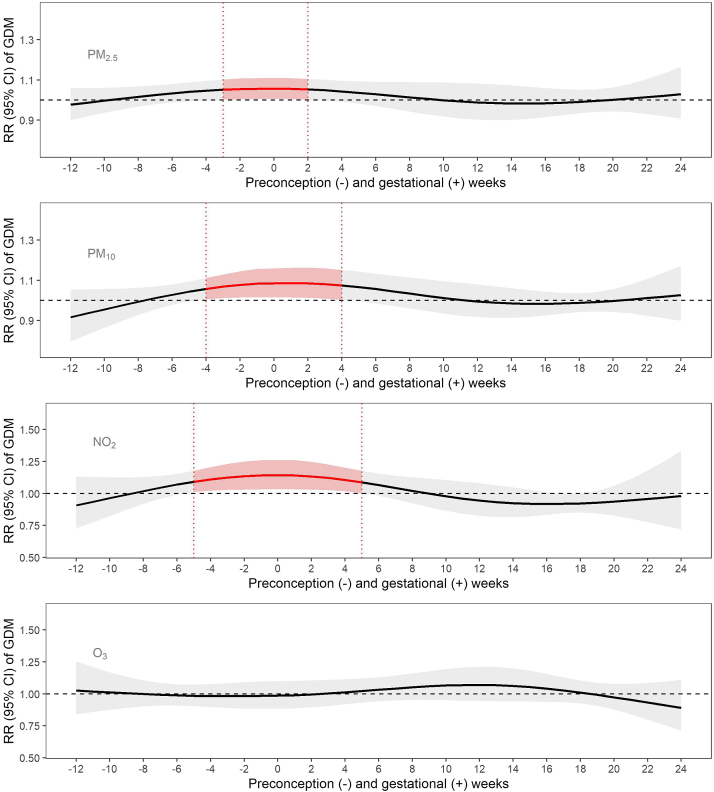
From DLM models (Fig. 1 and Supplementary Table S6), we found increased risk of GDM was associated with exposures to PM2.5, PM10, and NO2 in a similar susceptible window, spanning from 5 weeks before conception to 5 weeks after conception. Specifically, each IQR increase in PM2.5 exposure from 3 weeks before conception to 2 weeks after conception, PM10 exposure from 4 weeks before conception to 4 weeks after conception, and NO2 exposure from 5 weeks before conception to 5 weeks after conception were each significantly associated with greater GDM risk, with a weekly average RR of 1.06 (95% CI: 1.05–1.07) for PM2.5, 1.09 (95% CI: 1.08–1.10) for PM10 and 1.15 (95% CI: 1.14–1.16) for NO2. Weekly exposure to O3 was not associated with GDM risk. In a sensitivity analysis, adjusting these models for 1-year pre-conception average air pollutants did not meaningfully change the results (Supplementary Fig. S7). The results also did not meaningfully change in additional sensitivity analyses, including excluding participants with history of GDM (Supplementary Fig. S8), excluding non-physician diagnosed GDM (Supplementary Fig. S9), further adjusting for year of birth (Supplementary Fig. S10), or adjusting for gestational weeks at enrollment (Supplementary Fig. S11).
Fig. 1.
Associations of pre-conception and prenatal weekly exposure to PM2.5, PM10, NO2, and O3with risk of gestational diabetes among 617 pregnant women in the MADRES cohort. All results were from DLMs adjusted for year, season of conception, weekly temperature, household income, maternal age, pre-pregnancy body mass index, maternal ethnicity and nativity, parity, and enrollment timepoint. Effect estimation was based on per IQR increases in weekly air pollutant concentrations (i.e., 5 μg/m3, 12 μg/m3, 11 ppb, and 15 ppb for PM2.5, PM10, NO2, and O3 (8 hr max), respectively). Gray bands indicate 95% CI. Week 0 indicates conception.
In analyses stratified by probable prenatal depression (Fig. 2 and Supplementary Table S6), we generally found more pronounced associations and wider susceptible periconceptional windows among individuals with depression, and much attenuated or null associations among individuals without depression. Specifically, among individuals with depression, increased risk of GDM was associated with exposure to PM2.5 during 1 week before to 8 weeks after conception (RR, 95% CI: 1.30, 1.27–1.33), PM10 during 4 week before to 7 weeks after conception (1.48, 1.44–1.52), and NO2 during 2 week before to 13 weeks after conception (1.31, 1.29–1.33). Interestingly, early pre-conceptional exposures to PM2.5 during 12 to 11 weeks before conception and PM10 during 12 to 8 weeks before conception was associated with decreased risk of GDM (0.70, 0.59–0.82 for PM2.5; 0.39, 0.33–0.46 for PM10). Among individuals without probable depression, relatively smaller increased risk of GDM was associated with exposure to PM10 (1.09, 1.08–1.11) during 2 weeks before to 3 weeks after conception and NO2 (1.14, 1.12–1.17) during 2–5 weeks after conception.
Fig. 2.
Stratified analysis by prenatal depression for pre-conception and prenatal weekly exposure to PM2.5, PM10, NO2, and O3with risk of gestational diabetes. Left panels show the associations among 385 participants without prenatal depression; right panels show the associations among 179 participants with prenatal depression. Associations were adjusted for year, season of conception, weekly temperature, household income, maternal age, pre-pregnancy body mass index, maternal ethnicity and nativity, parity, and enrollment timepoint. Effect estimation was based on per IQR increases in each air pollutant (i.e., 5 μg/m3, 12 μg/m3, 11 ppb, and 15 ppb for PM2.5, and PM10, NO2, and O3, respectively). Gray bands indicate 95% CI. Week 0 indicates conception.
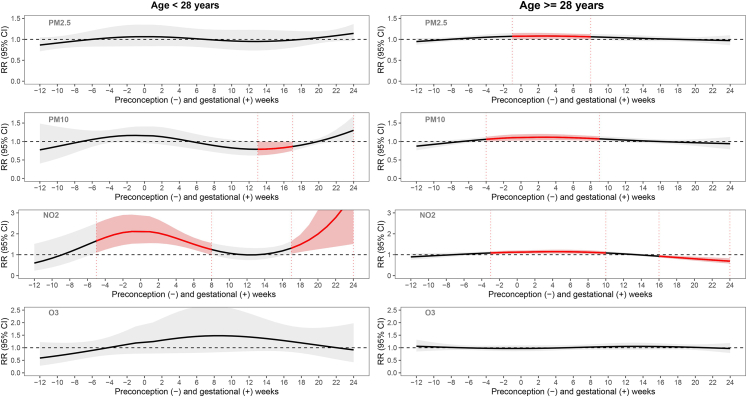
In analyses stratified by age (Fig. 3 and Supplementary Table S6), we similarly found more pronounced effects and wider periconceptional susceptible windows for PM2.5 and PM10 exposure with risk of GDM among the older age group (≥28 years) and attenuated to null associations among the younger age group (<28 years). However, a different pattern was observed for NO2 between the older and the younger group. Specifically, NO2 had pronounced effects in the younger group with two susceptible windows: during 5 weeks before to 8 weeks after conception (1.82, 1.77–1.86) and during 17–24 weeks after conception (2.21, 2.06–2.36). In the older group, GDM risk was increased with NO2 exposure during the periconceptional period from 3 weeks before to 10 weeks after conception (1.12, 1.11–1.13), but GDM risk was decreased with NO2 exposure in mid-pregnancy (0.80, 0.79–0.82). No other air pollutant was associated with GDM in this subgroup.
Fig. 3.
Stratified analysis by age at gestation for pre-conception and prenatal weekly exposure to PM2.5, PM10, NO2, and O3with risk of gestational diabetes. Left panels show the associations among 297 participants aged <28 years; right panels show the associations among 320 participants aged ≥28 years. Associations were adjusted for year, season of conception, weekly temperature, household income, pre-pregnancy body mass index, maternal ethnicity and nativity, parity, and enrollment timepoint. Effect estimation was based on per IQR increases in each air pollutant (i.e., 5 μg/m3, 12 μg/m3, 11 ppb, and 15 ppb for PM2.5, and PM10, NO2, and O3, respectively). Gray bands indicate 95% CI. Week 0 indicates conception.
In analyses stratified by ppBMI (Fig. 4 and Supplementary Table S6), we found association and periconceptional susceptible windows in the higher ppBMI group (≥27.5 kg/m2), although only PM10 exposure during 4 weeks before to 3 weeks after conception was significantly associated with increased risk of GDM (RR:1.11, 1.10–1.12). Similar to the stratification by age, more pronounced effects of NO2 exposure during 2 weeks before to 11 weeks after conception (RR: 1.24, 1.22–1.25) were observed in the relatively lower ppBMI group (<27.5 kg/m2), and null associations in the higher ppBMI group. No other air pollutant was associated with GDM risk in ppBMI stratified analyses.
Fig. 4.
Stratified analysis by pre-pregnancy BMI for pre-conception and prenatal weekly exposure to PM2.5, PM10, NO2, and O3with risk of gestational diabetes. Left panels show the associations among 306 participants aged <27.5 kg/m2; right panels show the associations among 312 participants aged ≥27.5 kg/m2. All results were from DLM adjusted for year, season of conception, weekly temperature, household income, maternal age, maternal ethnicity and nativity, parity, and enrollment timepoint. Effect estimation was based on per IQR increases in each air pollutant (i.e., 5 μg/m3, 12 μg/m3, 11 ppb, and 15 ppb for PM2.5, PM10, NO2, and O3, respectively). Week 0 indicates conception.
Discussion
GDM risk (9.7%) was higher in the MADRES cohort as compared to the prevalence in 2020 in the US (7.8%), California (8.1%), or US Hispanics (8.5%).1 By applying DLM to temporally fine measures of air pollution, we identified a susceptible exposure window from 5 weeks before to 5 weeks after conception, during which exposures to PM2.5, PM10, and NO2 were associated with increased risk of GDM. Moreover, we found probable prenatal depression may heighten the vulnerability of GDM in relation to air pollution exposure, similar to established risk factors of GDM, including age (≥28 years) and ppBMI (≥27.5 kg/m2). Taken together, our findings suggest air quality may be related to the risk of GDM, with potentially stronger effects among those with prenatal depression, as well as those with higher levels of traditional risk factors (i.e., age and BMI) for GDM.
Whereas the exact susceptible exposure windows slightly differed by air pollutants and subgroups, we consistently observed an increased risk of GDM for exposure to PM2.5, PM10, and NO2, all within a period from five weeks before conception to five weeks after conception, suggesting the time around last menstrual period to the first few weeks of pregnancy could be a susceptible window of air pollution exposure in determining GDM risk. This time window is in part in line with previous studies that reported significant associations of GDM risk with nitrogen oxides and PM2.5 exposure in the 3 months before conception9,12 or PM2.5 exposure in the first trimester.7,10 In a study from Guangzhou, China (2011–2014) that also used DLM, sensitive windows in 4–10 gestational weeks were identified for SO2 exposure with GDM risk, but PM2.5, PM10, or NO2 were not associated with GDM risk.11 Notably, the average PM2.5 concentration in that study was three-fold higher than ours (e.g., PM2.5: 53.6 μg/m3 vs. 18.5 μg/m3), although the prevalence of GDM (11.7%) was similar to ours (9.1%).11 A later study (2016–2020) in the same region found a significant association between PM2.5 exposure at 22 gestational weeks with GDM risk, but that population had a much higher GDM prevalence (24.4%) and relatively lower pregnancy-average PM2.5 exposure (mean 32 μg/m3).33 An US-based DLM analysis of birth records identified 21 to 24 gestational weeks as a sensitive window of PM2.5 exposure (mean: 8.78 μg/m3) for its association with GDM risk in Florida (2005–2015),34 while another study found exposure to PM2.5 and PM10 in the 1–5 weeks prior to the OGTT (typically 19–24 gestational weeks) were associated with rapid and significant increase in fasting glucose levels.35 Such differences in these previous and our studies suggest that both the association and the sensitive windows of air pollution exposure with GDM risk are likely modified by not only geographic heterogeneity, possibly due to different levels and mixtures of air pollution, but also by population characteristics that defined population GDM risk vulnerability, which has been understudied.
We found that the vulnerability to increased GDM risk from air pollutant exposures (PM2.5, PM10, and NO2) was modified by age, ppBMI, and prenatal depression. Generally, stronger effects and wider susceptible periconceptional windows were more pronounced among individuals with higher levels of the effect modifiers, including ppBMI (≥27.5 kg/m2), age (≥28 years), and prenatal depression. Our study is the first to identify the novel role of prenatal depression in modifying the vulnerability to GDM risk from air pollution exposure. Interestingly, our results highlighted a unique pattern for NO2, where significant associations were more pronounced in the periconceptional window in the subgroup with lower age or lower ppBMI. Such findings may be attributed to the specific properties of NO2, such as its gaseous form and predominate source from traffic pollution, but exact mechanisms remain elusive and warrant further investigation. However, caution is also needed when interpreting such findings because the prevalence of GDM was much lower in these subgroups (4%), which could render the effect estimation unstable. Nevertheless, prevalence of GDM was comparable by prenatal depression status. Unexpectedly, among those with prenatal depression, we found a decreased risk of GDM with PM2.5 and PM10 exposure in 8–12 weeks before conception, which needs further investigation. Such seemingly protective effect in earlier preconceptional exposure could be driven by a special case of selection bias—the live-birth bias,36 as air pollution may increase one’s risk of miscarriage,37 so that in studies of live births only, such as ours, those participants who keep the pregnancy would have some protective mechanisms that counteract the harm of air pollution and depression to keep viable pregnancies,36 and thus generate a seemingly protective effect of air pollution at an earlier time. Future pre-conceptional cohort studies with information on miscarriages are warranted.
The mechanism underlying the association of ambient air pollution with GDM remains unclear. Previous studies suggest mechanisms underlying the association of air pollution exposure with GDM are similar to those identified for type 2 diabetes, such as air pollution exposures triggered oxidative stress in the respiratory track, subsequent chronic systemic inflammation, and exhausted anti-oxidant defenses over time.38 These systemic effects disrupt insulin signaling pathways and limit glucose-consuming cells (e.g., skeletal muscle and liver) from adequate intake of circulating glucose.5 Furthermore, systemic effects lead to cellular apoptosis and dysfunction that diminish insulin production, thus also increasing glucose abundance.5,39 Interestingly, we found GDM risk was not associated with long-term air pollution exposure but was associated with air pollution exposure in a relatively short periconceptional window, stressing pregnancy as a sensitive window of air pollution exposure.
Mechanisms underlying the susceptible periconception window and heightened vulnerability by effect modifiers remain elusive. During pregnancy, the body undergoes drastic changes in glucose availability through hormonal regulation by the maternal-placental-fetal interface, with a stronger maternal influence in early pregnancy when the placenta and fetus are relatively small.19 Therefore, we suspect air pollution, particularly for exposure in a defined period of the eight weeks from last menstrual period through the first few weeks of pregnancy, could perturb physiological adaptations during early pregnancy that may predispose the pregnant women to an increased risk of GDM. Women with higher age or ppBMI may be more susceptible to such effects given their augmented insulin resistance and glucose insensitivity from aging and excess adipose tissue. Moreover, these physical changes related to aging and adiposity could disturb hormonal regulations throughout pregnancy, thus modifying an individual’s susceptibility to air pollution exposures. How depression might increase the susceptibility to GDM in response to air pollution exposure remains unclear, but may be due in part to shared mechanisms of impaired glucose metabolism, inflammation, and hormonal changes, as well as their interplay.40 Its effect modification of the susceptible window for air pollution exposure on GDM may also involve the dynamic physiological changes throughout pregnancy (e.g., immune responses and various hormones), similar to mechanisms underlying the effect modification by aging and adiposity. Future mechanistic studies are warranted to investigate potentially inter-related pathophysiological changes induced by air pollution exposure and depression with physiological and psychological changes during pregnancy.
Our study is strengthened by a well-characterized cohort with comprehensive measures of potential confounders, weekly air pollution estimates generated from complete maternal residential histories, and the use of DLM to identify sensitive windows of exposure. Our study has a few limitations. First, we may have missed severe GDM cases that could impede one from participating in our study, although no participants dropped out due to GDM. Second, our study focused on examining the individual effect of each air pollutant. Future studies are warranted to examine the interactive and overall mixture effect of the air pollutants. Third, we used the inverse-distance-squared weighted spatial interpolation to estimate ambient air pollutant levels, which is well suited for capturing temporal variability in ambient air pollution levels but may be limited in terms of spatial resolution. While this could introduce exposure measurement error, the higher temporal resolution was chosen to help align exposures more precisely with gestational windows of time based on daily residential timelines. In addition, the relatively high monitoring network density in Southern California compared to other regions in the United States further provided adequate prediction of regional, background air pollutant exposures. Fourth, depression was only measured during the prenatal period and may not fully capture pre-pregnancy depressive symptoms. Future studies that measure and assess the role of depression in both pre- and post-conception periods are warranted. Fifth, although DLM is an advanced approach to constrain multiple exposure modeling simultaneously that can evaluate correlated exposure measurements over time, our analyses involve many statistical tests based on a relatively small sample size., thus some of the significant associations, especially those not consistently seen in subgroup analyses, might have been identified by chance. The identification of susceptible window is also exploratory in nature and needs future replication with larger sample size. Like most observational studies, there is always a chance for our study have residual confounding and measurement error.
Conclusion
In a cohort of predominantly low-income Hispanic pregnant women, periconceptional period is a susceptible window of ambient PM2.5, PM10, and NO2 exposures with increased risk of GDM. Vulnerability to air pollution in relation to GDM risk was higher among women with probable prenatal depression, higher age or ppBMI.
Contributors
Z.N. researched data, wrote, reviewed and edited the manuscript. R.H. and T.Y. researched data and reviewed and edited the manuscript. S.F.F., T.M.B., and C.V.B. secured funding for this work, contributed to discussion and reviewed and edited the manuscript. B.H.G., S.P.E., C.M.T., J.J., G.F.D., N.L., L.A., F.L. and N.P. contributed to discussion and reviewed and edited the manuscript. All authors approved the final version of the manuscript.
Z.N. and S.F.F. are the guarantors of this work and, as such, have full access to all the data in the study and take responsibility for the integrity of the data, the accuracy of the data analysis, and have verified the data reported in the manuscript.
Data sharing statement
The data underlying this article are under restricted protection for participants’ privacy. Data are available by reasonable request on a case-by-case basis. Enquiries should be directed to Dr. Farzan.
Declaration of interests
The authors declare they have no actual or potential competing financial interests.
Acknowledgement
We would like to thank all MADRES participants, the study staff, and our community clinic partners for their many contributions to this work.
This work was supported by NIH grants P50MD015705, P50 ES026086, UH3OD023287, P30ES007048, R01ES027409 and EPA grant 83615801–0. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of these funding organizations. The funding organizations had no roles in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100575.
Appendix A. Supplementary data
References
- 1.Gregory E.C.W.E., Danielle M. 2022. Trends and characteristics in gestational diabetes: United States, 2016–2020; p. 71. [DOI] [PubMed] [Google Scholar]
- 2.Kim C., Newton K.M., Knopp R.H. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 3.Farahvar S., Walfisch A., Sheiner E. Gestational diabetes risk factors and long-term consequences for both mother and offspring: a literature review. Expert Rev Endocrinol Metab. 2019;14(1):63–74. doi: 10.1080/17446651.2018.1476135. [DOI] [PubMed] [Google Scholar]
- 4.Shah N.S., Wang M.C., Freaney P.M., et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326(7):660–669. doi: 10.1001/jama.2021.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H., Wang Q., He S., et al. Ambient air pollution and gestational diabetes mellitus: a review of evidence from biological mechanisms to population epidemiology. Sci Total Environ. 2020;719 doi: 10.1016/j.scitotenv.2020.137349. [DOI] [PubMed] [Google Scholar]
- 6.Choe S.A., Kauderer S., Eliot M.N., et al. Air pollution, land use, and complications of pregnancy. Sci Total Environ. 2018;645:1057–1064. doi: 10.1016/j.scitotenv.2018.07.237. [DOI] [PubMed] [Google Scholar]
- 7.Hu H., Ha S., Henderson B.H., et al. Association of atmospheric particulate matter and ozone with gestational diabetes mellitus. Environ Health Perspect. 2015;123(9):853–859. doi: 10.1289/ehp.1408456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jo H., Eckel S.P., Chen J.C., et al. Associations of gestational diabetes mellitus with residential air pollution exposure in a large Southern California pregnancy cohort. Environ Int. 2019;130 doi: 10.1016/j.envint.2019.104933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rammah A., Whitworth K.W., Symanski E. Particle air pollution and gestational diabetes mellitus in Houston, Texas. Environ Res. 2020;190 doi: 10.1016/j.envres.2020.109988. [DOI] [PubMed] [Google Scholar]
- 10.Kang J., Liao J., Xu S., et al. Associations of exposure to fine particulate matter during pregnancy with maternal blood glucose levels and gestational diabetes mellitus: potential effect modification by ABO blood group. Ecotoxicol Environ Saf. 2020;198 doi: 10.1016/j.ecoenv.2020.110673. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H., Dong H., Ren M., et al. Ambient air pollution exposure and gestational diabetes mellitus in Guangzhou, China: a prospective cohort study. Sci Total Environ. 2020;699 doi: 10.1016/j.scitotenv.2019.134390. [DOI] [PubMed] [Google Scholar]
- 12.Shen H.N., Hua S.Y., Chiu C.T., Li C.Y. Maternal exposure to air pollutants and risk of gestational diabetes mellitus in Taiwan. Int J Environ Res Public Health. 2017;14(12):1604. doi: 10.3390/ijerph14121604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleisch A.F., Gold D.R., Rifas-Shiman S.L., et al. Air pollution exposure and abnormal glucose tolerance during pregnancy: the project Viva cohort. Environ Health Perspect. 2014;122(4):378–383. doi: 10.1289/ehp.1307065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padula A.M., Yang W., Lurmann F.W., Balmes J., Hammond S.K., Shaw G.M. Prenatal exposure to air pollution, maternal diabetes and preterm birth. Environ Res. 2019;170:160–167. doi: 10.1016/j.envres.2018.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen M., Olsen S.F., Halldorsson T.I., et al. Gestational diabetes mellitus and exposure to ambient air pollution and road traffic noise: a cohort study. Environ Int. 2017;108:253–260. doi: 10.1016/j.envint.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Liu W.Y., Lu J.H., He J.R., et al. Combined effects of air pollutants on gestational diabetes mellitus: a prospective cohort study. Environ Res. 2022;204(Pt D) doi: 10.1016/j.envres.2021.112393. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y., Li X., Benmarhnia T., et al. Exposure to air pollutant mixture and gestational diabetes mellitus in Southern California: results from electronic health record data of a large pregnancy cohort. Environ Int. 2022;158 doi: 10.1016/j.envint.2021.106888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.OuYang H., Chen B., Abdulrahman A.M., Li L., Wu N. Associations between gestational diabetes and anxiety or depression: a systematic review. J Diabetes Res. 2021;2021 doi: 10.1155/2021/9959779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McIntyre H.D., Catalano P., Zhang C., Desoye G., Mathiesen E.R., Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5(1):47. doi: 10.1038/s41572-019-0098-8. [DOI] [PubMed] [Google Scholar]
- 20.Gershon E., Dekel N. Newly identified regulators of ovarian folliculogenesis and ovulation. Int J Mol Sci. 2020;21(12):4565. doi: 10.3390/ijms21124565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choe S.A., Eliot M.N., Savitz D.A., Wellenius G.A. Ambient air pollution during pregnancy and risk of gestational diabetes in New York City. Environ Res. 2019;175:414–420. doi: 10.1016/j.envres.2019.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson A., Chiu Y.M., Hsu H.L., Wright R.O., Wright R.J., Coull B.A. Potential for bias when estimating critical windows for air pollution in children's health. Am J Epidemiol. 2017;186(11):1281–1289. doi: 10.1093/aje/kwx184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gasparrini A. Distributed lag linear and non-linear models in R: the package dlnm. J Stat Softw. 2011;43(8):1–20. [PMC free article] [PubMed] [Google Scholar]
- 24.Bastain T.M., Chavez T., Habre R., et al. Study design, protocol and profile of the maternal and developmental risks from environmental and social Stressors (MADRES) pregnancy cohort: a prospective cohort study in predominantly low-income hispanic women in urban Los Angeles. BMC Pregnancy Childbirth. 2019;19(1):189. doi: 10.1186/s12884-019-2330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P.J.T.L. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 26.Niu Z., Habre R., Chavez T.A., et al. Association between ambient air pollution and birth weight by maternal individual- and neighborhood-level Stressors. JAMA Netw Open. 2022;5(10) doi: 10.1001/jamanetworkopen.2022.38174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Diabetes A. 13. Management of diabetes in pregnancy. Diabetes Care. 2017;40(Suppl 1):S114–S119. doi: 10.2337/dc17-S016. [DOI] [PubMed] [Google Scholar]
- 28.Abatzoglou J.T. Development of gridded surface meteorological data for ecological applications and modelling. Int J Climatol. 2013;33(1):121–131. [Google Scholar]
- 29.ACOG ACOG practice bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):e49–e64. doi: 10.1097/AOG.0000000000002501. [DOI] [PubMed] [Google Scholar]
- 30.Greenland S., Pearl J., Robins J.M. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 31.Radloff L.S. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977 Jun;1(3):385–401. [Google Scholar]
- 32.Wilson A., Chiu Y.M., Hsu H.L., Wright R.O., Wright R.J., Coull B.A. Bayesian distributed lag interaction models to identify perinatal windows of vulnerability in children's health. Biostatistics. 2017;18(3):537–552. doi: 10.1093/biostatistics/kxx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen G., Sun X., Wang J., et al. The association between maternal exposure to fine particulate matter (PM2. 5) and gestational diabetes mellitus (GDM): a prospective birth cohort study in China. Environ Res Lett. 2021;16(5) doi: 10.1088/1748-9326/abe4f8. [DOI] [Google Scholar]
- 34.Zheng Y., Wen X., Bian J., Lipkind H., Hu H. Associations between the chemical composition of PM2.5 and gestational diabetes mellitus. Environ Res. 2021;198 doi: 10.1016/j.envres.2020.110470. [DOI] [PubMed] [Google Scholar]
- 35.Hu Q., Wang D., Yue D., et al. Association of ambient particle pollution with gestational diabetes mellitus and fasting blood glucose levels in pregnant women from two Chinese birth cohorts. Sci Total Environ. 2021;762 doi: 10.1016/j.scitotenv.2020.143176. [DOI] [PubMed] [Google Scholar]
- 36.Raz R., Kioumourtzoglou M.A., Weisskopf M.G. Live-birth bias and observed associations between air pollution and autism. Am J Epidemiol. 2018;187(11):2292–2296. doi: 10.1093/aje/kwy172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ha S., Sundaram R., Buck Louis G.M., et al. Ambient air pollution and the risk of pregnancy loss: a prospective cohort study. Fertil Steril. 2018;109(1):148–153. doi: 10.1016/j.fertnstert.2017.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haberzettl P., O'Toole T.E., Bhatnagar A., Conklin D.J. Exposure to fine particulate air pollution causes vascular insulin resistance by inducing pulmonary oxidative stress. Environ Health Perspect. 2016;124(12):1830–1839. doi: 10.1289/EHP212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plows J.F., Stanley J.L., Baker P.N., Reynolds C.M., Vickers M.H. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19(11):3342. doi: 10.3390/ijms19113342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinkle S.N., Buck Louis G.M., Rawal S., Zhu Y., Albert P.S., Zhang C. A longitudinal study of depression and gestational diabetes in pregnancy and the postpartum period. Diabetologia. 2016;59(12):2594–2602. doi: 10.1007/s00125-016-4086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.