Abstract
Background:
Patients with chronic lymphocytic leukemia (CLL) have an increased risk of developing second primary cancers (SPC). The aim of this study is to determine the frequency of SPC in CLL patients and determine the relationship between these cancers and their treatment status, cytogenetic factors, and other risk factors.
Methods:
The study was designed as multicenter and retroprospective. The sample comprised 553 subjects with a CLL diagnosis. Data collection commenced in August 2016, and completed at May 2021.
Results:
Fifty one of 553 patients followed for CLL, had a history of SPC. SPC development rate was 9.2%. Epithelial tumors were mostly observed. According to the incidence skin, lymphoma, renal, breast, lung, gastrointestinal system, thyroid, malignant melanoma, prostate, Kaposi’s sarcoma, neuroendocrine tumor, ovarian, larynx and salivary gland cancers were detected respectively. The 13q deletion was the most common genetic abnormality in those who developed SPC, and the frequency of 13q deletion was found to be increased statistically significant in those with malignancy, compared to those who did not.
Conclusion:
In CLL patients with SPC, the age of diagnosis, 13q and CD38 positivity, and treatment rates with fludarabine and monoclonal antibodies were found to be higher. Also, we determined that SPC frequency increased independently from hemogram values (except hemoglobin values), ß2 microglobulin level on admission, number of treatment lines, and genetic mutations other than 13q, in CLL patients. In addition, the mortality rate was higher in CLL patients with SPC and they were prone to be in advanced stages at the time of diagnosis.
Key Words: Chronic lymphocytic leukemia, epidemiology, genetic, second cancer, treatment
Introduction
Chronic lymphocytic leukemia (CLL) is defined as a clonal expansion of mature B lymphocytes. CLL is the most common hematological malignancy in adults, accounting for 25–30% of all leukemias (Johnston et al., 2009). The causes of CLL are unknown but both constitutional and environmental factors are blamed for CLL development (Yuille et al., 2000). Primary causes of death include disease progression, second primary cancers (SPC), infections, and immune deficiency (Tsimberidou et al., 2009; Yoon et al., 2012; Molica, 2005; Greene et al., 1978).
In several studies, an increased incidence of SPC in patients with CLL is reported (Callea et al., 2006; Cheson et al., 1999; Zheng et al., 2019; Morrison et al., 2002; Robak et al., 2004; Schollkopf et al., 2007; Ishdorj et al., 2019; Clarke et al., 2004). Population-based studies revealed that CLL patients have two-to-three-fold elevated risk of developing a SPC than the age and sex matched general population (Manusow and Weinerman, 1975). Even in the period of Monoclonal B cell lymphocytosis, which are considered as the early phase of CLL, it has been shown that the incidence of some non-hematological cancers increases when compared to the normal population (Solomon et al., 2012). CLL patients with SPC have poorer survival rates. CLL patients have two-to-five-fold higher risk for lymphoid malignancies, four-to-eight-fold for skin cancers, five-fold for connective tissue cancers, two-fold for lung cancers, three-fold for thyroid cancers (Tsimberidou et al., 2009; Ishdorj et al., 2019; Ries et al., 2003; Kumar et al., 2019).
In the literature, the largest retrospective studies revealed that the highest increase rates for Kaposi’s sarcoma, malignant melanoma, laryngeal cancer, and lung cancer are in CLL patients (Ishdorj et al., 2019; Ries et al., 2003; Kumar et al., 2019). The increased incidence of SPC was associated with those listed below; late effects of systemic chemotherapies are especially alkylating agents, disease/therapy related immune deficiency, inherited chromosomal abnormalities which creates tumorigenic microenvironment, lifestyle, epigenetic modifications, chronic infections (HIV), paracrine effects of leukemic cells and autoimmune diseases (Robak et al., 2004; Clarke et al., 2004; Smoller and Warnke, 1998; Harrop et al., 2021).
It is still unclear whether fludarabine and cyclophosphamide increase second primary cancer development. Different results have been obtained in this regard. Hisada et al (1979). reported that the use of nucleoside in treatment did not lead to an increase in the risk of SPC (Kumar et al., 2019). Schölkopf et al., (2007) also found similar results with Hishada et al., (1979). As result fludarabine alone is not responsible for the development of SPC, but cancers that developed in those receiving fludarabine treatment were more aggressive (Schollkopf et al., 2007). Likewise, other studies have reported aggressive clinical course of second malignancies and increased risk for lung cancer, AML, Non-Hodgkin’s lymphoma in CLL patients (Schollkopf et al., 2007; Harrop et al., 2021).
Genetic alterations in CLL are also considered a contributory factor for SPC. However, there are very limited studies in this area and not enough data with predictive value. Tsimberidou et al., (2009) examined relationship between SPC risk and frequent cytogenetic alterations of CLL and found that certain cytogenetic abnormalities (17p, 6q, or 11q deletion, trisomy 12) may be associated with an increased risk (Tsimberidou et al., 2009). Bernues et al., (2014) also found increased risk with 13q deletion in a small group.
It has been demonstrated in studies that the risk of developing SPC increases in patients with CLL. The aim of our study is to determine the frequency of SPC, type of SPC, its relationship with treatments and the conditions that may be related.
Materials and Methods
Patients
Obtained retrospectively were the data of patients diagnosed and immunophenotypically confirmed as having CLL by three centers between 2016 and 2021. Patients diagnosed with small lymphocytic lymphoma (SLL) were excluded from the study. The gender, the age of diagnosis, the disease stage, the type of SPC, the treatment history, and their cytogenetic Fluorescent In Situ Hybridization (FISH) results were noted from clinical records. ZAP-70 and CD38 status determined by the flow cytometry.
SPC
Patients who had SPC proven by biopsy during the CLL follow-up period were included in the study. Two patients (0.6%) were diagnosed with a second cancer within 3 months of their CLL diagnosis. Since these patients had lymphocytosis a year before the time of diagnosis of CLL, they remained in the study. Patients with a history of cancer before the diagnosis of CLL were excluded. Secondary lymphatic malignancies that may represent transformation of CLL were also included for the analyses. For patients with multiple malignancies, only the first cancer other than CLL was considered as SPC.
Therapy
For patients who were treated with a systemic therapy less than 6 months prior to SPC diagnosis, their SPC was assumed to not be associated with treatment. During the comparison of the number of treatment lines and treatment protocols of CLL patients with or without SPC, only the treatment lines and protocols received prior to SPC development were included.
Ethical approval was waived by the local Ethics Committee of Gulhane Educational and Research Hospital (2021/281) in the view of the retrospective nature of the study and all the procedures being performed were part of routine care.
Statistical Methods
Pairwise Comparisons
In univariate analysis of categorical and continuous variables, appropriate ones were used according to their assumptions from Chi Kare, Fisher Exact and Wilcoxon Rank-Sum tests.
Survival analysis
Survival was estimated using the Kaplan-Meier analysis, and survival between groups were compared using the bilateral log-rank test. The multivariate Cox Proportional Hazards Model was used to study risk factors related to survival.
Cancer risk analyzes
Standard incidence rates (SIR) were calculated as the ratio of observed SPC number to expected cancer number (O/E). The expected incidence of each of the SPCs in the normal population during the study period was calculated according to the age and gender of the patients using the Turkish Public Health Cancer Statistics data. For SIR, 95% confidence intervals were calculated, and p values were calculated by accepting Poisson distribution.
In calculations, type 1 error rate was accepted as alpha 0.05. Statistical analyzes were performed using R 3.5.0 (R Core Team, 2018) software.
Results
Fifty one of 553 CLL patient (9.22%) developed SPC, of whom 33 (64.7%) were male and 18 were (35.3%) female. Mean diagnosis age is 65.28±11.90 years and the average follow-up time 4.3 years. Laboratory data were given in table-1. In terms of gender and CBC values at diagnosis, no statistically significant difference was observed between those who developed SPC and those who did not, except for patients ages and hemoglobin levels.
Table 1.
Characteristics of 553 CLL Patient
| Variable Mean (SD) | CLL patients without SPC (n=502) | CLL patients with SPC (n=51) | Overall (n=553) |
p.value |
|---|---|---|---|---|
| Age (Year) | 69.29 (±11.88) | 72.78 (±10.27) | 69.61 (±11.78) | 0.026 |
| Gender (%) | ||||
| Male | 283 (56.37) | 33 (64.70) | 316 (57.14) ) | 0.3 |
| Female | 219 (43.63) | 18 (35.30) | 237 (42.86 | |
| Age Of Diagnosis (Year) | 65.00 (±11.93) | 68.05 (±11.35) | 65.28 (±11.90) | 0.073 |
| WBC (/mm3) (4,000-10,400) | 34992.46 (±40729.44) | 31845.49 (±27418.66) | 34696.89 (±39660.94) | 0.5 |
| ALC (/mm3) (1,000-4,800) | 26880.13 (±34280.93) | 24559.61 (±25331.86) | 26662.18 (±33529.97) | 0.6 |
| Hb (gr/dL) (13.5-17.5) | 13.16 (±2.02) | 12.54 (±2.05) | 13.10 (±2.03) | 0.044 |
| PLT (/mm3) (150,000-450,000) | 201835.60 (±80592.69) | 209403.90 (±83399.67) | 202546.40 (±80811.80) | 0.5 |
| β2-microglobulin (mg/L) (1.09-2.53) | 3.60 (±3.25) | 3.50 (±1.56) | 3.59 (±3.12) | 0.8 |
Sixty one CLL patients had been experiencing lymphocytosis (absolute lymphocyte count> 5,000/mm3) before the diagnosis of CLL. For these patients, the average delay in the CLL diagnosis is 2.48±1.35 years. The duration of CLL disease was 2.66±3.19 years on average when SPC was detected.
Significantly increased risks were observed respectively for malignant melanoma (MM) (O/E=8.22), renal cell carcinoma (O/E=7.25), thyroid cancer (O/E: 7.14), lymphomas (O/E=6.74), female breast (O/E: 3.17), and non-melanoma cancer of the skin (O/E:2.64). Although statistically not significant, other cancers that increased relatively were salivary gland (O/E=13.8), Kaposi sarcoma (KS) (O/E=7.25), ovarian (O/E: 4) and larynx cancer (O/E=1.74). Cancers detected at a lower rate than expected were gastrointestinal malignancies, (O/E: 0.95) lung (O/E: 0.89) and prostate cancer (O/E=0.46) (Table 2). Since neuroendocrine tumor frequency is unknown, it wasn’t included in the statistical analysis. Apart from these, two patients developed more than one SPC. In a female patient, acute myeloid leukemia possibly related to breast cancer treatment occurred during the CLL follow-up period, and a male patient was diagnosed with lung cancer after a colon cancer diagnosis.
Table 2.
Second Primary Cancer Risks of CLL Patients
| Solid Tumors | Observed (O) | Expected (E) | Standardized Incidence Ratio | p.value |
|---|---|---|---|---|
| Salivary gland | 1 | 0.07 | 13.8 | 0.070 |
| Melanoma of the skin | 2 | 0.24 | 8.22 | 0.025 |
| Kidney | 5 | 0.69 | 7.25 | 0.001 |
| Kaposi’s sarcoma | 1 | 0.14 | 7.22 | 0.129 |
| Thyroid | 4 | 0.56 | 7.14 | 0.003 |
| Lymphoma | 6 | 0.89 | 6.74 | 0.000 |
| Overian | 1 | 0.25 | 4 | 0.220 |
| Female breast | 5 | 1.58 | 3.17 | 0.022 |
| Non-melanoma cancer of the skin | 13 | 4.93 | 2.64 | 0.002 |
| Larynx | 1 | 0.58 | 1.74 | 0.438 |
| Cancer of the gastrointestinal tract (stomach and colon) | 4 | 4.22 | 0.95 | 0.609 |
| Lung, Bronchus | 5 | 5.62 | 0.89 | 0.661 |
| Prostate | 2 | 4.35 | 0.46 | 0.931 |
When the disease stages in the diagnosis were examined, there was a statistically significant increase in SPC development in RAI advanced stage (stage 3-4) and BINET advanced stage (stage C) (Table 3). As seen in Table 4, CD38 positivity and 13q deletion were found at a statistically significant higher frequency in CLL patients with SPC. There was no statistically significant difference between patients with or without SPC according to other FISH and flow cytometry results.
Table 3.
Comparison of Disease Stages at Diagnosis Patients with and without SPC
| Stage | CLL patients without SPC (n=502) | CLL patients with SPC(n=51) | Overall (n=553) | p.value | |||
|---|---|---|---|---|---|---|---|
| RAI | No | % | No | % | No | % | 0.003 |
| 0 | 135 | 26.89 | 10 | 19.61 | 145 | 26.22 | |
| 1-2 | 297 | 59.16 | 26 | 50.98 | 323 | 58.40 | |
| 3-4 | 70 | 13.95 | 15 | 29.41 | 85 | 15.38 | |
| BINET | No | % | No | % | No | % | 0.044 |
| A | 326 | 64.94 | 25 | 49.02 | 351 | 63.47 | |
| B | 109 | 21.71 | 12 | 23.53 | 121 | 21.88 | |
| C | 67 | 13.35 | 14 | 27.45 | 81 | 14.65 | |
Table 4.
Comparison of FISH and Flow Cytometry Positivity of CLL Patients with or without SPC
| Fluorescence in situ hybridization (FISH) | CLL patients without SPC No (%) | CLL patients with SPC No (%) | Overall No (%) | p.value |
|---|---|---|---|---|
| 17p deletion | 24/185 (12.97) | 3/24 (12.50) | 27/209 (12.91) | 0.3 |
| 11q deletion | 11/97 (11.34) | 0/13 (0) | 11/110 (10) | 0.2 |
| Trisomy 12 | 5/87 (5.75) | 0/12 (0) | 5/99 (5.15) | 0.4 |
| 13q deletion | 33/98 (33.67) | 9/16(56.25) | 42/114 (36.84) | 0.024 |
| ZAP70-positive | 13/87 (14.94) | 0/4 (0) | 13/91 (14.28) | 0.3 |
| CD38-positive | 55/298 (18.45) | 6/21(28.57) | 61/319 (19.12) | 0.022 |
We found that the number of previous treatment lines had no effect on the development of SPC. But alkylating agents and monoclonal antibody usage especially were more common in CLL patients with SPC, and this was statistically significant when the relationship between SPC development and treatment lines-agents received was investigated (Table 5).
Table 5.
Comparison of the Treatment Lines and Agents of CLL Patients with or without SPC
| CLL patients without SPC (n=502) | CLL patients with SPC (n=51) | Overall (n=553) | p.value | |||||
|---|---|---|---|---|---|---|---|---|
| No | % | No | % | No | % | |||
| The number of treatment lines | 0 | 392 | 78.09 | 33 | 64.71 | 425 | 76.86 | 0.2 |
| 1 | 72 | 14.34 | 13 | 25.49 | 85 | 15.37 | ||
| 2 | 24 | 4.78 | 4 | 7.84 | 28 | 5.06 | ||
| 3 | 12 | 2.39 | 1 | 1.96 | 13 | 2.35 | ||
| 4 | 2 | 0.40 | 0 | 0 | 2 | 0.36 | ||
| Drugs | Fludarabine | 65 | 12.95 | 12 | 23.53 | 77 | 13.92 | 0.027 |
| Bendamustine | 30 | 5.98 | 1 | 1.96 | 31 | 5.60 | ||
| Chlorambucil | 32 | 6.37 | 3 | 5.88 | 35 | 6.33 | ||
| Ibrutinib | 20 | 3.98 | 2 | 3.92 | 22 | 3.97 | ||
| Venetoclax | 2 | 0.39 | 0 | 0 | 2 | 0.36 | ||
| Monoclonal Antibody* | 93 | 18.52 | 16 | 31.37 | 109 | 19.71 | ||
*(Rituximab, Alemtuzumab, Obinutuzumab)
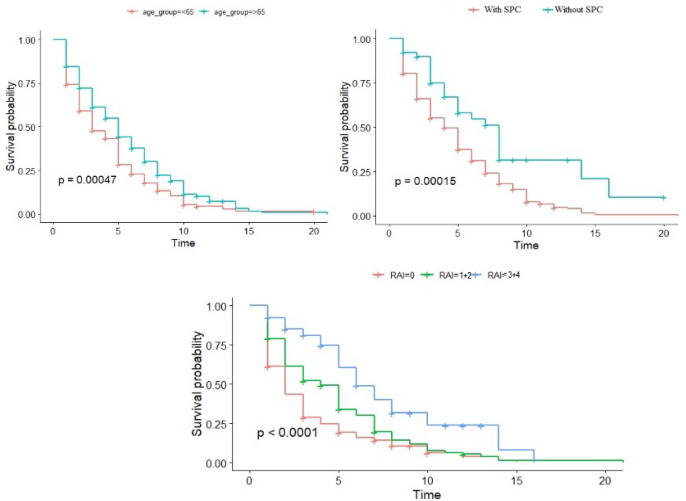
When evaluating patient groups in terms of prognosis, all causes of death were included, and it was found that mortality was higher in CLL patients with SPC (94 (18.73%) versus 25 (49.02%), p.value<0.001). Other survival data were given in the graphic.
Figure 1.
Survival Data of Patients
Discussion
In our study, there was an increase in the incidence of SPC in CLL patients compared to the general population. It was found that 9.22% of CLL patients developed a SPC on average within a 2.7 years of follow-up time. This rate is almost the same as the study by M.D. Anderson in an Australian population-based study (Tsimberidou et al., 2009; Royle et al., 2011).
Prior studies of patients with CLL have reported that various forms of skin cancer, including malignant melanoma, are the most common second cancers in patients with CLL (Greene et al., 1978; Ishdorj et al., 2019; Levi et al., 1996; Mehrany et al., 2004). We too found that the most common SPC among our patients was skin cancers; non-melanoma cancer of the skin and malignant melanoma was detected in 13 (SIR:2.64) and 2 (SIR:8.22) patients respectively. Skin cancers especially increase with age and are often associated with sunlight exposure. In addition, the increase in skin cancer incidence in immunosuppressive conditions such as organ transplantation suggests that immunosuppression as in CLL is a risk factor for skin cancers (Garcia et al., 2013). Although it has been reported that skin cancers are associated with more aggressive and increased mortality, especially in CLL patients, no mortality has been observed in our patients due to skin cancer.
Despite Kaposi’s sarcoma in only one of our patients, there is a 7.25-fold increase in incidence compared to the general population. There are only a few studies related to the frequency of Kaposi’s sarcoma in CLL patients, and an increased risk of three-to-five-fold was detected (Zheng et al., 2019; Kumar et al., 2019).
The biggest difference from the other studies is the high frequency of renal carcinoma. Although previous studies indicated an increase in the risk of renal carcinoma in the range of 1-2, a risk increase of 7.25 times was detected in five patients in our study (Schollkopf et al., 2007; Morton et al., 2010). Since there is no epidemiological study on CLL and secondary cancer in our country, no comment exists on whether this increase is related to our country. None of these patients received treatment and were diagnosed with renal carcinoma approximately 2.2 years after the diagnosis of CLL. In addition, two of these patients died due to renal carcinoma.
In our study, the second hematological malignancy developed in six of the patients, and five of them had diffuse large B cell lymphoma, (DLBCL), Hodgkin’s lymphoma, and acute myeloid leukemia in one patient. The patient who developed acute myeloid leukemia was not included in the statistical analysis due to the development of prior breast cancer. Second hematological malignancy could be due the transform of CLL into aggressive large-cell lymphoma, also known as Richter syndrome. The frequency of Richter transformation detected in our study is similar to what has been previously reported large cohort studies (Christianson et al., 2007). It is thought that prolonged immunosuppression due to treatment or CLL itself is responsible for this transformation. It has been claimed that the development of new clonal lymphoid populations or Epstein-Barrvirus (EBV) driven clones due to prolonged immunosuppression could cause this. Furthermore, it has been reported in some studies that CLL is associated with autoimmune thyroiditis. Again, the frequency of thyroid cancer has been reported to increase in some studies, but its mechanism has not been fully understood. In our study, it was found that the incidence of thyroid cancer increased at a higher rate than the rate previously reported (Schollkopf et al., 2007; Polliack and Lugassy, 1992). A slight increase in the risk of female breast and overian cancer was observed in our study after CLL. Although Schollkopf et al., (2007). reported a decreased risk of breast and gynecological malignancy after CLL, other evidence supports our finding that CLL patients may have a slight increase in the risk of female breast and ovarian cancer. While the increase in female breast cancer was statistically significant in our study, the increase in the incidence of ovarian cancer was not statistically significant.
We found a decrease in the frequency of gastrointestinal, lung and prostate cancer in CLL patients. In general, studies have reported an increase in its incidence, and smoking is also mentioned as an etiological factor (Chow et al., 1999). Schollkopf et al., (2007). notes that the observed relative risk estimate was the highest for adenocarcinoma. Although not statistically significant, salivary gland cancer was observed; in our single case it was found to be 13.8 times more common than expected. An increase in the incidence of salivary gland cancer in CLL patients has also been shown in previous large studies (Morton et al., 2010).
The most curious question is which factors contribute to the development of second cancers in CLL patients. The foremost claim is that the treatments of CLL patients could cause SPC. But the treatment approach has changed over time. History started with alkylating agents, purine nucleoside analogs and continues with targeted therapies such as monoclonal antibodies and Bruton’s tyrosine kinase (BTK) and B-cell lymphoma 2 (BCL-2) inhibitors. Another point to pondered is if the treatment has an effect on SPC, this effect would be eliminated with current treatment approaches or not. The effect of recent treatment approaches on the development of SPC has not been investigated in previous studies, and in our study, patients treated with obinuzumab, ibrutinib and venetoclax were included. However, due to the small size of the patient groups and that most of the treatment periods arelimited to one to two years, statistical subgroup analysis was not clear. However, what is clear is that the frequency of SPC is increased in CLL patients treated with fludarabine and monoclonal antibodies. Although there are studies supporting an increased risk of developing SPC after fludarabine, there are not many data reporting an increase in the frequency of SPC in patients treated with monoclonal antibodies. In our study, it was found that the number of treatment lines received by CLL patients did not have a statistically significant effect on the development of SPC. In one of the publications reporting that treatment had no effect on the development of SPC, CLL patients receiving fludarabine treatment had an increased risk of SPC compared to the healthy population, while no significant difference was found compared to other CLL patients not receiving treatment (Cheson et al., 1999). A study by Callea et al., (2006) found little evidence to suggest an association between treatment with chlorambucil for CLL and SPC.Recent publications also showed that treatment is a risk factor for the development of SPC in patients with CLL (Fürstenau et al., 2021; Cunha-Bang et al., 2021; Lenartoa et al., 2020).
The 13q deletion is the most common genetic abnormality found in CLL patients with SPC. A study by Marta et al. found that the genetic alterations including del(13)(q14.3) increases the risk of developing second cancer in patients with CLL (Bernués et al. 2014). Since 13q deletion contains BRCA2 gene, it is also common in breast cancer and prostate cancer. In our study, a genetic panel was examined in two of the four patients with breast cancer and 13q and 17p deletions were detected in one patient.
It was determined that the increased frequency of SPC was independent of the patient’s ages, hemogram values or ß2 microglobulin at the time of admission. But a study by Tsimberidou et al. found that elevated levels of ß2 microglobulin, creatinine and LDH were significantly associated with the risk of SPC (Tsimberidou et al., 2009). It was also revealed in our study that CLL patients who developed SPC were in an advanced stage at the time of admission and the mortality rate due to all causes increased.
Although it is a three-centers study, the number of patients was limited for some analyzes. This is a retrospective study, and some data were not available. One of the other limitations is that since genetic tests have especially been available in the last four-five years, the number of patients examined has decreased and the statistical evaluation has limited. Immunoglobulin heavy chain mutation could not be studied except in certain centers in our country. Because of this, it could not be included in the parameters examined in our study.
In conclusion, our study emphasizes that the risk of SPC in CLL patients is greater than the general population. Clinicians should be careful regarding SPC that may develop due to the lifelong expectancy, even if CLL is a disease of advanced age. Although there is not any screening program in terms of SPC screening in CLL patients, it is thought that it may be appropriate to evaluate the features in terms of cancers that can be evaluated by examination such as skin cancers. In addition, recommendations may be made to warn patients to report new symptoms and not to smoke in terms of general cancer development measures, and to prevent UV exposure, especially for skin cancers.
Author Contribution Statement
Selim Sayın and Murat Yıldırım contributed to the writing of the article, Emrah Kılıçaslan and Hacer Berna Afacan Öztürk contributed to the collection of patient data, Esra Şafak Yılmaz contributed to the statistical analysis, Murat Albayrak and M.Kürşat Kaptan and Meltem Aylı contributed to the determination of the subject and the final evaluation of the article..
Acknowledgements
Ethical Issue
This study has been evaluated and approved by the local Ethics Committee of Gulhane Educational and Research Hospital (2021/281)
Availability of Data and Material
Patient data is available in databases of 3 different hospitals.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Bernués M, Durán MA, Puget G, et al. Genetics of lymphocytes influences the emergence of second cancer in chronic lymphocytic leukemia. Anticancer Res. 2014;34:2311–4. [PubMed] [Google Scholar]
- Callea V, Brugiatelli M, Stelitano C, et al. Incidence of second neoplasia in patients with B-cell chronic lymphocytic leukemia treated with chlorambucil maintenance chemotherapy. Leuk Lymphoma. 2006;47:2314–20. doi: 10.1080/10428190600880977. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Vena DA, Barrett J. Second malignancies as a consequence of nucleoside analog therapy for chronic lymphoid leukemias. J Clin Oncol. 1999;17:2454–60. doi: 10.1200/JCO.1999.17.8.2454. [DOI] [PubMed] [Google Scholar]
- Chow WH, Swanson CA, Lissowska J, et al. Risk of stomach cancer in relation to consumption of cigarettes, alcohol, tea and coffee in Warsaw, Poland. Int J Cancer. 1999;81:871–6. doi: 10.1002/(sici)1097-0215(19990611)81:6<871::aid-ijc6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Christianson KM, Slager SL, Zent CS, et al. Risk factors for development of a second lymphoid malignancy in patients with chronic lymphocytic leukaemia. Br J Haematol. 2007;139:398–404. doi: 10.1111/j.1365-2141.2007.06801.x. [DOI] [PubMed] [Google Scholar]
- Clarke CA, Glaser SL. Population-based surveillance of HIV-associated cancers: Utility of cancer registry data. J Acquir Immune Defic Syndr. 2004;36:1083–91. doi: 10.1097/00126334-200408150-00012. [DOI] [PubMed] [Google Scholar]
- Cunha-Bang C, Rostgaard K, Andesen MA, et al. Risk of new malignancies among patients with CLL treated with chemotherapy: results of a Danish population-based study. Br J Haematol. 2021;193:339–45. doi: 10.1111/bjh.17337. [DOI] [PubMed] [Google Scholar]
- Fürstenau M, Giza A, Stumpf T, et al. Second primary malignancies in treated and untreated patients with chronic lymphocytic leukemia. AJH. 2021;96:457–60. doi: 10.1002/ajh.26363. [DOI] [PubMed] [Google Scholar]
- Garcia JB, Suarez-Varela MM, Vilata JJ, et al. Risk factors for non-melanoma skin cancer in kidney transplant patients in a Spanish population in the Mediterranean region. Acta Derm Venereol. 2013;93:422–7. doi: 10.2340/00015555-1525. [DOI] [PubMed] [Google Scholar]
- Greene MH, Hoover RN, Fraumeni JF. Subsequent cancer in patients with chronic lymphocytic leukemia: A possible immunologic mechanism. J Natl Cancer Inst. 1978;61:337–40. [PubMed] [Google Scholar]
- Harrop S, Polliack A, Tam CS. Chronic lymphoproliferative disorders and secondary cancers in the era of purine analogues and beyond. Leuk Lymphoma. 2021;62:771–8. doi: 10.1080/10428194.2020.1849682. [DOI] [PubMed] [Google Scholar]
- Hisada M, Biggar RJ, Greene MH et al. Solid tumors after chronic lymphocytic leukemia. Blood. 2001;98:1979–81. doi: 10.1182/blood.v98.6.1979. [DOI] [PubMed] [Google Scholar]
- Ishdorj G, Beiggi S, Nugent Z, et al. Risk factors for skin cancer and solid tumors in newly diagnosed patients with chronic lymphocytic leukemia and the impact of skin surveillance on survival. Leuk Lymphoma. 2019;60:3204–13. doi: 10.1080/10428194.2019.1620941. [DOI] [PubMed] [Google Scholar]
- Kumar V, Ailawadhi S, Bojanini L, et al. Trends in the risk of second primary malignancies among survivors of chronic lymphocytic leukemia. Blood Cancer J. 2019;9:75. doi: 10.1038/s41408-019-0237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CR, Hansen PB, Clausen NT. Aggressive growth of epithelial carcinomas following treatment with nucleoside analogues. Am J Hematol. 2002;70:48–50. doi: 10.1002/ajh.10080. [DOI] [PubMed] [Google Scholar]
- Lenartoa A, Johannesen TB, Tjonnfjord GE. Chronic lymphocytic leukemia and secondary hematological malignancies: A nation-wide cancer registry study. Eur J Haematol. 2020;104:546–53. doi: 10.1111/ejh.13396. [DOI] [PubMed] [Google Scholar]
- Levi F, Randimbison L, Te VC, La Vecchia C. Non Hodgkin’s lymphomas, chronic lymphocytic leukemias and skin cancers. Br J Cancer. 1996;74:1847–50. doi: 10.1038/bjc.1996.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manusow D, Weinerman BH. Subsequent neoplasia in chronic lymphocytic leukemia. JAMA. 1975;232:267–9. [PubMed] [Google Scholar]
- Mehrany K, Weenig RH, Pittelkow MR, Roenigk RK, Otley CC. High recurrence rates of Basal cell carcinoma after mohs surgery in patients with chronic lymphocytic leukemia. Arch Dermatol. 2004;140:985–8. doi: 10.1001/archderm.140.8.985. [DOI] [PubMed] [Google Scholar]
- Molica S. Second neoplasms in chronic lymphocytic leukemia: incidence and pathogenesis with emphasis on the role of different therapies. Leuk Lymphoma. 2005;46:49–54. doi: 10.1080/10428190400007524. [DOI] [PubMed] [Google Scholar]
- Morrison VA, Rai KR, Peterson BL, et al. Therapy-related myeloid leukemias are observed in patients with chronic lymphocytic leukemia after treatment with fludarabine and chlorambucil: Results of an Intergroup Study, Cancer and Leukemia Group B 9011. J Clin Oncol. 2002;20:3878–84. doi: 10.1200/JCO.2002.08.128. [DOI] [PubMed] [Google Scholar]
- Morton LM, Curtis RE, Linet NS, et al. Second Malignancy Risks After Non-Hodgkin’s Lymphoma and Chronic Lymphocytic Leukemia: Differences by Lymphoma Subtype. J Clin Oncol. 2010;28:4935–44. doi: 10.1200/JCO.2010.29.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polliack A, Lugassy G. Autoimmunity and auto-immune syndromes associated with and preceding the development of lymphoproliferative disorders. Leukemia. 1992;6:152–4. [PubMed] [Google Scholar]
- Ries LA, Eisner MP, Kosary C, et al. SEER cancer statistics review. 2003:1975–2000. [Google Scholar]
- Robak T, Blonski JZ, Gora-Tybor J, et al. Second malignancies and Richter’s syndrome in patients with chronic lymphocytic leukaemia treated with cladribine. Eur J Cancer. 2004;40:383–9. doi: 10.1016/j.ejca.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Royle JS, Baade P, Joske D, Fritschi L. Risk of second cancer after lymphohematopoietic neoplasm. Int J Cancer. 2011;129:910–9. doi: 10.1002/ijc.25706. [DOI] [PubMed] [Google Scholar]
- Schollkopf C, Rosendahl D, Rostgaard K, Pipper C, Hjalgrim H. Risk of second cancer after chronic lymphocytic leukemia. Int J Cancer. 2007;121:151–6. doi: 10.1002/ijc.22672. [DOI] [PubMed] [Google Scholar]
- Smoller BR, Warnke RA. Cutaneous infiltrate of chronic lymphocytic leukemia and relationship to primary cutaneous epithelial neoplasms. J Cutan Pathol. 1998;25:160–4. doi: 10.1111/j.1600-0560.1998.tb01709.x. [DOI] [PubMed] [Google Scholar]
- Solomon BM, Rabe KG, Moreira J, et al. Risk of Cancer in Patients with Clinical Monoclonal B- Cell Lymphocytosis (MBL): A Cohort Study of Newly Diagnosed Patients Compared to Controls. ASH Annual Meeting Abstracts. 2012;120:2893. [Google Scholar]
- Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904–10. doi: 10.1200/JCO.2008.17.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J-Y, Lafarge S, Dawe D, et al. Association of interleukin-6 and interleukin-8 with poor prognosis in elderly chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53:1735–42. doi: 10.3109/10428194.2012.666662. [DOI] [PubMed] [Google Scholar]
- Yuille MR, Matutes E, Marossy A, et al. Familial chronic lymphocytic leukaemia: a survey and review of published studies. Br J Haematol. 2000;109:794–9. doi: 10.1046/j.1365-2141.2000.02111.x. [DOI] [PubMed] [Google Scholar]
- Zheng G, Chattopadhyay S, Sud A, et al. Second primary cancers in patients with acute lymphoblastic, chronic lymphocytic and hairy cell leukaemia. Br J Cancer. 2019;105:1076–81. doi: 10.1111/bjh.15777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Patient data is available in databases of 3 different hospitals.