Abstract
Background:
Gastric cancer remains one of the leading causes of death, and a burden of public health in the United States (US). The aim of the study was to provide updates to gastric cancer estimates, and analyzed the long-term trends in incidence, survival, and mortality of gastric cancer in the US, which was helpful for the monitoring of the screening program and the prevention strategy.
Methods:
The incidence, and long-term trends in incidence, survival, and mortality of gastric cancer in the US from 2001 to 2015 were analyzed. The data were obtained from the Surveillance, Epidemiology, and End Results (SEER) Database. Age-adjusted incidence rates were calculated, joinpoint regression and age-period-cohort analyses were conducted. All statistical tests were 2-sided.
Results:
The overall age-adjusted incidence of gastric cancer decreased over the study period, with an annual percentage change (APC) of -1.4% (95% confidence interval [CI] = -1.1 to 13.3; P < 0.001). The incidence rates leveled off at an earlier age (< 45 years) and creased obviously with age. The age rate deviations increased sharply before the age of 47.5 years (age rate deviation = 0.92; 95% CI = 0.71 to 1.13). The 5-year mortality attributed to gastric cancer declined from 65.98% to 56.29% over the study period. The trend of 5-year mortality attributed to gastric cancer showed no significant fluctuation. The hazard ratio for 5-year all-cause death increased with cancer stage from 1.22 (95% CI = 1.13 to 1.33; P < 0.001) to 4.71 (95% CI = 4.40 to 5.06; P < 0.001).
Conclusion:
During the study period, the incidence decreased, while the survival rate increased slightly. Specially, the trend of 5-year mortality attributed to gastric cancer did not vary significantly. The data demonstrated that the prognosis of gastric cancer in the US remained challenging.
Key Words: Gastric cancer, incidence, prognosis
Introduction
Gastric cancer is an important contributor to the global burden of cancer, and it used to be the most common carcinoma in the world, less than a century ago (World Health, 2021). As estimated in 2020, the incidence rate of gastric cancer was 5.6%, ranking the fifth, and the mortality rate of gastric cancer was 7.7%, ranking the fourth (Sung et al., 2021). The epidemiological characteristics of gastric cancer were with substantial geographical heterogeneity, and the variation of incidence between high-risk and low-risk countries could reach 5-fold to 10-fold (Abengozar et al., 2021). Several risk factors contributed to the incidence of gastric cancer. Helicobacter pylori infection was one of the leading causes of gastric cancer. The other risk factors of gastric cancer included tobacco use, obesity, alcohol consumption, salt-preserved food and so on (Inoue, 2021).
The incidence and mortality rates of gastric cancer have fallen in recent years. However, this trend has shown some signs of changes; for example, some researchers believed that in the US, the incidence and mortality rates of gastric cancer might be increasing among young population (i.e., < 50 years) and they predicted that the increase might reverse the overall decline in the incidence of gastric cancer (Anderson et al., 2018). Although the survival rates had generally improved over the last decades, the prognosis remained poor (Huang et al., 2021). The 5-year survival rate was around 20%, with notable exceptions of 68.9% in Japan (Nashimoto et al., 2013) and 74.5% in South Korea (Jung et al., 2018), where population-based screening has led to effective diagnosis of tumors at an early stage (Isobe et al., 2011). Additionally, the costs of gastric cancer were generally higher than other cancers. The expenses on gastrointestinal diseases in the US has been estimated at $142 billion per year, including both direct and indirect costs (Everhart and Ruhl, 2009).
Given the poor survival rate and the considerable burden associated with gastric cancer, we evaluated the incidence, and long-term trends in incidence, survival, and mortality of gastric cancer in the US from 2001 to 2015 based on the Surveillance, Epidemiology, and End Results (SEER) Database. We highlighted the trends that can help to inform global and local interventions to lower the disease burden and, perhaps, curtail the increasing number of incident cases.
Materials and Methods
Data Source and Variables
Population-based data of cancer was available for the SEER program, which was publicly and freely accessible (https://seer.cancer.gov/). The data of patients diagnosed with gastric cancer from 2001 and 2015 were obtained from the SEER-9 Registries. The SEER-9 Registries included nine areas (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco- Oakland, Seattle-Puget Sound, and Utah) and three races (white, black, and other), covering approximately 9.4% of the US population (Scott et al., 2020). The age-adjusted incidence, survival and mortality of gastric cancer were obtained via SEER*Stat software. Due to the absence of direct access to the survival and mortality of gastric cancer, we exported the individual patient data, which included survival outcomes, and calculated the survival and mortality rates of gastric cancer. The long-term trends of the incidence, survival and mortality of gastric cancer and the differences between subgroups [including sex, age, race (white, black, and other), stage, the year of diagnosis, and survival outcome (alive, dead attributed to gastric cancer)] were evaluated in this study.
Statistical Analysis
Age-adjusted gastric cancer incidence rates could be directly calculated by SEER*Stat software (version 8.3.9). Age-adjusted incidence rates were standardized according to the US standard population in 2000. To identify changes in gastric cancer incidence rate trends, joinpoint regression was conducted for every age, sex, stage, and race by using Joinpoint software (version 4.9.0). The software calculated the annual percentage change (APC) in incidence rates between trend-change points and average annual percentage change (AAPC) in the whole period studied. t test and Z test were respectively used to test whether the APC and AAPC were statistically different from zero. All statistical tests were 2-sided. The P-value was considered statistically significant when it was less than 0.05.
To help understand the differences in gastric cancer incidence rates and trends by age, age-period-cohort analysis was conducted by using a web tool for age-period-cohort analysis (Rosenberg et al., 2014). We divided gastric cancer incidence cases extracted from the SEER-9 Registries into 5-year age groups (ages 5-9 through ≥ 85 years), 5-year calendar periods (2001-2005, 2006-2010, 2011-2015), and 5-year birth cohorts.
Cox proportional hazards models were used to estimate the effect of different predefined factors on 5-year survival, including age, sex, race, stage, tumor site, and histologic grade, by R software (version 4.0.0).
Results
Baseline Characteristics
Demographic and clinical characteristics of gastric cancer diagnosed from 2001 to 2015 in SEER-9 Registries are shown in Table 1. 32,029 patients have been diagnosed with gastric cancer during the study period. Among all cases, patients diagnosed with gastric cancer were mainly over the age of 45, and the number of patients in each age group increased with age. Patients were mainly male (61.4%) and white (68.49%). Except for the unknown stages, more than one-quarter (28.56%) of the patients were diagnosed at stage IV. 30.30% (9719/32029) of the gastric carcinomas occurred in gastric cardia. The average tumor size was 44 mm (interquartile range = 25 to 68).
Table 1.
Characteristics of Gastric Cancer Patients in SEER Registries from 2001 to 2015
| Characteristic | All gastric cancerN=32029 |
|---|---|
| Age at diagnosis, no. (%) | |
| 0-14 | 10 (0.03) |
| 15-44 | 1810 (5.64) |
| 45-54 | 3654 (11.39) |
| 55-64 | 6277 (19.57) |
| 65-74 | 7950 (24.78) |
| ≥75 | 12378 (38.59) |
| Sex no. (%) | |
| Male | 19698 (61.40) |
| Female | 12381 (38.60) |
| Race, no. (%) | |
| White | 21970 (68.49) |
| Black | 4443 (13.85) |
| Other | 5579 (17.39) |
| Unknown | 87 (0.27) |
| Stagea, no. (%) | |
| I A | 3412 (10.64) |
| I B | 2349 (7.32) |
| II | 2589 (8.07) |
| III A | 1901 (5.93) |
| III B | 466 (1.45) |
| IV | 9163 (28.56) |
| Unknown / Blank | 12199 (38.03) |
| Year of diagnosis, no. (%) | |
| 2001 | 1990 (6.20) |
| 2002 | 2073 (6.46) |
| 2003 | 2066 (6.44) |
| 2004 | 2123 (6.62) |
| 2005 | 2043 (6.37) |
| 2006 | 2106 (6.57) |
| 2007 | 2076 (6.47) |
| 2008 | 2103 (6.56) |
| 2009 | 2203 (6.87) |
| 2010 | 2140 (6.67) |
| 2011 | 2255 (7.03) |
| 2012 | 2247 (7.00) |
| 2013 | 2218 (6.91) |
| 2014 | 2203 (6.87) |
| 2015 | 2233 (6.96) |
| Tumor size(cm), median (Q25, Q75) | 44 (25, 68) |
| Primary tumor site, no. (%) | |
| Cardia | 9719 (30.30) |
| Fundus of stomach | 1494 (4.66) |
| Body of stomach | 2895 (9.02) |
| Gastric antrum | 5369 (16.74) |
| Pylorus | 713 (2.22) |
| Lesser curvature of stomach | 2304 (7.18) |
| Characteristic | All gastric cancerN=32029 |
| Primary tumor site, no. (%) | |
| Greater curvature of stomach | 1321 (4.12) |
| Overlapping lesion of stomach | 2089 (6.51) |
| Unclassified | 6175 (19.25) |
| Histologic grade, no. (%) | |
| Grade I | 1778 (5.54) |
| Grade II | 6466 (20.16) |
| Grade III | 14843 (46.27) |
| Grade IV | 704 (2.19) |
| Unknown | 8288 (25.84) |
a, The gastric cancer stage from 2004 to 2105 was grouped by 6th AJCC Stage Group Criteria (2004-2015), and stage from 2001 to 2003 stage by 3rd AJCC Stage Group Criteria (1988-2003). To ensure consistent standards and reliable results, the data from 2004-2015 were used for analysis.
Incidence Trends of Gastric Cancer
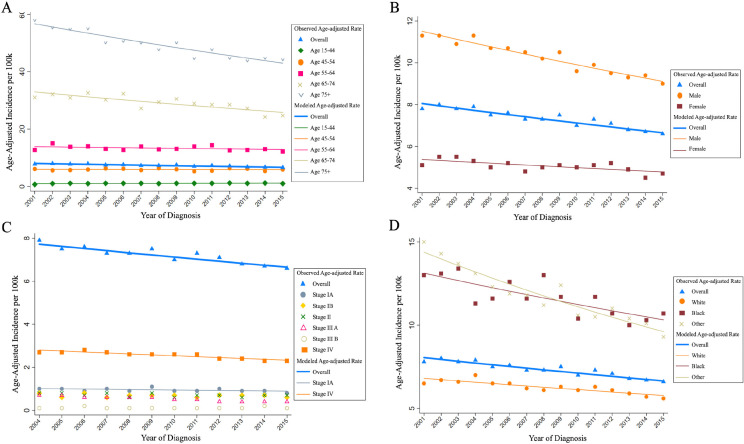
The overall and subgroups age-adjusted incidence rates of gastric cancer are shown in Supplemental Table 1. The overall age-adjusted incidence of gastric cancer decreased over the study period with an APC of -1.4% [95% CI = -1.1% to 13.3%; P < 0.001]. We further estimated the trends in different subgroups, including age, sex, stage, and race, presented in Figure 1 and Table 2.
Figure 1.
Trends in Age-Adjusted Incidence Rates of Gastric Cancer from 2001 to 2015 by Age (A), Sex (B), Stage (C) and Race (D). a The gastric cancer stage from 2004 to 2105 was grouped by 6th AJCC Stage Group Criteria (2004-2015), and stage from 2001 to 2003 stage by 3rd AJCC Stage Group Criteria (1988-2003). To ensure consistent standards and reliable results, the data from 2004-2015 were used for analysis
Table 2.
Gastric Cancer Incidence Rate Trends with Joinpoint Analyses from 2001 to 2015 (%)
| Characteristic | Years | Trend 1 | 2001-2015 AAPC | ||
|---|---|---|---|---|---|
| APC (95% CI) | P | AAPC (95% CI) | P | ||
| Overall | 2001-2015 | -1.4 (-1.1 to 13.3) | <0.001 | -1.4 (-1.1 to 13.3) | < 0.001 |
| Sex | |||||
| Male | 2001-2015 | -1.7 (-1.9 to -1.4) | <0.001 | -1.7 (-1.9 to -1.4) | < 0.001 |
| Female | 2001-2015 | -0.8 (-1.3 to -0.3) | 0.004 | -0.8 (-1.3 to -0.3) | 0.004 |
| Age, year | |||||
| 15-44 | 2001-2015 | 1.1 (-0.1 to 2.3) | 0.063 | 1.1 (-0.1 to 2.3) | 0.063 |
| 45-54 | 2001-2015 | -0.1 (-0.9 to 0.7) | 0.829 | -0.1 (-0.9 to 0.7) | 0.829 |
| 55-64 | 2001-2015 | -0.5 (-1.3 to 0.2) | 0.124 | -0.5 (-1.3 to 0.2) | < 0.100 |
| 65-74 | 2001-2015 | -1.7 (-2.4 to -1.1) | < 0.001 | -1.7 (-2.4 to -1.1) | < 0.001 |
| ≥75 | 2001-2015 | -2.0 (-2.3 to -1.6) | < 0.001 | -2.0 (-2.3 to -1.6) | < 0.001 |
| Race | |||||
| White | 2001-2015 | -1.2 (-1.6 to -0.8) | < 0.001 | -1.2 (-1.6 to -0.8) | < 0.001 |
| Black | 2001-2015 | -1.7 (-2.4 to -1.0) | < 0.001 | -1.7 (-2.4 to -1.0) | < 0.001 |
| Other | 2001-2015 | -2.8 (-3.4 to -2.3) | < 0.001 | -2.8 (-3.4 to -2.3) | < 0.001 |
| Stagea | |||||
| I A | 2001-2015 | -1.2 (-2.6 to 0.3) | 0.107 | -1.2 (-2.6 to 0.3) | 0.107 |
| I B b | — | — | — | — | — |
| II b | — | — | — | — | — |
| III A b | — | — | — | — | — |
| III B b | — | — | — | — | — |
| IV | 2001-2015 | -1.6 (-2.2 to -1.1) | < 0.001 | -1.6 (-2.2 to -1.1) | < 0.001 |
a, Th,e gastric cancer stage from 2004 to 2105 was grouped by 6th AJCC Stage Group Criteria (2004-2015), and stage from 2001 to 2003 stage by 3rd AJCC Stage Group Criteria (1988-2003). To ensure consistent standards and reliable results, the data from 2004-2015 were used for analysis; b, APC and AAPC wasn’t estimated. Joinpoint cannot records with weight variable ≤ 0.
The age-adjusted incidence rates in the 55 - 64, 65 - 74, > 75 age groups were all above the overall level and higher than those in the 15 - 44 and 45 - 64 age groups (Figure 1A). There were significant decreases in the 65 - 74, > 75 age groups (APC = -1.7% and -2.0%, respectively; P < 0.001 for both). In the > 75 age group, the incidence rate was the highest, while the decreased trend in incidence was the most obvious. The trends in other age groups leveled off during the study period (P > 0.05 for each group).
The age-adjusted incidence rates in both males and females decreased, but with a different incidence rate (Figure 1B). The incidence rate in males decreased with an APC of -1.7% per year (95% CI = -1.9% to -1.4%; P < 0.001), which was higher than that in females (APC = -0.8%; 95% CI = -1.3% to -0.3%; P = 0.004). The incidence rates in males were all about one time higher than those in females in each year.
The trend of incidence was the most evident in stage IV compared with other stage groups (Figure 1C). The incidence of the stage IV decreased with an APC of -1.6% (95% CI = -2.2% to -1.1%; P < 0.001). And the incidence rates of the stage IV were the highest from 2004 to 2015, compared with other stage groups. The incidence decreased significantly in white, black, and other population (APC = -1.2%, -1.7%, and -2.8%, respectively; P < 0.001 for each group). The incidence rates in white population were far lower than those in black and other population (Figure 1D).
To explore the age distribution of gastric cancer diagnosed varied across sex, stage, and race groups, we also calculated age-specific incidence, presented in Figure 2 and Supplemental Table 2. The incidence rates leveled off at an earlier age (< 45 years) and creased obviously with age. Although gastric cancer incidence rates generally increased with age, the trends of incidence also varied by sex (Figure 2A), race (Figure 2B), and stage (Figure 2C) groups for patients aged 80 - 84 years and ≥ 85 years. Compared with black and other population, the increase of incidence was smaller in white population. Except for the stage I A, the increases of incidence in other stage groups were smaller than before.
Figure 2.
Trends in Age-Specific Gastric Cancer Age-Adjusted Incidence Rates from 2001 to 2015 by sex (A), Stage (B) and Stage (C). The effect of age on gastric cancer incidence by age-period-cohort analysis(D). a The gastric cancer stage from 2004 to 2105 was grouped by 6th AJCC Stage Group Criteria (2004-2015), and stage from 2001 to 2003 stage by 3rd AJCC Stage Group Criteria (1988-2003). To ensure consistent standards and reliable results, the data from 2004-2015 were used for analysis
Figure 2. D illustrated the results of age-period-cohort analysis: the age rate deviations and 95% CIs for gastric cancer incidence. The age rate deviations increased sharply before the age of 47.5 years (age rate deviation = 0.92; 95% CI = 0.71 to 1.13). The age rate deviations of incidence peaked at 47.5 years old and then decreased.
Trends of 5-year Survival of Gastric Cancer
The trends of 5-year survival were analyzed by age, sex, stage, and race (as shown in Figure 3 and Supplemental Table 3). The trends of 5-year survival varied similarly across groups, including age, sex, stage, and race, that is, the 5-year survival improved slightly before 2012 when the patients diagnosed and the peaked in 2012. The 5-year survival rate of patients diagnosed between 2014 and 2015 sharply declined to 0%. Among all age groups, the 5-year survival rate of the ≥ 75 years age group was the lowest, and those for other age groups were all higher than overall 5-year survival rate (Figure 3A). For the long-term trends, the 5-year survival rates of each stage group from the lowest to the highest were IV, III B, III A, II, I B, and I A, respectively (Figure 3C). The 5-year survival by sex and race increased continuously with little fluctuation, during 2001 through 2013 diagnosed (Figure 3B and 3D). Additionally, the overall survival rates by different characteristics were shown in Supplemental Figure 1.
Figure 3.
Trends in Five-Year Survival Probability of Gastric Cancer from 2001 to 2015 by Age (A), Sex (B), Stage (C) and Race (D). a The gastric cancer stage from 2004 to 2105 was grouped by 6th AJCC Stage Group Criteria (2004-2015), and stage from 2001 to 2003 stage by 3rd AJCC Stage Group Criteria (1988-2003). To ensure consistent standards and reliable results, the data from 2004-2015 were used for analysis
Trends of 5-year Mortality Attributed to Gastric cancer
The trends of 5-year mortality attributed to gastric cancer were analyzed by age, sex, stage, and race (as shown in Figure 4 and Supplemental Table 4). The trends of 5-year mortality attributed to gastric cancer were similar in each group and declined slowly over the period by age, sex, stage, and race. The 5-year mortality for the 75 years age group was the highest among all age groups (Figure 4A). The 5-year mortality in males was a little higher than that in females (Figure 4B). The distribution of 5-year mortality in stage was obviously related to the stage (Figure 4C). The 5-year mortality rates from the highest to the lowest were in stage IV, III B, III A,II, I B, and I A, respectively. The 5-year mortality in white population was relatively higher than those in black and other population (Figure 4D).
Figure 4.
Trends in Five-Year Mortality Attributed to Gastric Cancer from 2001 to 2015 by Age (A), Sex (B), Stage (C) and Race (D). a The gastric cancer stage from 2004 to 2105 was grouped by 6th AJCC Stage Group Criteria (2004-2015), and stage from 2001 to 2003 stage by 3rd AJCC Stage Group Criteria (1988-2003). To ensure consistent standards and reliable results, the data from 2004-2015 were used for analysis
The effect of different characteristics on 5-year all-cause mortality was analyzed by Cox regression (Table 3). The hazard ratio (HR) of 5-year all-cause mortality increased with age, but not statistically significant. The 5-year all-cause mortality in females was lower than that in males (HR = 0.91; 95% CI = 0.88 to 0.95; P < 0.001). Among stage groups, taking stage I A as the reference, the HR for 5-year all-cause mortality increased with stage from 1.22 (95% CI = 1.13 to 1.33; P < 0.001) to 4.71 (95% CI = 4.40 to 5.06; P < 0.001). Over the study period, the HR of death leveled off with the year. From 2004 to 2013, the HR of death decreased slightly, ranging from 0.58 to 0.68 (P < 0.001 for each group). Compared with the white population, the HR of the black population (HR = 1.12; 95% CI = 1.06 to 1.18; P < 0.001) was higher, while the HR of other population (HR = 0.86; 95% CI = 0.82 to 0.91; P < 0.001) was lower. Compared with tumors in gastric cardia, the HR of tumors at other sites varied similarly, ranging from 0.74 to 0.91 (P < 0.05 for each group). Taking grade I as the reference, the HR for other histologic grades ranged from 1.29 to 1.59 (P < 0.001 for each group).
Table 3.
Multivariable-Adjusted Hazard Ratios of the Influence Factors of 5-Year All-Cause Mortality from 2001 to 2015 in Cox Proportional Hazards Model
| Variable | N, (%) | 5-year mortality, (%) | Hazard ratio | P value |
|---|---|---|---|---|
| Age, year | ||||
| 0-14 | 10 (0.03) | 6 (60.00) | Ref | |
| 15-44 | 1810 (5.64) | 1316 (72.71) | 1.15 (0.37, 3.58) | 0.812 |
| 45-54 | 3654 (11.39) | 2665 (72.93) | 1.16 (0.37, 3.59) | 0.803 |
| 55-64 | 6277 (19.57) | 4671 (74.41) | 1.24 (0.40, 3.84) | 0.714 |
| 65-74 | 7950 (24.78) | 6175 (77.67) | 1.47 (0.47, 4.56) | 0.507 |
| ≥75 | 12378 (38.59) | 10811 (87.34) | 2.39 (0.77, 7.41) | 0.133 |
| Sex | ||||
| Male | 19698 (61.40) | 16076 (81.61) | Ref | |
| Female | 12381 (38.60) | 9568 (77.28) | 0.91 (0.88, 0.95) | <0.001 |
| Stagea | ||||
| I A | 3412 (10.64) | 2140 (62.72) | Ref | |
| I B | 2349 (7.32) | 1567 (66.71) | 1.22 (1.13, 1.33) | <0.001 |
| II | 2589 (8.07) | 1981 (76.52) | 1.62 (1.50, 1.75) | <0.001 |
| III A | 1901 (5.93) | 1566 (82.38) | 2.04 (1.87, 2.21) | <0.001 |
| III B | 466 (1.45) | 414 (88.84) | 2.60 (2.31, 2.93) | <0.001 |
| IV | 9163 (28.56) | 8928 (97.43) | 4.71 (4.40, 5.06) | <0.001 |
| Unknown | 12199 (38.03) | 9048 (74.17) | 1.23 (1.14, 1.34) | <0.001 |
| Year of diagnosis | ||||
| 2001 | 1990 (6.20) | 1584 (79.60) | Ref | |
| 2002 | 2073 (6.46) | 1639 (79.06) | 1.04 (0.94, 1.15) | 0.452 |
| 2003 | 2066 (6.44) | 1623 (78.56) | 0.97 (0.87, 1.07) | 0.539 |
| 2004 | 2123 (6.62) | 1658 (78.10) | 0.63 (0.56, 0.71) | <0.001 |
| 2005 | 2043 (6.37) | 1634 (79.98) | 0.68 (0.61, 0.76) | <0.001 |
| 2006 | 2106 (6.57) | 1597 (75.83) | 0.61 (0.54, 0.68) | <0.001 |
| 2007 | 2076 (6.47) | 1605 (77.31) | 0.65 (0.58, 0.73) | <0.001 |
| 2008 | 2103 (6.56) | 1584 (75.32) | 0.60 (0.54, 0.68) | <0.001 |
| 2009 | 2203 (6.87) | 1652 (74.99) | 0.59 (0.53, 0.67) | <0.001 |
| 2010 | 2140 (6.67) | 1603 (74.91) | 0.63 (0.57, 0.71) | <0.001 |
| 2011 | 2255 (7.03) | 1700 (75.39) | 0.60 (0.53, 0.67) | <0.001 |
| 2012 | 2247 (7.00) | 1624 (72.27) | 0.58 (0.52, 0.65) | <0.001 |
| 2013 | 2218 (6.91) | 1705 (76.87) | 0.63 (0.57, 0.71) | <0.001 |
| 2014 | 2203 (6.87) | 2203 (100.00) | 0.92 (0.83, 1.03) | 0.142 |
| 2015 | 2233 (6.96) | 2233 (100.00) | 1.08 (0.98, 1.21) | 0.136 |
| Race | ||||
| White | 21970(68.49) | 17743 (80.76) | Ref | |
| Black | 4443(13.85) | 3556 (80.04) | 1.12 (1.06, 1.18) | <0.001 |
| Other | 5579(17.39) | 4294 (76.97) | 0.86 (0.82, 0.91) | <0.001 |
| Unknown | 87(0.27) | 0.55 (0.36, 0.84) | 0.006 | |
| Tumor size, mm | — | — | 1.00 (1.00, 1.00) | 0.016 |
| Primary tumor site | ||||
| Cardia | 9719 (30.30) | 8188 (84.25) | Ref | |
| Fundus of stomach | 1494 (4.66) | 1086 (72.69) | 0.88 (0.80, 0.96) | 0.003 |
| Body of stomach | 2895 (9.02) | 2179 (75.27) | 0.81 (0.76, 0.87) | <0.001 |
| Gastric antrum | 5369 (16.74) | 4165 (77.57) | 0.77 (0.73, 0.81) | <0.001 |
| Pylorus | 713 (2.22) | 574 (80.50) | 0.89 (0.79, 0.99) | 0.033 |
| Lesser curvature of stomach | 2304 (7.18) | 1684 (73.09) | 0.74 (0.70, 0.79) | <0.001 |
| Greater curvature of stomach | 1321 (4.12) | 910 (68.89) | 0.76 (0.70, 0.83) | <0.001 |
| Variable | N, (%) | 5-year mortality, (%) | Hazard ratio | P value |
| Primary tumor site | ||||
| Overlapping lesion of stomach | 2089 (6.51) | 1795 (85.93) | 0.91 (0.85, 0.99) | 0.021 |
| Unclassified | 6175 (19.25) | 5063 (81.99) | 0.86 (0.81, 0.92) | <0.001 |
| Histologic grade | ||||
| Grade I | 1778 (5.54) | 1039 (58.44) | Ref | |
| Grade II | 6466 (20.16) | 5044 (78.01) | 1.29 (1.19, 1.41) | <0.001 |
| Grade III | 14843 (46.27) | 12750 (85.90) | 1.59 (1.47, 1.73) | <0.001 |
| Grade IV | 704 (2.19) | 565 (80.26) | 1.49 (1.31, 1.70) | <0.001 |
| Unknown | 8288 (25.84) | 6246 (75.36) | 1.26 (1.16, 1.38) | <0.001 |
Discussion
Gastric cancer is an important cause of morbidity and mortality in many parts or the world. According to the study, the decrease in incidence was observed in the US from 2001 to 2015, which were in accordance with the global trend. The overall incidence of gastric cancer decreased from 7.8% to 6.6% with an APC of -1.4%. The APC of the decrease in the US was lower than in most countries of the world (Scott et al., 2020). The epidemiological characteristics of incidence over the study period were in accordance with previous global reports (Karimi et al., 2014; Colquhoun et al., 2015; Scott et al., 2020): the incidence rates increased continuously with age before 84 years old, either in overall, sex, stage, and race. The incidence rates in males were about one time higher than those in females, and the decrease of incidence in males was also higher than in females. Most of the gastric cancer patients were in advanced stages when diagnosed. The incidence rates of stage IV were higher than those in other stages. The incidence rates of black and other population were 1.5 - 2 folds of the white. Additionally, 30.3% of the gastric carcinomas were in cardia, while 69.7% were non-cardia located.
The decreasing trend of gastric cancer incidence and mortality in most populations was due to the falling rates of non-cardia gastric cancer, which has been linked to the decline of H. pylori infection rates. (Etemadi et al., 2020) H. pylori was a known carcinogen for non-cardia gastric cancer, which most adults probably once infected during their life course (de Martel et al., 2013). From 1990 to 2020, gastric cancer dropped from the fourth leading incident cancer worldwide to the fifth, and from the second leading cause of cancer deaths to the fourth (following lung, colorectal, and breast cancers) (Sung et al., 2021). As a result, gastric cancer accounted for the fourth highest mortality cancer, however, this decline in burden relative to other cancers and the dramatic decline in age-standardized rates might not necessarily led to a lower burden of gastric cancer on the health systems in high-risk countries. That is due to the changes in the age structure and growth of the population, which means that numbers of incident cases and deaths of gastric cancer have continued to increase in many locations (Etemadi et al., 2020). Thus, a further decrease in the absolute number of cases and deaths could be possible if the rates in east Asia, where almost half of the incident cases and deaths occur, are further reduced (Etemadi et al., 2020). However, the increasing trend of incidence occurred in the younger population (age ≤ 44 years). Obesity has been confirmed to be a risk factor and related to the increasing incidence rates in western population (Petryszyn et al., 2020). The prevalence rates of H. pylori infection have decreased notably in the younger populations in most parts of the world. The increase in obesity among the younger population may be one of the main causes of the increasing trend in the younger population.
In previous literature (Smith et al., 2013; Li, 2020; Scott et al., 2020; Wong et al., 2021), the researchers used the age of 40 or 50 years as the cutoff point to analyze the incidence risk of gastric cancer in subgroups. They also used this cutoff point to design the screening strategy for early gastric cancer. In this study, the results of age-period-cohort analyses may support to take the age of 47.5 years as the exact age cutoff point adjusting period and cohort. Our findings were generally in accordance with those recommendation for cutoff point and it can help to provide guidance for more accurate screening and prevention for gastric cancer.
The prognosis of gastric cancer in the US remained challenge. The 5-year survival ranged from 20% to 30%, with a little increase in recent years. Compared with the 5-year survival of 20% in most regions of the world (Kim et al., 2016), there was no gap in 5-year survival in the US. On the contrary, the 5-year survival was higher in high-incidence regions (for example, the survival in Japan and Korea approximately achieved 70%) (Nashimoto et al., 2013; Jung et al., 2018). The prognosis in high-incidence countries have improved significantly due to the adoption of population-based mass screening, which makes it possible for more and more patients to be diagnosed and treated at an early stage. In contrast, the incidence of gastric cancer when diagnosed at stage I A or I B ranged from 1.4% to 1.8% in the US.
The prognosis of gastric cancer strongly depended on the stage when diagnosed. The 5-year survival and mortality rates distributed differently among stage groups. The prognosis of gastric cancer could be worse when diagnosed in advanced stage, comparing with in early stage. In all stage groups, the incidence in stage IV was the highest, ranging from 2.3% to 2.8%, while the 5-year survival in stage IV was the lowest, ranging from 2.09% to 4.06%. On one hand, the early stage of gastric cancer generally present with minimal or no symptoms; on the other hand, the incidence of H. pylori infection and gastric cancer was stably low, the awareness of population was insufficient, and a national population-based screening program has not been established in the US. Those reasons resulted in the fact that the patients with gastric cancer in the US were more likely to be diagnosed at an advanced stage. That may provide one possible explanation for the current condition of little improvement in the prognosis in the US, although treatment has been greatly improved. Application of three-stage prevention was an effective strategy for reducing mortality and improving survival rate. The primary prevention was the test and eradication of H. pylori. The secondary prevention was endoscopic screening in at-risk populations. The population-based screening has been proved to be cost-effective in high-incidence countries (i.e., Korea and Japan), but in the US, which was a low-incidence country, it was thought costly. (Casamayor et al., 2018) The cost-effective of population-based screening in the US was controversial and needed to be further studied.
Particularly, it was found that the 5-year survival of cases diagnosed in 2014 and 2015 was zero. However, the 5-year mortality rates attributed to gastric cancer of cases diagnosed in 2014 and 2015 decreased lightly, which were 61.46% and 58.69%, respectively. It indicated that most of the deaths of the cases were caused by other reasons, instead of gastric cancer. In 2019 and 2020, the event with the greatest risk to health and life was the outbreak of COVID-19 pandemic. It was assumed that the condition of zero 5-year survival was related to the COVID-19 pandemic. Cancer patients with COVID-19 co-infection were at an increased risk of mortality. Data showed that gastrointestinal cancer were with the top three pooled COVID-19 in-hospital all-cause mortality rate (Venkatesulu et al., 2021).
For resectable gastric cancer, guidelines recommended endoscopic resection at an early stage. Additionally, guidelines recommended chemotherapy as the first-line therapy for advanced gastric cancer. For patients with stage II or III gastric cancer, guidelines recommended tumor resection/lymphadenectomy D2, and adjuvant chemotherapy. For resectable gastric cancer at stage IV, perioperative chemotherapy was recommended (Casamayor et al., 2018). Our study demonstrated that the decrease in mortality occurred in gastric cancer patients diagnosed at an early stage, which could likely reflect the improvements in curative treatment modalities, while the 5-year mortality attributed to gastric cancer remained at high levels (ranging from 56.29% to 65.98%) and declined slowly. Therefore, it was necessary to enhance the monitoring to improve the prognosis of gastric cancer, and increase investments to seek for better treatment methods.
In conclusion, the incidence of gastric cancer has been decreasing from 2001 to 2015. However, most of the patients with gastric cancer were still diagnosed at an advanced stage. Even though slight improvements of the prognosis of gastric cancer have been made, it was still far from satisfaction. In order to improve the prognosis of the gastric cancer and lower the burden of disease, it is necessary to establish a national, population-based screening program and prevention strategy. Future iterations of the research by providing annual updates to regional and country-level gastric cancer estimates, would be helpful for the monitoring of the screening program and the prevention strategy.
Author Contribution Statement
Concept and Design, D.X.C.; Methodology, C.Y.L.; Statistic Analysis, C.Y.L, D.X.C.; Writing Original Draft, C.Y.L., Review and Editing, D.X.C., H.Y.; Supervision, H.Y.
Acknowledgements
Availability of Data
The data used in current study are observed from SEER database (https://seer.cancer.gov/) which is a public database.
Conflict of Interest
The authors declare that there are no conflicts of interest associated with this article.
References
- Abengozar R, Sharma A, Sharma R. Gastric cancer: lessons learned from high-incidence geographic regions. J Gastrointest Oncol. 2021;12:350–60. doi: 10.21037/jgo-2019-gi-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WF, Rabkin CS, Turner N, et al. The Changing Face of Noncardia Gastric Cancer Incidence Among US Non-Hispanic Whites. J Natl Cancer Inst. 2018;110:608–15. doi: 10.1093/jnci/djx262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamayor M, Morlock R, Maeda H, et al. Targeted literature review of the global burden of gastric cancer. Ecancermedicalscience. 2018;12:883. doi: 10.3332/ecancer.2018.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun A, Arnold M, Ferlay J, et al. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut. 2015;64:1881–8. doi: 10.1136/gutjnl-2014-308915. [DOI] [PubMed] [Google Scholar]
- de Martel C, Forman D, Plummer M. Gastric cancer: epidemiology and risk factors. Gastroenterol Clin North Am. 2013;42:219–40. doi: 10.1016/j.gtc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Etemadi A, Safiri S, Sepanlou SG, et al. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol. 2020;5:42–54. doi: 10.1016/S2468-1253(19)30328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. 2009;136:376–86. doi: 10.1053/j.gastro.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Huang RJ, Epplein M, Hamashima C, et al. An Approach to the Primary and Secondary Prevention of Gastric Cancer in the United States. Clin Gastroenterol Hepatol. 2021:2021. doi: 10.1016/j.cgh.2021.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M. Public Health Interventions for Gastric Cancer Control. Gastrointest Endosc Clin N Am. 2021;31:441–9. doi: 10.1016/j.giec.2021.03.002. [DOI] [PubMed] [Google Scholar]
- Isobe Y, Nashimoto A, Akazawa K, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011;14:301–16. doi: 10.1007/s10120-011-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KW, Won YJ, Kong HJ, et al. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2015. Cancer Res Treat. 2018;50:303–16. doi: 10.4143/crt.2018.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–13. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GH, Liang PS, Bang SJ, et al. Screening and surveillance for gastric cancer in the United States: Is it needed? Gastrointest Endosc. 2016;84:18–28. doi: 10.1016/j.gie.2016.02.028. [DOI] [PubMed] [Google Scholar]
- Li J. Gastric Cancer in Young Adults: A Different Clinical Entity from Carcinogenesis to Prognosis. Gastroenterol Res Pract. 2020;2020:9512707. doi: 10.1155/2020/9512707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashimoto A, Akazawa K, Isobe Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1–27. doi: 10.1007/s10120-012-0163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryszyn P, Chapelle N, Matysiak-Budnik T. Gastric Cancer: Where Are We Heading? Dig Dise. 2020;38:280–5. doi: 10.1159/000506509. [DOI] [PubMed] [Google Scholar]
- Rosenberg PS, Check DP, Anderson WF. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2014;23:2296–302. doi: 10.1158/1055-9965.EPI-14-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AR, Stoltzfus KC, Tchelebi LT, et al. Trends in Cancer Incidence in US Adolescents and Young Adults, 1973-2015. JAMA Netw Open. 2020;3:e2027738. doi: 10.1001/jamanetworkopen.2020.27738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AW, Bellizzi KM, Keegan THM, et al. Health-Related Quality of Life of Adolescent and Young Adult Patients With Cancer in the United States: The Adolescent and Young Adult Health Outcomes and Patient Experience Study. J Clin Oncol. 2013;31:2136–45. doi: 10.1200/JCO.2012.47.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Venkatesulu BP, Chandrasekar VT, Girdhar P, et al. A Systematic Review and Meta-Analysis of Cancer Patients Affected by a Novel Coronavirus. JNCI Cancer Spectr. 2021;5:pkaa102. doi: 10.1093/jncics/pkaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MCS, Huang J, Lok V, et al. Differences in Incidence and Mortality Trends of Colorectal Cancer Worldwide Based on Sex, Age, and Anatomic Location. Clin Gastroenterol Hepatol. 2021;19:955–66. doi: 10.1016/j.cgh.2020.02.026. [DOI] [PubMed] [Google Scholar]
- World Health O. World health statistics 2021: monitoring health for the SDGs, sustainable development goals, Geneva. World Health Organization; 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in current study are observed from SEER database (https://seer.cancer.gov/) which is a public database.