Abstract
Background:
This study aimed to determine whether nutritional education, from the preoperative to postoperative period, and nutritional management designed to improve nutritional status alone, could improve patients’ health-related self-management and nutritional management skills during the postoperative period.
Methods:
We evaluated 101 hospitalised patients with oesophageal cancer who underwent surgery between 2015 and 2016 and received perioperative nutritional education (PERIO-N). The control group included 52 patients who underwent surgery between 2014 and 2015 and were supported only by normal interventions according to the Enhanced Recovery After Surgery protocol. The PERIO-N group paid specific attention to nutrition risk screening, nutrition assessment, nutrition monitoring, and lifestyle education.
Results:
The patients in the PERIO-N group were 1.8 times more likely to be able to consume food orally than the control group (p=0.010). In the PERIO-N group, 50.5% of the patients could orally consume food, 42.6% received a combination of oral and enteral nutrition, and 6.9% only underwent enteral nutrition. In comparison, in the control group, 28.8% of the patients could orally consume food, 53.8% received a combination of oral and enteral nutrition, and 17.3% were only administered enteral nutrition (p=0.004). In addition, patients in the PERIO-N group were discharged at a 1.5 times higher rate than those in the control group (p=0.027). The readmission rate for malnutrition within 3 months was 4% in the PERIO group (5.4% for home discharge only) and 5.8% in the control group (10.5% for home discharge only) (p=0.61).
Conclusion:
This study found that perioperative nutrition education in patients who underwent oesophageal cancer surgery led to increase in the amount of oral intake at discharge. Moreover, the group that received nutrition education did not have an increased probability of hospitalisation due to the risk of malnutrition within 3 months after discharge.
Key Words: Perioperative management, nutritional education, oesophageal cancer
Introduction
Patients with cancer often experience malnutrition (Sharma et al., 2015). Patients with oesophageal cancer are particularly vulnerable to malnutrition (Anandavadivelan and Lagergren, 2016). In Japan, gastric or jejunal reconstruction is the gold standard for managing complications after oesophagectomy for squamous cell carcinoma. This results in reduced space in the gastrointestinal tract and poses a risk in these patients for dumping syndrome, anorexia, and malnutrition. Even when preoperative nutritional status was nearly ideal, there were significant differences in hospital discharge time and the amount of oral intake after surgery. In addition, enteral nutrition is used in the early postoperative period to prevent malnutrition after oesophageal cancer surgery (Weijs et al., 2015). However, long-term enteral nutrition decreases patient quality of life (QOL) unless oral intake increases. The optimal approach to increase postoperative oral intake or improve nutritional status in patients while maintaining their QOL remains unclear.
Many cancer patients are vulnerable to poor nutritional status and may benefit from nutrition education (Melnic et al., 2022). The application value of self-transcendence theory combined with comprehensive nursing care under oncological nutritional education is more significant in elderly patients with gastric cancer. This is more helpful in enhancing symptoms and nutritional status and controlling the incidence of malnutrition. (Cui et al., 2022)
Therefore, this study aimed to determine whether nutritional education, from the preoperative to postoperative period, and nutritional management designed to improve nutritional status alone could improve patients’ health-related self-management and nutritional management skills during the postoperative period. We also aimed to assess whether preoperative and perioperative nutritional education could be more effective than ERAS-only nutritional management in increasing postoperative oral intake. We also aimed to determine the incidence of subsequent undernutrition after increasing the amount of oral intake. Therefore, we conducted this controlled historical study of 155 patients with oesophageal cancer who were indicated for surgery.
Materials and Methods
Participants
Patients were recruited from the Okayama University Hospital for this historically controlled study. The study included consecutive patients with oesophageal cancer who underwent video-assisted thoracic surgery between April 2015 and December 2016 and received perioperative nutritional education (PERIO-N). The control group comprised patients who underwent video-assisted thoracic surgery between April 2014 and March 2015 and were supported only by normal interventions according to the ERAS protocol. Patients who expired in the hospital were excluded from the study. The study was designed and conducted per the Declaration of Helsinki and approved by the Okayama University Hospital Ethics Committee (ID:1706-029). An opt-out method was used.
Protocol design
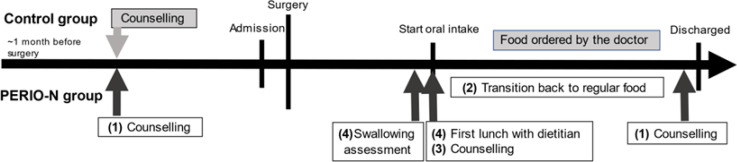
Based on the different nutrition intervention methods, we divided the patients into the PERIO-N and control groups. Both groups received the intervention for a month before surgery. A dietician specialising in perioperative nutritional management was involved in managing the PERIO-N group. Specific attention was paid to nutritional risk screening, assessment, monitoring, and lifestyle education. We implemented the dietitian program in April 2015. The control period was defined as before this implementation, after which the PEIRO-N period was implemented. Specifically, the dietitian implemented four important changes from those in the control group (Figure 1). First, we focused on preoperative interventions to promote good behaviour, create positive postoperative lifestyle changes, and promote self-management of daily life using a self-management notebook. Second, after surgery, we shortened the transition time to regular food intake and started patients on porridge immediately instead of thin gruel. Third, we provided nutritional counselling for the patients and their families according to the patient’s characteristics and level of consciousness. Fourth, the dietitian was present during the swallowing examination. The surgeons, dentists, and dietitians discussed suitable food textures to prevent aspiration pneumonia. The dietitian was present for the first postoperative lunch to observe the patient’s eating habits, communicate with the patient frequently, and manage the risk of nutrition-related complications. In contrast, the control group underwent a normal intervention following the ERAS protocol that physicians managed.
Figure 1.
Intervention Bar Chart
Data collection
The primary outcome was the success rate of nutritional management determined by the amount of oral intake at discharge from the hospital. The definition of successful oral nutritional management implied that the patients could be managed without enteral nutrition. The success rate of home discharge was also assessed. Home discharge was defined as spending the postoperative period at home without being transferred to a facility or convalescent hospital.
The secondary outcome was the incidence of complications. We classified the incidence of postoperative complications using the Clavien–Dindo classification. Grade 1 or higher was defined as having complications.
The readmission rate for malnutrition within 3 months after hospital discharge was also assessed. All data were collected from the patient case records. Data from the study participants were collected upon admission. The data obtained through an interview were regarding the dates on which patients lived alone. Body mass index (BMI) was calculated by the formula BMI = kg/m². Serum albumin was measured in the peripheral blood. The cancer stage was the preoperative diagnosis using the Union for International Cancer ControlTNM Classification of Malignant Tumours.
Statistical analysis
Data were analysed using Statistical Package for the Social Sciences Statistics 20.0 package for Windows (IBM Japan, Tokyo, Japan). Values were expressed as the mean ± standard deviation. Epidemiological analyses were performed using the chi-square test. A p-value < 0.05 was considered statistically significant. A logistic regression model was used to calculate the odds ratios (OR), with 95% confidence intervals (CI), for discharge by oral intake only. It was adjusted for intervention, age, stage, recurrent nerve paralysis, pneumonia, and leakage.
Sample size
We followed the standard methods to estimate the sample size for multiple logistic regression, with at least 10 outcomes needed for each independent variable. With an expected oral intake success rate of 43%, 153 patients were required to perform a multiple logistic regression analysis of six variables appropriately.
Results
Baseline patient characteristics
After excluding two deaths, 153 patients with oesophageal cancer were enrolled in this study (Figure 2), from whom demographic data were collected. There were two in-patient hospital deaths (mortality, 1.29%). The demographic data of the study participants are presented in Table 1. Postoperative complications and length of hospital stay are shown in Table 2. Only leakage was significantly different between the two groups. No other significant differences were observed between the PERIO-N and control groups. The median length of hospital stay was 25 days in the PERIO-N group and 22 days in the control group. The median length of postoperative stay was 20 days in the PERIO-N group and 18 days in the control group.
Figure 2.
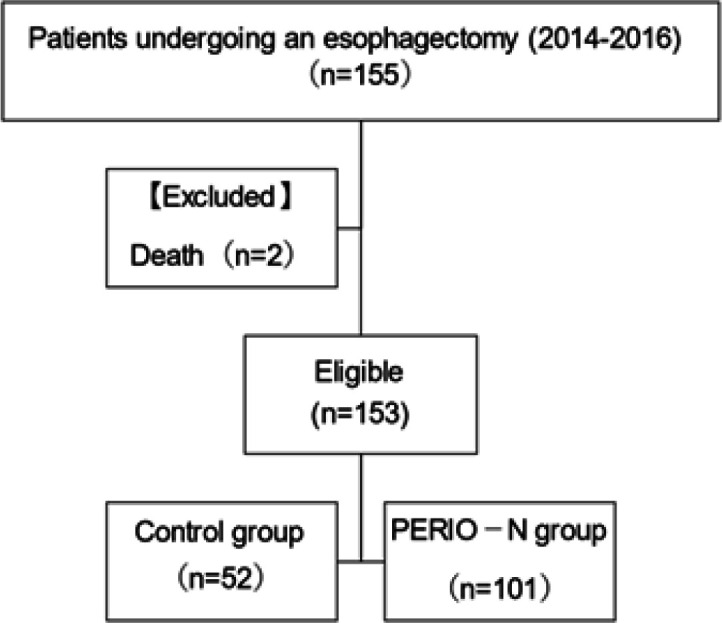
Participant Flow
Table 1.
Participant Demographics
| PERIO-N (n=101) |
Control (n=52) |
p-value | |
|---|---|---|---|
| Sex (Male : Female) | 80:21 | 42:10 | 0.820 |
| Age (years) | 64.2±8.6 | 65.0±8.1 | 0.540 |
| Body Mass Index (kg/m2) | 21.9±2.8 | 22.3±3.4 | 0.453 |
| Serum Albumin (g/dl) | 3.9±0.4 | 4.0±0.4 | 0.317 |
| Stage ( 0 : 1 : 2 : 3 : 4 ) | 3:38:29:32:10 | 0:23:14:14:1 | 0.067 |
| Living alone (%) | 12.9 | 9.6 | 0.554 |
| Route of reconstruction (Antethoracic route: Posterior mediastinal route: Retrosternal route) |
8:13:80 | 10:3:39 | 0.797 |
| Organ for substitution (Stomach:jejunum) | 92:9 | 46:6 | 0.605 |
| neoadjuvant chemotherapy (%) | 62.4 | 48.1 | 0.090 |
| preoperative radiotherapy (%) | 2.0 | 2.0 | 0.981 |
| Comorbidities (%) | |||
| Heart disease | 14.9 | 5.8 | 0.099 |
| Diabetes | 19.8 | 11.5 | 0.197 |
| Hypertension | 43.6 | 36.5 | 0.403 |
| Kidney disease | 2.0 | 2.0 | 0.981 |
| Liver disease | 8.9 | 0 | 0.026 |
Table 2.
Length of Hospital Stay and Complication Incidence
| PERIO-N (n=101) |
Control (n=52) |
p-value | |
|---|---|---|---|
| Length of Hospital Stay (days) | 28.6±13.6 | 28.6±17.6 | 0.914 |
| Leakage (%) | 9.9 | 27.0 | 0.006 |
| Chylothorax (%) | 3.0 | 0.0 | 0.209 |
| Recurrent nerve paralysis(%) | 28.8 | 26.7 | 0.816 |
| Pneumonia (%) | 15.4 | 13.9 | 0.696 |
Outcomes
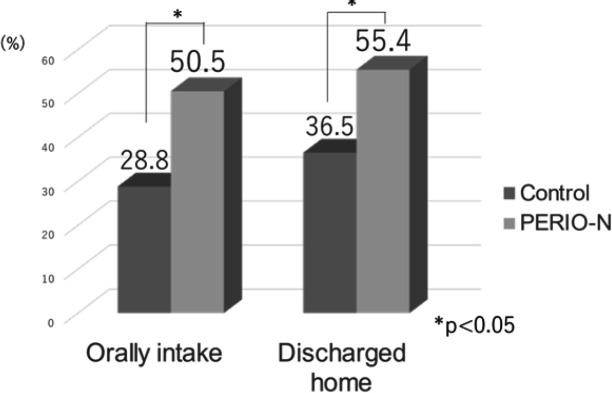
The patients in the PERIO-N group were 1.8 times more likely to tolerate oral intake of food than those in the control group (p=0.010) (Figure 3). In the PERIO-N group, 50.5% of the patients could orally intake food, 42.6% received a combination of oral food intake and enteral nutrition, and 6.9% underwent only enteral nutrition. In comparison, in the control group, 28.8% of the patients could orally intake food, 53.8% received a combination of oral and enteral nutrition, and 17.3% underwent only enteral nutrition (p=0.004).
Figure 3.
Main Outcome
The patients in the PERIO-N group were discharged at a 1.5 times higher rate than those in the control group (p=0.027) (Figure 3). Additionally, 77.3% of the patients discharged home could orally intake food. Still, only 10.3% of discharged patients could orally intake food. The readmission rate for low malnutrition within 3 months was 4% in the PERIO-N group (5.4% for home discharge only) and 5.8% in the control group (10.5% for home discharge only) (p=0.61).
In the PERIO-N group, 58 patients (57.4%) did not develop postoperative complications. In the control group, 25 (48.1 %) patients did not develop complications. In particular, complications associated with oral food intake included pneumonia. The PERIO-N group had pneumonia (13.9%), but only 4.0% of the patients developed pneumonia after starting oral food intake. In contrast, 15.4% of the patients in the control group had pneumonia, and 7.7% experienced it after oral food intake.
Factor prediction according to the amount of oral intake
The results of the multivariate logistic regression analysis are presented in Table 3. The multivariate analysis included PERIO-N intervention, age, disease stage, recurrent nerve palsy, pneumonia, and leakage. These analyses indicated that the PERIO-N intervention was more likely to allow oral food intake (odds ratio [OR]: 2.31; 95% confidence interval [CI]: 1.04–5.13). In addition, there were poorer outcomes regarding recurrent nerve paralysis (OR: 0.24; 95% CI: 0.10–0.58) and ageing (OR: 0.92; 95% CI: 0.88–0.97).
Table 3.
Multivariate Logistic Regression Analysis for Oral Intake at Time of Discharge
| Factors | Adjusted odd ratio | 95% Cl | p-value |
|---|---|---|---|
| Intervention (with or without) | 2.31 | 1.04–5.13 | 0.040 |
| Age (years) | 0.92 | 0.88–0.97 | 0.001 |
| Stage (0–4) | 1.04 | 0.72–1.50 | 0.853 |
| Recurrent nerve paralysis(positive or negative) | 0.24 | 0.10–0.58 | 0.002 |
| Pneumonia (positive or negative) |
0.86 | 0.30–2.46 | 0.774 |
| Leakage (positive or negative) | 0.82 | 0.55–1.22 | 0.334 |
Discussion
Our findings indicated that perioperative nutritional education increased the amount of oral intake at discharge and the probability of discharging patients undergoing oesophageal cancer surgery. Furthermore, the patients who received nutrition education only had oral intake and were discharged without enteral nutrition. However, this did not increase the probability of hospitalisation for risk of malnutrition within 3 months after discharge. The multivariate analysis showed that nutritional interventions independently affected the amount of oral intake.
First, in patients undergoing oesophageal cancer surgery, perioperative nutritional education led to increase in the amount of oral intake at discharge and the probability of discharge from the hospital.
Decreased oral intake after oesophagal cancer surgery affects postoperative nutritional status and overall prognosis (Okada et al., 2019). Reflux is one of the most significant factors hindering the postoperative amount of oral intake (Gockel et al., 2010) (Ouattara et al., 2012). Reflux can be reduced in frequency by changing eating behaviours, such as how food is eaten. However, changing eating habits requires behavioural change to occur. Important factors to promote behaviour change include individualised advice based on symptoms, social/biopsychosocial explanations, therapeutic relationships, concurrent treatment of behaviour-limiting symptoms, and physical involvement of the patient in the intervention. (Pinto et al., 2021) In the present study, nutrition education for esophageal cancer patients was also initiated preoperatively, and early and frequent postoperative patient education from the start of diet therapy provided individualised advice based on symptoms and social/biopsychosocial explanations. This led to behavioural changes and improved postoperative self-management skills. Education helps patients manage side effects and complications and improves adherence. (Chelf et al., 2001{Chelf, 2001 #30})
Second, even when patients were discharged from the hospital without enteral nutrition and oral intake only, the hospitalisation rate for malnutrition risk within 3 months of discharge did not increase in the group that received nutrition education. It has been reported that some weight loss occurs almost without exception after esophagectomy (Donohoe et al., 2017). Early postoperative weight loss is a useful marker of poor prognosis in oesophagal cancer patients. (Yamamoto et al., 2022) The results of the current study indicate that nutritional status of difficult-to-treat patients could not be improved regardless of nutritional supplementation methods. Nutrition education is not effective for cancer patients with complex metabolic changes and systemic inflammation but may be effective in other cases.
Third, nutritional interventions independently affect the amount of oral intake. Malnutrition is a common problem in cancer patients, yet about half of the medical staff do not conduct nutrition screening (Sharma et al., 2015). In many cases, providing nutrition information to patients and assessing nutritional adherence in cancer patients is suboptimal. Nutritional status must be assessed promptly, and reasonable nutritional interventions must be implemented as soon as possible (Guo et al., 2020). Individualised counselling on nutritional status and quality of life has also been effective in nutrition intervention compared to non-intervention and standard nutrition advice. (Langius et al., 2013) In oesophagal cancer, in particular, higher educational achievement is associated with higher postoperative survival (Linder et al., 2018). Nutritional interventions can identify and resolve nutrition-related risks earlier, encouraging patients to change their eating behaviours.
This study had three limitations. First, the frequency of nutrition education varied according to health and medical history. Second, the evaluation of hospitalisation owing to undernutrition emphasised assessing only the presence of severe malnutrition. Therefore, it is expected that there may have been mild malnutrition. However, this study was limited to conducting a detailed nutritional evaluation. Third, since we recruited all eligible patients before and after the dietitian’s intervention, the possibility of selection bias was not high. However, we cannot rule out the possibility that there might have been a bias of which we were unaware.
This study found that perioperative nutritional education in patients undergoing oesophageal cancer surgery led to increase in oral intake at discharge and the probability of discharge. The group that received nutritional education did not have an increased probability of hospitalisation due to the risk of malnutrition within 3 months after discharge, even if they had oral intake only without enteral nutrition.
Author Contribution Statement
S. M. and M.H. contributed substantially to study conceptualisation. S.M. and M.H. contributed significantly to data analysis and interpretation. N.K., T. S. S.Y, and M.N. contributed substantially to the manuscript drafting. All authors critically reviewed and revised the manuscript and approved the final version for submission
Acknowledgements
We extend our greatest appreciation to the staff of the Okayama University Hospital. We would like to thank Editage (www.editage.com) for English language editing.
Ethical Declaration
The study protocol was approved by the Ethics Committee of Okayama University Hospital.
Study Registration
The study was not registered in any dataset due to the retrospective nature of the study.
Conflict of Interest
The authors declare no conflicts of interest.
References
- Anandavadivelan P, Lagergren P. Cachexia in patients with oesophageal cancer. Nat Rev Clin Oncol. 2016;13:185–98. doi: 10.1038/nrclinonc.2015.200. [DOI] [PubMed] [Google Scholar]
- Cui X, Shan T, Qiao L. Effect of Self-Transcendence Theory Combined with Comprehensive Nursing Intervention under Tumor Nutrition Education on Symptom Improvement, Nutritional Status, and Positive Psychology of Elderly Patients with Gastric Cancer. Contrast Media Mol Imaging. 2022;2022:6084732. doi: 10.1155/2022/6084732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe CL, Healy LA, Fanning M, et al. Impact of supplemental home enteral feeding postesophagectomy on nutrition, body composition, quality of life, and patient satisfaction. Dis Esophagus. 2017;30:1–9. doi: 10.1093/dote/dox063. [DOI] [PubMed] [Google Scholar]
- Gockel I, Gönner U, Domeyer M, Lang H, Junginger T. Long-term survivors of esophageal cancer: disease-specific quality of life, general health and complications. J Surg Oncol. 2010;102:516–22. doi: 10.1002/jso.21434. [DOI] [PubMed] [Google Scholar]
- Guo ZQ, Yu JM, Li W, et al. Survey and analysis of the nutritional status in hospitalised patients with malignant gastric tumors and its influence on the quality of life. Support Care Cancer. 2020;28:373–80. doi: 10.1007/s00520-019-04803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langius JA, Zandbergen MC, Eerenstein SE, et al. Effect of nutritional interventions on nutritional status, quality of life and mortality in patients with head and neck cancer receiving (chemo)radiotherapy: a systematic review. Clin Nutr. 2013;32:671–8. doi: 10.1016/j.clnu.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Linder G, Sandin F, Johansson J, et al. Patient education-level affects treatment allocation and prognosis in esophageal- and gastroesophageal junctional cancer in Sweden. Cancer Epidemiol. 2018;52:91–8. doi: 10.1016/j.canep.2017.12.008. [DOI] [PubMed] [Google Scholar]
- Melnic I, Alvarado AE, Claros M, et al. Tailoring nutrition and cancer education materials for breast cancer patients. Patient Educ Couns. 2022;105:398–406. doi: 10.1016/j.pec.2021.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada G, Momoki C, Habu D, et al. Effect of Postoperative Oral Intake on Prognosis for Esophageal Cancer. Nutrients. 2019:11. doi: 10.3390/nu11061338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouattara M, D’Journo XB, Loundou A, et al. Body mass index kinetics and risk factors of malnutrition one year after radical oesophagectomy for cancer. Eur J Cardiothorac Surg. 2012;41:1088–93. doi: 10.1093/ejcts/ezr182. [DOI] [PubMed] [Google Scholar]
- Pinto JW, Bradbury K, Newell D, Bishop FL. Lifestyle and Health Behavior Change in Traditional Acupuncture Practice: A Systematic Critical Interpretive Synthesis. J Altern Complement Med. 2021;27:238–54. doi: 10.1089/acm.2020.0365. [DOI] [PubMed] [Google Scholar]
- Sharma D, Kannan R, Tapkire R, Nath S. Evaluation of Nutritional Status of Cancer Patients during Treatment by Patient-Generated Subjective Global Assessment: a Hospital-Based Study. Asian Pac J Cancer Prev. 2015;16:8173–6. doi: 10.7314/apjcp.2015.16.18.8173. [DOI] [PubMed] [Google Scholar]
- Sharma D, Kannan R, Tapkire R, Nath S. Evaluation of Nutritional Status of Cancer Patients during Treatment by Patient-Generated Subjective Global Assessment: a Hospital-Based Study. Asian Pac J Cancer Prev. 2015;16:8173–6. doi: 10.7314/apjcp.2015.16.18.8173. [DOI] [PubMed] [Google Scholar]
- Weijs TJ, Berkelmans GH, Nieuwenhuijzen GA, et al. Routes for early enteral nutrition after esophagectomy. A systematic review. Clin Nutr. 2015;34:1–6. doi: 10.1016/j.clnu.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Tanaka K, Yamasaki M, et al. Early postoperative weight loss is associated with poor prognosis in patients with esophageal cancer. Esophagus. 2022;19:596–603. doi: 10.1007/s10388-022-00937-2. [DOI] [PubMed] [Google Scholar]