Abstract
Following large-scale radiation events, an overwhelming number of people will potentially need mitigators or treatment for radiation-induced injuries. This necessitates having methods to triage people based on their dose and its likely distribution, so life-saving treatment is directed only to people who can benefit from such care. Using estimates of victims following an improvised nuclear device striking a major city, we illustrate a two-tier approach to triage. At the second tier, after first removing most who would not benefit from care, biodosimetry should provide accurate dose estimates and determine whether the dose was heterogeneous. We illustrate the value of using in vivo electron paramagnetic resonance nail biodosimetry to rapidly assess dose and determine its heterogeneity using independent measurements of nails from the hands and feet. Having previously established its feasibility, we review the benefits and challenges of potential improvements of this method that would make it particularly suitable for tier 2 triage. Improvements, guided by a user-centered approach to design and development, include expanding its capability to make simultaneous, independent measurements and improving its precision and universality.
Introduction: defining the needs
The emphasis of biodosimetry for response to a large event has to a considerable extent focused on facilitating triage in the first week following the event. The underlying assumption is that biodosimetry, with its ability to sample from the victims’ own body to assess the dose they received, offers far better information for medical decision-making than would be a population-based estimate for the victim or using generalized external dosimetry methods. Especially in large radiation events, the availability of life-saving mitigators or treatments is likely to be severely limited and thus should only be offered to those for whom it offers survival benefits.
In the USA, government agencies charged with promoting the development of biodosimetry for large radiation events have emphasized the need to have methods that can rapidly sort out those whose dose does or does not warrant using mitigators or treatment for acute radiation syndrome (ARS), so they can be triaged into or away from the medical response system.
For example, one interagency group developed the Radiation-specific TRiage, TReatment, TRansport system (‘RTR’) for emergency responders nearby the event to quickly sort out people who have physical injuries requiring immediate medical treatment, irrespective of sustaining radiological injuries. The second tier in this plan then focuses on assessing their risk of having radiological injuries. The goal at this second stage is to identify those whose dose warrants receiving mitigators or treatment for the first effects of ARS, generally described as doses of 1.5–8.3 Gy(1) (Note: ‘ARS care’ hereafter refers to mitigators and/or treatments for short-term effects of radiation injury.)
The Biomedical Advanced Research and Development Agency (BARDA), somewhat in contrast, focuses its radiation-related development efforts on the role that biodosimetry and countermeasures can play in responding to ARS, albeit understanding these roles occur in the larger context of assessing and meeting all needs for immediate care. Their triage-related goal is to develop methods that can distinguish between two broad radiation-based categories of victims: those who can survive without needing immediate health care for ARS versus those for whom ARS care would offer significant survival benefits. In their target product profile (TPP) for biodosimetry methods, the initial tier for triage emphasizes the method’s portability and ease of being operated at temporary sites to distinguish between these two groups. Additionally, first tier methods must be capable of surveying within 1 week up to 1 million people presenting for evaluation of radiation exposure. The dose estimate needed at this first tier is dichotomous, i.e. is it below or above the minimum threshold to treat, generally described as 2 Gy. The intent is to have a sensitivity within 0.5 Gy, although some recognize this may not be achievable(1).
The expectation is that this strategy will result in triaging most victims out of needing medical response for ARS. While ‘most’ is not defined, the experience of people in radiation accidents led to the widespread assumption that most people seeking evaluation in a large event will simply be the ‘worried well’ who were never exposed and do not need ARS care.
A low false positive rate in screening is desirable (and should be as low as feasible). Nevertheless, the results of initial tier biodosimetry are expected to generate a substantial number of false positives among those sent on to the next tier for possible care. This is because of the statistical tradeoff between minimizing false positives versus false negatives. At the initial tier, where biodosimetry methods are less sensitive, the more appropriate public health goal is to minimize false negatives and thereby tolerate a relatively higher rate of false positives. That is, there is little harm to life when people are found at tier 2 to not have a dose that warrants ARS care (i.e. false positives from tier 1) because they are being reevaluated at tier 2 with more sensitive methods. In contrast being designated as false negative at tier 1 implies that such victims may die because they were falsely triaged away from any care or further evaluation.
Consequently, at the second tier in BARDA’s TPP, because of the relatively high false positive rate and the need to decide on using scarce radiation-related treatment or mitigators, biodosimetry methods should have a much greater sensitivity, i.e. provide more accurate and more specific dose estimates, including identifying doses that are too high to survive the short-term effects of radiation.
To obtain more refined dose estimates, BARDA’s TPP for second-tier methods assumes the need to use more sophisticated personnel and resources located in offsite laboratories. However, because there are now significantly fewer victims to process (the TPP says tier 2 methods need ‘only’ to be able to evaluate 600 000 samples within 10 days), tier 2 biodosimetry relies on high-throughput methods to be able to process what is still a very large number of samples.
Note that, while virtually all who refer to a second stage of dosimetry uniformly assume it implies using more refined methods with more accurate estimates, not all assume the second stage provides definitive information for initiating and planning ARS-related care. Instead, some like BARDA argue that the purpose of ‘tier 2 dosimetry’ should be to identify and eliminate as many false positives from tier 1 as possible and, for the true positives, to identify more accurately what dose was received and to assess its biological implications, either in terms of predicting organ-specific damage or for predicting the potential for spontaneous recovery from damage to the hematopoietic system. We adopt this approach in this paper.
Improvised nuclear device as a scenario to evaluate the role of biodosimetry
We recognize that not all radiation events are ‘large’ (and some European planners refer to >100 victims as a ‘large’ event compared with BARDA’s 1 million). Likewise, some very large radiation events will not involve an improvised nuclear device (IND)(2, 3).
Nonetheless, considering the case of a very large IND, while arguably being a ‘worst-case scenario’, provides a useful context to evaluate the role of biodosimetry in responding to large radiation disasters.
In particular, this scenario, and the concept of using two tiers for evaluating the dose, raises important contextual complexities. Chief among these is acknowledging the high likelihood of needing to carry out the processes of biodosimetry and medical response to ARS while using severely compromised infrastructures for health care, transportation and communication. Yet, in this context, biodosimetry methods still need to be capable of processing a huge volume of samples, from sampling to delivering results to the decision-maker for a victim, in a very short timeframe.
Thus, we propose to use this scenario and these broad plans for radiation-based response to an IND to argue the value of biodosimetry for second tier dose evaluation. While arguments can be made for other types of biodosimetry, we focus here on a new method under development, in vivo nail dosimetry, to illustrate the value of two tiers of evaluation for triage. We chose to use in vivo nail dosimetry because, although it still under development, it is uniquely qualified to be able to provide both a refined dose estimate and an indication that the hematopoietic system can be salvaged by autologous regeneration. (When the goal is instead to decide on ARS-related treatments following triage, we assume that medical responses would be based on a combination of all available biodosimetry results along with clinical signs, e.g. number of lymphocytes; clinical symptoms, e.g. vomiting; and hematological measurements.)
Additional considerations affecting biodosimetry
The reality for planning medical response following an IND of course involves much more than radiation injury. Many survivors are likely to have concomitant (or even preexisting) physical injuries that may both complicate dose estimates and magnify the effects of ARS, thereby changing decisions about ARS care (and of course about treating the other trauma and injuries).
Even though we limit our discussion to planning the response to radiation effects and focusing on biodosimetry, there are other factors that influence the variability in a person’s exposure besides distance from the blast and wind patterns affecting fallout. For example, the exposed population may have been in shelters that partially protected them from the initial blast and/or resulted in partial body exposure; they may have been exposed to radiative fallout in addition to or instead of the initial explosion(1, 4). Thus, it is important to assess not only the individual’s exposure but whether it was whole or partial body.
There are also social-psychological considerations, including the public health need to offer help regardless of payment or socioeconomic status. Other issues include the need to communicate well when ARS care is refused, whether because the dose was below the threshold to begin to treat or above the threshold to offer active treatment or mitigators(2, 5).
Finally, as discussed elsewhere(6–8), there are significant time-dependent complexities regarding when samples may be valid to collect (both how soon and how long after the exposure). The time urgency at both tiers of evaluation is also a function of the need to begin timely administration of any mitigating or ARS treatments to prevent acute life-threatening radiation injuries. Time also factors into transporting stockpiled equipment or the victims or their samples for analysis off-site. Likewise, if the results take many hours or days to become available, contacting the victims matched to the results, who may not be able to return home or have a reliable means to be reached, and communicating the matched results to their appropriate medical decision-makers will occur in a chaotic and very compromised transportation and communication infrastructure.
The scenario: IND, risk of ARS, types of dosimetry
Buddemeier and colleagues(1, 9, 10) have produced several simulations to estimate the distribution of ionizing radiation following an IND and the numbers and likelihood of risk from injury for the affected population. The scenario we adopt is based on their estimates following a ground-burst 10 kT bomb set off in Times Square in Manhattan, under atmospheric conditions that carried dangerous fallout southward(1, 10). (Note: See estimates summarized in Figure 1. To obtain survivors, we eliminated only ‘prompt fatalities’. ‘Expectant’ is used to estimate those whose dose is too high to treat. To obtain the number ‘below treatment threshold of 2 Gy’, we combine ‘uninjured’ and ‘recover’ without treatment. ‘At risk’ is used to estimate doses between 2 and 8 Gy who, if exposure was homogeneous, could potentially benefit from ARS treatment or mitigators.)
Figure 1.
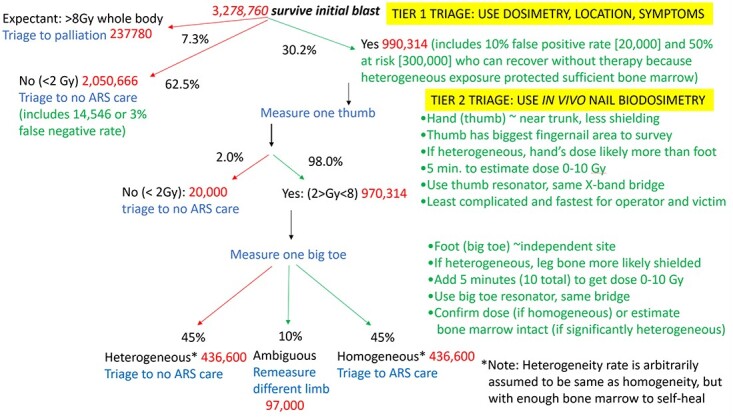
Schema to estimate dose and population to be triaged at tier 1 and 2. Numbers based on Buddemeier’s simulation of actual injuries following an IND in Times Square NYC(1, 10) and using in vivo nail dosimetry at tier 2 triage.
In their simulation, almost 4 million people would be present in either light, moderate or severe damage areas or the dangerous fallout zone, and most would survive the immediate effects of the IND. Of note, Buddemeier’s estimates do not include any ‘worried well’ who may live in other parts of New York City (NYC) (population in 2020 was over 8 million) or in the larger metropolitan area (over 18 million). Thus, Buddemeier’s 3 278 760 survivors that we use in our estimate of the population needing dosimetry are those who were actually in the area of the IND explosion and dangerous fallout (Figure 1).
The actual number of people directly in the area of the blast and fallout is already four times higher than BARDA’s TPP. If the worried well in the remainder of NYC or the metropolitan region were included, it would add up to several million more people needing to be evaluated. Therefore, however fast and however easy an individual biodosimetry assessment would be, it is not feasible to process all survivors individually at the first tier.
Instead, as shown in Buddemeier’s simulation, it would be feasible to very rapidly identify those geographic areas where the population has a significant potential to have been exposed to levels of radiation that determine its likely lethality. This information, along with physical dosemeters in the region and strategic use of some rapid biodosimetry methods and signs and symptoms following exposure to very high dose, could lead to a rapid first tier assessment to identify:
Lethal doses (>8 Gy whole body; Buddemeier’s ‘expectant’ group); this group would receive palliative care but not ARS care;
No or minimal exposure (≤2 Gy; Buddemeier’s uninjured group or those expected to survive without ARS care); this group would be triaged away from ARS care and any further evaluation; and
Those warranting further evaluation at tier 2 (Buddemeier’s at risk group and any false positives; we further assume his at risk group contains people with partial body [heterogeneous] exposure who, after confirmation, may be reassigned to the group who can recover without ARS care; we arbitrarily assume half of the at risk group received partial body exposure).
The top of Figure 1 shows these decisions at tier 1, using Buddemeier’s estimates of people in each injury category. We also assumed that 2% who were triaged to ‘no care’ will be false negatives while 10% triaged to tier 2 will be false positives.
As illustrated in Figure 1, by using such physical dosimetry methods and calculations and limited use of rapid biodosimetry and medical signs, it should be possible to reduce the number to be screened by biodosimetry from the original several million to one-third of this. Nevertheless, the number needing biodosimetry at tier 2 would still be almost 1 million.
Toxic effects of ionizing radiation
Identification of radiation dose exposures is critical to initiate currently available supportive care surrounding the early pathophysiology of disease. ARS care (both mitigators and treatment of symptoms) is largely under development, with only a few methods approved(1, 11–13).
Another very important role for biodosimetry is to help assess partial versus whole body exposure, particularly because partial exposure which results in sparing of bone marrow can allow the victim’s body to regenerate blood cells and self-recover from the earliest effects of ARS(14). Additionally, understanding radiation injury and recovery is also useful to understand the relationship between biodosimetry and the toxicological effects caused by ionizing radiation.
Although we focus on the hematopoietic effects, toxic effects of ionizing radiation include gastrointestinal and cardio/neurovascular syndromes. Each syndrome depends on the dose received as well as the duration and homogeneity of exposure (whole body versus regional anatomical sites)(15). Blood forming and supportive tissues are highly sensitive to radiation induced cellular apoptosis and necrosis through mechanisms mediated by reactive oxygen species(16) The suppressive effects of these tissues occur at exposures between 2 and 7.5 Gy in bone marrow and at exposures between 0.1 and 1.0 Gy in thymus and lymph nodes(17).
Radiation-induced hematotoxicity occurs acutely (within days to weeks) in primary (bone marrow and thymus) and secondary lymphoid tissues (lymph nodes and spleen)(15). The former is critical for the hematopoiesis and maturation of leucocytes, platelets and red blood cells from myeloid progenitor cells as well T cells, B cells and natural killer cells from lymphoid progenitor cells. The latter is critical for removing bacteria, viruses and fungi as well as preserving immune cells’ quantity and function.
The hematopoietic syndrome follows a continuum from lymphocytopenia, granulocytopenia and monocytopenia to thrombocytopenia and finally to red blood cell aplasia(18). Infection and hemorrhage are important sequela of reduced circulating white blood cells and platelets, respectively. As a result, administration of granulocyte colony stimulating factor is approved for human use in radiation syndrome under the FDA Animal Rule to improve immune cell function and increase survival(15, 19). Furthermore, aberrant coagulopathy requires blood platelet component or whole blood transfusions to attenuate hemorrhage caused by thrombocytopenia and loss of platelet function(11, 13).
The gastrointestinal and cardio/neuro vascular syndromes occur at moderate (>5 Gy) to high (>20 Gy) dose whole body irradiation, respectively. Both because they occur at higher doses and may manifest later than the hematopoietic effects, a major emphasis regarding biodosimetry’ role at tier 2 includes establishing if the exposure is partial body (heterogeneous), since spared bone marrow is crucial in determining the likelihood of the victim’s surviving the initial toxic effects of ARS hematotoxicity as well as the delayed acute effects. When warranted, the outcomes can be significantly improved by active supportive treatment, and/or supplements of active bone precursors, and/or radiation mitigators that improve bone marrow function.
It should be noted that the alternative of administering mitigators or active ARS care to the entire group of people, regardless of determining whether they are at risk, is very undesirable as well as unlikely. The logistics for doing this would be very difficult, requiring very large stockpiles at many sites. More importantly, mitigators and other countermeasures for ARS, however, effective they might be when needed, almost certainly have potentially significant side effects, especially for some subgroups(1, 4, 11). Therefore, administration of a drug to someone who cannot benefit from it only increases the risk of undesirable effects. The role for biodosimetry is thus a critical diagnostic to determine the risk to benefit ratio of therapeutic management.
Types of biodosimetry
Biodosimetry methods, while sharing using the person’s own biology to estimate dose, are based on two very different principles of how ionizing radiation affects the body: biologically based and physically based. Biologically based methods take advantage of the injury caused by ionizing radiation and assay evidence of injury or repair ongoing in cells or genes that is proportionate to the absorbed dose. Physically based methods take advantage of stable changes that are produced in biological tissue (such as bone, teeth or fingernails) as a consequence of and proportionate to exposure to ionizing radiation. A companion paper(20) discusses this in detail, noting which methods are more feasible to use, depending on the number of victims.
Because of the overwhelming numbers of individuals who would need to be screened in a large radiological event such as an IND, all types of biodosimetry struggle with how to measure so many people quickly and get results back to the decision makers if done offsite. A high degree of ingenuity has been employed to increase capacity and decrease processing time to meet the screening goals. Despite these efforts, very significant challenges remain(12, 14, 21, 22) and we argue that the initial evaluation should include means other than biodosimetry.
Requirements of tier 2 biodosimetry
Assuming the initial triage results in identifying the population at risk of dying without ARS care, the need for biodosimetry at tier 2 is to determine the dose with high sensitivity and whether the dose was heterogeneous to the extent that the bone marrow can recover without ARS care. With these higher demands for accuracy, tier 2 biodosimetry requires more sophisticated and specialized equipment, personnel and facilities. Nonetheless, as Figure 1 illustrates, the number of victims at tier 2 may still be quite large and the time urgency to begin ARS care is still great. Therefore, it is important to identify biodosimetry methods that are very rapid, relatively simple to operate even if needing sophisticated equipment, and which can result in information both about the dose and the whole versus partial body exposure.
We next illustrate a protocol that uses one such biodosimetry method for tier 2 evaluation. We begin with a brief description of in vivo EPR nail biodosimetry.
In vivo nail EPR biodosimetry: current status
In vivo EPR nail dosimetry is based on measuring the radiation induced free radicals generated in the keratin of human nails. (Note: EPR biodosimetry based on clipped nails offers potential advantages but recent developments suggest it is not suitable for this purpose(23).) It can be done in both fingernails and toenails and each nail can be an independent site for assessing dose. Because each nail/limb acts as an independent ‘dosemeter’, it can be used to determine if the dose was homogeneous (e.g. same for measurements made on several limbs) or heterogeneous (e.g. differing significantly in different limbs)(14).
Measurements can be performed simultaneously or consecutively on more than one limb and on multiple nails on the same limb. Tradeoffs in choosing between simultaneous or consecutive options include the total time needed to complete each measurement, the number of different resonators or magnets that would be required, the likely ‘independence’ of the digits on the same limb, the greater sensitivity by assessing a larger sample, comfort or convenience for the subject (especially if nails need to have polish removed, etc.). Preliminary work suggests measurement sessions can be accomplished with 5 min of data acquisition and made relatively simple for the operator to accomplish. Therefore, if the method can achieve sufficient sensitivity with short measurement times, it has great potential for obtaining the data needed for tier 2 triage.
In vivo EPR nail biodosimetry shares the favorable properties of other EPR biodosimetry including
Measurements can be made immediately after the event since the radiation-induced signals (RIS) occur immediately upon absorption of the radiation.
The signal is relatively stable, lasting for thousands of years in teeth and bone; the signal in nails appears to be stable for several weeks which is long enough for tier 1 and tier 2 evaluation of dose. (Note: This length of time has been verified in clipped nails but needs to be tested for in vivo nails once the improved device described here has been developed.)
The device can be highly automated so that measurements can be made by non-expert operators.
Results are available immediately after processing the data acquired during a measurement session.
It can resolve doses within the specifications needed, i.e. 1.5–8.3 Gy.
Measurements are specific to the site, so they can be compared to measurements at other sites or can be compared to biologically based biodosimetry which is not site specific, to provide an indication of the homogeneity of the exposure.
Unlike biologically based biodosimetry, EPR biodosimetry is relatively unaffected by biological factors such as stress, wounds (especially other than burns) and pre-existing conditions and does not evolve with injury-repair cycles.
The site for conducting tier 2 triage is likely to be in a temporary or permanent structure with access to utilities and at least some supplies and trained personnel. Complementary to this scenario, in vivo EPR nail biodosimetry has minimal requirements for operation and placement, requiring only ordinary electrical sources either through a grid or a generator or powered by batteries. It has a low strength permanent magnet (about 350 millitesla within a small volume) presenting essentially no hazards or incompatibility with the presence of other devices. Therefore, it is feasible to place spectrometers in virtually any environment with access to any source of power.
In contrast to the needs for tier 1 triage, there is less need for the equipment to be completely transportable and operable by individuals with no prior training. Fewer instruments would be needed because of there are fewer victims being screened at tier 2. Operators could rapidly gain the skills needed to operate the instrument because the instrument will be highly automated and incorporate automated quality assurance checks and processes.
A possible protocol using in vivo nail biodosimetry at tier 2
Returning to Figure 1, we use Buddemeier’s estimates of radiation injury following an IND to illustrate how a protocol using in vivo nail biodosimetry could be used to meet the two goals for tier 2 biodosimetry. In our hypothetical protocol, one thumbnail would be measured for each victim at tier 2. Measuring the thumb has the advantage of being the largest fingernail and so offers the largest sample. The hand, compared to the foot, is closest to the trunk and most likely to reflect the dose to the torso. Using the hand is also the simplest logistical set-up for the operator and victim, and ≤5 min measurement can provide a dose estimate.
Assuming tier 1 dosimetry worked as described earlier, only ~20 000 people will be found at tier 2 to be false positive (and therefore not in need of ARS care); the overwhelming majority at tier 2 will be true positive and so would need a second measurement to establish whether their exposure was heterogeneous or not. Continuing our hypothetical protocol, the big toe would be used for the second measurement, again because its nail has the largest surface of the toes and, if the dose was truly heterogeneous, feet are more likely to have been shielded than hands.
In Figure 1, we arbitrarily assumed half of the people at tier 2 will have had heterogeneous exposures where the second measurement was <2 Gy. Designating an exposure as heterogeneous in this example implies evidence of bone marrow sufficient to self-renew blood cells, obviating the need for hematopoietic care. This is consistent with defining ‘heterogeneity for triage purposes’ as finding that one dose estimate on nails (e.g. from the big toe) was <2 Gy, while the other (e.g. from the thumb) was <8 Gy but >2 Gy.
Other protocols using in vivo nail dosimetry could be proposed. For example, since most people in this scenario will need two digits measured at tier 2, it may be more efficient to measure both digits simultaneously. This would require two bridges or multiplexing a single bridge to accommodate the use of two resonators. However, such measurements could be done in ≤5 min total instead of 10 min.
Alternatively, if there are ambiguities or uncertainties in the measurements, more digits or limbs could be measured to increase the sensitivity. It may also be prudent to consider context. For example, if the affected person was in an area where building flash was more likely, the protocol could be to measure both sites. However, if the person was outside and directly witnessed a flash, it could be more safely assumed they received a homogeneous dose, so multiple measurements would not be necessary.
While our hypothetical protocol capitalizes on the unique capability of in vivo nail biodosimetry to assess heterogeneity as well as assessing dose, it still faces several challenges before it can be used. Several types of challenges for the device development and current progress toward solutions are briefly reviewed next.
While feasibility studies have been conducted successfully in the prototype device, in vitro studies and in vivo studies using these improvements (such as studies to test the impact of removing nail polish prior to measurement and testing the final device in patients whose treatment includes in vivo irradiation of nails) remain to be conducted and are not discussed here.
Optimizing the resonator for measurements
The usual method for making measurements in vivo using low frequency L-band EPR (e.g. 1 GHz) is expected not to have sufficient resolution or sensitivity to resolve the RIS in the dose range of interest as compared to EPR methods using higher microwave frequencies (i.e. X-band)(24). Therefore, measurements in vivo need to be made using more sensitive (higher) microwave frequencies such as X-band EPR (nominally 9.5 GHz).
The technical problem is that the electrical field of 9.5 GHz microwaves incident on lossy media (such as liquid water and tissues) limits the applicability of X-band EPR for in vivo applications. Nails present an anomaly. Within the nail, the water content is low, but the nailbed underneath is a normal, lossy tissue. Therefore, an approach is needed to selectively sample the nail while avoiding the underlying lossy tissues.
When conventional resonators are used, a sample is placed inside the cavity where the electric field is within a volume much larger than the nails; therefore, signal to noise (S/N) is severely degraded by microwave losses and local heating. To achieve sufficient sensitivity for data acquisition in vivo, new resonator technology was required. Specifically, new geometry was needed to limit the electrical field distribution within lossy tissue while maximizing the magnetic field within the nail.
Additional requirements for in vivo EPR biodosimetry to be successful include making the measurements quickly and comfortably with non-expert operators. This necessitates positioning the nails quickly and comfortably while maintaining a high degree of automation. Two potential types of resonators have the required characteristics: aperture resonators and surface resonator array (SRA) resonators.
The aperture resonator was originally developed by Ikeya and colleagues at X-band but had limited sensitivity(25–27). The aperture resonator they used was based on a rectangular TE102 cavity with an aperture, either a hole or slot, cut in the far wall, illustrated in Figure 2A. (Digit with nail is placed outside the slot on the left of the ‘box’ illustrated in Figure 2A and B). The aperture geometry can be optimized, but it remains a small perturbation on the cavity (approx. 1 × 7 mm). From this perturbation the magnetic field ‘leaks’ out as an evanescent wave. The sample is placed on the aperture where an evanescent wave couples the spin system to the resonator. This structure is limited by the total microwave magnetic field at the aperture, which is only half of the magnetic field at the center of the cavity.
Figure 2.
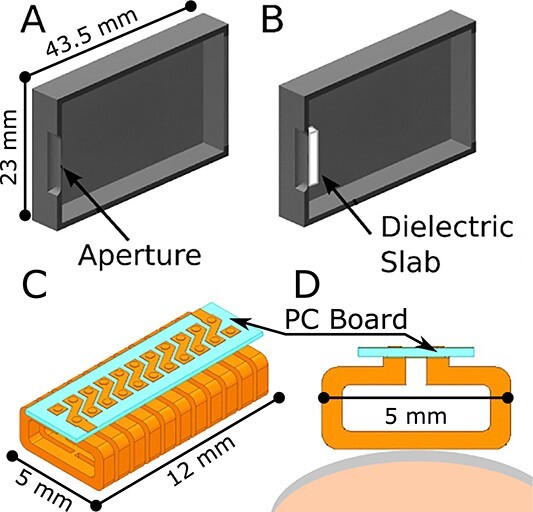
Resonator geometries. (A) Rectangular TE102-based aperture resonator by Ikeya et al.(25–27) (B) Dielectric-backed aperture resonator(28). (C) Surface resonator array (SRA), showing array of 11 elements and a printed circuit (PC) board. (D) Cross section of SRA geometry showing a single element placed on a nail (gray) ready for in vivo measurement.
To improve sensitivity, Grinberg et al. developed versions of the aperture resonator that increased the microwave magnetic field at the aperture using a non-resonant dielectric slab(28). Figure 2B illustrates this resonator.
Grinberg et al.(28) speculated that further improvements could come from changing the overall geometry from a rectangular TE102 to the less conventional semi-spherical TE011 geometry. The semi-spherical geometry is attractive due to having twice the incident microwave magnetic field at the aperture for a fixed input power. Moreover, placing a slab of dielectric material (KTaO3) at the aperture inside the rectangular TE102 cavity was found to result in a 20-fold increase in EPR signal intensity over a non-backed aperture configuration(28).
More recently, Guo et al.(29) designed and tested a custom-built X-band spectrometry instrument for performing in vivo EPR analysis of RIS in nails. Guo’s in vivo EPR nail dosemeter incorporates a rectangular TE101 mode cavity with a rectangular detection aperture opened on the cavity wall. This design allows for the cusp of the fingernail tip to be inserted into the aperture. This nail insertion method accommodates sampling of the nail only, without the interference of the lossy tissues underlying the nail. As with the TE102 cavity aperture resonator, the aperture was transfixed through the cavity body. In this way, the magnet field and its modulation can be applied into the detecting aperture.
The current prototype of the aperture resonator could detect the RIS signal following a 6 Gy dose, with RIS signal detection limits equivalent to 2–3 Gy due to the presence of background signals. Of note, this method depends on subjects having a cusp at the tip of the fingernail and for the cusp to have sufficient length to detect and reproducibly quantify the RIS.
This approach remains a viable solution to the need for sensitivity. However, due to geometrical benefits, compactness and temperature dependance of dielectric materials, new studies try to optimize SRA geometry.
Figure 2C and D illustrate the array structure and basic element of the SRA respectively as designed for use in in vivo nail dosimetry. (Digit with nail is placed along bottom of array, as illustrated in Figure 2D). The use of SRAs to produce a local magnetic field has been successfully implemented in MRI for enhanced sensitivity on a surface(30). From the principles discussed by Hyde et al.(31) at an operating frequency of 64 MHz, Sidabras et al.(32) designed a SRA optimized for EPR at 9.5 GHz. SRA geometry can be designed to limit the depth sensitivity to a region of interest on a surface, which is ideal for making measurements in the nail, minimizing the non-resonant absorption of the microwave by tissue beneath the nail.
The SRA has been used in several EPR biodosimetry studies where both in vitro nail models and in vivo test samples have been studied(33). Such experiments provide useful information regarding the overall sensitivity of the SRA, its usability and its ability to be repeatably placed on healthy volunteers.
Table 1 lists potential changes to the SRA geometry and the benefits and challenges of each. Since the SRA geometry can be formed with two or more segments, many geometric variations can exist to increase its sensitivity to RIS. To date, the active region cross section (5 × 12 mm; Figure 2C) has been held constant and the number of elements has been varied as 7-, 9- and 11-elements. By maintaining a constant cross section, the EPR sensitivity can be varied to match the depth of the nail. For instance, 7-element SRA has 90% of the EPR sensitivity at a depth of 0.8 mm, while the 11-element SRA has 90% of the EPR sensitivity at a depth of 0.4 mm. Although initial studies show promise, it is difficult to optimize the SRA geometry with computer simulations or in vitro models alone, due to characteristics of the RIS within the nail.
Table 1.
Benefits and challenges of optimizing surface array resonator (SRA) geometry.
| Feature optimized: | Benefits | Challenges |
|---|---|---|
| Geometric shaping of field of SRA | Minimizes electric field loss in nail/nailbed Minimizes cross section (reduces curvature effect) Increases SNR Allows variable depth sensitivity (maximizes signal from nail) |
Needs in vivo studies to optimize, i.e. cannot use simulations or in vitro models |
| Shaping for nail curvature | Ensures greater volume of nail measured | Curvature varies greatly; may need several shapes of resonators |
| Increased operating frequency | Improves SNR | Must increase static magnetic field |
| Hand/foot holding apparatus | Minimizes movement and physiological effects | Difficult to use on injured individuals |
| Automatic tuning/matching | Significantly decreases physiologically induced noise in data collection | Complex circuitry Requires feedback control software (available) |
One promising geometric optimization involves reducing the width from 5 to 3 mm. Simulations show this size modification increases the efficiency of the resonator, i.e. increases the EPR signal sensitivity, while reducing the sampled volume. This is beneficial for biodosimetry because it would reduce the nail sample size needed, thereby accommodating a wider variety of nail curvatures.
Optimizing the spectrometer for measurements
In addition to changes made to the resonator, other features of the spectrometer can be optimized to improve the sensitivity of this method. Table 2 summarizes the benefits and challenges in changing from continuous wave to rapid scan microwave, in modulating the frequency, in using synthesizer technology and in adopting rare-earth static magnets. Not summarized here are proposals to optimize the spectrometer for miniaturization or automation and to optimize the software for automation, e.g. automatic matching control (which has been shown to significantly reduce physiological artifacts(34–37)) and for simplifying operation.
Table 2.
Benefits and challenges of optimizing spectrometer.
| Feature optimized | Benefits | Challenges |
|---|---|---|
| Rapid scan vs continuous wave (CW) | Significantly increases SNR Eliminates many physiological artifacts Reduces data acquisition time |
Needs field modulation coils to output  15 gauss 15 gaussCoils may need water cooling Vibrations from coils may be problematic |
| Frequency modulation | Removes need for external modulation coils (simpler design) No vibrational issues (increases robustness) |
Limited by spectrometer’s frequency response (limits applicability) Techniques less straight-forward than CW |
| Synthesizer technology | Performs as well as single-mode oscillator More robust; not susceptible to vibrations Many built-in functions |
Cost in the 10 GHz range is about $20 000 |
| Rare-earth static magnets | Small size (good for portability) Uniformity fits resonator |
Magnetic field ‘always on’ (but confined to volume immediately around it) Needs electromagnet for field sweeping |
Optimizing the ergonomics and human factors
A user-centered approach to design and engineering of devices seeks to consider ergonomic and human factors ‘early and often’(38, 39). We have already mentioned the need to optimize the resonator for in vivo nail dosimetry to consider the comfort of the victim during 5 min of measurement and simplifying the operation of the equipment for the operator. These are not only practical considerations that allow the repetitive use of the device to measures thousands of victims. They also increase the quality of the measurements by reducing physiological artifacts and eliminating common mistakes.
Rather than ignoring these factors during development of the device and having to back-design a laboratory-ready spectrometer, we attempt to be cognizant of these issues and adopt principles that embrace human factors at all phases of design and implementation(40, 41). These include
Modularizing both hardware and software so that subparts can be modified without redesigning all.
Simplifying the process for the users by taking into account the protocol requirements for measurements, common mistakes, safety, time and comfort during measurement sessions, the ability to re-use the device rapidly and repeatedly, and the intended clinical use of the data.
Figure 3 illustrates one such consideration: how should the subject be set-up for measurement? Four options are presented and advantages and disadvantages are considered in context, such as the complexity and stability of the device as a whole, ease of use by all sizes and agility levels of subjects, consideration of access to the magnet and placement of the resonator for both the subject and operator, hygiene and safety for both the subject and operator, and special consideration of likely circumstances in a disaster such as having no internet, no power, subjects vomiting, burns and injuries of subjects, obesity, etc. Option 3.C (fixed chair with platform holding resonator and magnet swiveling in place after the subject sits), for example, increases the complexity of the device to be able to swivel into place and move up and down after the subject is seated. However, it is more able to accommodate sitting obese or injured subjects and allows the measurement to take place at the right height for them to be comfortable and stable. It also allows easier access for the operator to help place the resonator on the subject’s hand or foot and move them into a magnet for measurement.
Figure 3.
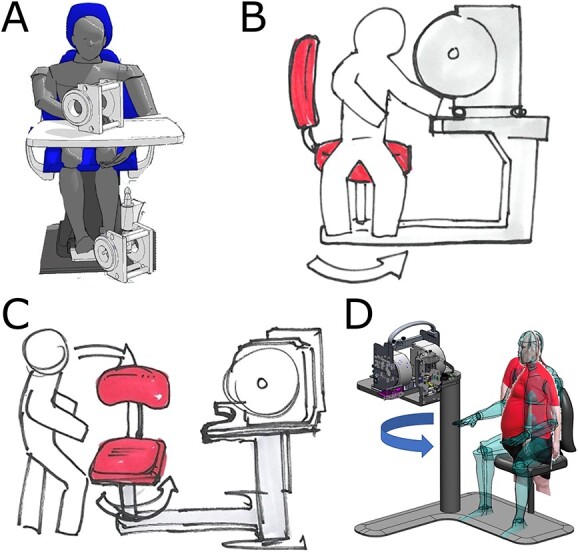
Basic options for sitting subject during in vivo dosimetry. (A) Subject sits in any chair and table available on site; two magnets are used. In B–D, chair and stand are built into spectrometer. (B) Subject sits sideways on stationary chair and moves body into place. (C) Chair swivels after subject sits down. (D) Magnet stand swivels and moves up and down to fit any body size after subject sits in stationary chair.
Conclusions
The most effective use of biodosimetry is likely to be after other means (probably based on location) are used initially to screen out subjects who are unlikely to have had exposures to ionizing radiation that could lead to ARS. Then there will be a need to evaluate thousands of subjects to determine if they have had an exposure that puts them at significant risk for having ARS. The unique characteristics of in vivo EPR nail biodosimetry may be especially useful. It appears to have the capability of providing the dose sensitivity needed at tier 2. It also offers the unique capability among biodosimetry methods to rapidly determine the homogeneity of the exposure, information that will impact therapeutic decisions for some subjects.
The most effective use of EPR in vivo nail dosimetry will be in coordination with other sources of information on the exposure and status of the subjects. While the feasibility of this approach has already been demonstrated, there remains a need to advance and optimize the technology. Such developments can be carried out initially using volunteers with paramagnetic materials placed on the surface of their nails. Ultimately, the robustness of the technique can be validated in patients undergoing therapeutic whole-body irradiation (or other therapeutic treatments involving exposure to the nails).
The challenge for all biodosimetry methods is to evaluate their use in realistic scenarios involving large-scale radiation. We propose including the IND as one such scenario that can help clarify the role of biodosimetry at different tiers of triage and identify the magnitude of the problem.
Contributor Information
Ann Barry Flood, Radiology Department, Geisel School of Medicine at Dartmouth, Dartmouth College, Hanover, NH, USA; Clin-EPR, LLC, Lyme, NH, USA.
Jason W Sidabras, Department of Biophysics, Medical College of Wisconsin, Milwaukee, WI, USA.
Steven G Swarts, Department of Radiation Oncology, University of Florida, Gainesville, FL, USA.
Paul W Buehler, Department of Pathology, University of Maryland, Baltimore, MD, USA.
Wilson Schreiber, Clin-EPR, LLC, Lyme, NH, USA.
Oleg Grinberg, Clin-EPR, LLC, Lyme, NH, USA.
Harold M Swartz, Radiology Department, Geisel School of Medicine at Dartmouth, Dartmouth College, Hanover, NH, USA; Clin-EPR, LLC, Lyme, NH, USA.
Conflict of interest
Ann Barry Flood and Harold M Swartz are co-owners of Clin-EPR, LLC of Lyme, NH that manufactures clinical EPR instruments for investigational use only.
Funding
Funding for initial resonator development was from NIH (NIAID) U19AI091173.
Data availability
Data are reported in this paper are public, as cited.
References
- 1. Satyamitra, M. M., Perez-Horta, Z., Dicarlo, A. L., Cassatt, D. R., Rios, C. I., Price, P. W. and Taliaferro, L. P. NIH policies and regulatory pathways to U.S. FDA licensure: strategies to inform advancement of radiation medical countermeasures and biodosimetry devices. Radiat. Res. 197(5), 533–553 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Federal Emergency Management Agency . Planning Guidance for Response to a Nuclear Detonation, Third Edition . (2022). https://www.fema.gov/sites/default/files/documents/fema_nuc-detonation-planning-guide.pdf Accessed 9 Jan. 2023.
- 3. Rump, A., Eder, S., Hermann, C., Lamkowski, A., Ostheim, P., Abend, M. and Port, M. Estimation of radiation-induced health hazards from a “dirty bomb” attack with radiocesium under different assault and rescue conditions. Mil. Med. Res. 8, 65, 1–20 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DiCarlo, A. L., Maher, C., Hick, J. L., Hanfling, D., Dainiak, N., Chao, N., Bader, J. L., Coleman, C. N. and Weinstock, D. M. Radiation injury after a nuclear detonation: medical consequences and the need for scarce resources allocation. Disaster Med. Public Health Prep. 5(Suppl 1), S32–S44 (2011). 10.1001/dmp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Swartz, H. M., Wilkins, R. C., Ainsbury, E., Port, M., Flood, A. B., Trompier, F., Roy, L. and Swarts, S. G. What if a major radiation incident happened during a pandemic? Considerations of the impact on biodosimetry. Int. J. Radiat. Biol. 98(5), 825–830 (2022). [DOI] [PubMed] [Google Scholar]
- 6. Flood, A. B., Boyle, H. K., Du, G., Demidenko, E., Nicolalde, R. J., Williams, B. B. and Swartz, H. M. Advances in a framework to compare bio-dosimetry methods for triage in large-scale radiation events. Radiat. Prot. Dosimetry 159(1–4), 77–86 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flood, A. B., Nicolalde, R. J., Demidenko, E., Williams, B. B., Shapiro, A., Wiley, A. L. Jr. and Swartz, H. M. A framework for comparative evaluation of dosimetric methods to triage a large population following a radiological event. Radiat. Meas. 46(9), 916–922 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Swartz, H. M., Swarts, S. G., Ainsbury, E., Wilkins, R. C., Port, M., Trompier, F., Flood, A. B. and Roy, L. Complementary lessons learned from testing strategies used for radiation emergencies and COVID-19: a white paper from the International Association of Biological and Electron Paramagnetic Resonance (EPR) Radiation Dosimetry (IABERD). Radioprotection 57(3), 217–231 (2022). [Google Scholar]
- 9. Buddemeier, B. R., Suski, N. Preparing for the aftermath of a nuclear detonation; an analytic framework for disaster management LLNL-CONF-664395. (Los Angeles, CA: Lawrence Livermore National Laboratory; ) (2014). https://www.osti.gov/servlets/purl/1184108 Accessed 9 Jan. 2023. [Google Scholar]
- 10. Greater New York Hospital Association . Summit: Improvised Nuclear Device (IND) Response Planning Workshop, May 29, 2019. https://www.gnyha.org/wp-content/uploads/2020/01/Summit_IND-Response-Planning-Workgroup_for-postingII.pdf Accessed 9 Jan. 2023.
- 11. DiCarlo, A. L., Poncz, M., Cassatt, D. R., Shah, J. R., Czarniecki, C. W. and Maidment, B. W. Development and licensure of medical countermeasures for platelet regeneration after radiation exposure. Radiat. Res. 176(1), 134–137 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singh, V. K., Swartz, H. M. and Seed, T. M. Radiation medical countermeasures and use of EPR biodosimetry to facilitate effectiveness of applied clinical procedures. Appl. Magn. Reson. 53(1), 289–303 (2022). [Google Scholar]
- 13. DiCarlo, A. L., Poncz, M., Cassatt, D. R., Shah, J. R., Czarniecki, C. W. and Maidment, B. W. Medical countermeasures for platelet regeneration after radiation exposure. Report of a workshop and guided discussion sponsored by the National Institute of Allergy and Infectious Diseases, Bethesda, MD, March 22–23, 2010. Radiat. Res. 176(1), e0001–e0015 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Swartz, H. M., Flood, A. B., Singh, V. K. and Swarts, S. G. Scientific and logistical considerations when screening for radiation risks by using biodosimetry based on biological effects of radiation rather than dose: the need for prior measurements of homogeneity and distribution of dose. Health Phys. 119(1), 72–82 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Waselenko, J. K. et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann. Intern. Med. 140(12), 1037–1051 (2004). [DOI] [PubMed] [Google Scholar]
- 16. Shao, L., Luo, Y. and Zhou, D. Hematopoietic stem cell injury induced by ionizing radiation. Antioxid. Redox Signal. 20(9), 1447–1462 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dainiak, N., Waselenko, J. K., Armitage, J. O., MacVittie, T. J. and Farese, A. M. The hematologist and radiation casualties. Hematology Am. Soc. Hematol. Educ. Program 2003(1), 473–496 (2003). [DOI] [PubMed] [Google Scholar]
- 18. Dainiak, N. Hematologic consequences of exposure to ionizing radiation. Exp. Hematol. 30(6), 513–528 (2002). [DOI] [PubMed] [Google Scholar]
- 19. Singh, V. K., Newman, V. L. and Seed, T. M. Colony-stimulating factors for the treatment of the hematopoietic component of the acute radiation syndrome (H-ARS): a review. Cytokine 71(1), 22–37 (2015). [DOI] [PubMed] [Google Scholar]
- 20. Swartz, H. M. and Flood, A. B. EPR biodosimetry: challenges and opportunities. Radiat. Prot. Dosimetry (in press (2023). [DOI] [PubMed] [Google Scholar]
- 21. Swartz, H. M., Williams, B. B. and Flood, A. B. Overview of the principles and practice of biodosimetry. Radiat. Environ. Biophys. 53(2), 221–232 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Swartz, H. M., Williams, B. B., Nicolalde, R. J., Demidenko, E. and Flood, A. B. Overview of biodosimetry for management of unplanned exposures to ionizing radiation. Radiat. Meas. 46(9), 742–748 (2011). [Google Scholar]
- 23. Tkatchenko, N., Romanyukha, A., Reyes, R., Swarts, S. G., Gourier, D. and Trompier, F. EPR dosimetry in human fingernails: investigation of the origin of the endogenous signal and implications for estimating dose from nail signals. Appl. Magn. Reson. 53(1), 319–334 (2022). [Google Scholar]
- 24. 4 Electron Paramagnetic Resonance Dosimetry. J. ICRU 19(1), 46–68 (2019). [Google Scholar]
- 25. Ikeya, M. and Furusawa, M. A portable spectrometer for ESR microscopy, dosimetry and dating. Int. J. Radiat. Appl. Instrum. Part A: Appl. Radiat. Isotopes 40(10), 845–850 (1989). [Google Scholar]
- 26. Hara, H. and Ikeya, M. Frequency-sweep ESR spectrometer for dosimetry and dating. Int. J. Radiat. Appl. Instrum. Part A: Appl. Radiat. Isotopes 40(10), 841–843 (1989). [DOI] [PubMed] [Google Scholar]
- 27. Ishii, H. and Ikeya, M. An electron spin resonance system for in-vivo human tooth dosimetry. Japanese J. Appl. Phys. 29, 871–875 (1990). [Google Scholar]
- 28. Grinberg, O., Sidabras, J. W., Tipikin, D., Krymov, V., Swarts, S. G. and Swartz, H. M. Dielectric-backed aperture resonators for X-band depth-limited in vivo epr nail dosimetry. Appl. Magn. Reson. 51(9–10), 1093–1101 (2020). [Google Scholar]
- 29. Guo, J. et al. The design of X-band EPR cavity with narrow detection aperture for in vivo fingernail dosimetry after accidental exposure to ionizing radiation. Sci. Rep. 11(1), 2883 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Froncisz, W., Jesmanowicz, A., Kneeland, J. B. and Hyde, J. S. Counter rotating current local coils for high-resolution magnetic resonance imaging. Magn. Reson. Med. 3(4), 590–603 (1986). [DOI] [PubMed] [Google Scholar]
- 31. Hyde, J. S., Strangeway, R. A. and Sidabras, J. W. Dispersion EPR: considerations for low-frequency experiments. Appl. Magn. Reson. 53(1), 193–206 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sidabras, J. W., Varanasi, S. K., Mett, R. R., Swarts, S. G., Swartz, H. M. and Hyde, J. S. A microwave resonator for limiting depth sensitivity for electron paramagnetic resonance spectroscopy of surfaces. Rev. Sci. Instrum. 85(10), 104707 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Swarts, S. G. et al. Developments in biodosimetry methods for triage with a focus on x-band electron paramagnetic resonance in vivo fingernail dosimetry. Health Phys. 115(1), 140–150 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. He, G., Evalappan, S. P., Hirata, H., Deng, Y., Petryakov, S., Kuppusamy, P. and Zweier, J. L. Mapping of the B1 field distribution of a surface coil resonator using EPR imaging. Magn. Reson. Med. 48(6), 1057–1062 (2002). [DOI] [PubMed] [Google Scholar]
- 35. Hirata, H. and Fujii, H. Automatic matching control circuit based on phase-sensitive detection for in vivo CW-EPR spectroscopy. Measure Sci. Tech. 18(5), N27–N31 (2007). [Google Scholar]
- 36. Hirata, H., Yamaguchi, Y., Takahashi, T. and Luo, Z.-W. Control characteristics of an automatic matching control system for in vivo EPR spectroscopy. Magn. Reson. Med. 50(1), 223–227 (2003). [DOI] [PubMed] [Google Scholar]
- 37. Hirata, H. and Swartz, H. M. RF/microwave resonators for preclinical and clinical EPR applications: current status and challenges. Appl. Magn. Reson. 53(1), 167–191 (2022). [Google Scholar]
- 38. Sauer, J. and Sonderegger, A. Methodological issues in product evaluation: the influence of testing environment and task scenario. Appl. Ergon. 42(3), 487–494 (2011). [DOI] [PubMed] [Google Scholar]
- 39. Flood, A. B., Wood, V. A., Schreiber, W., Williams, B. B., Gallez, B. and Swartz, H. M. Guidance to transfer ‘bench-ready’ medical technology into usual clinical practice: case study – sensors and spectrometer used in EPR oximetry. Adv. Exp. Med. Biol. 1072, 233–239 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Money, A. G., Barnett, J., Kuljis, J., Craven, M. P., Martin, J. L. and Young, T. The role of the user within the medical device design and development process: medical device manufacturers’ perspectives. BMC Med. Inform. Decis. Mak. 11(1), 15 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vincent, C., Li, Y. and Blandford, A. Integration of human factors and ergonomics during medical device design and development: It’s all about communication. Appl. Ergon. 45, 413–419 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are reported in this paper are public, as cited.


