Abstract
Purpose/Aim:
Glaucomatous optic neuropathy (GON) remains the world’s leading cause of irreversible blindness. Treatments including topical medications are directed at reducing intraocular pressure (IOP), the most significant risk factor for GON. Current medications, while generally effective, are limited by insufficient response and side-effects in some patients. In search of a more targeted therapy that acts downstream of existing medications that has a potential for a lower side effect profile, our laboratory has identified Stanniocalcin-1 (STC-1), a multifunctional hormone, as an effector molecule in latanoprost-mediated IOP reduction with similar IOP-lowering efficacy as latanoprost in normotensive mice.
Materials and methods:
To investigate whether STC-1 can also reduce IOP in ocular hypertensive mice, we used a steroid-induced ocular hypertensive mouse model characterized by trabecular meshwork dysfunction as well as the DBA/2J mouse as an inherited model of pigment dispersion and secondary angle closure. Steroid-induced ocular hypertension was induced by weekly injections of dexamethasone into the conjunctival fornix of wild-type C57BL/6J mice (6–8 months old). After confirmation of the steroid response, mice were administered STC-1 or phosphate buffered saline (PBS) topically once daily for six weeks. For DBA/2J mice (14 months old), after baseline IOP measurements, mice were treated topically once daily with STC-1 or PBS for 5 days and IOP was assessed twice daily.
Results:
In steroid-induced ocular hypertensive mice, STC-1 lowered IOP by 26% (P < .001, week three) and maintained this level of IOP reduction throughout the remainder of the treatment period (P < .001, week six). In DBA/2J mice, STC-1 lowered IOP by 37% (P < .001).
Conclusions:
Together, these data show that STC-1 reduced IOP in two models of ocular hypertension with different mechanisms of outflow obstruction.
Keywords: Glaucoma, ocular hypertension, intraocular pressure, steroid glaucoma, pigmentary glaucoma, DBA/2J, angle closure, stanniocalcin
Introduction
Glaucomatous optic neuropathy (GON) remains the world’s leading cause of irreversible blindness.1 Currently, the only reliable therapeutic target is the reduction of intraocular pressure (IOP) by means of pharmacologic, laser, or surgical intervention. Of these, topical eye drop monotherapy is generally the initial treatment of choice for patients with GON or ocular hypertension2 of the available medication classes, Prostaglandin F2 alpha analogues (PGF2α) such as latanoprost are often used as first-line therapy3 given their low systemic side-effect profile, once daily dosing, and greater IOP-lowering effects compared to other classes of medications.4–6 However, up to 20% of patients have a diminished response to PGF2α analogues which has been associated with single polymorphisms in the prostaglandin F (FP) receptor.7–12 Additionally, patients can also be intolerant of the medication due to ocular side-effects including conjunctival hyperemia, surface irritation, pigmentation of the iris and periocular skin, orbital fat atrophy, hypertrichosis,13 intraocular inflammation,14,15 reactivation of herpes simplex keratitis and macular edema.16
With the goal of being able to maintain the IOP-lowering properties of latanoprost, avoid the side-effects, and offer a novel therapy to PGF2α non-responders, our laboratory identified Stanniocalcin-1 (STC-1) as a downstream effector molecule in latanoprost-mediated IOP reduction.17 STC-1 is a multifunctional hormone with anti-apoptotic18–20 and anti-oxidative stress properties,18,20–27 and has been shown to provide neuroprotection to cerebral neurons,28–30 retinal photoreceptors,25 and retinal ganglion cells.20 The importance of STC-1 in latanoprost-mediated IOP reduction was demonstrated by the fact that STC-1 knockout mice were unresponsive to latanoprost treatment.17 In addition, STC-1 was also identified as an independent ocular hypotensive agent as it lowered pressure in the human anterior segment perfusion culture model and in wild-type C57BL/6J mice.17 Additionally, unlike latanoprost, STC-1-mediated IOP reduction did not require the FP receptor.31 Though the point of signaling overlap and divergence has not yet been defined, our current data suggests that latanoprost induces expression of STC-1 downstream of the FP receptor in the pathway responsible for IOP reduction. Since previous data regarding STC-1 and IOP regulation were obtained from normotensive mouse models, in the present study, we examined whether STC-1 would also reduce IOP in two separate mouse models of ocular hypertension characterized by different mechanisms of aqueous outflow obstruction.
Materials and methods
All studies were approved by the Mayo Clinic (Rochester, MN) IACUC and adhered to ARVO guidelines. To develop the steroid-induced ocular hypertension model, IOP was measured in both eyes of wild-type C57BL/6J mice (n = 7, 6–8 months old) twice daily with an iCare rebound tonometer and averaged for 3 consecutive days to obtain baseline pressure as previously described.17 At this point, dexamethasone acetate suspension (200 μg in 20 μl volume) was injected weekly into the inferior conjunctival fornix of one eye in a slow release formulation (sodium chloride [0.667 g/100 mL], edetate disodium USP dehydrate [0.05 g/100 mL], sodium bisulfate [0.1 g/100 mL], and creatinine [0/5 g/100 mL], pH 7) as previously described.32 The fellow eye received a weekly injection with slow release formulation (vehicle) without the dexamethasone. IOP was obtained 48 and 72 hrs post-injection, averaged, and recorded as the weekly IOP. After a sustained and elevated IOP response was observed in the dexamethasone injected group (experimental weeks 1–3), mice were randomized into two groups and dexamethasone injections were continued weekly for the duration of the experiment: in Group 1, both eyes were treated once daily with topical PBS (5 μL, n = 8; experimental weeks 4–6, treatment weeks 1–3), and in Group 2, both eyes were treated once daily with topical STC-1 (Biovender, Asheville, NC, 5 μL; 0.5 μg/μL, n = 10; experimental weeks 4–6, treatment weeks 1–3). In the final phase of the experiment (experimental weeks 7–9, treatment weeks 4–6), dexamethasone-injected animals in Group 2 continued to receive topical STC-1 while treatment was halted in the vehicle-injected fellow eye for a medication wash-out period (Table 1). For statistical purposes, the final week of each condition was selected (i.e. weeks 3, 6, and 9) for analysis. To determine if a difference in IOP was present among groups at week 6 and 9, a Kruskal-Wallis test was performed. An unpaired t-test was used to directly compare the two groups. Values for all statistical tests were considered significant at P < .05.
Table 1.
Experimental design for dexamethasone-injected mice. In all animals, one conjunctival fornix received a vehicle injection while the fellow conjunctival fornix received a dexamethasone injection weekly for the duration of the experiment. In the initial treatment phase of the experiment, both eyes of the animals in the control group received topical PBS treatment while both eyes of animals in the treatment group received topical STC-1. In the second treatment phase of the experiment, the vehicle-injected eyes of the STC-1 treatment had a washout of STC-1 treatment.
| Condition | Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Left eye, group 1 (n = 8) | Topical | - | - | - | - | PBS | PBS | PBS | PBS | PBS | PBS |
| Injection | - | Dex | Dex | Dex | Dex | Dex | Dex | Dex | Dex | Dex | |
| Right eye, group 1 (n = 8) | Topical | - | - | - | - | PBS | PBS | PBS | PBS | PBS | PBS |
| Injection | - | Veh | Veh | Veh | Veh | Veh | Veh | Veh | Veh | Veh | |
| Left eye, group 2 (n = 10) | Topical | - | - | - | - | STC-1 | STC-1 | STC-1 | STC-1 | STC-1 | STC-1 |
| Injection | - | Dex | Dex | Dex | Dex | Dex | Dex | Dex | Dex | Dex | |
| Right eye, group 2 (n = 10) | Topical | - | - | - | - | STC-1 | STC-1 | STC-1 | - | - | - |
| Injection | - | Veh | Veh | Veh | Veh | Veh | Veh | Veh | Veh | Veh |
For examination of STC-1 treatment in a chronic model of ocular hypertension and GON, DBA/2J mice aged 14 months (n = 10) were selected. Since variability in IOP is high between eyes and among mice,33 for expression of data in a longitudinal fashion, IOP was expressed normalized to an average of the baseline IOPs. Utilizing mice without corneal calcification,33 baseline IOP measurements were obtained, and then one eye was treated once daily for 5 days with topical STC-1 (5 μL; 0.5 μg/μL) and the fellow eye received topical PBS (5 μL). For statistical purposes, at full treatment response, experimental days 6–8 (treatment days 3–5) were averaged and taken as a single value of IOP. A paired T-test was performed, and values were considered significant at P < .05. For both models, a second laboratory member unfamiliar with the experimental design independently validated the IOP measurements at multiple time-points during the experiment.
For both models histologic analysis was performed. At the conclusion of each experiment, whole eyes were enucleated, fixed, processed, sectioned, stained with toluidine blue, and examined under a light microscope as previously described.17
Results
For the steroid-induced ocular hypertension model, prior to injection of dexamethasone, baseline IOP measurements were assessed and found to be similar between left and right eyes (16.1 ± 1.1 vs 16.2 ± 1.2 mmHg, P > .8, n = 18, Figure 1a,b). Following 3 weekly injections of dexamethasone, a significant increase in IOP in the dexamethasone-injected eyes compared to vehicle-injected eyes occurred (18%, 16.6 ± 1.0 vs 19.7 ± 1.8 mmHg, P < .05, n = 18, Figure 1a,b). This was maintained with weekly dexamethasone injections for 3 additional weeks comparing PBS-treated vehicle-injected mice (n = 8, 15.6 ± 1.1 mmHg) to PBS-treated dexamethasone-injected mice (n = 8, 19.5 ± 1.7 mmHg, P < .001, 25% change, Figure 1a, Group 1). With the steroid-induced ocular hypertensive model established, treatment with topical STC-1 resulted in a 25% IOP reduction when compared to PBS-treatment of dexamethasone-injected eyes (19.5 ± 1.7, n = 8, vs 14.6 ± 1.2 mmHg, n = 10, P < .001, Figure 1a, Group 2, week 6). This was maintained through 3 additional weeks of dexamethasone-injections and topical STC-1 treatments (26%, 19.7 ± 1.3, n = 8, vs 14.6 ± 0.9 mmHg, n = 10, P < .001, Figure 1a, Group 2, week 9). When comparing STC-1-treated dexamethasone-injected eyes (n = 10, Figure 1a, Group 2) to PBS-treated vehicle-injected eyes (n = 8, Figure 1a, Group 1), there was no significant difference in IOP (6%, 14.6 ± 1.0 vs 15.6 ± 1.2 mm Hg, P = .08, Figure 1b) indicating that STC-1 treatment reduced IOP in the steroid-induced ocular hypertension eye to levels seen in normotensive mice.
Figure 1.
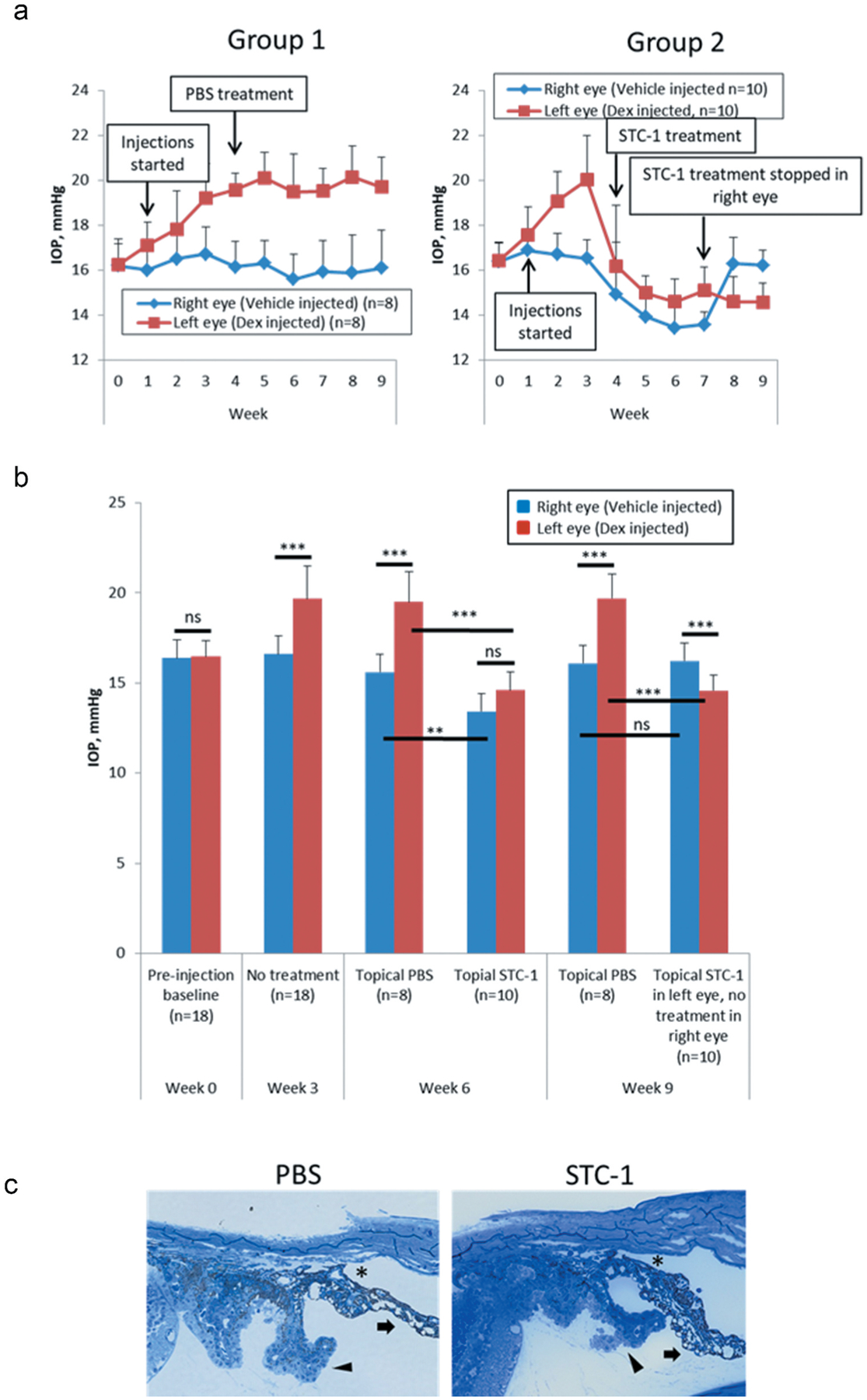
STC-1 reduces IOP in the dexamethasone-injected mouse. (a) Complete experiment displayed in a longitudinal fashion for Group 1 (PBS treatment) and Group 2 (STC-1 treatment). (b) At week 0, there was no difference in IOP between right and left eyes. IOP measurements at week 3 show confirmation of dexamethasone-injected steroid-induced ocular hypertension prior to treatment randomization. At experimental week 6, with topical PBS treatment in both groups, IOP remained higher in dexamethasone-injected mice compared to vehicle-injected mice. STC-1 treatment of vehicle-injected mice showed significantly lower IOP compared to PBS-treated, vehicle-injected mice. STC-1 treatment of dexamethasone-injected mice showed significantly lower IOP than PBS-treated, dexamethasone-injected mice. STC-1-treated, vehicle-injected mice showed no significant difference in IOP compared to STC-1-treated, dexamethasone-injected mice. At experimental week 9, with topical PBS treatment in both groups, IOP remained significantly higher in dexamethasone-injected mice compared to vehichle-injected mice. Vehicle-injected mice with no topical treatment showed no significant difference in IOP compared to PBS-treated vehicle injected mice. STC-1-treated, dexamethasone-injected mice showed significantly lower IOP compared to PBS-treated, dexamethasone-injected mice. STC-1-treated, dexamethasone-injected mice showed lower IOP compared to untreated, vehicle-injected mice. (c) Representative toluidine blue-stained sections of steroid-induced ocular hypertension mice were examined following treatment with PBS and STC-1. In both treatment groups, normal-appearing open angles (asterisk), iris (arrow), and ciliary body (chevron) were observed. *P < .05, **P < .005, ***P < .001. Error bars represent mean ± standard deviation.
In addition to STC-1 lowering IOP in steroid induced ocular hypertension eyes, STC-1 also reduced pressure in normotensive eyes as previously reported.17,31 STC-1 treatment of vehicle-injected eyes (n = 8) reduced IOP from 15.6 ± 1.1 mmHg to 13.4 ± 1.2 mmHg (14% decrease, P < .005, Figure 1a, Group 2). Once STC-1 was washed out from the vehicle-injected eye, IOP returned to baseline levels (16.2 ± 0.7, n = 10, vs 16.1 ± 1.7 mmHg, n = 8, P > .1, Figure 1a Group 2). Interestingly, the IOP was even lower in the STC 1-treated dexamethasone-injected eyes when comparing to the untreated vehicle-injected eyes (10%, 16.2 ± 0.7 vs 14.6 ± 1.0 mmHg, P < .001, Figure 1b).
Representative toluidine blue-stained eye sections of steroid-induced ocular hypertension mice were examined following treatment with PBS and STC-1. In both treatment groups, normal-appearing open angles (asterisk), iris (arrow), and ciliary body (chevron) were observed.
For DBA/2J mice, eyes treated with STC-1 showed a steady decrease in IOP until the treatment plateaued at treatment days 3–5 (Figure 2a). No detectable change in IOP was observed in PBS treated eyes. Using the combined average IOP from treatment days 3–5 compared to baseline, STC-1 lowered IOP compared to vehicle control (37%, 20.7 ± 2.6 mmHg vs 13.1 ± 1.7 mm Hg, P < .001, Figure 2b). Representative toluidine blue-stained sections of 14-month old DBA/2J mice treated with PBS in one eye and STC-1 in the fellow eye showed age-appropriate angle anatomy in this model34 including angle closure with synechiae formation (asterisk), iris atrophy (arrow), and pigment-laden macrophages (chevron). Though subtle variations in elements of the disease phenotype were observed in this chronic model, significant differences in angle morphology were not observed when comparing PBS-treated eyes with STC-1-treated eyes.
Figure 2.
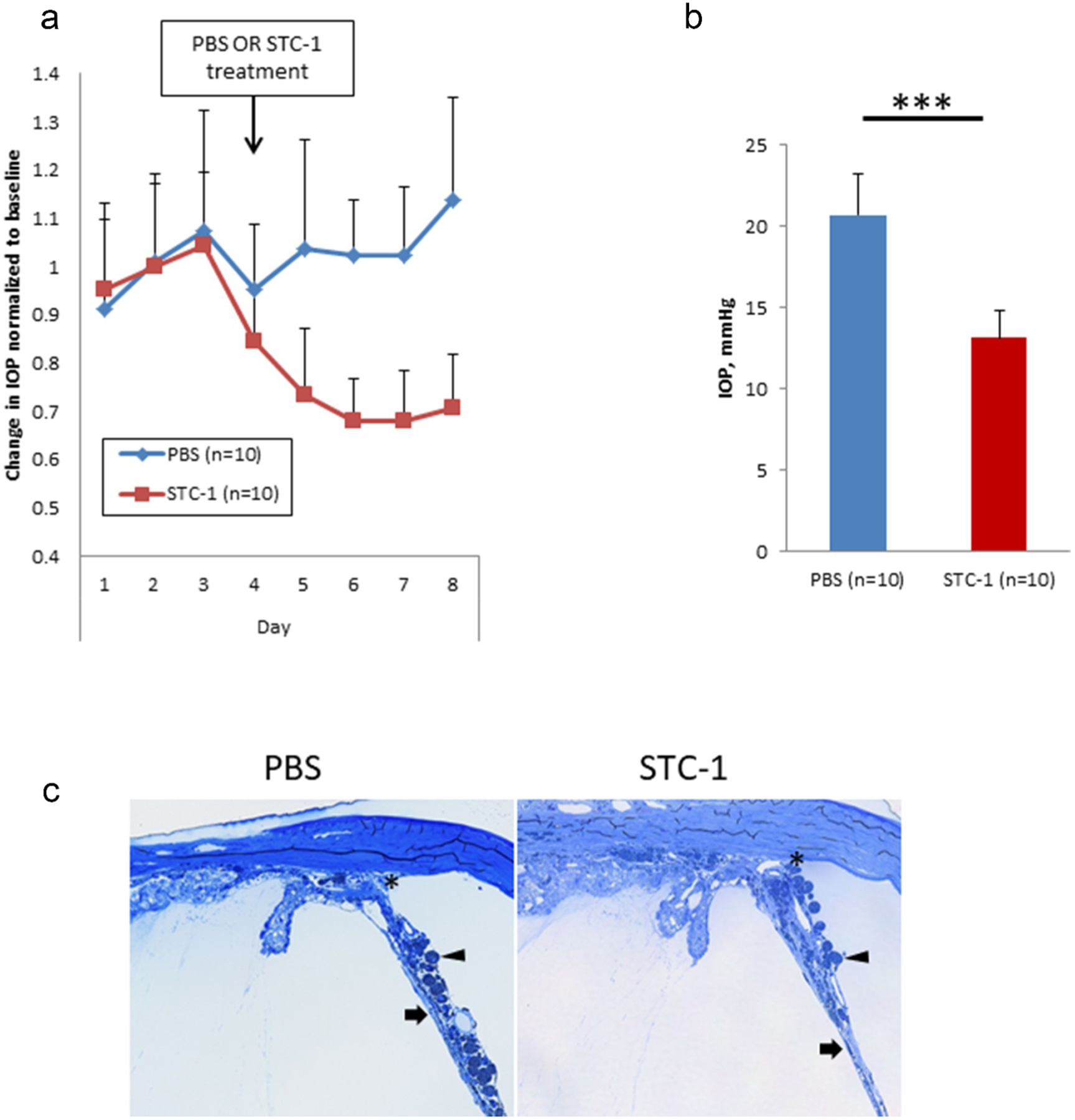
STC-1 reduces IOP in the DBA/2J mouse. (a) Complete experiment displayed in a longitudinal fashion. (b) At experimental days 6–8, STC-1 lowered IOP compared to PBS (n = 10). (c) Representative toluidine blue-stained sections of 14-month old DBA/2J mice treated with PBS in one eye and STC-1 in the fellow eye show angle closure with synechiae formation (asterisk), iris atrophy (arrow), and pigment-laden macrophages (chevron). ***P < .001. Error bars represent mean ± standard deviation.
Discussion
Together, these data show that topical STC-1 reduces IOP in two distinct models of ocular hypertension. The dexamethasone-injected mouse represents a model of secondary open-angle glaucoma characterized by trabecular meshwork dysfunction.32 STC-1 lowered IOP by 26% at treatment weeks 3 and 6 compared to PBS-treated ocular hypertensive mice. This is a much greater IOP reduction than the 14% we observed in the STC-1 treated vehicle-injected eyes in this study and the 15–20% we previously observed when treating wild-type normotensive mice.17,31 Consistent with this observation, latanoprost also has a greater effect clinically at higher IOP.35 While it is unclear as to whether STC-1 is affecting aqueous humor formation, episcleral venous pressure, outflow facility, uveoscleral outflow, or combinations thereof, we hypothesize that the mechanism of action is most likely similar to that of latanoprost (uveoscleral outflow) since it is a downstream effector molecule in latanoprost-mediated IOP reduction.17 Interestingly, STC-1-treated, dexamethasone injected mice had even lower IOP than vehicle-injected normotensive control mice treated with PBS and also had similar IOP as vehicle-injected mice treated with STC-1. While the mechanism for this is not apparent, the data suggests that the effect of STC-1 may be on a general mechanism of IOP reduction (e.g. enhanced outflow) rather than reversal of the pathology induced by dexamethasone.
The DBA/2J inbred mouse is an inherited model of ocular hypertension and GON36 resulting from pigment dispersion.34 Most mice develop elevated IOP by 9 months of age likely due to a combination of trabecular meshwork dysfunction, presence of posterior synechiae, and abnormal proliferation of corneal endothelium which may partially block the flow of aqueous, and ultimately result in a complete, acquired secondary angle closure.33,34,37 In 14 month old DBA/2J mice, STC-1 reduced IOP by 37% compared to PBS. It is important to note that latanoprost has been effective in the treatment of angle closure38 and in DBA/2J mice.39 Therefore, we hypothesize that the mechanism of action of IOP reduction by STC-1 is likely similar to that of latanoprost in this model. While DBA/2J mice develop RGC loss, we did not investigate this as the focus was on the early IOP response. Future experiments will address this issue given the neuroprotective functions of STC-1.25,40
In summary, topical STC-1 reduced IOP in two separate models of ocular hypertension with different mechanisms of outflow obstruction. Further pre-clinical studies including aqueous humor dynamics are needed to understand the mechanism of action of STC-1 in these models.
Funding
Supported by Mayo Foundation (GWR), American Glaucoma Society (GWR), and NEI grant EY21727 (MPF).
Footnotes
Declaration of interest
The authors report no conflicts of interest.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linden C, Heijl A, Johannesson G, Aspberg J, Andersson Geimer S, Bengtsson B. Initial intraocular pressure reduction by mono- versus multi-therapy in patients with open-angle glaucoma: results from the glaucoma intensive treatment study. Acta Ophthalmol. 2018;96:567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doucette LP, Walter MA. Prostaglandins in the eye: function, expression, and roles in glaucoma. Ophthalmic Genet. 2017;38:108–16. [DOI] [PubMed] [Google Scholar]
- 4.King A, Azuara-Blanco A, Tuulonen A. Glaucoma. BMJ. 2013;346 (f3518). [DOI] [PubMed] [Google Scholar]
- 5.Tanna AP, Lin AB. Medical therapy for glaucoma: what to add after a prostaglandin analogs? Curr Opin Ophthalmol. 2015;26(116–120). [DOI] [PubMed] [Google Scholar]
- 6.Winkler NS, Fautsch MP. Effects of prostaglandin analogues on aqueous humor outflow pathways. J Ocul Pharmacol Ther. 2014;30:102–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui XJ, Zhao AG, Wang XL. Correlations of afap1, gmds and ptgfr gene polymorphisms with intra-ocular pressure response to latanoprost in patients with primary open-angle glaucoma. J Clin Pharm Ther. 2017;42:87–92. [DOI] [PubMed] [Google Scholar]
- 8.Zhang P, Jiang B, Xie L, Huang W. Ptgfr and slco2a1 gene polymorphisms determine intraocular pressure response to latanoprost in han chinese patients with glaucoma. Curr Eye Res. 2016;41:1561–65. [DOI] [PubMed] [Google Scholar]
- 9.Ussa F, Fernandez I, Brion M, Carracedo A, Blazquez F, Garcia MT, Sanchez-Jara A, De Juan-Marcos L, Jimenez-Carmona S, Juberias JR, et al. Association between snps of metalloproteinases and prostaglandin f2alpha receptor genes and latanoprost response in open-angle glaucoma. Ophthalmology. 2015;122(5):1040–1048 e1044. [DOI] [PubMed] [Google Scholar]
- 10.Sakurai M, Higashide T, Ohkubo S, Takeda H, Sugiyama K. Association between genetic polymorphisms of the prostaglandin f2alpha receptor gene, and response to latanoprost in patients with glaucoma and ocular hypertension. Br J Ophthalmol. 2014;98:469–73. [DOI] [PubMed] [Google Scholar]
- 11.Sakurai M, Higashide T, Takahashi M, Sugiyama K. Association between genetic polymorphisms of the prostaglandin f2alpha receptor gene and response to latanoprost. Ophthalmology. 2007;114:1039–45. [DOI] [PubMed] [Google Scholar]
- 12.Peng HB, Zahary MN, Tajudin LS, Lin CL, Teck CM, Sidek MR, Zulkifli A, Zilfalil BA. A novel single nucleotide polymorphism, ivs2–97a>t, in the prostaglandin f2alpha receptor gene was identified among the malaysian patients with glaucoma. Kobe J Med Sci. 2007;53:49–52. [PubMed] [Google Scholar]
- 13.Toris CB, Gabelt BT, Kaufman PL. Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv Ophthalmol. 2008;53:S107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith SL, Pruitt CA, Sine CS, Hudgins AC, Stewart WC. Latanoprost 0.005% and anterior segment uveitis. Acta Ophthalmol Scand. 1999;77:668–72. [DOI] [PubMed] [Google Scholar]
- 15.Warwar RE, Bullock JD, Ballal D. Cystoid macular edema and anterior uveitis associated with latanoprost use. Experience and incidence in a retrospective review of 94 patients. Ophthalmology. 1998;105:263–68. [DOI] [PubMed] [Google Scholar]
- 16.Razeghinejad MR. The effect of latanaprost on intraocular inflammation and macular edema. Ocul Immunol Inflamm. 2019;27(2):181–8. [DOI] [PubMed] [Google Scholar]
- 17.Roddy GW, Viker KB, Winkler NS, Bahler CK, Holman BH, Sheikh-Hamad D, Roy Chowdhury U, Stamer WD, Fautsch MP. Stanniocalcin-1 is an ocular hypotensive agent and a downstream effector molecule that is necessary for the intraocular pressure-lowering effects of latanoprost. Invest Ophthalmol Vis Sci. 2017;58:2715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang SE, Wu CP, Wu SY, Peng CK, Perng WC, Kang BH, Chu SJ, Huang KL. Stanniocalcin-1 ameliorates lipopolysaccharide-induced pulmonary oxidative stress, inflammation, and apoptosis in mice. Free Radic Biol Med. 2014;71(321–331). [DOI] [PubMed] [Google Scholar]
- 19.Huang L, Belousova T, Pan JS, Du J, Ju H, Lu L, Zhang P, Truong LD, Nuotio-Antar A, Sheikh-Hamad D. Aki after conditional and kidney-specific knockdown of stanniocalcin-1. J Am Soc Nephrol. 2014;25:2303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SJ, Ko JH, Yun JH, Kim JA, Kim TE, Lee HJ, Kim SH, Park KH, Oh JY. Stanniocalcin-1 protects retinal ganglion cells by inhibiting apoptosis and oxidative damage. PLoS One. 2013;8:e63749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu D, Shang H, Liu Y. Stanniocalcin-1 protects a mouse model from renal ischemia-reperfusion injury by affecting ros-mediated multiple signaling pathways. Int J Mol Sci. 2016;17(1051). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang L, Zhang L, Ju H, Li Q, Pan JS, Al-Lawati Z, Sheikh-Hamad D. Stanniocalcin-1 inhibits thrombin-induced signaling and protects from bleomycin-induced lung injury. Sci Rep. 2015;5(18117). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu LM, Guo R, Hui L, Ye YG, Xiang JM, Wan CY, Zou M, Ma R, Sun XZ, Yang SJ, et al. Stanniocalcin-1 protects bovine intestinal epithelial cells from oxidative stress-induced damage. J Vet Sci. 2014;15(4):475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi X, Wang J, Qin Y. Recombinant adeno-associated virus-delivered hypoxia-inducible stanniocalcin-1 expression effectively inhibits hypoxia-induced cell apoptosis in cardiomyocytes. J Cardiovasc Pharmacol. 2014;64:522–29. [DOI] [PubMed] [Google Scholar]
- 25.Roddy GW, Rosa RH Jr., Oh JY, Ylostalo JH, Bartosh TJ Jr., Choi H, Lee RH, Yasumura D, Ahern K, Nielsen G, et al. Stanniocalcin-1 rescued photoreceptor degeneration in two rat models of inherited retinal degeneration. Mol Ther. 2012;20(4):788–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang L, Belousova T, Chen M, DiMattia G, Liu D, Sheikh-Hamad D. Overexpression of stanniocalcin-1 inhibits reactive oxygen species and renal ischemia/reperfusion injury in mice. Kidney Int. 2012;82:867–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen A, Chang AC, Reddel RR. Stanniocalcin-1 acts in a negative feedback loop in the prosurvival erk1/2 signaling pathway during oxidative stress. Oncogene. 2009;28:1982–92. [DOI] [PubMed] [Google Scholar]
- 28.Durukan Tolvanen A, Westberg JA, Serlachius M, Chang AC, Reddel RR, Andersson LC, Tatlisumak T. Stanniocalcin 1 is important for poststroke functionality, but dispensable for ischemic tolerance. Neuroscience. 2013;229:49–54. [DOI] [PubMed] [Google Scholar]
- 29.Westberg JA, Serlachius M, Lankila P, Penkowa M, Hidalgo J, Andersson LC. Hypoxic preconditioning induces neuroprotective stanniocalcin-1 in brain via il-6 signaling. Stroke. 2007;38:1025–30. [DOI] [PubMed] [Google Scholar]
- 30.Zhang K, Lindsberg PJ, Tatlisumak T, Kaste M, Olsen HS, Andersson LC. Stanniocalcin: a molecular guard of neurons during cerebral ischemia. Proc Natl Acad Sci U S A. 2000;97:3637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roddy GW, Rinkoski TA, Monson KJ, Chowdhury UR, Fautsch MP. Stanniocalcin-1 (stc-1), a downstream effector molecule in latanoprost signaling, acts independent of the fp receptor for intraocular pressure reduction. PLoS One. 2020;15:e0232591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel GC, Phan TN, Maddineni P, Kasetti RB, Millar JC, Clark AF, Zode GS. Dexamethasone-induced ocular hypertension in mice: effects of myocilin and route of administration. Am J Pathol. 2017;187:713–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner AJ, Vander Wall R, Gupta V, Klistorner A, Graham SL. Dba/2j mouse model for experimental glaucoma: pitfalls and problems. Clin Exp Ophthalmol. 2017;45:911–22. [DOI] [PubMed] [Google Scholar]
- 34.John SW, Smith RS, Savinova OV, Hawes NL, Chang B, Turnbull D, Davisson M, Roderick TH, Heckenlively JR. Essential iris atrophy, pigment dispersion, and glaucoma in dba/2j mice. Invest Ophthalmol Vis Sci. 1998;39:951–62. [PubMed] [Google Scholar]
- 35.Denis P, Baudouin C, Bron A, Nordmann JP, Renard JP, Rouland JF, Sellem E, Amrane M. First-line latanoprost therapy in ocular hypertension or open-angle glaucoma patients: a 3-month efficacy analysis stratified by initial intraocular pressure. BMC Ophthalmol. 2010;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheldon WG, Warbritton AR, Bucci TJ, Turturro A. Glaucoma in food-restricted and ad libitum-fed dba/2nnia mice. Lab Anim Sci. 1995;45:508–18. [PubMed] [Google Scholar]
- 37.Chang B, Smith RS, Hawes NL, Anderson MG, Zabaleta A, Savinova O, Roderick TH, Heckenlively JR, Davisson MT, John SW. Interacting loci cause severe iris atrophy and glaucoma in dba/2j mice. Nat Genet. 1999;21:405–09. [DOI] [PubMed] [Google Scholar]
- 38.Chen R, Yang K, Zheng Z, Ong ML, Wang NL, Zhan SY. Meta-analysis of the efficacy and safety of latanoprost monotherapy in patients with angle-closure glaucoma. J Glaucoma. 2016;25:e134–144. [DOI] [PubMed] [Google Scholar]
- 39.Sawada K, Hiraoka M, Ohguro H. Effect of antiglaucoma medicine on intraocular pressure in dba/2j mice. Ophthalmic Res. 2016;55:205–11. [DOI] [PubMed] [Google Scholar]
- 40.Roddy GW, Yasumura D, Matthes MT, Alavi MV, Boye SL, Rosa RH Jr., Fautsch MP, Hauswirth WW, LaVail MM. Longterm photoreceptor rescue in two rodent models of retinitis pigmentosa by adeno-associated virus delivery of stanniocalcin-1. Exp Eye Res. 2017;165(175–181). [DOI] [PMC free article] [PubMed] [Google Scholar]


