Abstract
Alzheimer’s disease (AD) is a progressive and neurodegenerative illness which results in alterations in cognitive development. It is characterized by loss/dysfunction of cholinergic neurons, and formation of amyloid plaques, and formation of neurofibrillary tangles, among other changes, due to hyperphosphorylation of tau-protein. Exposure to pesticides in humans occurs frequently due to contact with contaminated food, water, or particles. Organochlorines, organophosphates, carbamates, pyrethroids and neonicotinoids are associated with the most diagnosed incidents of severe cognitive impairment. The aim of this study was to determine the effects of these pesticides on the phosphorylation of tau protein, and its cognitive implications in the development of AD. It was found that exposure to pesticides increased the phosphorylation of tau protein at sites Ser198, Ser199, Ser202, Thr205, Ser396 and Ser404. Contact with these chemicals altered the enzymatic activities of cyclin-dependent kinase 5 and glycogen synthase kinase 3 beta, and protein phosphatase-2A. Moreover, it altered the expression of the microtubule associated protein tau gene, and changed levels of intracellular calcium. These changes affected tau protein phosphorylation and neuroinflammation, and also increased oxidative stress. In addition, the exposed subjects had poor level of performance in tests that involved evaluation of novelty, as test on verbal, non-verbal, spatial memory, attention, and problem-solving skills.
Keywords: Organochlorines, Organophosphates, Carbamates, Pyrethroids, Neonicotinoids, Tau protein
Core Tip: Exposure to pesticides occurs frequently through contact with contaminated particles, food, or water. In 2022, the Alzheimer’s Association emphasized that contact with these pollutants is a risk factor for Alzheimer diseases. This study showed that contact with organochlorines, organophosphates, carbamates, pyrethroids and neonicotinoids modified mechanisms related to tau hyperphosphorylation and neuroinflammation. In cognitive findings, these chemicals altered memory, attention, and problem-solving processes. Few published studies have evaluated the effect of these pesticides on tau protein. Therefore, this review is novel in the sense that it presents an analysis for each pesticide class.
INTRODUCTION
Alzheimer’s disease (AD) represents 60%-70% of the cases of major cognitive disorders (MCD) worldwide. In 2022, data from the World Health Organization[1] showed that approximately 55 million people were diagnosed with MCD. Additional data from Alzheimer’s Disease International indicate that by 2030, this figure may increase to 78 million, with most cases expected to come from developing countries[2]. Amongst the risk factors associated with the development and progression of AD, exposure to pollutants has been linked to poor cognitive impairment[3]. These pollutants comprise organochlorine (OCs) pesticides, organophosphates (OPs), carbamates (Cs), pyrethroids (Ps) and neonicotinoid insecticides (Ns). Majority of them have neurotoxic potential. Environmental pollution by pesticides occurs through airborne dust particles, resulting in frequent contamination of air, water and food. Approximately, 30% of these chemicals are dispersed in powder form, while the remaining 70% are volatilized into the environment from surfaces where they are applied[4-7]. Another indirect form of contact with these chemicals is through consumption of contaminated water and food. Previous studies from India, Brazil, Lithuania, Egypt, Turkey, Mexico, and Venezuela revealed presence of contamination in vegetables, fruits, cereals, and water via exposure to OCs, OPs, Cs, Ps and Ns[5,8-10].
Pesticides are organic and hydrophobic molecules which are easily absorbed through different routes of exposure in humans. Pesticides are distributed mainly in lipid tissues of the body where they bioaccumulate as residues[5,11]. It is important to note that the brain and central nervous system (CNS) are rich in lipids, mainly sphingolipids, cholesterol, glycerophospholipids and omega-3 and omega-6 polyunsaturated fatty acids[12]. Thus, the brain and CNS are anatomical sites vulnerable to pesticides due to physicochemical affinity[5,11]. Previous studies indicate that in CNS, exposure to pesticides alters neurogenesis and leads to cognitive impairment[13,14]. However, there is still doubt about the involvement pesticides in the development of AD, and the underlying pathophysiological mechanisms. Most of the published studies on patients with AD or in experimental models were focused mainly on evaluation of the effects of exposure to two classes of pesticides: OC and OP. There are limited reports on effect of exposure to Cs, Ps and Ns, and the impact of the pesticides on tau protein phosphorylation. Tau protein, which is expressed in the distal extremity of the axon, controls the stability of microtubules. Hyperphosphorylation of tau protein stimulates the dissociation of microtubules, interrupts axonal extension, and enhances the aggregation of insoluble tau, leading to alterations in the synapse, and hence tauopathy[15,16]. Therefore, the present this study was aimed at investigating the effects of OC, OP, C, P and N pesticides on the phosphorylation of tau protein, and the associated cognitive implications in the development of AD.
PESTICIDES AND CNS EFFECTS
In a general way, the major reported effects of pesticide exposure on CNS are changes in enzymatic activity of acetylcholinesterase (AchE), blockage of receptors, blockage of transport channels, changes in steroidal hormonal responses, mitochondrial damage, and increased oxidative stress, all of which affect motor, sensory, autonomous, and cognitive functions[14,17,18]. Specifically, OC pesticides block calcium-dependent sodium-potassium pump and chloride channels, a phenomenon that generates antagonistic effect on the neurotransmitter gamma aminobutyric acid (GABA), leading to increases in CNS excitotoxicity[4,17,19]. On the other hand, OP and C pesticides inhibit AchE: OPs bind irreversibly to the active site of AchE, while Cs binds reversibly to AchE[4,17,18]. The inhibition of AchE increases the concentration of acetylcholine, thereby overstimulating postsynaptic muscarinic receptors[4,19,20]. Therefore, exposures to OP and C pesticides have been linked to the development of MCD through changes in acetylcholine levels[4]. The class P pesticides act via 3 different mechanisms. Firstly, they bind to voltage-dependent sodium channels, thereby modifying their conformations. This affects the transition from ion to non-conductive state which results in a higher sodium input. Secondly, they block the binding of calcium to calmodulin, resulting in increases in calcium ion concentration which alter the neurotransmission and depolarization of the N-methyl-D-aspartate receptor. Thirdly, they bind to chloride-dependent GABA receptors. These 3 mechanisms alter muscarinic, adrenergic, and serotonergic neurotransmissions, resulting in symptoms such as tremor, prostration, sensitivity to stimuli, choreoathetosis, salivation and clonic seizures[4,17,21]. The N pesticides are nicotinic receptor agonists in CNS postsynaptic neurons. The nicotinic receptors are part of important ion channels in the neurotransmission functions of acetylcholine, GABA, glycine, and glutamate. Therefore, exposure to N pesticides triggers nicotinic syndrome which involves the respiratory and cardiovascular functions, as well as CNS[4,17,22].
Other disturbances induced by pesticide exposure are linked to estrogen steroid receptors and steroid nuclear receptors. Interactions of pesticides with these receptors alter various metabolic and genetic pathways, which may lead to multiple pathologies, including AD[18,23]. It has been reported that several pesticides act as endococcal disruptors due to their ability to inhibit cytochrome P450 enzyme complex in the brain, with adverse impact on the synthesis of steroid hormones, vitamins, retinoic acid, and thyroid hormones[18]. It has been reported that exposure to hydroxychlor, an OC pesticide, resulted in antagonism of estrogen receptors alpha and beta, a situation which may lead to neurological alterations[24]. Besides, due to their hydrophobic characteristics, the OC and OP pesticides are easily incorporated into the mitochondria along with mitochondrial respiratory chain translocating proteins, leading to enhanced oxidative damage, increased mitochondrial permeability, induction of apoptosis, and decreased synthesis of ATP[25].
Additionally, pesticide-induced overactivation of cholinergic/glutamatergic responses and increased concentration of intracellular calcium ion, are associated with increased free radicals, mainly reactive nitrogen species and reactive oxygen species, thereby tilting the oxidant-antioxidant balance towards pro-oxidants[25,26]. A break in the mitochondrial oxidant-antioxidant homoeostasis results in loss of neuronal synapses, as well as neuropathological, neurochemical, and neurobehavioral alterations[4].
PESTICIDES AND THEIR IMPACTS ON TAU PROTEIN
Tau protein and tauopathies
AD is characterized by loss of cholinergic neurons or dysfunctional cholinergic neurons, formation of amyloid plaques, and formation of neurofibrillary tangles, due to hyperphosphorylation of tau-protein[15,27]. Based on the objective of this work, the characteristics of tau protein and its response to exposure to pesticides are highlighted in this review.
Physiologically, the tau protein is involved in myelination processes[16,28], regulation of glucose metabolism[29], rearrangement of microtubules[16], axonal transport[16], iron homeostasis[16], as well as neurogenesis and processes related to learning and memory[16,28]. However, exposure to pesticides may affect the phosphorylation of tau protein and the formation of neurofibrils, resulting in morphological changes in CNS[30,31].
Tau protein is expressed in six different isoforms: 2N4R, 1N4R, 0N4R, 2N3R, 1N3R and 0N3R, depending on their amino and carboxyl groups. These isoforms come from the alternative cutting and splicing of the microtubule associated protein tau (MAPT) gene located on position q21 of chromosome 17[16,28]. Tau is a 441-amino acid hydrophilic protein sub-classified into 4 domains. The sites susceptible to phosphorylation correspond to the amino acids Ser198, Ser199 and Ser202[32]. The amino acids Thr181, Thr205 and Thr217 are associated with the early stages in the development of AD[16,28], while amino acid residues Ser262, Ser396 and Ser404 are associated with formation of aggregates[31].
Tauopathies are pathologies that arise as a result of alterations in the phosphorylation of tau. Often, these alterations are the result of imbalance involving two kinases: Cyclin-dependent kinase 5 (Cdk5) and glycogen synthase kinase 3 beta (GSK-3β), and protein phosphatase-2A (PP2A). The enzymes Cdk5 and GSK-3β are responsible for phosphorylating tau protein, whereas PP2A dephosphorylates the protein. A close relationship has been reported between GSK-3β and PP2A, both of which regulate each other. For example, when GSK-3β is activated, PP2A is inactivated by auto-phosphorylation at its amino acid residue Tyr-307[28,32-34]. Moreover, the regulation of GSK-3β also depends on routes modulated by calcium/calmodulin, and on the ratio guanosine-5'-triphosphate/guanosine diphosphate. Another important alteration in tauopathy is the phosphorylation of proteins associated with microtubules-2 (MAP-2) which are regulated by cyclic adenosine monophosphate. The MAP-2 is important in stabilizing microtubule assembly, and it is intimately bound to tau protein. Thus, it has been reported that multiple axopathies are associated with abnormalities in these pathways[35,36].
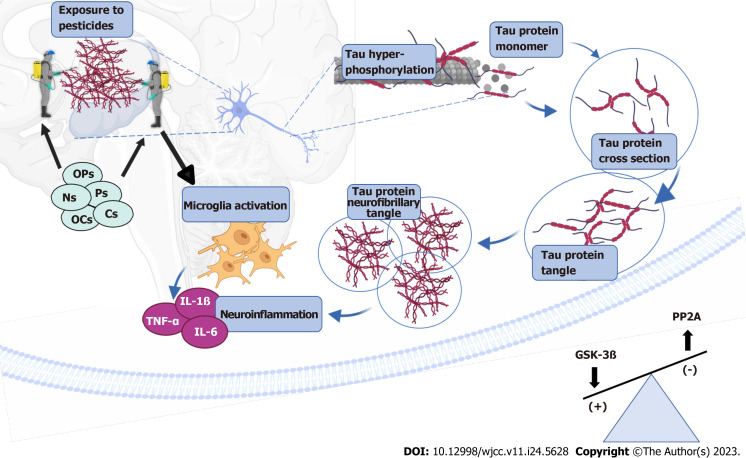
In AD, increased hyperphosphorylation of tau forms aggregates in neuronal cytoplasm, resulting in generation of the so-called neurofibrillary tangles and neurotrophic neurites, which are responsible for neurodegeneration[28,31,37,38]. This phenomenon induces morphological changes in dendrites and causes axonal shortening which alters neuronal plasticity and response to neurotransmitters, leading to problems associated with spatial memory, motor skills and learning, all of which are characteristics of AD[33,34,39]. In addition, the release of tau aggregates in the cytoplasm increases immunoreactivity and oxidative stress which influence neuroinflammation[34,40]. Increased oxidative stress over-activates GSK-3β, thereby making tau protein more vulnerable to formation of aggregates and new neurofibrils[41]. Results from multiple studies indicate that exposure to pesticides modifies the mechanisms involved in tau phosphorylation[32-34,42,43] (Figure 1).
Figure 1.
Pesticide effect on phosphorylation in tau. Exposure to pesticides organochlorine, organophosphate, carbamate, pyrethroid and neonicotinoid alters the balance of enzymatic activity of glycogen synthase kinase 3 beta (GSK-3β) and protein phosphatase-2A (PP2A). Especially the increase of GSK-3β and the decrease of PP2A favors tau hyperphosphorylation and the formation of neurofibrillary tangle which induce the activation of microglia and neuroinflammations. Created with Biorender.com. OCs: Organochlorine; OPs: Organophosphate; Cs: Carbamate; Ps: Pyrethroid; Ns: Neonicotinoid; GSK-3β: Glycogen synthase kinase 3 beta; PP2A: Protein phosphatase-2A; TNF: Tumor necrosis factor; IL: Interleukin.
Effect of OCs on the tau protein
Table 1 shows 7 studies in which the effect of OCs on tau protein phosphorylation was determined[42,44-49]. Two clinical studies reported that exposure to these pesticides may be associated with polymorphisms in MAPT and microtubule associated protein 1B gene which are related to the formation of tau aggregates[44,45]. Studies have demonstrated that dichlorodiphenyltrichloroethan exposure to an OC altered mitochondrial function, resulting in the formation of tau aggregates, with up-regulations in the expressions of proteins such as synaptosome-associated protein 25 kDa, cytochrome C, enolase A, hemoglobin alpha chain and histone cluster 1, which are characteristic of AD[42,46,47]. Finally, Mir et al[48] has shown that exposure to 2,3,7,8 tetrachlorodibenzo-p-dioxin induced overexpression of GSK-3β, and hence tau phosphorylation. In all, 4 of the 7 studies described in Table 1 reported increases in tau phosphorylation[42,44,46,48]. Therefore, OCs have been associated with development of tauopathy which leads to axonal instability, mitochondrial dysfunction and neuroinflammation[44].
Table 1.
Effect of organochlorine pesticides on tau protein
|
Type of study
|
Sample
|
Type of pesticide
|
Exposure data
|
Tau phosphory-lation
|
GSK-3β
|
PP2A
|
Other mechanisms
|
Ref.
|
| Clinical/epidemiological studies. Cohort | 13 postmortem brains of humans without exposure, and 4 postmortem brains of humans with exposure | OCs | Concentration: NA. Exposure time: From 0 to 10 yr | Increased | NA | NA | Exposure altered mitochondrial genes encoding MAPT and MAP1B. These are associated with MAPT phosphorylation and neurite formation that contributed to the development of tauopathies | [44] |
| Clinical/epidemiological studies. Cross-sectional | 90 subjects with PD, and 90 healthy subjects | δ-HCH | Concentration: NA. Exposure time: NA | NA | NA | NA | Exposure was associated with MAPT rs16940758 polymorphism which was related to tau aggregation | [45] |
| Experimental studies | Strains of Caenorhabditis elegans (N2 BR5270) | DDT | Concentration: 3 μM. Exposure time of 2 h | Increased | NA | NA | DDT exacerbated tau protein toxicity, reduced mitochondrial respiration, and induced apoptosis | [42] |
| Experimental studies | Strains of Caenorhabditis elegans (N2 BR5271) | DDT | Concentration: 3 μM. Exposure time of 2 h | Increased | NA | NA | Exposure to DDT increased tau protein aggregation and modified mitochondrial respiration | [46] |
| Experimental studies | Female largemouth bass | Dieldrin | Concentration: 3.0 mg/kg. Exposure time of 57 d | NA | NA | Decreased | Increased expression of proteins in hypothalamus such as Snap25, Cytc, Eno1, Hba1, and H2bb. These proteins were elevated in the pathophysiology of mice with AD and were associated with tau protein. Additionally, downregulation of MAPT was observed, which affected phosphatase activity | [47] |
| Experimental studies | Wistar rats | Chlordane | Range of concentration: 1 to 100 nM. Chronic exposure | Not modified | NA | NA | No significant changes in tau protein levels from exposure to chlordane | [49] |
| Review studies | Multiple studies | TCDD | Range of concentration: 5-23 ppt. Single dose | Increased | Increased | NA | Increased intracellular calcium levels and tau phosphorylation in neurons through overexpression of GSK-3β and hence its enzymatic activity | [48] |
OCs: Organochlorine; NA: Not available; MAPT: Microtubule associated protein tau; δ-HCH: δ-hexachlorocyclohexane; DDT: Dichlorodiphenyltrichloroethan; AD: Alzheimer’s disease; TCDD: 2,3,7,8 tetrachlorodibenzo-p-dioxin; GSK-3β: Glycogen synthase kinase 3 beta; PD: Parkinson disease; ppt: Parts per thousand; PP2A: Protein phosphatase-2A.
Effect of OPs on the tau protein
Exposure to OPs (chlorpyrifos, paraquat and malathion) increases the level of hyperphosphorylated tau protein, overstimulates glial cells, and increases the levels tumor necrosis factor-α, interleukin (IL)-6, IL-β, chemokines, NADPH oxidase 2, NADPH oxidase and COX-2, thereby inducing neuroinflammation. Intensified inflammation accelerates pathologies associated with neurodegenerative processes[33,40,50]. Similarly, when OP pesticides are transported through the blood-brain barrier, a process regulated by Na+ dependent transporters, microglia are activated, resulting in redox imbalance which affects the mitochondrial respiratory chain at mitochondrial complex I, leading to deterioration of CNS function[51,52]. Table 2 provides a breakdown of 18 studies on the effect of OPs on tau protein[31,33,36,37,39,41,49,50,51,53-61]. The only report on cases and controls with OP exposure for more than 2 years showed higher levels of tau phosphorylation in exposed subjects[53]. On the other hand, eight out of eleven studies indicate that exposure to these pesticides increased tau phosphorylation through different mechanisms involving GSK-3β overexpression, increased Cdk5 activity, and decreased expression of PP2A, among other factors (Table 2). Six review studies described in Table 2 reported increases in tau phosphorylation related to greater Cdk5 activity, with changes in regulatory proteins MAPT and MAP-2, and increased oxidative stress, among other changes.
Table 2.
Effect of organophosphates pesticides on tau protein
|
Type of study
|
Sample
|
Type of pesticide
|
Exposure data
|
Tau phosphory-lation
|
GSK-3β
|
PP2A
|
Other mechanisms
|
Ref.
|
| Clinical/epidemiological studies. Cases and controls, unpaired | 33 humans exposed to OPs and 33 humans without exposure | OPs | Concentration: NA. Exposure time 2 yr | Increased | NA | NA | Subjects exposed to OPs for more than 10 yr showed 97% higher serum concentration of phosphorylated tau, when compared to the control group | [53] |
| Experimental studies | C57BL/6 and 129/Sv mice | Paraquat | Concentration: 10 mg/kg. Exposure time for 6 wk | Increased | Increased | NA | Exposed mice showed a 67% increase in hyperphosphorylation of tau in Ser262, Ser396 and Ser404 in striata region, suggesting that paraquat may inhibit the proteosome 20S as tau overexpression occurs. Thus, it was inferred that the proteosomal activity was reduced by exposure to paraquat | [31] |
| Experimental studies | Wistar rats | Malathion | Concentration: 100 mg/kg. Exposure time of 14 d | Increased | Increased | Decreased | The level of hyperphosphorylated tau protein in rats with exposure was increased in Thr205 and Ser404. This result may be related to phosphatase inactivation and increased GSK-3β activity. In addition, a decrease in the expression of mRNA of PP2A was reported due to the exposure to malathion | [33] |
| Experimental studies | MAP-rich tubulin from Sus Scrofa from porcine brain | Chlorpyrifo- oxon, paraoxon and diazoxon | Concentration: 100 μM. Exposure time of 48 h | Increased | NA | NA | Cross-link was formed between MAP-tubulin (alpha), at residues Lys163, Lys336 and Asp98 of MAP with residues Glu158 and Lys115 of tubulin beta. Lys336 and 163 cross-links covalently joined with tau protein, forming Lys-adduct, which resulted in unstable microtubules | [36] |
| Experimental studies | FVB and C57BL/6 mice | DFP | Concentration: 5 mg/kg. Exposure time of 15 d | Increased | NA | NA | Exposure increased Cdk5 activity by converting p35 to p25. Exposure to DFP increased 15.5 ± 2 times the phosphorylation of Cdk5 in Thr205 and therefore of tau protein, thereby inducing neurological effects in the striatum and hippocampus | [37] |
| Experimental studies | Wistar rats | Chlorpyrifos- oxon | Range of concentration: 1 to 100 nM. Chronic exposure | Not modified | NA | NA | No significant changes in tau protein levels from chlorpyrifos exposure | [49] |
| Experimental studies | Transgenic AD model rats | Chlorpyrifos | Concentration: 3 and 10 mg/kg. Exposure time of 21 d | Not modified | NA | NA | No changes in hyperphosphorylation of rat tau protein with exposure to control rats | [50] |
| Experimental studies | Wistar rats | Paraquat | Concentration: 0.1 mg/kg. Exposure time of 4 mo | Increased | NA | NA | In exposed rats, neurofibrillary tangle was formed in the compact pars of the substantia nigra region and in extracellular neuritic plaques as a result of a neuroinflammatory cascade by the activation of microglia and astrocytes, which increased tau phosphorylation | [51] |
| Experimental studies | NMRI mouse | Chlorpyrifos | Concentration: 0.1, 1.0, 5.0 mg/kg. Single dose | Not modified | NA | NA | No significant differences were observed in tau levels due to exposure to this pesticide | [54] |
| Experimental studies | Cell culture in septal SN56 basal forebrain cholinergic neurons | Chlorpyrifos | Concentration: 30 μM. 24 h and 14 d exposure time | Increased | Increased | NA | Exposure to OPs upregulated the expression of GSK-3β and its activity, thereby increasing the phosphorylation of tau | [55] |
| Experimental studies | Cell culture hiPSC and Wistar rats | DFP | Cell culture concentration: 200 nM. Exposure time for 2 d. Murine concentration: 1.5 mg/kg. Exposure time for 7 d | Increased | NA | NA | Exposure was associated with increased tau phosphorylation and decreased microtubule acetylation which decreased its stability. In the CA3 region of the hippocampus, an increase in tau phosphorylation was observed, indicating that it is a vulnerable site for the action of OPs | [56] |
| Experimental studies | Wistar rats | Dichlorvos | Concentration: 200 mg/kg. Single dose | Increased | NA | NA | Increased phosphorylation of MAP-2 and tubulin. Exposure increased phosphorylation and stimulated increased activity of calcium-dependent kinases/calmodulin and cAMP. Microtubules were destabilized, resulting in changes in morphology and increased neurotoxicity in exposed rats | [57] |
| Review studies | Multiple studies | Malathion | Range of concentration: 97 to 775 μM in model MCF-7. Concentration: 100 mg/kg in Wistar rats. Single dose | Increased | Increased | Decreased | MAP-2 hyperphosphorylation was observed, especially of KGS amino acids. This may be related to ubiquitination and protein degradation with these amino acids. Tau hyperphosphorylation is associated with GSK-3β kinase activation and phosphatase inhibition | [39] |
| Review studies | Multiple studies | Paraquat | NA | Increased | NA | NA | Paraquat raised levels of oxidative stress, thereby inducing phosphorylation of tau, based on several studies conducted in cell cultures | [41] |
| Review studies | Multiple studies | OPs | NA | Increased | NA | NA | Exposure to OPs increased Cdk5 hyperactivity and tau hyperphosphorylation. This disrupted the structure and function of microtubules in patients with AD, thereby affecting axonal transport. Even low levels of exposure caused changes in microtubules | [58] |
| Review studies | Multiple studies | OPs | NA | Increased | Increased | NA | Increased the level of reactivity autoantibodies against microtubule-associated proteins and tau-regulatory proteins (MAPT and MAP-2) | [59] |
| Review studies | Multiple studies | OPs ester | Different conditions | Increased | NA | NA | The activities of kinase enzymes were altered phosphorylation of Ser or Thr. This enhanced the aggregation of proteins and the formation of neurofibrils, thereby inducing neurodegeneration. The target enzymes are calcium/calmodulin dependent kinases that increase phosphorylation of MAP-2 and tau protein | [60] |
| Review studies | Multiple studies | Methamido-phos, trichlorfon, dichlorvos, chlorpyrifos | Different conditions | Increased | NA | NA | Increased activity of calcium-dependent kinases/calmodulin, forming aberrations in the phosphorylation of cytoskeleton proteins, a common feature in neurodegenerative diseases | [61] |
OPs: Organophosphates; NA: Not available; DFP: Diisopropyl fluorophosphate; Thr: Threonine; K: Lysine; G: Glycine; S: Serine; Ser: Serine; mRNA: Messenger ribonucleic acid; hiPSC: Human-induced pluripotent stem cells; Lys: Lysine; Asp: Aspartate; Glu: Glutamate; MAPT: Microtubule associated protein tau; AD: Alzheimer’s disease; TCDD: 2,3,7,8 tetrachlorodibenzo-p-dioxin; GSK-3β: Glycogen synthase kinase 3 beta; MAP-2: Microtubules-2; Cdk5: Cyclin-dependent kinase 5; cAMP: Cyclic adenosine monophosphate; PP2A: Protein phosphatase-2A.
Effect of Cs on the tau protein
There are only a few studies on the effect of C pesticides on tau phosphorylation. It is important to highlight that there are no clinical or epidemiological studies on this topic, to date. Most of the studies analyzed in this review indicate that exposure to Cs led to hyperphosphorylation of tau[32,54,62,63]. Only two studies, reported otherwise[31,64]. Increased hyperphosphorylation may be mediated by increased GSK-3β activity and PP2A inhibition (Table 3). In a murine model, exposure to carbofuran, a C pesticides resulted in neuronal death at the cortex and hippocampus, as well as alterations in spatial memory and learning processes[65]. It is interesting to note that C pesticides are currently being used for their therapeutic potential as AchE inhibitors in different pathologies[64,66,67]. More details associated with the effect of exposure to C pesticides on tau protein are presented in Table 3.
Table 3.
Effects of carbamate pesticides on tau protein
|
Type of study
|
Sample
|
Type of pesticide
|
Exposure data
|
Tau phosphorylation
|
GSK-3β
|
PP2A
|
Other mechanisms
|
Ref.
|
| Experimental studies | C57BL/6 and 129/Sv mice | Maneb | Concentration: 30 mg/kg. Exposure time for 6 wk | Decreased | Decreased | NA | No changes in tau phosphorylation. However, if combined with paraquat, tau phosphorylation was enhanced in Ser202 (38% more), Ser262 (28% more) and Ser396/404 (141% more) | [31] |
| Experimental studies | Sprague-Dawly rats | Carbofuran | Concentration: 1 mg/kg. Exposure time of 28 d | Increased | Increased | Decreased | Increased phosphorylation of tau was observed in Ser198/199/202, Thr205 and Ser404. In addition, there was an increase in GSK-3β and a decrease in PP2A | [32] |
| Experimental studies | NMRI mouse | Carbaryl | Concentrations: 0.5, 5.0, 20.0 mg/kg. Single dose | Increased | NA | NA | In the hippocampus, levels of phosphorylated tau increased by 135% in rats exposed to low, medium and high doses. In cerebral cortex, there was oscillating increase of 155% to 210% in tau phosphorylation | [54] |
| Experimental studies | Sprague-Dawly rats | Deltametrin (P)/carbofuran (Cs) | Concentration: NA. Exposure time for 28 d | Increased | Increased | Decreased | Exposure induced tau hyperphosphorylation and GSK-3β activation, as well as PP2A phosphatase inhibition | [63] |
| Review studies | Multiple studies | Cs | NA | Increased | Increased | NA | Exposure induced increased activity of kinase, thereby increasing phosphorylation of tau protein | [62] |
| Review studies | Multiple studies | Pyridine carbamate | Concentrations: 15.7 μM. Single dose | Decreased | NA | NA | An inhibitory effect on phosphorylation was observed. This prevented the aggregation of tau protein | [64] |
Ser: Serine; Thr: Treonine; GSK-3β: Glycogen synthase kinase 3 beta; PP2A: Protein phosphatase-2A; NA: Not available; Cs: Carbamate; Ps: Pyrethroids.
Effect of Ps on the tau protein
Exposure to P pesticides also increases tau protein phosphorylation by modifying the activity of kinase enzymes through over-activating. Contact with P pesticides is associated with increased immunoreactivity that affects cognitive processes, spatial memory, and learning, which are alterations consistent with the development of AD[32,65,68]. Three out the few studies that have been so far published on the effect of Ps on tau protein, and one review, are shown in Table 4. Amongst the most relevant results reported are increased activity of GSK-3β[34,69], increased neuroinflammation[34,69] and decreased activity of PP2A[34].
Table 4.
Effects of pyrethroid pesticides on tau protein
|
Type of study
|
Sample
|
Type of pesticide
|
Exposure data
|
Tau phosphorylation
|
GSK-3β
|
PP2A
|
Other mechanisms
|
Ref.
|
| Experimental studies | Sprague-Dawly rats | Deltamethrin | Concentration: 12.5 mg/kg. Exposure time for 28 d | Increased | Increased | Decreased | Increased phosphorylation of tau was observed in Ser198/199/202, Thr205 and Ser404 | [32] |
| Experimental studies | Wistar rats | Cyfluthrin, imiprothrin, prallethrin | Concentrations:25%, 50% and 75%. Exposure time of 45 d | Increased | Increased | Decreased | Higher immunoreactivity of tau occurred in the hippocampus with high exposures to Ps. For medium and low doses, low immunoreactivity occurred. On the other hand, the activity of GSK- 3β was increased, while that of PP2A 2 was decreased | [34] |
| Experimental studies | Wistar rats | Cypermethrin | Concentration: 10 mg/kg and 25 mg/kg. Exposure time for 2, 3 and 6 wk | Increased | Increased | NA | In weaned exposed rats, tau phosphorylation increased in frontal cortex and hippocampus. This was induced by an increase in GSK-3β activity. Furthermore, increased neuroinflammation was observed with increased production of IL-1β | [69] |
| Review studies | Multiple studies | Ps | NA | Increased | Increased | NA | Exposure to Ps induced increased kinase activity, thereby increasing the phosphorylation of tau protein | [62] |
Ps: Pyrethroid; IL: Interleukin; GSK-3β: Glycogen synthase kinase 3 beta; PP2A: Protein phosphatase-2A; NA: Not available.
Effect of Ns on the tau protein
Studies on the effect of N pesticides on tau protein in humans or experimental models are very few in number. The few reports available highlight the work of Kimura-Kuroda et al[70] who found that exposure to 1-100 μM acetamiprid or imidacloprid (both N pesticides) increased intracellular Ca2+ influx in cerebellar neurons by activating calcium/calmodulin-dependent kinases, thereby over-stimulating tau protein phosphorylation. In a clinical case report on accidental ingestion of imidacloprid and thiamethoxam, the resultant increase in Ca2+ influx altered the kinase response[22]. Another mechanism involved activation of the Wnt pathway, leading to apoptosis[71]. More details are shown in Table 5.
Table 5.
Effects of neonicotinoid pesticides on tau protein
|
Type of study
|
Sample
|
Type of pesticide
|
Exposure data
|
Tau phosphorylation
|
GSK-3β
|
PP2A
|
Other mechanisms
|
Ref.
|
| Clinical/epidemiological studies. Clinical case | Accidental intake with Ns | Imidacloprid and thiamethoxam | Concentration: NA. Single dose | NA | NA | NA | The metabolite desnitro-imidacloprid activated the flow of intracellular calcium, thereby altering the response of kinase enzymes, and causing an excitatory neurological phase | [22] |
| Experimental studies | Primary cultures of cerebellar neurons from neonatal Sprague-Dawly rats | Acetamiprid imidacloprid | Concentrations: 1-100 μM. Exposure time of 600 s | NA | NA | NA | Exposure to Ns increased the influx of Ca2+ in cerebellar neurons. These pesticides excited cerebellar neurons to a degree similar to that from nicotine exposure. The influx of calcium ions activated the VDCC | [70] |
| Experimental studies | Human neural cells | Desnitro-imidacloprid | Concentration: 50 μM. Exposure time of 48 h | Increased | Increased | Decreased | Activation of Wnt signal pathway. Exposure induced tau hyperphosphorylation by a GSK-3β response, this enzyme is associated with Beta catenin activity. Exposure to this Ns induced watered-down expression that regulated tau hyperphosphorylation and apoptotic responses that impacted synaptotoxicity | [71] |
Ns: Neonicotinoid; GSK-3β: Glycogen synthase kinase 3 beta; PP2A: Protein phosphatase-2A; NA: Not available; VDCC: Voltage-dependent calcium channels.
PESTICIDES AND THEIR COGNITIVE IMPLICATIONS
Epidemiological studies have associated pesticide exposure with increased risks of cognitive impairment and AD[72,73]. For example, exposure to OC pesticides has been associated with low scores in the mini-mental test, and with severe cognitive decline[74-77]. Singh et al[78] reported that exposure to δ-hexachlorocyclohexane), dieldrin and pp’-dichlorodiphenyldichloroethylene were associated with AD (odds ratio = 2.064, 2.086 and 4.8, respectively; 95% confidence interval). These results are consistent with the cognitive findings[32-34,44,46-48,50,53,54,56,58,60,63,69,79] reported in Table 6, where contact with OCs was associated with increased cognitive impairment, decreased scores in tests evaluating spatial memory, decreased scores in results of tests on evaluation of novelty, and poor performance in tests evaluating attention and problem solving, except for two studies that did not report differences in scores in the tests applied[46,49].
Table 6.
Effect of pesticides on cognitive processes
|
Type of study
|
Sample
|
Type of pesticide
|
Exposure data
|
Cognitive implications
|
Ref.
|
| Clinical/epidemiological studies. Cross-sectional | 90 subjects with PD and 90 healthy subjects | OCs: δ-HCH | Concentration: NA. Exposure time NA | MMSE1 values in subjects with PD and without exposure to OCs: 27.66 ± 4.63. MMSE1 values in healthy subjects with exposure to OCs: 24.33 ± 4.31 | [45] |
| Clinical/epidemiological studies. Cohort | 13 postmortem brains of subjects without exposure and 4 postmortem brains of subjects with exposure | OCs | Concentration: NA. Exposure time: from 0 to 10 yr | Last CASI1 score of subjects without exposure: 67.9 ± 24.4. Last CASI1 score of subjects with exposure: 41.6 ± 22.8 | [44] |
| Experimental studies | Strains of Caenorhabditis elegans (N2 BR5271) | OCs: DDT | Concentration: 3 μM. Exposure time of 2 h | No significant differences reported in Associative Learning Paradigm tests | [46] |
| Experimental studies | Wistar rats | OCs: Chlordane | Range of concentration: 1 to 100 nM. Chronic exposure | Exposure did not affect results of tests that measured spatial memory | [49] |
| Review studies | Multiple studies | OCs: TCDD | Range of concentration: 5 to 23 ppt. Single dose | Decreased performance in verbal and nonverbal memory tests | [48] |
| Clinical/epidemiological studies. Cases and controls, unpaired | 33 subjects exposed to OPs and 33 subjects without exposure | OPs | Concentration: NA. Exposure time 2 yr | 87% of exposed subjects had cognitive impairment. Exposure to OPs for 10 yr increased the risk of cognitive decline 17 times | [53] |
| Experimental studies | Wistar rats | OPs: Chlorpyrifos- oxon | Range of concentration: 1 to 100 nM. Chronic exposure | Rats exposed to OPs showed deterioration of spatial memory | [49] |
| Experimental studies | NMRI mouse | OPs: Chlorpyrifos | Concentration: 0.1, 1.0, 5.0 mg/kg. Single dose | Decreased locomotion response and novelty were observed in rats exposed to this OPs | [54] |
| Experimental studies | Transgenic AD model rats | OPs: Chlorpyrifos | Concentrations: 3 and 10 mg/kg. Exposure time of 21 d | Exposure was associated with accelerated cognitive impairment in male rats, as indicated in memory and recognition tests | [50] |
| Experimental studies | Wistar rats | OPs: Malathion | Concentration: 100 mg/kg. Exposure time of 14 d | Decrease in spatial memory (evaluated using Morris water maze). This decrease was related to tau hyperphosphorylation | [33] |
| Experimental studies | Cell culture hiPSC and Wistar rats | OPs: DFP | Cell culture concentration: 200 nM. Exposure time for 2 d. Murine concentration: 1.5 mg/kg. Exposure time for 7 d | Slight decreases in learning and memory tests in the Morris water maze tests, and in 0 new object recognition | [56] |
| Review studies | Multiple studies | OPs | Different conditions | Lower perfomance in MMSE was associated with the exposure, with a modestly increased risk of MCD | [58] |
| Review studies | Multiple studies | OPs | NA | Exposure produced psychotic episodes, and alterations in attention, memory, problem solving, abstraction and cognitive flexibility | [59] |
| Review studies | Multiple studies | OPs ester | Different conditions | Decreased attention, visual memory, persistent and longer cognitive dysfunction and short-term memory | [60] |
| Experimental studies | Sprague-Dawly rats | Cs: Carbofuran | Concentration: 1 mg/kg. Exposure time of 28 d | Rats exposed to this pesticide took longer time to solve the Morris water maze, relative to the control group | [32] |
| Experimental studies | NMRI mouse | Cs: Carbaryl | Concentrations: 0.5, 5.0, 20.0 mg/kg. Single dose | Decreased locomotion response and response to novelty test in rats exposed to this pesticide | [54] |
| Experimental studies | Sprague-Dawly rats | Cs: Carbofuran | Concentration: NA. Exposure time for 28 d | Exposed rats had longer escape latency time in the Morris water maze test. Exposure was related to spatial memory deficit | [63] |
| Experimental studies | Sprague-Dawly rats | Ps: Deltamethrin | Concentration: 12.5 mg/kg. Exposure time for 28 d | Rats exposed to this pesticide took longer time to solve the Morris water maze, relative to the control group | [32] |
| Experimental studies | Wistar rats | Ps: Cypermethrin | Concentration: 10 mg/kg and 25 mg/kg. Exposure time for 2, 3 and 6 wk | Cognitive impairment was induced; deficiencies in learning and memory of exposed rats occurred. These changes may be related to changes in calcium-dependent kinases and calmodulin | [69] |
| Experimental studies | Wistar rats | Ps: Cyfluthrin. Imiprothrin and Prallethrin | Concentrations: 25%, 50% and 75%. Exposure time of 45 d | Rats with the highest P exposure had higher cognitive impairment, when compared to control rats and other concentrations, possibly via increased activation of astrocytes in hippocampus | [34] |
| Clinical/epidemiological studies. Retrospective study of suicidal patients | Accidental intake of different Ns | Ns: Imidacloprid | Different concentrations. Single dose | Disorientation, altered mental status, lack of coordination and confusion | [79] |
mean ± SD.
OCs: Organochlorines; OPs: Organophosphates; NA: Not available; DFP: Diisopropyl fluorophosphate; MMSE: Mini Mental State Examination; CASI: Cognitive Abilities Screening Instrument; Ps: Pyrethroids; Ns: Neonicotinoids; Cs: Carbamates; MCD: Major cognitive disorders; hiPSC: Human-induced pluripotent stem cells; δ-HCH: δ-hexachlorocyclohexane; DDT: Dichlorodiphenyltrichloroethan; AD: Alzheimer’s disease; TCDD: 2,3,7,8 tetrachlorodibenzo-p-dioxin; PD: Parkinson disease.
Lin et al[80] reported that workers exposed to OPs had cognitive impairment (hazard ratio = 2.21; 95% confidence interval). Hayden et al[81] reported that exposure to OPs increased the risks of MCD and AD (hazard ratios = 1.38 and 1.42, respectively; 95% confidence interval), and Paul et al[82] reported that exposure to OPs has been associated with rapid progression to MCD (hazard ratio = 1.94, 95% confidence interval). In the studies analyzed in Table 6, it was found that contact with OPs was associated with decreased MMSE testing and deficiencies in tests involving evaluation of verbal, non-verbal and spatial memory (Table 6). These cognitive findings are related to the results presented by Lin et al[80], Hayden et al[81] and Paul et al[82] referred to above.
Exposure to Cs is also associated with increased risk of developing MCD (odds ratio = 1.98; 95% confidence interval)[80]. Kamboj et al[83] showed that exposure of rats to Cs for 28 d performed poorly in Active Avoidance Task, indicating deterioration of cognitive function. Additionally, findings from studies on effect of Cs exposure on cognition have been linked to deterioration in spatial memory and decreased scores in results of tests involving evaluation of novelty (Table 6).
Very few studies have been done on the risk of cognitive impairment and AD due to exposure to P and N pesticides. In Taiwan workers exposed to Ns, approximately 2.9% mortality was reported, which is similar to the value of 3.1% reported for pyrethrins and Ps[22,79]. The results reported in Table 6 indicate that exposure to Ps is related to a deterioration in spatial memory, alteration in problem solving capacity, and increase in cognitive impairment similar to that reported by Estrada Atehortúa et al[22]. Besides, according to Phua et al[79], exposure to N pesticides resulted in aggravated disorientation, altered mental status, and confusion (Table 6).
Previously, the difficulty in studying the association between exposure to pesticides and prevalence of MCD and AD in humans was attributed to the complexity of obtaining separate data for each pesticide classification, because human exposure usually results from poisoning from multiple pesticides in conjunction with different chemical vehicles[65].
CONCLUSION
Pesticide exposure is associated with increased hyperphosphorylation of the tau protein amino acid residues Ser198, Ser199 and Ser202, Thr205, Ser396 and Ser404. This occurs through the following mechanisms: Increased enzyme activity of Cdk5 or GSK-3β, decreased PPA2, mutations associated with the MAPT gene, increased neuroinflammatory response, enhanced influx of intracellular Ca2+, and impairment of oxidative phosphorylation. These mechanisms may be related to the pathogenesis of AD. In addition, exposure to pesticides may be involved in lower performance in mini-mental tests, alteration in verbal, non-verbal and spatial memory, decreased response to novelty tests, and reductions in attention and problem-solving potential. One limitation in this study is the small number of publications on Cs, Ps and Ns pesticides and their effects on tau protein. Therefore, there is need to carry out a broader study on these variables in subsequent investigations.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: May 22, 2023
First decision: July 3, 2023
Article in press: August 8, 2023
Specialty type: Medicine, research and experimental
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Toledano A, Spain; Vaz M, Portugal S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
Contributor Information
Erandis D Torres-Sánchez, Department of Medical and Life Sciences, University Center of la Cienega, University of Guadalajara, Ocotlan 47820, Jalisco, Mexico.
Genaro G Ortiz, Department of Philosophical and Methodological Disciplines and Service of Molecular Biology in Medicine Hospital Civil, University of Guadalajara, Guadalajara 44340, Jalisco, Mexico.
Emmanuel Reyes-Uribe, Department of Medical and Life Sciences, University Center of la Cienega, University of Guadalajara, Ocotlan 47820, Jalisco, Mexico.
Juan H Torres-Jasso, Department of Biological Sciences, CUCOSTA, University of Guadalajara, Puerto Vallarta 48280, Jalisco, Mexico.
Joel Salazar-Flores, Department of Medical and Life Sciences, University Center of la Cienega, University of Guadalajara, Ocotlan 47820, Jalisco, Mexico. joel.salazar@academicos.udg.mx.
References
- 1.World Health Organization. Dementia. [cited 20 March 2023]. Available from: https://www.who.int/es/news-room/fact-sheets/detail/dementia .
- 2.Alzheimer’s Disease International. Dementia statistics. [cited 6 April 2023]. Available from: https://www.alzint.org/about/dementia-facts-figures/dementia-statistics/
- 3.2023 Alzheimer's disease facts and figures. Alzheimers Dement. 2023;19:1598–1695. doi: 10.1002/alz.13016. [DOI] [PubMed] [Google Scholar]
- 4.Eldin Bayoumi A. Deleterious effects of banned chemical pesticides on human health in developing countries. In: Larramendy LM, Soloneski S. Pesticides - Updates on Toxicity, Efficacy and Risk Assessment. London: IntechOpen, 2022. [Google Scholar]
- 5.Shah R. Pesticides and human health. In: Nuro A. Emerging contaminants. London: IntechOpen, 2021. [Google Scholar]
- 6.Klaimala P, Khunlert P, Chuntib P, Pundee R, Kallayanatham N, Nankongnab N, Kongtip P, Woskie S. Pesticide residues on children's hands, home indoor surfaces, and drinking water among conventional and organic farmers in Thailand. Environ Monit Assess. 2022;194:427. doi: 10.1007/s10661-022-10051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Degrendele C, Prokeš R, Šenk P, Jílková SR, Kohoutek J, Melymuk L, Přibylová P, Dalvie MA, Röösli M, Klánová J, Fuhrimann S. Human Exposure to Pesticides in Dust from Two Agricultural Sites in South Africa. Toxics. 2022;10 doi: 10.3390/toxics10100629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fatunsin OT, Oyeyiola AO, Moshood MO, Akanbi LM, Fadahunsi DE. Dietary risk assessment of organophosphate and carbamate pesticide residues in commonly eaten food crops. Scientific African. 2020;8:e00442. [Google Scholar]
- 9.Silva-Madera RJ, Salazar-Flores J, Peregrina-Lucano AA, Mendoza-Michel J, Ceja-Gálvez HR, Rojas-Bravo D, Reyna-Villela MZ, Torres-Sánchez ED. Pesticide contamination in drinking and surface water in the Cienega, Jalisco, México. Water Air Soil Pollut. 2021;232 [Google Scholar]
- 10.Tang W, Wang D, Wang J, Wu Z, Li L, Huang M, Xu S, Yan D. Pyrethroid pesticide residues in the global environment: An overview. Chemosphere. 2018;191:990–1007. doi: 10.1016/j.chemosphere.2017.10.115. [DOI] [PubMed] [Google Scholar]
- 11.Kalyabina VP, Esimbekova EN, Kopylova KV, Kratasyuk VA. Pesticides: formulants, distribution pathways and effects on human health - a review. Toxicol Rep. 2021;8:1179–1192. doi: 10.1016/j.toxrep.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain G, Anwar H, Rasul A, Imran A, Qasim M, Zafar S, Imran M, Kamran SKS, Aziz N, Razzaq A, Ahmad W, Shabbir A, Iqbal J, Baig SM, Ali M, Gonzalez de Aguilar JL, Sun T, Muhammad A, Muhammad Umair A. Lipids as biomarkers of brain disorders. Crit Rev Food Sci Nutr. 2020;60:351–374. doi: 10.1080/10408398.2018.1529653. [DOI] [PubMed] [Google Scholar]
- 13.Rossetti MF, Stoker C, Ramos JG. Agrochemicals and neurogenesis. Mol Cell Endocrinol. 2020;510:110820. doi: 10.1016/j.mce.2020.110820. [DOI] [PubMed] [Google Scholar]
- 14.Rehman K, Irshad K, Kamal S, Imran I, Akash MSH. Exposure of Environmental Contaminants and Development of Neurological Disorders. Crit Rev Eukaryot Gene Expr. 2021;31:35–53. doi: 10.1615/CritRevEukaryotGeneExpr.2021037550. [DOI] [PubMed] [Google Scholar]
- 15.Kent SA, Spires-Jones TL, Durrant CS. The physiological roles of tau and Aβ: implications for Alzheimer's disease pathology and therapeutics. Acta Neuropathol. 2020;140:417–447. doi: 10.1007/s00401-020-02196-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo T, Noble W, Hanger DP. Roles of tau protein in health and disease. Acta Neuropathol. 2017;133:665–704. doi: 10.1007/s00401-017-1707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Food Safety Authority (EFSA), Crivellente F, Hart A, Hernandez-Jerez AF, Hougaard Bennekou S, Pedersen R, Terron A, Wolterink G, Mohimont L. Establishment of cumulative assessment groups of pesticides for their effects on the nervous system. EFSA J. 2019;17:e05800. doi: 10.2903/j.efsa.2019.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Upadhayay J, Rana M, Juyal V, Bisht SS, Joshi R. Impact of pesticide exposure and associated health effects. In: Srivastava PK, Singh VP, Singh A, Tripathi DK, Singh S, Prasad SM, Chauhan DK. Pesticides in Crop Production: Physiological and Biochemical Action. Milwaukee: John Wiley & Sons Ltd, 2020: 69-88. [Google Scholar]
- 19.Vellingiri B, Chandrasekhar M, Sri Sabari S, Gopalakrishnan AV, Narayanasamy A, Venkatesan D, Iyer M, Kesari K, Dey A. Neurotoxicity of pesticides - A link to neurodegeneration. Ecotoxicol Environ Saf. 2022;243:113972. doi: 10.1016/j.ecoenv.2022.113972. [DOI] [PubMed] [Google Scholar]
- 20.van Melis LVJ, Heusinkveld HJ, Langendoen C, Peters A, Westerink RHS. Organophosphate insecticides disturb neuronal network development and function via non-AChE mediated mechanisms. Neurotoxicology. 2023;94:35–45. doi: 10.1016/j.neuro.2022.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Pitzer EM, Williams MT, Vorhees CV. Effects of pyrethroids on brain development and behavior: Deltamethrin. Neurotoxicol Teratol. 2021;87:106983. doi: 10.1016/j.ntt.2021.106983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estrada Atehortúa AF, Berrouet Mejía MC, Giraldo JA. Toxicidad por neonicotinoides: revisión de tema y reporte de dos casos. Med UPB. 2016;35:41–46. [Google Scholar]
- 23.Muñoz-Mayorga D, Guerra-Araiza C, Torner L, Morales T. Tau Phosphorylation in Female Neurodegeneration: Role of Estrogens, Progesterone, and Prolactin. Front Endocrinol (Lausanne) 2018;9:133. doi: 10.3389/fendo.2018.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anlar HG, Bacanlı M, Basaran N. Endocrine disrupting mechanisms and effects of pesticides. Arch Pharm. 2021;71:480–490. [Google Scholar]
- 25.Reddam A, McLarnan S, Kupsco A. Environmental Chemical Exposures and Mitochondrial Dysfunction: a Review of Recent Literature. Curr Environ Health Rep. 2022;9:631–649. doi: 10.1007/s40572-022-00371-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farkhondeh T, Mehrpour O, Forouzanfar F, Roshanravan B, Samarghandian S. Oxidative stress and mitochondrial dysfunction in organophosphate pesticide-induced neurotoxicity and its amelioration: a review. Environ Sci Pollut Res Int. 2020;27:24799–24814. doi: 10.1007/s11356-020-09045-z. [DOI] [PubMed] [Google Scholar]
- 27.van der Kant R, Goldstein LSB, Ossenkoppele R. Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat Rev Neurosci. 2020;21:21–35. doi: 10.1038/s41583-019-0240-3. [DOI] [PubMed] [Google Scholar]
- 28.Arriagada J, Álvarez R. Proteína tau como biomarcador en Alzheimer preclínico: Tau protein as a biomarker in preclinical Alzheimer’s disease. Ars Medica: Rev Ciênc Méd. 2022;47:56–67. [Google Scholar]
- 29.González A, Calfío C, Churruca M, Maccioni RB. Glucose metabolism and AD: evidence for a potential diabetes type 3. Alzheimers Res Ther. 2022;14:56. doi: 10.1186/s13195-022-00996-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan D, Zhang Y, Liu L, Yan H. Pesticide exposure and risk of Alzheimer's disease: a systematic review and meta-analysis. Sci Rep. 2016;6:32222. doi: 10.1038/srep32222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wills J, Credle J, Oaks AW, Duka V, Lee JH, Jones J, Sidhu A. Paraquat, but not maneb, induces synucleinopathy and tauopathy in striata of mice through inhibition of proteasomal and autophagic pathways. PLoS One. 2012;7:e30745. doi: 10.1371/journal.pone.0030745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen NN, Luo DJ, Yao XQ, Yu C, Wang Y, Wang Q, Wang JZ, Liu GP. Pesticides induce spatial memory deficits with synaptic impairments and an imbalanced tau phosphorylation in rats. J Alzheimers Dis. 2012;30:585–594. doi: 10.3233/JAD-2012-111946. [DOI] [PubMed] [Google Scholar]
- 33.Mohammadzadeh L, Abnous K, Razavi BM, Hosseinzadeh H. Crocin-protected malathion-induced spatial memory deficits by inhibiting TAU protein hyperphosphorylation and antiapoptotic effects. Nutr Neurosci. 2020;23:221–236. doi: 10.1080/1028415X.2018.1492772. [DOI] [PubMed] [Google Scholar]
- 34.Iteire KA, Sowole AT, Ogunlade B. Exposure to pyrethroids induces behavioral impairments, neurofibrillary tangles and tau pathology in Alzheimer's type neurodegeneration in adult Wistar rats. Drug Chem Toxicol. 2022;45:839–849. doi: 10.1080/01480545.2020.1778020. [DOI] [PubMed] [Google Scholar]
- 35.Wheeler JR. Investigations into higher order assemblies of RNA and protein in health and disease. 2018. Available from: https://scholar.colorado.edu/concern/graduate_thesis_or_dissertations/8k71nh22q . [Google Scholar]
- 36.Schopfer LM, Onder S, Lockridge O. Organophosphorus Pesticides Promote Protein Cross-Linking. Chem Res Toxicol. 2022;35:1570–1578. doi: 10.1021/acs.chemrestox.2c00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres-Altoro MI, Mathur BN, Drerup JM, Thomas R, Lovinger DM, O'Callaghan JP, Bibb JA. Organophosphates dysregulate dopamine signaling, glutamatergic neurotransmission, and induce neuronal injury markers in striatum. J Neurochem. 2011;119:303–313. doi: 10.1111/j.1471-4159.2011.07428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baltazar MT, Dinis-Oliveira RJ, de Lourdes Bastos M, Tsatsakis AM, Duarte JA, Carvalho F. Pesticides exposure as etiological factors of Parkinson's disease and other neurodegenerative diseases--a mechanistic approach. Toxicol Lett. 2014;230:85–103. doi: 10.1016/j.toxlet.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez-Toledano D, Vega L. The cytoskeleton as a non-cholinergic target of organophosphate compounds. Chem Biol Interact. 2021;346:109578. doi: 10.1016/j.cbi.2021.109578. [DOI] [PubMed] [Google Scholar]
- 40.Sarailoo M, Afshari S, Asghariazar V, Safarzadeh E, Dadkhah M. Cognitive Impairment and Neurodegenerative Diseases Development Associated with Organophosphate Pesticides Exposure: a Review Study. Neurotox Res. 2022;40:1624–1643. doi: 10.1007/s12640-022-00552-0. [DOI] [PubMed] [Google Scholar]
- 41.Nunomura A, Moreira PI, Lee HG, Zhu X, Castellani RJ, Smith MA, Perry G. Neuronal death and survival under oxidative stress in Alzheimer and Parkinson diseases. CNS Neurol Disord Drug Targets. 2007;6:411–423. doi: 10.2174/187152707783399201. [DOI] [PubMed] [Google Scholar]
- 42.Kalia V, Niedzwiecki MM, Bradner JM, Lau FK, Anderson FL, Bucher ML, Manz KE, Schlotter AP, Fuentes ZC, Pennell KD, Picard M, Walker DI, Hu WT, Jones DP, Miller GW. Cross-species metabolomic analysis of tau- and DDT-related toxicity. PNAS Nexus. 2022;1:pgac050. doi: 10.1093/pnasnexus/pgac050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanini A, Rjeb A, Abdelmelek H. Alzheimer theories: Tau pathologies induced by brain protein nanoparticles agglomeration. Arch Neurol Neurol Disord. 2018;1:101. [Google Scholar]
- 44.Go RCP, Corley MJ, Ross GW, Petrovitch H, Masaki KH, Maunakea AK, He Q, Tiirikainen MI. Genome-wide epigenetic analyses in Japanese immigrant plantation workers with Parkinson's disease and exposure to organochlorines reveal possible involvement of glial genes and pathways involved in neurotoxicity. BMC Neurosci. 2020;21:31. doi: 10.1186/s12868-020-00582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu SQ, Yang XD, Qian YW, Wan DY, Sun FH, Luo Q, Song YY, Xiao Q. Interaction Between Genetic Variants and Serum Levels of Organochlorine Pesticides Contributes to Parkinson’s Disease. Proceedings of the MDS Virtual Congress; 2020 Sep 12-16; Milwaukee: Wiley, 2020. [Google Scholar]
- 46.Kalia V, Niedzwiecki MM, Bradner JM, Lau FK, Anderson FL, Bucher ML, Manz KE, Fuentes ZC, Pennell KD, Picard M, Walker DI, Hu WT, Jones DP, Miller GW. Cross-species metabolomic analysis of DDT and Alzheimer’s disease-associated tau toxicity. 2021 Preprint. Available from: bioRxiv 2021.06.14.448355v1.
- 47.Martyniuk CJ, Kroll KJ, Doperalski NJ, Barber DS, Denslow ND. Genomic and proteomic responses to environmentally relevant exposures to dieldrin: indicators of neurodegeneration? Toxicol Sci. 2010;117:190–199. doi: 10.1093/toxsci/kfq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mir RH, Sawhney G, Pottoo FH, Mohi-Ud-Din R, Madishetti S, Jachak SM, Ahmed Z, Masoodi MH. Role of environmental pollutants in Alzheimer's disease: a review. Environ Sci Pollut Res Int. 2020;27:44724–44742. doi: 10.1007/s11356-020-09964-x. [DOI] [PubMed] [Google Scholar]
- 49.López-Merino E, Cuartero MI, Esteban JA, Briz V. Perinatal exposure to pesticides alters synaptic plasticity signaling and induces behavioral deficits associated with neurodevelopmental disorders. Cell Biol Toxicol. 2022 doi: 10.1007/s10565-022-09697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voorhees JR, Remy MT, Erickson CM, Dutca LM, Brat DJ, Pieper AA. Occupational-like organophosphate exposure disrupts microglia and accelerates deficits in a rat model of Alzheimer's disease. NPJ Aging Mech Dis. 2019;5:3. doi: 10.1038/s41514-018-0033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaalan EAA, Qassem M, Bilal MM, Tahan ZS. Effect of oral administration of Paraquat pesticide on the hippocampus and substantia nigra in Wistar rats' brains. J Agr Environ Vet Sci. 2021;5:71–84. [Google Scholar]
- 52.Li Y, Fang R, Liu Z, Jiang L, Zhang J, Li H, Liu C, Li F. The association between toxic pesticide environmental exposure and Alzheimer's disease: A scientometric and visualization analysis. Chemosphere. 2021;263:128238. doi: 10.1016/j.chemosphere.2020.128238. [DOI] [PubMed] [Google Scholar]
- 53.Putu Dirasandhi Semedi PNL, Ayu Putri Laksmidew AA, Oka Adnyana IM. Paparan organofosfat kronik sebagai faktor risiko gangguan kognitif berdasarkan kadar phosphorylated Tau serum. Majalah Kedokteran Neurosains Perhimpunan Dokter Spesialis Saraf Indonesia. 2020;37 [Google Scholar]
- 54.Lee I, Eriksson P, Fredriksson A, Buratovic S, Viberg H. Developmental neurotoxic effects of two pesticides: Behavior and biomolecular studies on chlorpyrifos and carbaryl. Toxicol Appl Pharmacol. 2015;288:429–438. doi: 10.1016/j.taap.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 55.Moyano P, Frejo MT, Anadon MJ, García JM, Díaz MJ, Lobo M, Sola E, García J, Del Pino J. SN56 neuronal cell death after 24 h and 14 days chlorpyrifos exposure through glutamate transmission dysfunction, increase of GSK-3β enzyme, β-amyloid and tau protein levels. Toxicology. 2018;402-403:17–27. doi: 10.1016/j.tox.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Yates PL, Patil A, Sun X, Niceforo A, Gill R, Callahan P, Beck W, Piermarini E, Terry AV, Sullivan KA, Baas PW, Qiang L. A cellular approach to understanding and treating Gulf War Illness. Cell Mol Life Sci. 2021;78:6941–6961. doi: 10.1007/s00018-021-03942-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choudhary S, Joshi K, Gill KD. Possible role of enhanced microtubule phosphorylation in dichlorvos induced delayed neurotoxicity in rat. Brain Res. 2001;897:60–70. doi: 10.1016/s0006-8993(00)03222-4. [DOI] [PubMed] [Google Scholar]
- 58.Zaganas I, Kapetanaki S, Mastorodemos V, Kanavouras K, Colosio C, Wilks MF, Tsatsakis AM. Linking pesticide exposure and dementia: what is the evidence? Toxicology. 2013;307:3–11. doi: 10.1016/j.tox.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 59.Naughton SX, Terry AV Jr. Neurotoxicity in acute and repeated organophosphate exposure. Toxicology. 2018;408:101–112. doi: 10.1016/j.tox.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abou-Donia MB. Organophosphorus ester-induced chronic neurotoxicity. Arch Environ Health. 2003;58:484–497. doi: 10.3200/AEOH.58.8.484-497. [DOI] [PubMed] [Google Scholar]
- 61.Emerick GL, DeOliveira GH, dos Santos AC, Ehrich M. Mechanisms for consideration for intervention in the development of organophosphorus-induced delayed neuropathy. Chem Biol Interact. 2012;199:177–184. doi: 10.1016/j.cbi.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 62.Sharma S, Wakode S, Sharma A, Nair N, Dhobi M, Wani MA, Pottoo FH. Effect of environmental toxicants on neuronal functions. Environ Sci Pollut Res Int. 2020;27:44906–44921. doi: 10.1007/s11356-020-10950-6. [DOI] [PubMed] [Google Scholar]
- 63.Luo D, Chen N. P3-005: Pesticide exposure induces cognitive abnormalities and tau hyperphosphorylation in male rats. Alzheimer's Dementia. 2012;8:458. [Google Scholar]
- 64.Bortolami M, Pandolfi F, Tudino V, Messore A, Madia VN, De Vita D, Di Santo R, Costi R, Romeo I, Alcaro S, Colone M, Stringaro A, Espargaró A, Sabatè R, Scipione L. Design, Synthesis, and In Vitro, In Silico and In Cellulo Evaluation of New Pyrimidine and Pyridine Amide and Carbamate Derivatives as Multi-Functional Cholinesterase Inhibitors. Pharmaceuticals (Basel) 2022;15 doi: 10.3390/ph15060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci. 2015;9:124. doi: 10.3389/fncel.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh M, Kaur M, Kukreja H, Chugh R, Silakari O, Singh D. Acetylcholinesterase inhibitors as Alzheimer therapy: from nerve toxins to neuroprotection. Eur J Med Chem. 2013;70:165–188. doi: 10.1016/j.ejmech.2013.09.050. [DOI] [PubMed] [Google Scholar]
- 67.Zhang H, Wang Y, Li X, Wang S, Wang Z. Recent advance on carbamate-based cholinesterase inhibitors as potential multifunctional agents against Alzheimer's disease. Eur J Med Chem. 2022;240:114606. doi: 10.1016/j.ejmech.2022.114606. [DOI] [PubMed] [Google Scholar]
- 68.Yegambaram M, Manivannan B, Beach TG, Halden RU. Role of environmental contaminants in the etiology of Alzheimer's disease: a review. Curr Alzheimer Res. 2015;12:116–146. doi: 10.2174/1567205012666150204121719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maurya SK, Mishra J, Abbas S, Bandyopadhyay S. Correction to: Cypermethrin Stimulates GSK3β-Dependent Aβ and p-tau Proteins and Cognitive Loss in Young Rats: Reduced HB-EGF Signaling and Downstream Neuroinflammation as Critical Regulators. Mol Neurobiol. 2019;56:7905–7906. doi: 10.1007/s12035-019-01746-y. [DOI] [PubMed] [Google Scholar]
- 70.Kimura-Kuroda J, Komuta Y, Kuroda Y, Hayashi M, Kawano H. Nicotine-like effects of the neonicotinoid insecticides acetamiprid and imidacloprid on cerebellar neurons from neonatal rats. PLoS One. 2012;7:e32432. doi: 10.1371/journal.pone.0032432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Girish Kumar S. A Proteomic Profile of Imidacloprid and its Active Metabolites Desnitro-Imidacloprid and Imidacloprid-Olefin in Human Neural Cells. 2020. Available from: https://mars.gmu.edu/bitstream/handle/1920/13051/GirishKumar_thesis_2022.pdf?sequence=1&isAllowed=y .
- 72.Tang BL. Neuropathological Mechanisms Associated with Pesticides in Alzheimer's Disease. Toxics. 2020;8 doi: 10.3390/toxics8020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elonheimo HM, Andersen HR, Katsonouri A, Tolonen H. Environmental Substances Associated with Alzheimer's Disease-A Scoping Review. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph182211839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Medehouenou TCM, Ayotte P, Carmichael PH, Kröger E, Verreault R, Lindsay J, Dewailly É, Tyas SL, Bureau A, Laurin D. Exposure to polychlorinated biphenyls and organochlorine pesticides and risk of dementia, Alzheimer's disease and cognitive decline in an older population: a prospective analysis from the Canadian Study of Health and Aging. Environ Health. 2019;18:57. doi: 10.1186/s12940-019-0494-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim KS, Lee YM, Lee HW, Jacobs DR Jr, Lee DH. Associations between organochlorine pesticides and cognition in U.S. elders: National Health and Nutrition Examination Survey 1999-2002. Environ Int. 2015;75:87–92. doi: 10.1016/j.envint.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 76.Richardson JR, Roy A, Shalat SL, von Stein RT, Hossain MM, Buckley B, Gearing M, Levey AI, German DC. Elevated serum pesticide levels and risk for Alzheimer disease. JAMA Neurol. 2014;71:284–290. doi: 10.1001/jamaneurol.2013.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Steenland K, Mora AM, Barr DB, Juncos J, Roman N, Wesseling C. Organochlorine chemicals and neurodegeneration among elderly subjects in Costa Rica. Environ Res. 2014;134:205–209. doi: 10.1016/j.envres.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh N, Chhillar N, Banerjee B, Bala K, Basu M, Mustafa M. Organochlorine pesticide levels and risk of Alzheimer's disease in north Indian population. Hum Exp Toxicol. 2013;32:24–30. doi: 10.1177/0960327112456315. [DOI] [PubMed] [Google Scholar]
- 79.Phua DH, Lin CC, Wu ML, Deng JF, Yang CC. Neonicotinoid insecticides: an emerging cause of acute pesticide poisoning. Clin Toxicol (Phila) 2009;47:336–341. doi: 10.1080/15563650802644533. [DOI] [PubMed] [Google Scholar]
- 80.Lin JN, Lin CL, Lin MC, Lai CH, Lin HH, Yang CH, Kao CH. Increased Risk of Dementia in Patients With Acute Organophosphate and Carbamate Poisoning: A Nationwide Population-Based Cohort Study. Medicine (Baltimore) 2015;94:e1187. doi: 10.1097/MD.0000000000001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hayden KM, Norton MC, Darcey D, Ostbye T, Zandi PP, Breitner JC, Welsh-Bohmer KA Cache County Study Investigators. Occupational exposure to pesticides increases the risk of incident AD: the Cache County study. Neurology. 2010;74:1524–1530. doi: 10.1212/WNL.0b013e3181dd4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paul KC, Ling C, Lee A, To TM, Cockburn M, Haan M, Ritz B. Cognitive decline, mortality, and organophosphorus exposure in aging Mexican Americans. Environ Res. 2018;160:132–139. doi: 10.1016/j.envres.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kamboj SS, Kumar V, Kamboj A, Sandhir R. Mitochondrial oxidative stress and dysfunction in rat brain induced by carbofuran exposure. Cell Mol Neurobiol. 2008;28:961–969. doi: 10.1007/s10571-008-9270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]