Abstract
For patients with atrial fibrillation with an increased risk of stroke and contraindications to long-term anticoagulation, percutaneous left atrial appendage closure (LAAC) has become an important alternative to long-term oral anticoagulation. Incomplete closure of the LAAC during the procedure leads to faster blood flow in the interstitial space around the device, resulting in peri-device leak (PDL), which is not uncommon. Studies are still inconclusive in determining the incidence, long-term safety, and management of PDL. Therefore, this article reviewed the progress made in the research and treatment of PDL after LAAC.
Keywords: Atrial fibrillation, Left atrial appendage closure, Peri-device leak, Thromboembolism, Cardiac computed tomography angiography, Treatment
Core Tip: For patients with atrial fibrillation with an increased risk of stroke and contraindications to long-term anticoagulation, percutaneous left atrial appendage closure has become an important alternative to long-term oral anticoagulation. Incomplete closure of the left atrial appendage closure during the procedure leads to faster blood flow in the interstitial space around the device, resulting in peri-device leak. The incidence, long-term safety, and management of peri-device leak are still inconclusive.
INTRODUCTION
As one of the most common type of arrhythmias, atrial fibrillation (AF) can lead to the development of atrial thrombi. After a thrombus detaches from the vessel wall, it travels with the blood to the brain and the whole body. In nonvalvular AF, 90% of thrombi are formed in the left atrial appendage (LAA)[1]. Therefore, LAA closure (LAAC) has become an effective alternative to long-term oral anticoagulation for the prevention of stroke in AF patients with increased stroke risk or contraindications to long-term anticoagulation therapy[2]. LAAC uses a catheter delivery system to transport and fix the prefabricated and preinstalled LAA occlusion device to plug or seal off the LAA to block the blood flow between the LAA and the left atrium. Variability in the anatomical morphology, size, and orientation of LAAs between patients can result in the failure of the occluder in sealing the LAA, causing high-velocity blood flow to pass through the gap around the occluder and can possibly cause peri-device leak (PDL). The clinical significance of PDL is now still a controversial issue, while the main concern is its relationship to thromboembolic events.
CAUSES, ASSESSMENT METHODS, AND GRADING CRITERIA OF PDL
Causes of PDL
Plugs and cups are the two widely used occluders in clinical practice, and they can lead to PDL for slightly different reasons. PDL occurs with plug occluders when they cannot fully cover the LAA. PDL occurs with cup occluders mostly due to the gap under the blocking disc that does not fully cover the wall of the LAA[3].
Methods of PDL assessment
Methods to assess PDL include digital subtraction angiography (DSA), intracardiac echocardiography, transesophageal echocardiography (TEE), and cardiac computed tomography angiography (CCTA). A single-center observational study in Denmark studied 415 patients who underwent LAAC with the Amplatzer occluder between 2010 and 2018[4]. In total, 346 patients who received CCTA and TEE at 8 wk postoperatively were included in the research. The results of the study showed that PDL was observed in 110 patients (32%) by TEE, of which 29 (8%) had PDL > 3 mm. PDL was present in 210 patients (61%) by CCTA, of which 63 patients (18%) had PDL > 3 mm. This study suggested that CCTA is more sensitive than TEE in detecting PDL, which was similar to the findings from other studies[5,6].
In contrast, a study from Zhang et al[7] included a total of 208 patients with nonvalvular AF who underwent LAAC. Among them, 101 patients received standard surgery (intraoperative TEE confirmation required, retrospective cohort) and 107 patients with fluoroscopy alone (prospective cohort). The study analyzed individual occluder position, anchorage, compression, and PDL to assess clinical outcomes in both cohorts and found that both DSA angiography and TEE assessment intraoperatively showed better performance in assessing the occluder position and anchorage. However, no significant improvement in assessing PDL was observed (P = 0.304). Other data on intracardiac echocardiography and DSA are lacking, and further studies are needed.
Grading criteria
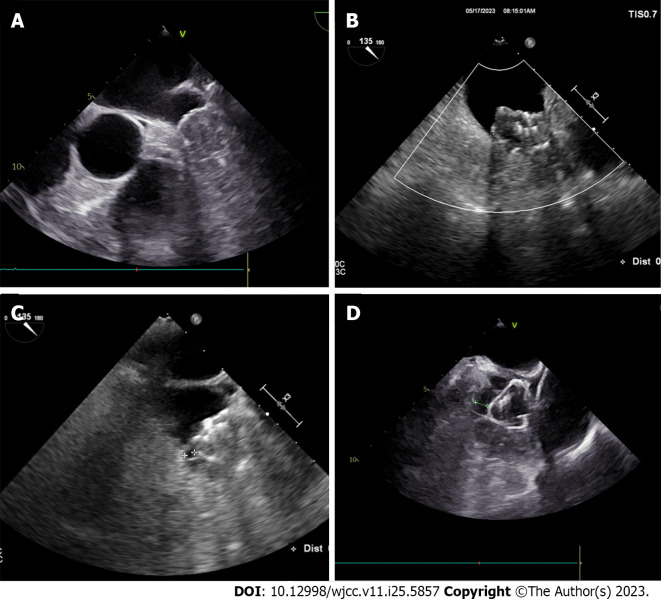
There are no well-recognized criteria for the assessment of PDL. For plugging occluders (e.g., Watchman[8]), a PDL ≤ 5 mm is generally considered a mild PDL, and PDL > 5 mm is considered severe PDL. PDL in some studies of cap occluders (e.g., LAmbre, ACP/Amulet[9]) strictly defined PDL< 1 mm as mild, PDL between 1-3 mm as moderate, and PDL > 3 mm as severe. Most experts agree that PDL of 5 mm is recommended as a grading criterion for either plug or cap occluders (Figure 1).
Figure 1.
Different grades of peri-device leak using transesophageal echocardiographic imaging. A: No peri-device leak (PDL); B: 0-3 mm PDL; C: 3-5 mm PDL; D: > 5 mm PDL.
INCIDENCE OF PDL
Most LAAC-related studies have statistically analyzed the incidence of PDL (Table 1). The PROTECT AF study was the first randomized controlled study of LAAC in which 468 patients successfully underwent LAAC (Watchman) with their PDL assessed by TEE[8]. If TEE found no thrombus on the surface of the occluder and the LAA was effectively occluded (complete occlusion or PDL < 5 mm), then the TEE criteria were met. Then, warfarin could be discontinued in favor of aspirin and clopidogrel for continued antithrombotic therapy. However, only 349 patients in this study met the TEE criteria at the 45-d follow-up, with the initial results of the study showing that the incidence of occluder surface thrombosis and PDL at 45 d (14%) might be higher than expected. In the subsequent PROTECT AF 2study, it was shown that during the follow-up after LAAC, 30 patients (7.5%), 14 patients (3.6%), and 10 patients (2.7%) continued warfarin after PDL (> 5 mm) was detected by TEE at 45 d, 6 mo, and 1 year after the procedure, respectively[10]. In contrast, the early PROTECT-AF study showed that the incidence of any degree of PDL was 40.9%, 33.8%, and 32.1% at 45 d, 6 mo, and 12 mo postoperatively, respectively[11]. Of these, mild (1 mm), moderate (1-3 mm), and severe (≥ 3 mm) PDL accounted for 7.7% of patients, 59.9% of patients, and 32.4% of patients, respectively.
Table 1.
Studies reporting the peri-device leak after left atrial appendage closure
|
Ref.
|
Study population
|
Type of study
|
Type of LAA closure
|
Incidence of PDL
|
Findings
|
| Holmes et al[8] | 485 | Randomized controlled study | Watchman | 14%: ≥ 5 mm | No difference between patients with any PDL in terms of primary effectiveness |
| Dukkipati et al[12] | 1205 | Randomized controlled study | Watchman | 0.7%: > 5 mm; 27.7%: 1-5 mm; and 71.6%: no PDL | PDL ≤ 5 mm was associated with an increased risk of stroke, systemic embolism, cardiovascular, unexplained death, or all-cause mortality |
| Korsholm et al[13] | 153 | Retrospective study | Amplatzer Amulet, Amplatzer Cardiac Plug | 61% | PDL did not increase the incidence of events related to thromboembolism. |
| Wang et al[14] | 152 | Retrospective study | LAmbre | 15.7%: > 3 mm | PDL was not associated with an increased risk for thromboembolic events |
| Alkhouli et al[15] | 51333 | Retrospective studies | Watchman, ACP | 73.4%: no PDL; 25.8%: moderate; and 0.7%: severe | Patients with PDL at 1 yr had a 2-fold increase in ischemic stroke/SE at 5 yr compared with patients without PDL |
| Miller et al[17] | 43 | Retrospective study | Watchman FLX | More than 40% | 3 TIAs (6.98%) and 3 strokes (6.98%) were documented each within the 6-mo to 1-yr period |
TIA: Transient ischemic attack; PDL: Peri-device leak; SE: Systemic embolization; LAA: Left atrial appendage.
A total of 1205 patients who were implanted with Watchman occluders from three studies, PROTECT-AF, PREVAIL, and CAP2, were included in another 5-year follow-up study[12]. By TEE at 45 d, 634 patients (60.2%) had no PDL, 404 patients (38.3%) had PDL < 5 mm, and 16 patients (1.5%) had PDL > 5 mm (TEE information was missing for the remaining 151 patients). Of the 404 patients with PDL < 5 mm, 255 patients (63.1%) had PDL ≤ 3 mm, and 149 patients (36.9%) had 3 mm < PDL ≤ 5 mm. At the 1-year TEE follow-up, a total of 983 patients reported PDL, of which 704 patients (71.6%) had no PDL, 272 patients (27.7%) had PDL ≤ 5 mm, and 7 patients (0.7%) had PDL > 5 mm.
A study from Denmark on the Amplatzer occluder included 153 patients who received LAAC and underwent 2-mo and 12-mo cardiac computed tomography at a single center between 2010 and 2017[13]. The Amplatzer Cardiac Plug Occluder was implanted in 43 patients (28%). The 2-mo and 12-mo follow-ups showed PDL in 103 patients (67%) and 93 patients (61%), respectively (P = 0.08). The mean PDL size at 2 mo and 12 mo was 2.9 ± 1.7 and 2.4 ± 1.4 mm, respectively (P = 0.07).
In a study from Wuhan University People’s Hospital, 152 AF patients were implanted with LAmbre occluders[14]. At the 3-mo follow-up, 123 patients underwent TEE, of whom 21 patients (17%) had PDL, 19 patients (15.4%) had mild PDL (1-3 mm), and 2 patients (1.6%) had moderate PDL (> 3 and ≤ 5 mm). Of the 121 patients who underwent TEE at the 12-mo follow-up, 19 patients (15.7%) had PDL (> 3 mm).
Another retrospective study through the NCDR-LAAO registry included all patients who underwent LAAO between January 1, 2016 and December 31, 2019[15]. Patients were classified according to PDL size on a 45-d echocardiogram: PDL= 0 mm, no PDL; PDL > 0-5 mm, moderate PDL; and PDL > 5 mm, severe PDL. A total of 51333 patients were included, of whom 37696 patients (73.4%) had no PDL, 13258 patients (25.8%) had moderate PDL, and 379 patients (0.7%) had severe PDL.
In the Amulet IDE study published in 2021, a head-to-head comparison of the safety and efficacy of the plug occluder (Watchman) vs the cap occluder (Amulet) was performed[16]. The study was randomized to the Amulet or Watchman groups, with 934 patients in the Amulet group and 944 patients in the Watchman group. It was discovered that the occluder blocking rate was better in the Amulet group than in the Watchman group at 45 d postoperatively (the proportion of those with PDL ≥ 3 mm was significantly lower in the Amulet group than in the Watchman group, 11% and 26%, respectively; P < 0.001) and lasted until 1 year postoperatively (9% and 22% in the Amulet group compared with the Watchman group for PDL ≥ 3 mm, respectively; P < 0.001). As seen in this study, the incidence of PDL was lower with the cap occluder than with the plug occluder.
However, the conclusion was different in another recent single-center retrospective study[17]. Miller et al[17] included 43 patients who underwent Watchman FLX implantation from July 2020 to September 2021, and the final results showed that more than 40% of patients (17/43) implanted with Watchman FLX had PDL. Only 53% of patients (23/43) had complete closure of the LAA. In this study, the incidence of PDL was not low for the Watchman FLX implantation group and still requires a long follow-up.
It can be easily seen from the above studies that the incidence of PDL ranges from 9% to 40%, and the incidence of PDL with different grades also differs. The incidence of PDL may be lower with a cap occluder than with a plug occluder.
PROGNOSTIC IMPACT OF PDL
The prognostic impact of PDL is currently the focus of attention in related studies. Data from the early PROTECT AF study suggested that patients without PDL were not statistically different than patients with any PDL in terms of primary effectiveness (P = 0.572) or ischemic stroke or systemic embolism (P = 0.669)[11]. In addition, further analysis found no statistically significant relationship between PDL severity and the primary endpoint [hazard ratio (HR): 0.84; P = 0.256].
Similarly, the aforementioned study on the Amplatzer occluder noted that with continued follow-up after completion of cardiac computed tomography at 12 mo and a median follow-up at 2.1 years, 52 patients (34%) had a composite endpoint of ischemic stroke, systemic embolism, transient ischemic attack, or all-cause death between the 12-mo visit and the last known follow-up[13]. The study divided PDL into four different grades: no PDL; grade 1; grade 2; and grade 3. The risk of the composite endpoint was 63% higher in the PDL group than in the no-PDL group, yet no statistical significance was found [HR: 1.63, 95% confidence interval: 0.90-2.93; P = 0.11]. The study further noted that PDL did not increase the incidence of events related to thromboembolism.
Although the two studies showed that PDL after Watchman and Amplatzer implantation did not increase the incidence of thromboembolic events, small sample sizes, small number of thromboembolic events, and short follow-up periods of both studies may undermine the statistical efficacy.
The retrospective NCDR-LAAO registry study also showed a trend toward increased ischemic stroke or systemic embolism in patients with any PDL at 45 d (5-year incidence 9.2% vs 6.6%), but this was not statistically significant (P = 0.14)[15]. However, patients with PDL at 1 year had a 2-fold increase in ischemic stroke/systemic embolism at 5 years compared with patients with no PDL (9.9% vs 5.1%; P = 0.008). This study is the first to show a correlation between PDL (although only detected at 1 year) and subsequent adverse ischemic events.
A similar view was shared in the 5-year follow-up study[12]. Compared with the no-PDL group, a 45-d TEE showing PDL ≤ 5 mm was not associated with an increased risk of ischemic stroke or systemic embolism (9.2% vs 6.6%; P = 0.141), cardiovascular or unexplained death (8.8% vs 10.3%; P = 0.547), or all-cause death (18.5% vs 21.5%; P = 0.350). However, 1-year TEE showing PDL ≤ 5 mm was associated with an increased risk of subsequent ischemic stroke or systemic embolism (9.9% vs 5.1%) with an unadjusted HR of 1.92 (95% confidence interval: 1.14-3.25; P = 0.0149), but 1-year TEE showed that PDL ≤ 5 mm was not associated with an increase in cardiovascular or unexplained death (10.1% vs 8.6%; P = 0.382) or all-cause mortality (16.2% vs 18.3%; P = 0.515).
A prospective observational registry study enrolled 1047 patients and classified PDL as < 1 mm, 1-3 mm, and > 3 mm[18]. After a mean follow-up period of 13 mo, the incidence of PDL at all levels was found to be 4.3%, 5.4% and 1.9%, respectively. There were nine strokes (0.9%) and nine transient ischemic attack (0.9%) during follow-up, which was not related to different grades of PDL.
Although the conclusions on the prognostic impact of PDL are mixed, the current findings favor that PDL can increase the risk of ischemic stroke or systemic embolism, especially in severe (≥ 5 mm) PDL.
CURRENT TREATMENT FOR PDL
PDL of more than 5 mm detected after LAAC will be considered a failure of occlusion, and TEE or CCTA are important follow-up tools to assess the PDL. Current treatment options for PDL include anticoagulation, spring-ring embolization, and radiofrequency ablation.
A study from the United States treated PDL by releasing a spring coil into the LAA[18]. The study included 30 patients with PDL after LAAC, of whom 10 patients (33.3%) had severe PDL (≥ 5 mm) and 20 patients (66.7%) had moderate PDL (3-4 mm). The immediate procedural success rate was 100%, and immediate angiographic and TEE results showed complete occlusion in 25 patients (83.3%), very mild PDL (1-2 mm) in 3 patients (10.0%), and partial occlusion with moderate PDL (3-4 mm) in 2 patients (6.7%). All patients underwent follow-up TEE after a median of 52 d (43–90 d). The majority of patients [n = 23 (76.7%)] showed complete occlusion, except for 2 patients (6.6%) with minor PDL (1-2 mm) and 5 patients (16.7%) with moderate PDL (3-4 mm), where spring coil placement resulted in a mean reduction in leak size of 86.3% (P < 0.001). This study provided a new means of treating PDL.
Another study shed light on the effectiveness of radiofrequency ablation for PDL management[19]. This study included 43 patients (PDL ≥ 4 mm) who underwent radiofrequency ablation of atrial tissue at the site of the PDL, and TEE was performed 48 ± 12 d after the procedure. The study found that 23 patients (53.5%) had complete occlusion of the LAA, 15 patients (34.9%) had mild or very mild (1-2 mm) PDL, and 5 patients (11.6%) had moderate (3-4 mm) PDL. The long-term success rate was 88.4% (n = 38). This study suggested that radiofrequency ablation may be effective in the treatment of PDL.
However, anticoagulation is still used clinically as the primary solution to treat large PDL. Although the small-sample study mentioned above has provided a new insight into the treatment of PDL, a large-scale clinical study is still needed to validate the results.
CONCLUSION
Since PDL after LAAC is not uncommon, it should be taken seriously by clinicians through achieving complete occlusion in LAA as much as possible. Both TEE and CCTA are powerful tools to assess PDLs after LAAC, with CCTA being more sensitive. With longer follow-up and larger sample sizes, it has been found that PDL may increase the risk of thromboembolic events, even though the overall incidence is low. Management of PDL includes anticoagulation, spring-ring embolization, and radiofrequency ablation, but further studies are needed to validate their safety and efficacy.
ACKNOWLEDGEMENTS
We appreciate all the medical staff for their efforts.
Footnotes
Conflict-of-interest statement: All authors declare having no potential conflicts of interests for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: February 18, 2023
First decision: May 8, 2023
Article in press: August 9, 2023
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mishra AK, United States; Patel L, United States S-Editor: Liu JH L-Editor: Filipodia P-Editor: Zhang XD
Contributor Information
Ying-Bo Qi, Department of Cardiology, Health Science Center, Ningbo University, Ningbo 315000, Zhejiang Province, China.
Hui-Min Chu, Department of Cardiology, Arrhythmia Center, Ningbo First Hospital, Ningbo 3153000, Zhejiang Province, China. epnbheart@163.com.
References
- 1.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–759. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 3.Jang SJ, Wong SC, Mosadegh B. Leaks after Left Atrial Appendage Closure: Ignored or Neglected? Cardiology. 2021;146:384–391. doi: 10.1159/000513901. [DOI] [PubMed] [Google Scholar]
- 4.Korsholm K, Jensen JM, Nørgaard BL, Samaras A, Saw J, Berti S, Tzikas A, Nielsen-Kudsk JE. Peridevice Leak Following Amplatzer Left Atrial Appendage Occlusion: Cardiac Computed Tomography Classification and Clinical Outcomes. JACC Cardiovasc Interv. 2021;14:83–93. doi: 10.1016/j.jcin.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 5.Goitein O, Fink N, Hay I, Di Segni E, Guetta V, Goitein D, Brodov Y, Konen E, Glikson M. Cardiac CT Angiography (CCTA) predicts left atrial appendage occluder device size and procedure outcome. Int J Cardiovasc Imaging. 2017;33:739–747. doi: 10.1007/s10554-016-1050-6. [DOI] [PubMed] [Google Scholar]
- 6.Saw J, Fahmy P, Spencer R, Prakash R, McLaughlin P, Nicolaou S, Tsang M. Comparing Measurements of CT Angiography, TEE, and Fluoroscopy of the Left Atrial Appendage for Percutaneous Closure. J Cardiovasc Electrophysiol. 2016;27:414–422. doi: 10.1111/jce.12909. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Jin Q, Kong D, Jiang Y, Chen S, Chen D, Hou CR, Zhang L, Pan C, Zhou D, Ge J. Comparison of fluoroscopy and transesophageal echocardiogram for intra-procedure device surveillance assessment during implantation of Watchman. Int J Cardiol. 2021;324:72–77. doi: 10.1016/j.ijcard.2020.08.070. [DOI] [PubMed] [Google Scholar]
- 8.Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P PROTECT AF Investigators. Percutaneous closure of the left atrial appendage vs warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 9.Urena M, Rodés-Cabau J, Freixa X, Saw J, Webb JG, Freeman M, Horlick E, Osten M, Chan A, Marquis JF, Champagne J, Ibrahim R. Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug device in patients with nonvalvular atrial fibrillation and contraindications to anticoagulation therapy. J Am Coll Cardiol. 2013;62:96–102. doi: 10.1016/j.jacc.2013.02.089. [DOI] [PubMed] [Google Scholar]
- 10.Reddy VY, Doshi SK, Sievert H, Buchbinder M, Neuzil P, Huber K, Halperin JL, Holmes D PROTECT AF Investigators. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-Year Follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) Trial. Circulation. 2013;127:720–729. doi: 10.1161/CIRCULATIONAHA.112.114389. [DOI] [PubMed] [Google Scholar]
- 11.Viles-Gonzalez JF, Kar S, Douglas P, Dukkipati S, Feldman T, Horton R, Holmes D, Reddy VY. The clinical impact of incomplete left atrial appendage closure with the Watchman Device in patients with atrial fibrillation: a PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) substudy. J Am Coll Cardiol. 2012;59:923–929. doi: 10.1016/j.jacc.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 12.Dukkipati SR, Holmes DR Jr, Doshi SK, Kar S, Singh SM, Gibson D, Price MJ, Natale A, Mansour M, Sievert H, Houle VM, Allocco DJ, Reddy VY. Impact of Peridevice Leak on 5-Year Outcomes After Left Atrial Appendage Closure. J Am Coll Cardiol. 2022;80:469–483. doi: 10.1016/j.jacc.2022.04.062. [DOI] [PubMed] [Google Scholar]
- 13.Korsholm K, Jensen JM, Nørgaard BL, Nielsen-Kudsk JE. Temporal changes and clinical significance of peridevice leak following left atrial appendage occlusion with Amplatzer devices. Catheter Cardiovasc Interv. 2022;99:2071–2079. doi: 10.1002/ccd.30178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang G, Kong B, Qin T, Liu Y, Huang C, Huang H. Incidence, risk factors, and clinical impact of peridevice leak following left atrial appendage closure with the LAmbre device-Data from a prospective multicenter clinical study. J Cardiovasc Electrophysiol. 2021;32:354–359. doi: 10.1111/jce.14824. [DOI] [PubMed] [Google Scholar]
- 15.Alkhouli M, Du C, Killu A, Simard T, Noseworthy PA, Friedman PA, Curtis JP, Freeman JV, Holmes DR. Clinical Impact of Residual Leaks Following Left Atrial Appendage Occlusion: Insights From the NCDR LAAO Registry. JACC Clin Electrophysiol. 2022;8:766–778. doi: 10.1016/j.jacep.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakkireddy D, Thaler D, Ellis CR, Swarup V, Sondergaard L, Carroll J, Gold MR, Hermiller J, Diener HC, Schmidt B, MacDonald L, Mansour M, Maini B, O'Brien L, Windecker S. Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE): A Randomized, Controlled Trial. Circulation. 2021;144:1543–1552. doi: 10.1161/CIRCULATIONAHA.121.057063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller T, Hana D, Patibandla S, Guzman DB, Avalon JC, Zeb I, Kadiyala M, Mills J, Balla S, Kim C, Lisle M, Kawsara M, Raybuck B, Daggubati R, Sengupta PP, Hamirani YS. Cardiac Computed Tomography Angiography for Device-Related Thrombus Assessment After WATCHMAN FLX™ Occluder Device Implantation: A Single-Center Retrospective Observational Study. Cardiovasc Revasc Med. 2022;41:35–46. doi: 10.1016/j.carrev.2022.01.028. [DOI] [PubMed] [Google Scholar]
- 18.Tzikas A, Shakir S, Gafoor S, Omran H, Berti S, Santoro G, Kefer J, Landmesser U, Nielsen-Kudsk JE, Cruz-Gonzalez I, Sievert H, Tichelbäcker T, Kanagaratnam P, Nietlispach F, Aminian A, Kasch F, Freixa X, Danna P, Rezzaghi M, Vermeersch P, Stock F, Stolcova M, Costa M, Ibrahim R, Schillinger W, Meier B, Park JW. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention. 2016;11:1170–1179. doi: 10.4244/EIJY15M01_06. [DOI] [PubMed] [Google Scholar]
- 19.Della Rocca DG, Horton RP, Di Biase L, Bassiouny M, Al-Ahmad A, Mohanty S, Gasperetti A, Natale VN, Trivedi C, Gianni C, Burkhardt JD, Gallinghouse GJ, Hranitzky P, Sanchez JE, Natale A. First Experience of Transcatheter Leak Occlusion With Detachable Coils Following Left Atrial Appendage Closure. JACC Cardiovasc Interv. 2020;13:306–319. doi: 10.1016/j.jcin.2019.10.022. [DOI] [PubMed] [Google Scholar]