Key Points
-
•
High level of the soluble macrophage marker CD163 is associated with shorter PFS and OS in MCL.
-
•
Soluble CD163 is prognostic in both rituximab-chemotherapy–treated and rituximab, ibrutinib and lenalidomide–treated MCL.
Visual Abstract
Abstract
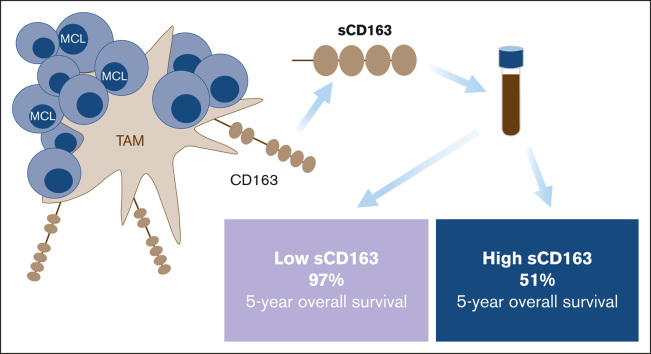
The outcome for patients with mantle cell lymphoma (MCL) has drastically improved with new treatments directed toward the tumor immune microenvironment, where macrophages play an important role. In MCL, the presence of M2 macrophages defined by CD163 expression in diagnostic biopsies has been associated with a worse prognosis. An alternative way to assess the abundance of M2 macrophages is by measuring the level of soluble CD163 in serum (sCD163). We aimed to investigate the prognostic value of sCD163 in 131 patients with MCL. We found that high sCD163 at diagnosis was associated with shorter progression-free survival (PFS) and shorter overall survival (OS) in 81 patients who were newly diagnosed and subsequently treated with chemoimmunotherapy. The same was seen in a cohort of 50 patients with relapsed MCL that were mainly treated within the phase 2 Philemon-trial with rituximab, ibrutinib, and lenalidomide. In patients who were newly diagnosed and had low levels of sCD163, 5-year survival was 97%. There was a moderate correlation between sCD163 and tissue CD163. The association with a poor prognosis was independent of MCL international prognostic index, Ki67, p53 status, and blastoid morphology, as assessed in a multivariable Cox proportional hazards model. In this study, high sCD163 was associated with both shorter PFS and shorter OS, showing that high levels of the M2 macrophage marker sCD163 is an independent negative prognostic factor in MCL, both in the chemoimmunotherapy and ibrutinib/lenalidomide era. In addition, low sCD163 levels identify patients with MCL with a very good prognosis.
Introduction
Mantle cell lymphoma (MCL) is a heterogeneous disease with a poor prognosis.1 New targeted treatments, such as Bruton tyrosine kinase (BTK) inhibitors, have drastically improved outcomes for patients with MCL.2 Another active treatment for MCL is the immunomodulatory drug lenalidomide. Both BTK inhibitors and lenalidomide affect tumor-associated macrophages (TAMs) in the tumor microenvironment.3, 4, 5 TAMs of M2 type are known to support angiogenesis, suppress T-cell response and promote tumor cell growth. The most established marker for M2 TAMs is CD163, a membrane-bound scavenger receptor. Carreras et al have shown prognostic significance of CD163 gene expression levels in diffuse large B–cell lymphoma (DLBCL) tumor tissue,6 and high levels of CD163+ cells in tumor tissue are associated with poor prognosis in several malignacies, including MCL.7, 8, 9, 10 MCL cells have been shown to polarize TAMs toward the M2 type, and thereby promoting their own growth.11 CD163 is of rising interest as a novel target for immunotherapy, and several different strategies are under investigation.12,13
CD163 can be shredded from the membrane into the blood upon macrophage activation and is then found in its soluble form, sCD163.14 One of the main functions of sCD163 is clearing plasma from hemoglobin-haptoglobin complexes.15 Increased levels of sCD163 in serum correlate with disease severity and progression in several malignancies, including lymphomas.12,16, 17, 18, 19, 20 Furthermore, sCD163 levels correlate positively with worse prognosis and decrease during successful treatment in patients with DLBCL21 and classical Hodgkin lymphoma (cHL).22 In chronic lymphocytic leukemia higher sCD163 levels correlate with a shorter time to the first treatment.23 It is not known if sCD163 measured in serum is associated with prognosis in MCL.
We aimed to evaluate the association between sCD163 and prognosis in MCL by analyzing serum samples from 131 patients with MCL.
Methods
Patients and cohorts
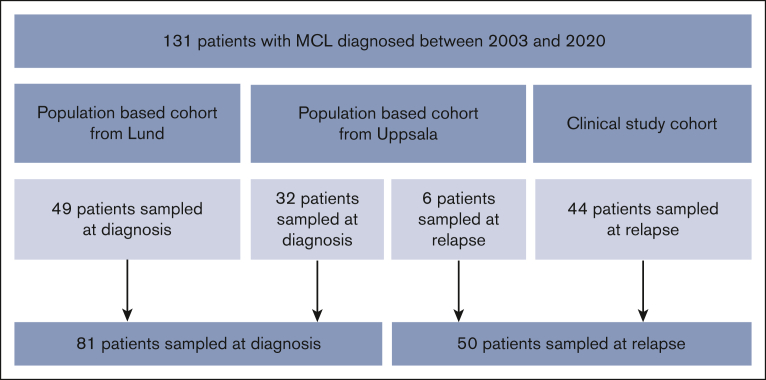
We used 3 cohorts with a total of 131 patients with MCL to evaluate sCD163 in different stages of the disease (Figure 1). First, 2 population-based cohorts in which samples were taken at diagnosis were used, from Lund (n = 49) and Uppsala (n = 32), Sweden. Next, to investigate if the results were valid also for patients with MCL who relapsed, a third cohort with 44 patients with MCL who relapsed was included from the phase 2 trial Philemon, the Nordic Lymphoma Group MCL6-study. Patients who relapsed were included between 2015 and 2016 in Sweden, Finland, Denmark, and Norway.24 Samples were taken at the inclusion in the study (newly verified relapse or progression was the inclusion criteria). These patients were evaluated together with 6 patients with MCL who relapsed from the Uppsala cohort.
Figure 1.
Flowchart of the MCL cohorts. The 38 patients from Uppsala also contributed with 29 samples taken during treatment, 33 samples taken in remission, and 6 samples taken at later relapses. Twenty-four of the patients from Uppsala had matching tissue samples from the time of diagnosis (n = 21) or relapse (n = 3).
Overall, 32 patients were sampled at diagnosis and 6 patients were sampled at relapse (38 patients in total) from Uppsala. In addition, 29 samples were taken during treatment, 33 samples in remission, and 6 samples at later relapses. Samples during treatment were used for analysis of sCD163 levels in relation to treatment, and samples from remission were used for evaluation of progression of disease within 24 months. The serum samples taken during treatment and in remission as well as the tissue samples were all from patients in the Uppsala cohort.
sCD163 levels and cutoffs
sCD163 was analyzed in pretreatment serum samples using an enzyme-linked immunosorbent assay with a human sCD163 Quantikine Kit from R&D Systems. All samples were analyzed in duplicates and the average value was used in the subsequent analysis. In ∼87% of the cases, the coefficient of variation (σ/μ × 100) was <25%.
There is no established cutoff for high vs low sCD163 levels in MCL, but the median level was used in a previous publication on sCD163 in patients with DLBCL21 and was a priori set out to be our cutoff for this study.
Association between sCD163 levels and baseline characteristics
Frequencies and proportions of clinical and biological factors by sCD163 levels were tested using Pearson χ2 test for categorical variables with cell counts ≥5, Fischer exact test for categorical variables with cell counts of <5, and Wilcoxon rank sum test for continuous variables. p53 abnormality was defined as either TP53 mutation, identified with genetic characterization in the study cohort or p53 overexpression identified with immunohistochemistry in >30% of the tumor cells in diagnostic biopsies. The concordance between TP53 mutation and p53 overexpression is considered high.25 Low hemoglobin (Hb) was defined as a Hb level below the reference value (<120 g/L for women and <130 g/L for men).
Association between sCD163 levels and tissue CD163 expression
To correlate sCD163 levels with tissue CD163, we analyzed CD163 in MCL tumor tissue in 29 patients from the Uppsala cohort. Samples were taken from bone marrow (n = 15), lymph nodes (n = 17), tonsil (n = 4), and others (liver and tongue, n = 2). Nine patients had samples from 2 locations of the body. Tissue CD163 and sCD163 from the same time point was available in 24 patients. Formalin-fixed, paraffin-embedded tumor tissue was stained for CD163 and evaluated manually for the percentage of positive cells within tumor areas. Methods have been described in detail in our previous publication7 and the same cutoff, ie, 0.6% positive cells was used in regression analysis. The Spearman rank correlation test was used to assess correlation between tissue CD163 and sCD163 as continuous variables. p53 overexpression in relation to dichotomized sCD163 values was analyzed with Wilcoxon rank sum test.
Association between sCD163 and outcome in univariable and multivariable analyses
Patients started being at risk from the date of MCL diagnosis or date of clinically detected relapse and were followed until the minimum of date of death (owing to any cause) in the analysis of overall survival (OS), date of verified next relapse/progression or death owing to any cause in the analysis of progression-free survival (PFS), or end of the study period (21 May 2021). The Kaplan-Meier method was used to calculate the survival function according to sCD163 level, and differences between survival curves were formally tested using the log-rank test. Univariable and multivariable Cox proportional hazards (PH) models were fitted to estimate hazard ratios (HRs) as a measure of the association between sCD163 and outcome, measured as OS or PFS. The PH assumption was formally tested using Schoenfeld residuals and was not violated in any of the analyses.
Variables used for adjustments in the multivariable model were chosen according to a directed acyclic graph26 (supplemental Figure 2). Because biological variables were incomplete in the population-based cohort from Lund, fully adjusted multivariable analysis was restricted to the Uppsala cohort and the clinical study cohort.
Defining a clinically significant sCD163 cutoff
To calculate the optimal cutoff, Maximally Selected Rank Statistics (survival::surv_cutpoint() using maxstat::maxstat) in R was used. Receiver operating characteristic curve was plotted with pROC package in R to determine the predictive ability of sCD163 levels at diagnosis on 5-year OS. This was based on the newly diagnosed cohort only as the follow-up was shorter in the relapsed cohort.
Statistical methods
All P-values were 2-sided and differences were considered statistically significant when P was <.05.
R version 4.2.0 with RStudio was used for all statistical analyses.
Ethical approval
Studies on the population-based cohorts were approved by the Ethical Regional Committee in the respective regions (Dnr 2014/233, Ethical Regional Committee in Uppsala, and Dnr 2011/539, Ethical Regional Committee in Lund). The clinical trial was approved by the national ethics committee in each participating country and the study was performed according to the International Conference on Harmonization Guidelines for Good Clinical Practice and Declaration of Helsinki. All patients in the clinical trial provided written informed consent.
Results
Patients and cohorts
To investigate if the macrophage marker sCD163 is associated with prognosis in MCL, we analyzed sCD163 in serum samples from a total of 131 patients with MCL. Eighty-one patients had their samples taken at diagnosis and 50 patients at the time of relapse (Figure 1). Ages ranged from 45 to 85 years with 53% being <70 years, and 73% of the patients being male (Table 1). The patients sampled at diagnosis represent a population-based cohort and were preferably treated with rituximab (R) bendamustin (n = 33) or the Nordic MCL 2 protocol with induction cytarabine–based chemotherapy and consolidation with high-dose chemotherapy with an autologous stem cell transplantation (n = 19). Other primary treatments are outlined in the legend of Table 1. Median follow-up time in this cohort was 4.3 years (range, 0.11-9.8), and 43% of the patients relapsed or died during follow-up.
Table 1.
Patient characteristics in all patients together and in the 2 cohorts
| Characteristic | All n = 131, n (%) |
Patients sampled at diagnosis n = 81, n (%) | Patients sampled at relapse n = 50, n (%) | OS HR∗, n (%) | |
|---|---|---|---|---|---|
| sCD163 (ng/mL)† | 3112 (1223-7611) | 3211 (1311-7611) | 2963 (1223-6002) | 3.8 (1.9-7.5)‡ | |
| Dx period | 2002-2015 | 59 (45) | 33 (41) | 26 (52) | ref |
| 2016-2020 | 72 (55) | 48 (59) | 24 (48) | 0.7 (0.39-1.3) | |
| Age | < 70 | 70 (53) | 42 (52) | 28 (56) | ref |
| ≥70 | 61 (47) | 39 (48) | 22 (44) | 1.4 (0.83-2.5) | |
| Sex | Female | 36 (27) | 22 (27) | 14 (28) | ref |
| Male | 95 (73) | 59 (73) | 36 (72) | 1.5 (0.76-3) | |
| ECOG | 0-1 | 83 (97) | 77 (96) | 6 (100) | ref |
| 2-4 | 3 (3.5) | 3 (3.8) | 0 (0) | 3.7 (0.88-16) | |
| Unknown | 45 | 1 | 44 | ||
| MIPI | 1 | 21 (17) | 11 (14) | 10 (20) | ref |
| 2 | 52 (41) | 35 (45) | 17 (34) | 3.2 (1.0-10.8) | |
| 3 | 54 (43) | 31 (40) | 23 (46) | 4.8 (1.4-15.8) | |
| Unknown | 4 | 4 | 0 | ||
| LDH | Normal | 70 (55) | 45 (57) | 25 (52) | ref |
| High | 57 (45) | 34 (43) | 23 (48) | 2.0 (1.2-3.5) | |
| Unknown | 4 | 2 | 2 | ||
| Hb | Normal | 70 (56) | 41 (55) | 29 (58) | ref |
| Low | 54 (44) | 33 (45) | 21 (42) | 3.3 (1.8-6.3) | |
| Unknown | 7 | 7 | 0 | ||
| Ki-67 | <30% | 27 (39) | 14 (45) | 13 (34) | ref |
| ≥30% | 42 (61) | 17 (55) | 25 (66) | 1.7 (0.76-3.6) | |
| Unknown | 62 | 50 | 12 | ||
| Histology | Classic | 52 (76) | 27 (84) | 25 (69) | ref |
| Blastoid | 16 (24) | 5 (16) | 11 (31) | 2.1 (0.9-4.8) | |
| Unknown | 63 | 49 | 14 | ||
| p53 or TP53 | Low or no mutation | 61 (79) | 24 (83) | 37 (77) | ref |
| High or mutated | 16 (21) | 5 (17) | 11 (23) | 2.2 (0.99-4.8) | |
| Unknown | 54 | 52 | 2 | ||
The patients sampled at diagnosis were treated with rituximab (R) and bendamustine (n = 33), the Nordic MCL 2 protocol with induction cytarabine-based chemotherapy and consolidation with high-dose chemotherapy with an autologous stem cell transplantation (n = 19), ibrutinib (n = 7), R-CHOP (R-cyclophosphamide, doxorubicin, vincristine and prednisone) (n = 3), rituximab only (n = 4), watch and wait (n = 1), or R-cytarabine (n = 1). For 13 patients the treatment was unknown. Patients from the clinical trial cohort were treated with rituximab, ibrutinib and lenalidomide within the trial. The 6 patients with MCL who relapsed from the Uppsala cohort were previously treated with in median 1 prior treatment (range 1-3), and after inclusion in this study they received bendamustine (n = 2), ibrutinib (n = 1), venetoclax (n = 1), Nordic MCL 2 protocol (n = 1), and cyclophosphamide (n = 1). Three of the patients received ibrutinib and 1 received venetoclax in later courses.
Univariable Cox PHs for all patients, showing change in OS rate by clinical or biological characteristic compared with its reference value, have been presented.
Boldface indicates statistically significant values.
Dx period, diagnostic period; ECOG, Eastern Cooperative Oncology Group performance status scale; LDH, lactate dehydrogenase; MIPI, mantle cell lymphoma international prognostic index; ref, reference.
Univariable Cox PHs (95% CI) for OS.
Median (minimum-maximum); n (col %).
HR for OS, sCD163 dichotomized by median.
The patients who relapsed from the study cohort had a median of 2 prior treatments (range, 1-5) and were subsequently treated with rituximab, ibrutinib, and lenalidomide within the trial. Median follow-up time among the patients who relapsed was 3.5 years (range, 0.1-4.2), and 59% of the patients relapsed or died during follow-up.
In univariable Cox PH models, high MCL international prognostic index (MIPI), lactate dehydrogenase above normal, and Hb levels below normal were associated with shorter OS.
sCD163 levels and cutoffs
The sCD163 cutoff for this study was a priori set to median level. A Kaplan-Meier analysis with sCD163 divided into quartiles showed a gradually increasing risk of progression or death with increasing sCD163 value, with the greatest difference between the upper and the lower half of patients, supporting the choice of median value as a cutoff (supplemental Figure 1). In the samples taken at diagnosis, the median level was 3211 ng/mL (range, 1311-7611) and in the samples taken at relapse the median value was 2963 ng/mL (range, 1223-6002). In analysis of all patients together, the overall median level was 3112 ng/mL (range, 1223-7611), and finally, as a suggestion for clinical implementation the cutoff of 3000 ng/mL was evaluated.
Association between sCD163 levels and baseline characteristics
sCD163 was higher in patients with p53 abnormalities. High sCD163 was also more common in patients with elevated lactate dehydrogenase and patients with low Hb, whereas there was no association with other well-known poor prognostic factors (Table 2).
Table 2.
Frequencies and proportions of clinical and biological factors, by sCD163 below or above median (3112 ng/mL)
| sCD163 low n = 65, n (%) |
sCD163 high n = 66, n (%) |
P value | ||
|---|---|---|---|---|
| Number of events | Died | 11 (24) | 34 (76) | |
| Relapsed | 21 (33) | 42 (67) | ||
| Time of sample | Diagnosis | 39 (48) | 42 (52) | .8∗ |
| Relapse | 26 (52) | 24 (48) | ||
| Age median (range) | 69 (46-85) | 67 (45-82) | .8† | |
| Sex | Female | 17 (47) | 19 (53) | .7∗ |
| Male | 48 (51) | 47 (49) | ||
| ECOG | 0-1 | 40 (48) | 43 (52) | .2‡ |
| 2-4 | 0 (0) | 3 (100) | ||
| Unknown | 25 | 20 | ||
| MIPI | 1 | 14 (67) | 7 (33) | .2∗ |
| 2 | 27 (52) | 25 (48) | ||
| 3 | 23 (43) | 31 (57) | ||
| Unknown | 1 | 3 | ||
| LDH | Normal | 45 (64) | 25 (36) | <.001∗ |
| High | 19 (33) | 38 (67) | ||
| Unknown | 1 | 3 | ||
| Hb | Normal | 46 (66) | 24 (34) | <.001∗ |
| Low | 15 (28) | 39 (72) | ||
| Unknown | 4 | 3 | ||
| Ki-67 | <30 | 13 (48) | 14 (52) | .7∗ |
| ≥30 | 22 (54) | 19 (46) | ||
| Unknown | 30 | 33 | ||
| Histology | Classic | 28 (54) | 24 (46) | .9∗ |
| Blastoid | 9 (56) | 7 (44) | ||
| Unknown | 28 | 35 | ||
| TP53/p53 | Low or no mutation | 36 (59) | 25 (41) | .015‡ |
| High or mutated | 4 (25) | 25 (75) | ||
| Unknown | 25 | 29 |
High sCD163 was more common in patients with high LDH, low Hb and p53 high/TP53 positive tumors.
ECOG, ECOG performance status scale; LDH, lactate dehydrogenase; MIPI, mantle cell lymphoma international prognostic index.
Data are presented as number (percentage), unless otherwise indicated.
Boldface indicates statistically significant values.
Pearson χ2 test.
Wilcoxon rank sum test.
Fisher exact test.
Association between sCD163 levels and tissue CD163 expression
Tissue CD163 was analyzed in lymph nodes vs bone marrow separately because CD163 levels differed by location.7 There was a moderate correlation between sCD163 levels and CD163 in tumor tissue sampled from lymph nodes (r = 0.64; P = .014; Spearman rank correlation) and a similar, but slightly weaker trend in tumor tissue sampled from bone marrow (r = 0.51; P = .052) (Figure 2).
Figure 2.
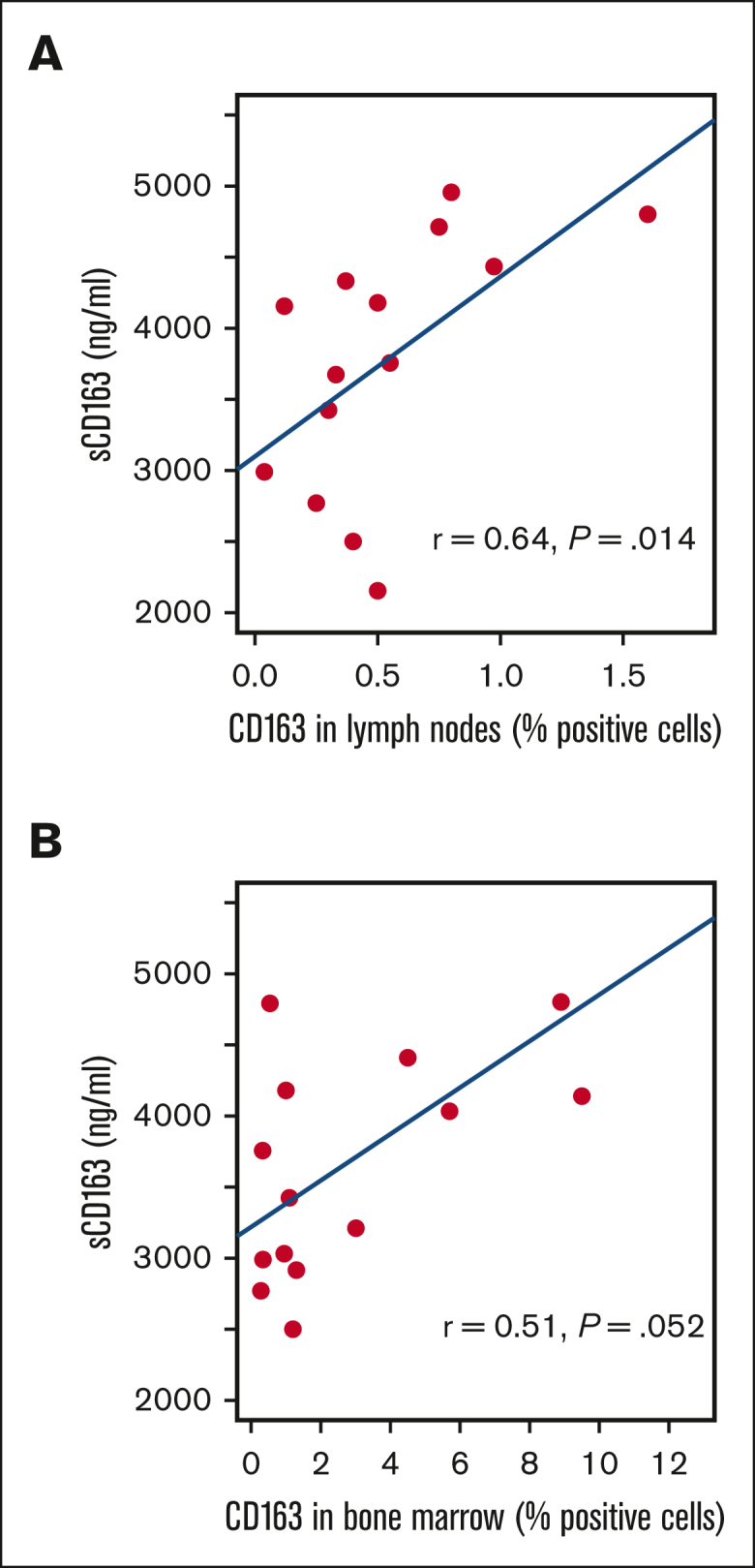
Correlation between sCD163 and CD163 in MCL tumor tissue. CD163 levels vary between different tissue types as indicated by the difference in the x-axis.
CD163 levels in MCL lymphoma tissue were analyzed for association with PFS in 29 patients and was confirmed as a poor prognostic marker in a multivariable Cox regression model (HR, 4.0; 95% confidence interval [CI], 1.05-15.16; adjusted for age >70 years, sex, and tissue sample site). Among the 3 cases (10% of the cohort) with p53 overexpression, a higher frequency of tissue CD163 was seen than in tumors with no p53 expression (average value 4.1% positive cells vs 0.9% positive cells; Wilcoxon rank sum test W = 79; P = .02).
Association between sCD163 and outcome in univariable analyses
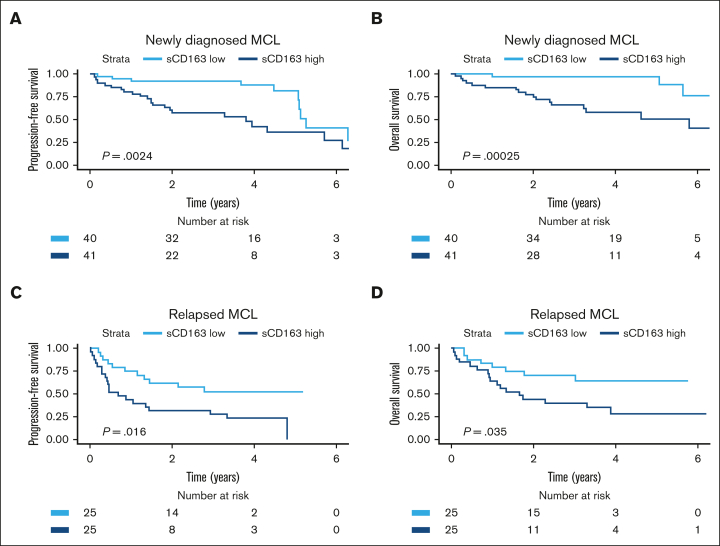
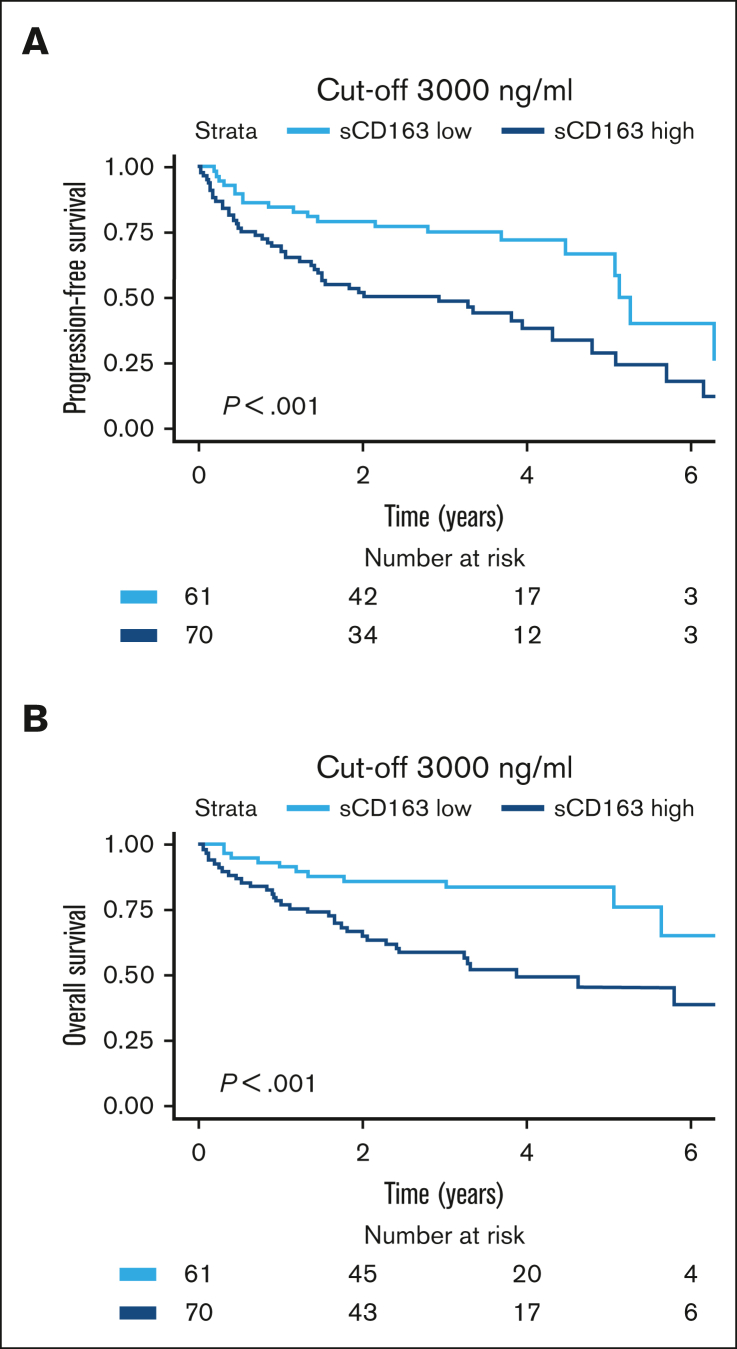
Patients with high sCD163 at diagnosis had shorter PFS and shorter OS than patients with low sCD163 (log-rank test P = .002 and P < .001, respectively) (Figure 3). Patients with low sCD163 at diagnosis had a 5-year OS of 97% (95% CI, 93-100) whereas the 5-year OS in patients with high sCD163 was 51% (95% CI, 34-75). In the cohort with patients with MCL who had relapsed, high sCD163 was also associated with shorter PFS and shorter OS (log-rank test P = .016 and P = .035) (Figure 3).
Figure 3.
Kaplan-Meier curves showing prognostic impact of sCD163 levels in the 2 cohorts. Probability of (A) PFS and (B) OS by sCD163 level (dichotomized by median level 3211 ng/mL) in patients newly diagnosed with MCL. Five-year OS was 97% vs 51% in patients with low sCD163 vs high sCD163. (C) probability of PFS and (D) Probability of OS by sCD163 level (dichotomized by median level 2963 ng/mL) in patients with relapsed MCL.
Association between sCD163 and outcome in multivariable analyses
Based on a Cox regression model including all 131 patients, adjusted for age and sex, and stratified by time of sampling (at diagnosis or relapse), high sCD163 was associated with shorter PFS and shorter OS (PFS: HR, 3.13 [95% CI, 1.83-5.36] and OS: HR, 4.10 [95% CI, 2.06-8.16]). sCD163 as a continuous variable (100 ng/mL intervals) also showed significant negative prognostic impact (PFS: HR, 1.04 [95% CI, 1.02-1.05; P < .001]; OS: HR, 1.04 [95% CI, 1.02-1.06; P < .001]).
Information on biological risk factors was available in 57 patients from the Uppsala and the clinical study cohort. In a fully adjusted regression model including MIPI, Ki67, histology and p53 status, high sCD163 was associated with shorter PFS (HR, 3.48; 95% CI, 1.42-8.54) and shorter OS (HR, 4.33; 95% CI, 1.32-14.2), suggesting that sCD163 is an independent risk factor for poor outcome in patients with MCL (Table 3).
Table 3.
Cox PHs (95% CI) for OS and PFS
| n | sCD163 high | P | sCD163 continuously | P | ||
|---|---|---|---|---|---|---|
| OS | a | 131 | 3.79 (1.91-7.48) | <.001 | 1.04 (1.02-1.06) | <.001 |
| b | 131 | 4.10 (2.06-8.16) | <.001 | 1.04 (1.02-1.06) | <.001 | |
| c | 57 | 4.33 (1.32-14.2) | .016 | 1.04 (0.996-1.08) | .075 | |
| PFS | a | 131 | 2.73 (1.16-4.62) | <.001 | 1.04 (1.02-1.05) | <.001 |
| b | 131 | 3.13 (1.83-5.36) | <.001 | 1.04 (1.02-1.05) | <.001 | |
| c | 57 | 3.48 (1.42-8.54) | .006 | 1.04 (1.003-1.07) | .034 |
The prognostic value of sCD163 is shown dichotomized at median level of sCD163 (3112 ng/mL) and as a continuous variable at intervals of 100 ng/mL. The model is stratified by time of sampling (at diagnosis or at relapse) and adjusted for: (a) univariable (b) age ≥ 70 years, sex. (c) MIPI, Ki-67 ≥30%, blastoid histology and presence of p53 overexpression or TP53 mutation. Model (c) is based on the population-based cohort from Uppsala and the clinical study cohort.
Boldface indicates statistically significant values.
MIPI, mantle cell lymphoma international prognostic index.
sCD163 levels and treatment
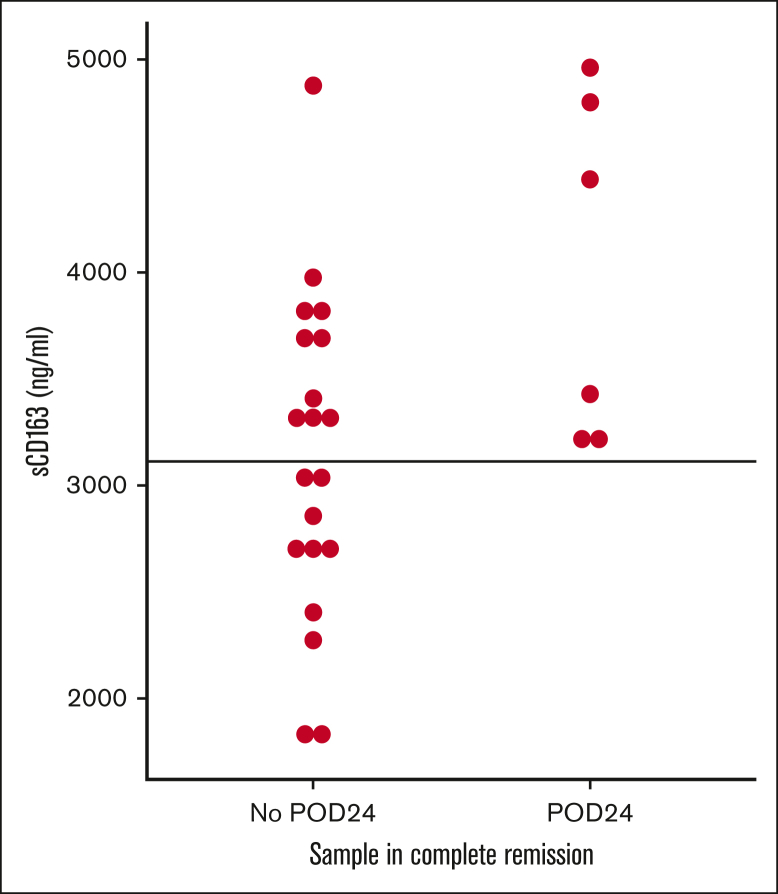
There was no systematic change in sCD163 levels during or after treatment on a group level (not shown) although variations can be seen on the individual levels (supplemental Figure 3) and specifically in ibrutinib treated patients (supplemental Figure 4). However, sCD163 levels measured in remission after treatment completion identified patients who did not experience progression of disease within 24 months. No patient with low sCD163 during remission relapsed within 24 months (Figure 4).
Figure 4.
sCD163 level in remission predicts progression of disease within 24 months. Samples were taken when patients were in remission after treatment and values are shown by outcome 24 months later. No patient with low sCD163 in remission relapsed within 24 months. POD24 is calculated from the time of each sample. Intercept line at median value for all patients (3112 ng/mL). POD24, progression of disease within 24 months.
Defining a clinically significant sCD163 cutoff
To refine the cutoff and provide a clinically useful measurement, samples taken at diagnosis and relapse were tested separately with maximally selected rank statistics. In the cohort of newly diagnosed MCL, the lowest P value for curve separation was found at a cutoff value of 4033 ng/mL. However, values between 2250 ng/mL (P = .044) and 4033 ng/mL (P < .0001) were all statistically significant with P < .05. Although suggested cutoffs are more flexible for the cohort with newly diagnosed MCL, the corresponding cutoff in the cohort with relapsed MCL was 2960 ng/mL (P = .035). For simplicity, the value was rounded up to the more intuitive number of 3000 ng/mL. In a receiver operating characteristic curve of newly diagnosed MCL the cutoff 3000 ng/mL resulted in 100% sensitivity and 67% specificity for 5-year survival. Area under curve was 83%. Figure 5 shows the prognostic implementation of this cutoff in patients who were newly diagnosed and those who were relapsed (log-rank P < .001 for both PFS and OS). Bearing in mind that values differ among the results of different enzyme-linked immunosorbent assay kits and we used the human sCD163 Quantikine Kit from R&D Systems, we propose a cutoff value at 3000 ng/mL to define patients with MCL who are at high risk.
Figure 5.
Prognostic value of the cutoff suggested for clinical implementation. Kaplan-Meier showing risk stratification based on clinically suggested cutoff at 3000 ng/mL for both pateints who were newly diagnosed with MCL and those who relapsed. (A) PFS (HR, 2.4; 95% CI, 1.4-4.1). (B) OS (HR, 3.1; 95% CI, 1.6-6.1).
Discussion
High levels of the soluble M2 macrophage marker sCD163 are associated with poor prognosis in several malignancies, and our results show that high sCD163 is also associated with poor prognosis in MCL. In both newly diagnosed and relapsed MCL, sCD163 levels above the median correlate with shorter PFS and OS, independent of other established risk factors. Furthermore, low levels of sCD163 at diagnosis are associated with a remarkably good prognosis considering the aggressive nature of MCL, with a 5-year survival of 97%.
Although, to our knowledge, this is the first study investigating sCD163 in MCL, the correlation of sCD163 levels with MCL prognosis is in line with the few previous studies of tissue CD163 in MCL conducted using immunohistochemistry7,8 and transcriptomic analysis of CD163 in MCL tissue10 as well as several studies of sCD163 in other malignancies.12,16, 17, 18 High levels of sCD163 also correlate with poor prognosis in most lymphoma subtypes21, 22, 23 although 1 study did not show prognostic implication of sCD163 in cHL,27 possibly because of the favorable prognosis of cHL.
In contrast to some previous studies of patients with DLBCL and cHL,21,22,28 we did not see any systematic change in sCD163 levels during or after treatment. However, our patients received varying treatments, and the time of sampling was not standardized according to the time after start of treatment. In line with our results, Plattel et al also found inconsistent change in sCD163 levels after treatment of cHL and suggested that sCD163 might be less useful for response evaluation.29 The fact that sCD163 levels are prognostic when measured in clinical remission suggests that levels are related to the immune microenvironment of the individual rather than solely to the tumor.
We found a moderate correlation (r = 0.64; P = .014) with CD163 levels in MCL regions of lymphoid tissue even with a limited number of samples. This is in line with the observations reported by Vajavaara et al who found a weak to moderate correlation between sCD163 and CD163 levels in DLBCL lymph nodes (r = 0.43; P = .005). The CD163 levels in bone marrow are generally higher than in lymph nodes, and different types of tissues need to be analyzed separately. The moderate correlation might be because of the relatively low number of matching tissue samples, as well as the fact that soluble CD163 represents all sCD163 shredding macrophages in the patient, whereas tissue levels are evaluated within the tumor only. The prognostic impact of soluble CD163 is similar to what was found for tissue CD163 by Rodrigues et al (HR, 2.83; 95% CI, 1.25-6.42, evaluating time to progression, adjusted for age and sex).7 Soluble CD163 however, has the advantage of being easily accessible through a blood sample, and it is also better at identifying patients with very good prognosis.
The correlation with p53 abnormality is interesting because genomic alteration in TP53 is one of the strongest risk factors in MCL, and drugs targeting the microenvironment could be a treatment option for these aggressive cases. Izquierdo et al recently proposed that extracellular vesicles from TP53-mutated tumor cells mediate suppression of macrophage phagocytic activity,30 possibly re-educating M1 macrophages into macrophages of M2 type. Their results are supported by an in vitro study showing polarization of macrophages by secretome from p53-positive hepatocytes.31 Although M1 macrophages were not investigated in this study, this would be consistent with our previous work in which p53-positive tumors tended to be more common among CD163 high cases than in CD163 low cases (20% vs 8% p53-positive cases; P = .06 from Pearson χ2 test), as well as our current results indicating that both CD163 and sCD163 are higher in patients with p53 aberrations.
Several established treatments affect M2 TAMs, some of the most successful being BTK inhibitors. In addition to blocking the BTK signaling pathway in MCL cells, BTK inhibitor ibrutinib shifts T cells toward the more therapeutically effective Th1 subset, disrupts communication between tumor cells and TAMs,3 and acts synergistically with monoclonal antibodies via JAK2 inhibition.32 Another active drug against MCL, lenalidomide, inhibits MCL cell growth by repressing macrophage recruitment.4 It also downregulates regulatory T cells and TAMs whereas it enhances antibody–dependent cellular cytotoxicity.5 sCD163 shows prognostic implications both in patients treated with chemotherapy and with BTK inhibitors. Newer immunomodulatory treatments, such as chimeric antigen receptor T cells and bispecific antibodies have also shown promising results in MCL,33,34 and different combinations of treatments that enhance antigen presentation and phagocytosis by TAMs are currently being tested to improve the efficacy of T-cell therapies.13
There is also increasing interest in the CD163 receptor itself as a novel target for immunotherapy, for example the depletion of CD163+ cells using monoclonal antibodies or antibody-drug conjugates, as well as the reprogramming of M2 to M1 by STAT3 inhibition and the delivery of glucocorticoids directly to CD163+ TAMs with dexamethasone-antibody conjugates.12 We hypothesize that these strategies are also worth further investigation in MCL.
The range of sCD163 levels in healthy individuals reported is wide (700-3900 ng/mL). Several factors can influence sCD163 levels, such as infection, inflammation (both chronic and acute), liver disease, and corticosteroid treatment.14 Levels also vary depending on what laboratory kit is used and standardization among laboratories is needed. Vajavaara et al used the same kit from R&D Systems in DLBCL and found prognostic implications with the median value of 2950 ng/mL (range, 870-30 000), which is very close to our results as we suggest a cutoff value of 3000 ng/mL for both diagnostic and relapsed MCL. Patients treated with immunochemotherapy as well as with targeted treatments with a sCD163 value >3000 ng/mL should be considered to have a high risk of progression or relapse and subsequent death.
Summary, significance, or implication
The results from this study further enhance the important role of M2-like macrophages in MCL. The emerging treatments directed toward these macrophages are of interest for patients with MCL with high sCD163. We propose that sCD163 could be used as a prognostic marker in both newly diagnosed and relapsed MCL, perhaps supporting clinicians in de-escalating treatment in older or fragile patients with low sCD163. It could also be an easily accessible biomarker used together with TP53 or blastoid or pleomorphic histology when selecting patients for more intense treatment.
Conflict-of-interest disclosure: I.G. has received honoraria from Janssen-Cilag and Takeda (not related to this study). M.J. has received research funding from AstraZeneca, Kite/Gilead, Roche, Janssen, Bristol Myers Squibb (BMS), and AbbVie, and honoraria from AstraZeneca, Genmab, Kite/Gilead, Incyte, Orion, Novartis, Roche, Janssen, BMS, and AbbVie (not related to this study). C.E.W. has membership on an entity’s board of directors or advisory committees for Red Door Analytics; reports research funding from Janseen Cilag (not related to this study); and is currently employed with War On Cancer. D.M. has received honoraria from Roche, Merck, BMS, and Takeda (not related to this study). G.E. has received honoraria from Gilead, Pierre Fabre, and Roche; has been on the advisory boards of Gilead and Pierre Fabre; and is a scientific adviser for Elicera Therapeutics AB, Sprint Bioscience AB, and XNK Therapeutics AB. The remaining authors declare no competing financial interests.
Acknowledgments
The study was supported by Swedish Cancer Society grant 22 2167 Pj (I.G.), Senior Clinical Investigator Award 19 0109 (I.G.), the Swedish Society of Medicine (I.G.), and the Lung Cancer Research Foundation (I.G.). Financial support for S.E. was granted by the European Union’s Horizon 2020 Framework Programme for Research and Innovation under agreement 754299, Cancerfonden grant 21 1561 Pj, Mats Paulsson’s Foundation for Research, Innovation Och Societal Development, Stefan Paulsson’s Cancer Foundation, and Create Health.
Authorship
Contribution: A.N. designed the research, performed statistical analysis, and wrote the manuscript; L.L. performed the research, statistical analysis, and wrote the manuscript; R.-M.A. collected and interpreted data; M.J., D.M., A.K., R.R., and M.H. collected data; A.P. collected and interpreted data; G.E. collected data and aquired ethical approval for blood sampling; C.E.W. performed statistical analysis; P.H. analyzed and interpreted data; S.E. interpreted data and wrote the manuscript; and I.G. designed the research, collected data, interpreted data, and wrote the manuscript.
Footnotes
Data are available upon reasonable request to the corresponding author, Ingrid Glimelius (ingrid.glimelius@igp.uu.se).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Jain P, Wang ML. Mantle cell lymphoma in 2022-a comprehensive update on molecular pathogenesis, risk stratification, clinical approach, and current and novel treatments. Am J Hematol. 2022;97(5):638–656. doi: 10.1002/ajh.26523. [DOI] [PubMed] [Google Scholar]
- 2.Silkenstedt E, Linton K, Dreyling M. Mantle cell lymphoma - advances in molecular biology, prognostication and treatment approaches. Br J Haematol. 2021;195(2):162–173. doi: 10.1111/bjh.17419. [DOI] [PubMed] [Google Scholar]
- 3.Svanberg R, Janum S, Patten PEM, Ramsay AG, Niemann CU. Targeting the tumor microenvironment in chronic lymphocytic leukemia. Haematologica. 2021;106(9):2312–2324. doi: 10.3324/haematol.2020.268037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song K, Herzog BH, Sheng M, et al. Lenalidomide inhibits lymphangiogenesis in preclinical models of mantle cell lymphoma. Cancer Res. 2013;73(24):7254–7264. doi: 10.1158/0008-5472.CAN-13-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saleh K, Cheminant M, Chiron D, Burroni B, Ribrag V, Sarkozy C. Tumor microenvironment and immunotherapy-based approaches in mantle cell lymphoma. Cancers. 2022;14(13):3229. doi: 10.3390/cancers14133229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carreras J, Roncador G, Hamoudi R. Artificial intelligence predicted overall survival and classified mature B-cell neoplasms based on immuno-oncology and immune checkpoint panels. Cancers (Basel) 2022;14(21):5318. doi: 10.3390/cancers14215318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigues JM, Nikkarinen A, Hollander P, et al. Infiltration of CD163-PD-L1- and FoxP3-positive cells adversely affects outcome in patients with mantle cell lymphoma independent of established risk factors. Br J Haematol. 2021;193(3):520–531. doi: 10.1111/bjh.17366. n/a. [DOI] [PubMed] [Google Scholar]
- 8.Li P, Yuan J, Ahmed FS, et al. High counts of CD68+ and CD163+ macrophages in mantle cell lymphoma are associated with inferior prognosis. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.701492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan KL, Scott DW, Hong F, et al. Tumor-associated macrophages predict inferior outcomes in classic Hodgkin lymphoma: a correlative study from the E2496 Intergroup trial. Blood. 2012;120(16):3280–3287. doi: 10.1182/blood-2012-04-421057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Bris Y, Normand A, Bouard L, et al. Aggressive, early resistant and relapsed mantle cell lymphoma distinct extrinsic microenvironment highlighted by transcriptome analysis. EJHaem. 2022;3(4):1165–1171. doi: 10.1002/jha2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le K, Sun J, Khawaja H, et al. Mantle cell lymphoma polarizes tumor-associated macrophages into M2-like macrophages, which in turn promote tumorigenesis. Blood Adv. 2021;5(14):2863–2878. doi: 10.1182/bloodadvances.2020003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skytthe MK, Graversen JH, Moestrup SK. Targeting of CD163(+) macrophages in inflammatory and malignant diseases. Int J Mol Sci. 2020;21(15):5497. doi: 10.3390/ijms21155497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe T. Approaches of the innate immune system to ameliorate adaptive immunotherapy for B-cell non-Hodgkin lymphoma in their microenvironment. Cancers (Basel) 2021;14(1):141. doi: 10.3390/cancers14010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Møller HJ. Soluble CD163. Scand J Clin Lab Invest. 2012;72(1):1–13. doi: 10.3109/00365513.2011.626868. [DOI] [PubMed] [Google Scholar]
- 15.Etzerodt A, Moestrup SK. CD163 and inflammation: biological, diagnostic, and therapeutic aspects. Antioxid Redox Signal. 2013;18(17):2352–2363. doi: 10.1089/ars.2012.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding D, Song Y, Yao Y, Zhang S. Preoperative serum macrophage activated biomarkers soluble mannose receptor (sMR) and soluble haemoglobin scavenger receptor (sCD163), as novel markers for the diagnosis and prognosis of gastric cancer. Oncol Lett. 2017;14(3):2982–2990. doi: 10.3892/ol.2017.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.No JH, Moon JM, Kim K, Kim YB. Prognostic significance of serum soluble CD163 level in patients with epithelial ovarian cancer. Gynecol Obstet Invest. 2013;75(4):263–267. doi: 10.1159/000349892. [DOI] [PubMed] [Google Scholar]
- 18.Waidmann O, Köberle V, Bettinger D, et al. Diagnostic and prognostic significance of cell death and macrophage activation markers in patients with hepatocellular carcinoma. J Hepatol. 2013;59(4):769–779. doi: 10.1016/j.jhep.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Lauridsen KM, Hokland M, Al-Karradi S, Møller HJ, Donskov F, Andersen MN. Soluble CD163: a novel independent prognostic biomarker in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2023;72(2):461–473. doi: 10.1007/s00262-022-03266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krijgsman D, De Vries NL, Andersen MN, et al. CD163 as a biomarker in colorectal cancer: the expression on circulating monocytes and tumor-associated macrophages, and the soluble form in the blood. Int J Mol Sci. 2020;21(16):5925. doi: 10.3390/ijms21165925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vajavaara H, Ekeblad F, Holte H, et al. Prognostic impact of soluble CD163 in patients with diffuse large B-cell lymphoma. Haematologica. 2021;106(9):2502–2506. doi: 10.3324/haematol.2020.278182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones K, Vari F, Keane C, et al. Serum CD163 and TARC as disease response biomarkers in classical Hodgkin lymphoma. Clin Cancer Res. 2013;19(3):731–742. doi: 10.1158/1078-0432.CCR-12-2693. [DOI] [PubMed] [Google Scholar]
- 23.Nederby L, Roug AS, Knudsen SS, et al. Soluble CD163 as a prognostic biomarker in B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2015;56(11):3219–3221. doi: 10.3109/10428194.2015.1026899. [DOI] [PubMed] [Google Scholar]
- 24.Jerkeman M, Eskelund CW, Hutchings M, et al. Ibrutinib, lenalidomide, and rituximab in relapsed or refractory mantle cell lymphoma (PHILEMON): a multicentre, open-label, single-arm, phase 2 trial. Lancet Haematol. 2018;5(3):e109–e116. doi: 10.1016/S2352-3026(18)30018-8. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigues JM, Hassan M, Freiburghaus C, et al. p53 is associated with high-risk and pinpoints TP53 missense mutations in mantle cell lymphoma. Br J Haematol. 2020;191(5):796–805. doi: 10.1111/bjh.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package 'dagitty'. Int J Epidemiol. 2016;45(6):1887–1894. doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 27.Hsi ED, Li H, Nixon AB, et al. Serum levels of TARC, MDC, IL-10, and soluble CD163 in Hodgkin lymphoma: a SWOG S0816 correlative study. Blood. 2019;133(16):1762–1765. doi: 10.1182/blood-2018-08-870915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riihijarvi S, Fiskvik I, Taskinen M, et al. Prognostic influence of macrophages in patients with diffuse large B-cell lymphoma: a correlative study from a Nordic phase II trial. Haematologica. 2015;100(2):238–245. doi: 10.3324/haematol.2014.113472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plattel WJ, Alsada ZN, van Imhoff GW, Diepstra A, van den Berg A, Visser L. Biomarkers for evaluation of treatment response in classical Hodgkin lymphoma: comparison of sGalectin-1, sCD163 and sCD30 with TARC. Br J Haematol. 2016;175(5):868–875. doi: 10.1111/bjh.14317. [DOI] [PubMed] [Google Scholar]
- 30.Izquierdo E, Vorholt D, Blakemore SJ, et al. Extracellular vesicles and PDL1 suppress macrophages inducing therapy resistance in TP53-deficient B-cell malignancies. Blood. 2022;139(25):3617–3629. doi: 10.1182/blood.2021014007. [DOI] [PubMed] [Google Scholar]
- 31.Sen B, Aggarwal S, Nath R, et al. Secretome of senescent hepatoma cells modulate immune cell fate by macrophage polarization and neutrophil extracellular traps formation. Med Oncol. 2022;39(9):134. doi: 10.1007/s12032-022-01732-w. [DOI] [PubMed] [Google Scholar]
- 32.Barbarino V, Henschke S, Blakemore SJ, et al. Macrophage-mediated antibody dependent effector function in aggressive B-cell lymphoma treatment is enhanced by ibrutinib via inhibition of JAK2. Cancers (Basel) 2020;12(8):2303. doi: 10.3390/cancers12082303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang M, Munoz J, Goy A, et al. Three-year follow-up of KTE-X19 in patients with relapsed/refractory mantle cell lymphoma, including high-risk subgroups, in the ZUMA-2 study. J Clin Oncol. 2022;41(3):555–567. doi: 10.1200/JCO.21.02370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eyre TA, Cheah CY, Wang ML. Therapeutic options for relapsed/refractory mantle cell lymphoma. Blood. 2022;139(5):666–677. doi: 10.1182/blood.2021013326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.