Abstract
Purpose:
The complex technological processes involved in radiation therapy can be intimidating to patients, causing increased treatment-related anxiety and reduced satisfaction. An intervention was implemented to provide direct consultations between patients and medical physicists to reduce patient anxiety and improve patient satisfaction. A randomized clinical trial was conducted to test the intervention’s effect on anxiety, distress, treatment adherence, technical understanding, and satisfaction in patients receiving radiation therapy.
Methods and Materials:
Eligible patients were recruited into “intervention” and “standard of care” arms within a phase 2 screening randomized trial. Intervention-arm patients met with a medical physicist who provided technical information and addressed patient questions or concerns at the time of treatment simulation and before the first treatment. In addition to baseline information collected before randomization, participants were surveyed (1) before simulation, (2) before the first treatment, and (3) before the completion of treatment to evaluate the study endpoints. Primary endpoints included patient anxiety and distress. Secondary endpoints included patient treatment adherence, overall satisfaction, and technical understanding of treatment. Patients in the intervention arm were surveyed before and after each physicist meeting.
Results:
Participant anxiety was significantly reduced in the intervention arm (difference, −0.29; 95% confidence interval, −0.57 to −0.02; P = .038). No differences in distress or treatment adherence were observed between groups. Although measures of technical understanding and satisfaction were evaluated as exploratory objectives, participants in the intervention group were more likely to feel that technical aspects of treatment were adequately explained (difference, 0.78; 95% confidence interval, 0.03–1.54), and all measures of technical understanding and satisfaction were considerably higher in the intervention group at the time of the first visit.
Conclusions:
The establishment of a direct patient-provider relationship with the medical physicist reduced anxiety in patients receiving radiation therapy. In addition, increases in patient understanding of the technical aspects of care and in satisfaction were observed at the initiation of treatment.
Introduction
Poor patient-provider communication in medicine represents an impediment to patient satisfaction, participation in care, safety, and adherence to treatment,1–3 and the field of radiation oncology is no exception.4,5 Technical treatment complexity in radiation oncology can lead to poor patient understanding of treatment and, in turn, difficulty making treatment decisions.6,7 Technical treatment complexity may also increase patient anxiety and distress, which are associated with poor prognosis.8 Improved clinical communication and improved patient understanding of the treatment process may enable patients to participate more actively in their own care. Research has demonstrated considerable benefits to encouraging patients to play an active role in their treatment, including improvements in patient confidence in the care team, increased belief in the efficacy of the treatment, and potential improvements in treatment adherence.9
Medical physicists play an instrumental role in the technical, quality, and safety aspects of radiation therapy. Expanding the role of the medical physicist by establishing a direct patient-provider relationship may potentially further improve the quality of patient care. Using patient-centered communication, medical physicists can potentially increase patients’ understanding of the treatment process, reduce their treatment-related anxiety and distress, and encourage them to become more actively involved in their care. The medical physicist, as the expert in the technical aspects of radiation therapy, may be the most appropriate team member to help patients understand the technical aspects of their treatment process. This enhanced understanding of the technical efforts and safety processes in support of treatment has the potential to improve patient satisfaction. Furthermore, increased patient understanding of the detrimental physical and biological effects of deviations from their personalized treatment plan may also improve patient adherence to treatment, and potentially, the accuracy of treatment delivery. Finally, the complexity of practice for radiation oncologists has increased dramatically with recent advances in treatment planning and delivery techniques. Time demands on radiation oncologists have also increased owing to the documentation required for electronic medical records and prior authorization. Thus, the medical physicist can potentially offset some of these demands by contributing directly to patient education and communication.
Unfortunately, most medical physicists do not participate in formal direct patient-physicist consultations, nor do they have formal training in patient-centered communication, which can optimize the effectiveness of clinical interactions. Fortunately, effective communication skills can be taught, learned, and assessed,10 and through practice and experience, medical physicists can develop high-quality patient-centered communication skills.11,12 Preliminary research findings demonstrate that when medical physicists play an active role in direct patient care, important patient outcomes, including anxiety, distress, and satisfaction, can be improved.13 However, this previous study was limited by its single-arm design. To advance this area of investigation, we implemented and evaluated a Medical Physics Direct Patient Care (MPDPC) program within the radiation oncology division of the Karmanos Cancer Institute (KCI), using a randomized controlled trial to overcome limitations in previous research. We evaluated the influence of the MPDPC on patient anxiety, distress, treatment adherence, technical understanding, and patient satisfaction.
Materials and Methods
Participants and setting
This study was approved by the institutional review board of Wayne State University as communication intervention protocol ID IRB-19–10-1298. Data were collected in the radiation therapy outpatient clinic of KCI/WSU, located in Detroit, MI, from March 2020 to May 2021. This included a 4-month pause in accrual because of institutional restrictions on the collection of clinical research data owing to the COVID-19 pandemic. Patients were eligible to participate if they had a diagnosis of breast, head and neck, or lung cancers and were being treated with curative intent.
Procedures and measures
As a part of a larger initiative at KCI to improve communication with patients receiving radiation therapy, we adapted a previously tested MPDPC training program designed to facilitate direct patient care with a medical physicist and improve effective patient communication regarding technical information related to radiation treatment.14 Four medical physicists at KCI participated in this evidence-based and systematic training. One of these physicists (JB) participated in an off-site workshop designed to improve patient–medical physicist communication,14 whereas the other 3 medical physicists were trained within a local patient communication program. This program was developed in collaboration between the physicist who participated in the off-site workshop and an investigator with expertise in clinical communication (LMH). Authors JB and LMH served as co–principal investigators of the clinic-based trial reported here.
All eligible patients seen in our clinic by 2 attending radiation oncologists who are members of our study team were approached about participation by their radiation oncologist (or their designee) before or after regularly scheduled treatment-related appointments. For simplicity in the coordination of clinical and research logistics, only 2 oncologists helped to recruit patients into this study. As a result, the patient cohort included patients with breast, head and neck, and lung cancers. Interested patients met with a member of the clinic staff or research staff, who explained the study and obtained consent. Upon consent, patients completed baseline measures including demographic information (included age, sex, race and ethnicity, income level, and insurance status). In addition, they completed measures of factors that may influence communication with their providers, including health literacy (3-item health literacy screening scale, eg, “How confident are you filling out forms by yourself?”),15 perceived efficacy in patient-provider interactions (10-item Perceived Efficacy in Patient-Physician Interactions [α, .91]; eg, “How confident are you in your ability to know what questions to ask your health care providers?”),16 and activation for managing health and health care, including their knowledge, skill, and confidence (13-item Patient Activation Measure [α, .81]; eg, “When all is said and done, I am the person who is responsible for managing my health condition”).17 Finally, they completed measures assessing outcomes of interest including anxiety (using a short form of the state scale of the Spielberger State-Trait Anxiety Inventory18), distress (using the single-item Distress Thermometer19), and technical understanding of treatment.
Patients were then randomized to receive either (1) printed materials describing the technical aspects of their treatment (control arm) or (2) printed materials and a minimum of 2 direct medical-physicist interactions to describe the technical aspects of their treatment (intervention arm). Randomization was stratified according to cancer type (breast, head and neck, or lung), and patients were randomly assigned in a 1:1 ratio in blocks of 4 to 1 of the 2 groups.
Patient intervention
Patients in the intervention arm met with a medical physicist either immediately before or immediately after treatment simulation to provide an opportunity for the physicist to (1) explain the simulation and treatment-planning process and (2) answer any technical questions from the patient. Patients met again with the physicist before the first treatment to facilitate discussion of any new or remaining questions or concerns. In this second meeting, the physicist described how the patient could best participate in their care from a technical perspective. Patients were informed at that time that they may request to meet with the physicist at any point during treatment if new questions should arise. Patients in the control arm did not meet with a medical physicist at the time of treatment simulation or before the first treatment but were informed that they could ask to meet with a physicist at any point during treatment. Patients in the control arm who asked for and participated in 1 or more meetings with a physicist would not have been included in the statistical analysis; however, no patients in the control arm asked for a meeting. Patients in both arms completed measures of their levels of treatment-related anxiety and distress and their satisfaction and technical understanding (1) before simulation, (2) before their first treatment, and (3) before their final treatment. The same measures were administered at each of these 3 time points. Immediately after each physicist interaction, patients again completed measures of anxiety, distress, and technical understanding of treatment and of their physicists’ patient-centered communication (12-item Patient Centered Communication [α, .75]; eg, “The medical physicist showed a genuine interest in my health”).20 Throughout the study, every effort was made to have each patient meet with the same physicist throughout the course of treatment, and this was achieved for 19 of 23 patients in the intervention arm. Patients’ treatment adherence, defined as 1 − [(number of treatment days missed) / (total prescribed treatment fractions)], was assessed via the patient’s medical record.
Data analysis
The primary objective of the study was to evaluate whether the MPDPC initiative would reduce anxiety and distress, and the secondary objectives were to assess whether the MPDPC initiative would improve patient treatment adherence, overall satisfaction, and technical understanding of their treatment across 3 time points. Additionally, as post hoc exploratory objectives, anxiety, distress, overall satisfaction, and technical understanding were evaluated at each time point; subgroup analysis was performed to examine the interactions between the group and a covariate of interest, and preinteraction outcomes were assessed.
A phase 2 screening randomized trial design was used with a power of 80% and a 2-sided type I error rate of 10% for 2 primary endpoints (anxiety and distress) and 2 secondary endpoints (adherence and satisfaction). The endpoints were evaluated using graphical sequential procedures to control the family-wise type I error rate at a 2-sided α of 10% across all primary and secondary endpoints.21 Sample size and power justification are provided in the supplementary Appendix E1.
Scores at visits 1, 2, and 3 were corrected by subtracting the patient’s baseline scores. The 6 State-Trait Anxiety Inventory questions were converted into a single score by averaging, as described elsewhere,18 to represent the patient’s state of anxiety. Distress was analyzed as a continuous variable. Health literacy (3 questions), perceived efficacy (10 questions), and patient activation (13 questions) were also averaged to provide single scores. The overall satisfaction question and 3 technical satisfaction questions were evaluated individually. Primary and secondary endpoints were assessed using linear mixed-effects models, considering patients as random effects along with the repeated measurements at 3 time points. The first secondary endpoint was assessed using a 2-sided, unpaired t test. Exploratory subgroup analysis was performed using multivariable linear mixed-effects models after continuous variables were dichotomized by their medians.
As shown in Table 1, the powered analyses were the overall comparisons between groups at all 3 visits for the 2 primary endpoints (anxiety and distress) and the first secondary endpoint (patient treatment adherence). All other analyses were exploratory and should be interpreted descriptively. For those exploratory analyses, no hypothesis testing was performed, so estimated group differences and associated 95% confidence intervals (CIs) are provided without P values.
Table 1.
Overview of analyses and outcomes.
| Analysis | Powered/Descriptive | 2-sided alpha | Outcome | p-value | Data |
|---|---|---|---|---|---|
|
| |||||
| Primary objectives | |||||
| Anxiety | Powered | 5% | Difference (95% CI): −0.29 (−0.57, −0.02) | 0.038 | Fig. 1 (A) |
| Distress | Powered | 10% | Difference (90% CI): 0.13 (−1.01, 1.26) | 0.851 | Fig. 1 (B) |
|
| |||||
| Secondary objectives | |||||
| Adherence | Powered | 5% | Difference (95% CI): −1.87 (−7.52, 3.78) | 0.507 | Table 4 |
| Overall satisfaction | Descriptive | - | Summarized descriptively by 95% CI | - | Fig. 2 (D) |
|
| |||||
| Exploratory (post-hoc) objectives | |||||
| Three technical satisfaction questions | Descriptive | - | Summarized descriptively by 95% CI | - | Fig. 2 (A, B, C) |
| Comparisons at each visit | Descriptive | - | - | Figs. 1, 2 | |
| Pre-interaction outcomes | Descriptive | - | - | Table 3 | |
| Subgroup analysis | Descriptive | - | - | Fig. 3 | |
CI, confidence interval
Results
An offer to participate in the trial was presented to 66 eligible patients (89% breast, 8% head and neck, and 3% lung cancer), of whom 51 agreed to participate (77% acceptance rate). Seven enrolled patients were lost to attrition; thus, the results presented here are based on 44 patients, including 40 with breast cancer (91%), 3 with head and neck cancers (7%), and 1 with lung cancer (2%). The intervention group consisted of 20 patients with breast cancer, 2 with head and neck cancers, and 1 with lung cancer. The control group consisted of 20 patients with breast cancer, 1 with head and neck cancer, and none with lung cancer. Most patients identified as Black or African American (73%), and 20% identified as White. Patient baseline characteristics are provided in Table 2, and preinteraction outcomes are provided in Table E2. Perceived efficacy in patient-provider interactions in the control group was higher than in the intervention group (difference, −0.45; 95% CI, −0.82 to −0.09; Table E2).
Table 2.
Patient baseline characteristics
| All (n=44) | Intervention (n=23) | Control (n=21) | |
|---|---|---|---|
|
| |||
| Age, year - mean (sd) | 56.56 (12.09) | 60 (10.63) | 52.95 (12.71) |
| Missing | 1 | 1 | 0 |
| Race - no. (%) | |||
| African-American or Black | 32 (73) | 16 (70) | 16 (76) |
| Caucasian or White | 9 (20) | 5 (22) | 4 (19) |
| Other race or multiple races | 3 (7) | 2 (9) | 1 (5) |
| Hispanic or Latina - no. (%) | |||
| Yes | 2 (5) | 1 (4) | 1 (5) |
| No | 41 (93) | 21 (91) | 20 (95) |
| Missing | 1 (2) | 1 (4) | 0 (0) |
| Gender - no. (%) | |||
| Male | 1 (2) | 1 (4) | 0 (0) |
| Female | 42 (95) | 21 (91) | 21 (100) |
| Other | 1 (2) | 1 (4) | 0 (0) |
| Education Level - no. (%) | |||
| Less than high school | 6 (14) | 5 (22) | 1 (5) |
| High School/GED | 13 (30) | 6 (26) | 7 (33) |
| Some College | 8 (18) | 4 (17) | 4 (19) |
| 2-year college degree | 8 (18) | 1 (4) | 7 (33) |
| 4-year college degree | 4 (9) | 3 (13) | 1 (5) |
| Graduate/professional degree | 5 (11) | 4 (17) | 1 (5) |
| Marital Status - no. (%) | |||
| Married | 9 (20) | 5 (22) | 4 (19) |
| Living with partner in a marriage-like relationship | 4 (9) | 1 (4) | 3 (14) |
| Widowed | 4 (9) | 3 (13) | 1 (5) |
| Divorced | 10 (23) | 5 (22) | 5 (24) |
| Separated | 3 (7) | 2 (9) | 1 (5) |
| Never married | 14 (32) | 7 (30) | 7 (33) |
| Household Income – no. (%) | |||
| Less than 20,000 | 12 (27) | 8 (35) | 4 (19) |
| 20,000–39,999 | 11 (25) | 4 (17) | 7 (33) |
| 40,000–59,999 | 9 (20) | 4 (17) | 5 (24) |
| 60,000–79,999 | 4 (9) | 2 (9) | 2 (10) |
| 80,000–99,999 | 2 (5) | 1 (4) | 1 (5) |
| 100,000–149,000 | 1 (2) | 0 (0) | 1 (5) |
| 150,000 or more | 1 (2) | 1 (4) | 0 (0) |
| Missing | 4 (9) | 3 (13) | 1 (5) |
| Insurance - no. (%) | |||
| No private insurance | 29 (66) | 17 (74) | 12 (57) |
| Private insurance | 13 30) | 6 (26) | 7 (33) |
| Missing | 2 (5) | 0 (0) | 2 (10) |
| Employment Status - no. (%) | |||
| Employed full time | 9 (20) | 2 (9) | 7 (33) |
| Employed part time | 6 (14) | 4 (17) | 2 (10) |
| Caring for home and/or family | 1 (2) | 1 (4) | 0 (0) |
| Unemployed and looking for work | 3 (7) | 2 (9) | 1 (5) |
| Unable to work due to illness or disability | 10 (23) | 4 (17) | 6 (29) |
| Retired | 12 (27) | 7 (30) | 5 (24) |
| Student | 3 (7) | 3 (13) | 0 (0) |
sd, standard deviation
Primary and secondary objectives
Baseline corrected participant anxiety was significantly reduced in the intervention group compared with the control group overall (difference, −0.29; 95% CI, −0.57 to −0.02; P = .038; Fig. 1A) and at the time of the first visit (difference, −0.31; 95% CI, −0.50 to −0.12). However, this effect diminished through the course of treatment. Participant distress was not significantly different between groups (difference, 0.13; 90% CI, −1.01 to 1.26; P = .851; Fig. 1B). No significant difference in patient treatment adherence was observed between arms (difference, −1.8; 95% CI, −7.5 to 3.8; p=0.51; Table 3).
Fig. 1.
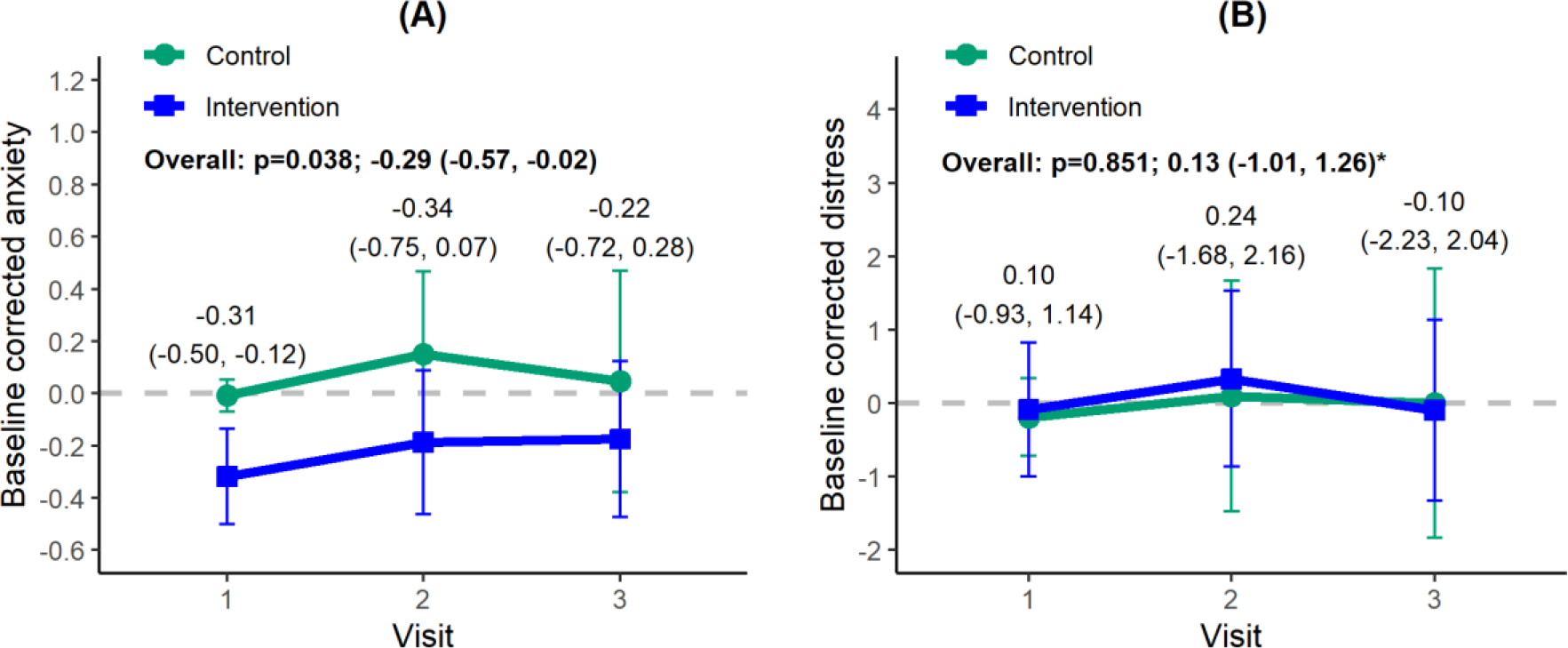
(A) Baseline corrected anxiety and (B) distress. These represent the primary study objectives and were obtained by subtracting the baseline scores from those at visit 1, 2, and 3 (simulation, first treatment, and last treatment, respectively). The data points indicate means, and the vertical lines, 95% confidence intervals (CIs). Overall P values were obtained from a linear mixed-effects model. Estimated group differences and 95% CIs are given for overall and each visit except for distress (90% CI, as indicated by an asterisk).
Table 3.
Adherence.
| All (n=44) | Intervention (n=23) | Control (n=21) | Difference (95% CI) | P value* | |
|---|---|---|---|---|---|
|
| |||||
| Adherence - mean (sd) | 94.85 (9.34) | 93.95 (10.73) | 95.82 (7.69) | −1.87 (−7.52, 3.78) | 0.507 |
Unpaired T-test; sd, standard deviation; CI, confidence interval.
Exploratory objectives
Baseline corrected technical aspects of care are presented in Fig. 2. As expected, patients in the intervention arm were considerably more likely to feel that adequate time was devoted to explaining the technical aspects of their treatment (difference, 0.78; 95% CI, 0.03–1.54; Fig. 2B). All technical measures were considerably higher in the intervention group at the time of the first visit. We note that the first-visit data points are not primary or secondary endpoints; rather, they are exploratory data points.
Fig. 2.
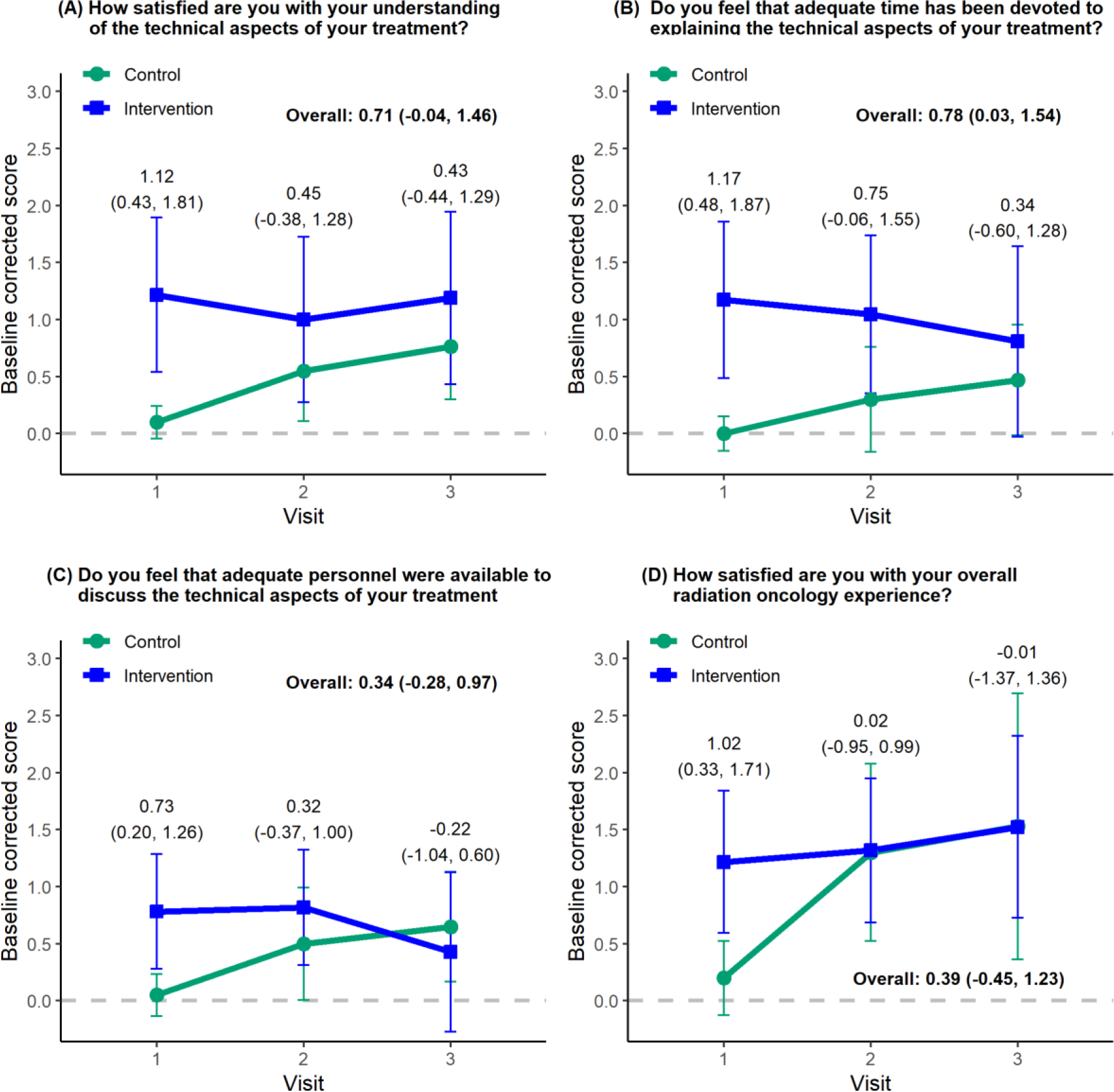
Baseline corrected technical aspects of care. The data points indicate mean values, and vertical lines represent 95% confidence intervals (CIs). Estimated group differences and 95% CIs are given overall and for each visit. Overall differences represent secondary study objectives, whereas individual time point differences were post hoc exploratory objectives. Overall group differences and 95% CIs were obtained from a linear mixed-effects model.
Subgroup analyses of baseline corrected anxiety, distress, satisfaction with technical aspects of care, and overall satisfaction are presented in Fig. 3. Here, “difference” represents intervention minus control; therefore, “control higher” means higher anxiety, distress, or satisfaction. The effect of intervention was homogeneous in all subgroups. Patients identifying as African American or Black in the intervention arm had a reduction in anxiety compared with the overall patient cohort, as did patients with lower health literacy and lower patient activation scores (Fig. 3A). In addition, patients with low income in the intervention arm had an increase in technical satisfaction (Fig. 3C), and patients without private health insurance in the intervention arm had an increase in overall satisfaction (Fig. 3D). The subgroup and interaction analyses were not powered and thus should be interpreted as exploratory analyses.
Fig. 3.
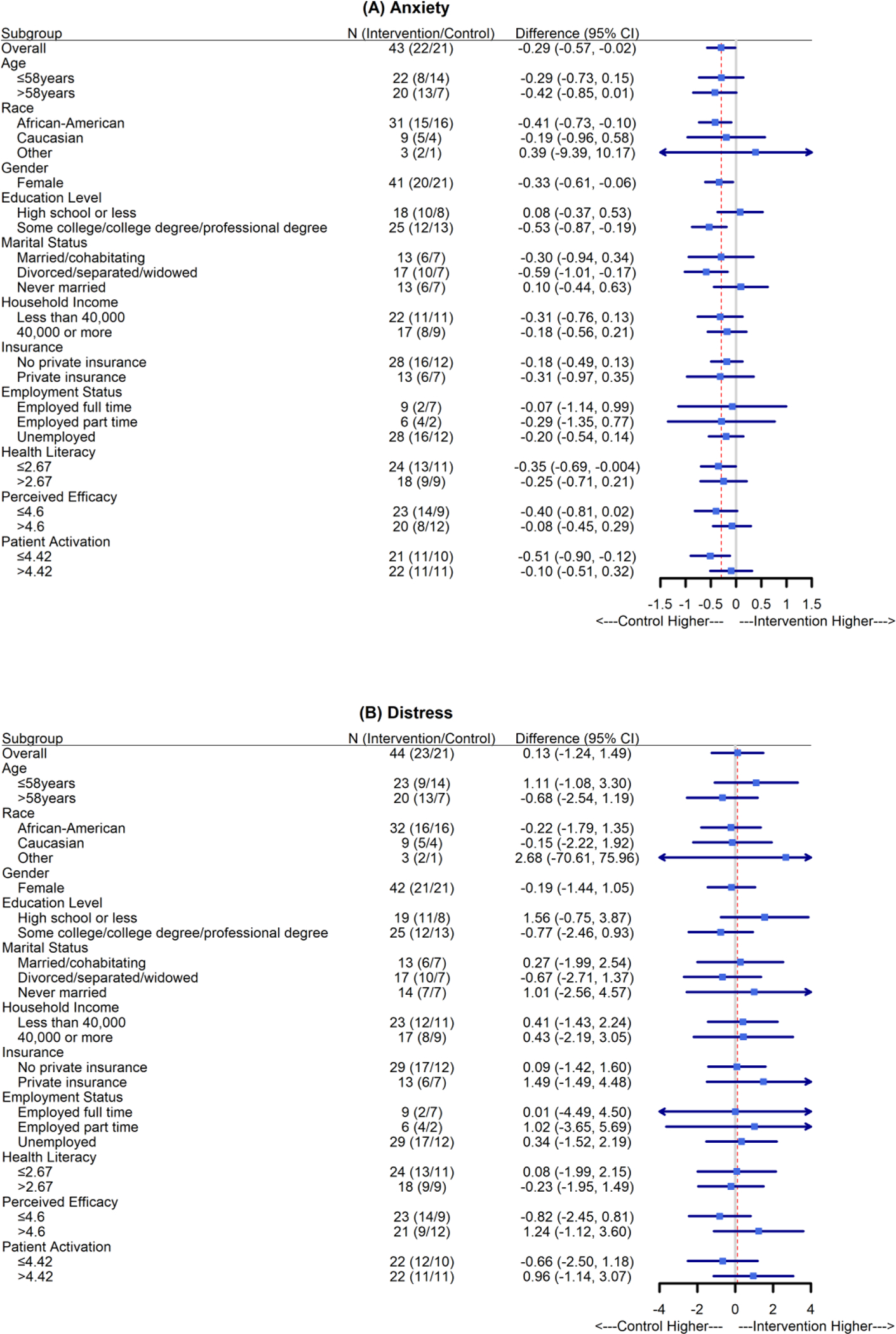
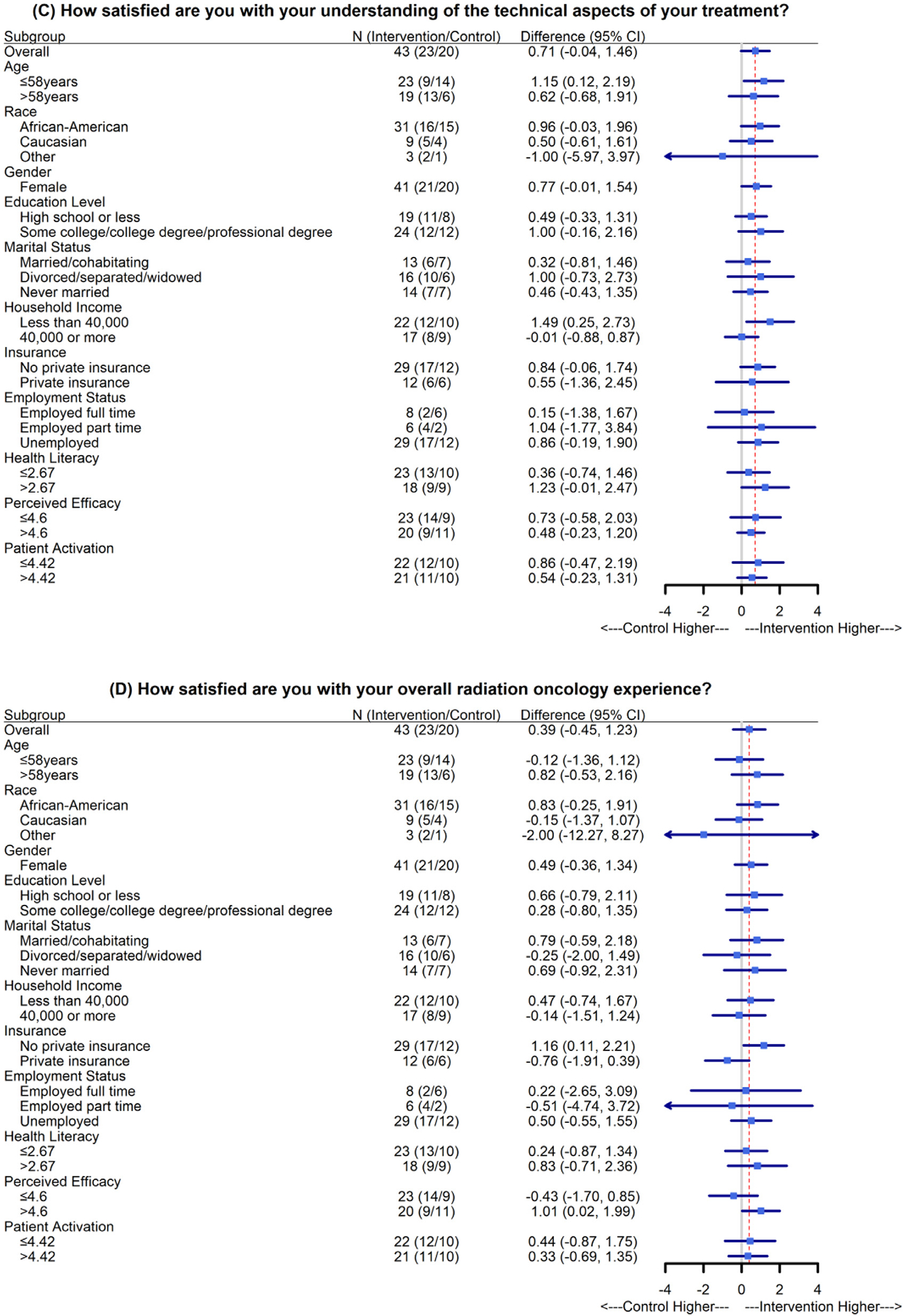
Subgroup analyses of baseline-corrected anxiety, distress, technical aspects of care, and overall satisfaction. The mean differences and 95% confidence intervals (CIs) were estimated using linear mixed-effects models. No subgroup analysis was carried out for “male” and “other” for “gender,” owing to the small sample sizes (n = 1 for each group). The red dotted lines indicate the overall estimated group differences. All subgroup analyses represent post hoc exploratory objectives.
Discussion
This study is among the first to implement an intervention designed to facilitate an independent relationship between medical physicists and patients receiving radiation therapy and to determine whether the intervention is effective at reducing treatment-related anxiety and distress and improving treatment adherence, patient technical understanding, and patient satisfaction. A recent prospective, single-arm, phase 2 clinical trial investigated the value of a direct patient-care role by the medical physicist and demonstrated a significant decrease in patient anxiety and increase in technical satisfaction.13 However, a major limitation to that study was its single-arm design, which did not include participant randomization or a control arm to allow for comparisons in patient outcomes. Results from our trial are in general agreement with those from the single-arm trial,13 including that whereas reported levels of anxiety and satisfaction were improved by the intervention, the relative improvement diminished with time during the course of treatment. We speculate that this may be owed, in both trials, to patients in the intervention arm establishing a relationship with the medical physicist, who provided important information and technical reassurance at the beginning of the treatment process but did not meet with patients again during treatment. Patient expectations for time devoted to and adequate personnel available for discussion of the technical aspects of treatment may be responsible for the apparent trends in panels B and C in Fig. 2. Patients in the intervention arm were provided with a substantial amount of time and personnel at the beginning of treatment, followed by a decrease in both through the course of treatment. The opposite was true for patients in the control arm. These trends could potentially also be influenced in this study by the fact that the perceived efficacy in patient-provider interactions was considerably higher in the control group.
We highlight that the sample in this trial was composed of a majority of patients identifying as Black or African American, again differentiating this trial from many clinic-based interventions, including the previously mentioned single-arm phase 2 trial. Overall, we observed improvements in patient-reported anxiety and understanding of technical aspects of treatment, even though the perceived efficacy in patient-provider interactions was considerably higher in the control group. Subgroup analyses showed that patients in the intervention group who identified as Black or African American had considerably reduced anxiety compared with those in the control arm (difference, −0.41; 95% CI, −0.73 to −0.10; Fig. 3A).
Data have shown persistent disparities in clinical communication experienced by patients who identify as Black or African American compared with patients who identify as White20,22–27 and that these communication disparities likely contribute to the well-documented racial inequities in cancer treatment and mortality.28 Previous research has also identified an association between poorer quality communication with racial discordance of the patient-clinician dyad (ie, the patient and physicist were of different races).25 All physicists in the current trial identified as White, which means all of the interactions for the Black or African American patients were racially discordant. Clinical communication interventions designed to improve communication in racially discordant oncology interactions have been developed and tested, and some have been shown to be effective with physician participants.29,30 We are among the first to test a communication intervention for a nonphysician provider population. Although our communication training program did not explicitly include improving racially discordant communication, our data indicate that this intervention designed for patient–medical physicist communication may also help reduce disparities in oncology care.
In addition, patients with some higher education had considerably reduced anxiety compared with control-arm patients, and patients without private insurance and those earning less than $40,000 per year had considerably higher technical satisfaction and overall satisfaction, respectively, compared with control patients. This intervention evidently benefitted different patients in different ways, reducing anxiety for Black or African American patients and those with higher education and improving satisfaction for patients with lower income and without private insurance. We note that 43 of the 44 patients in the study cohort were female. This is a result of the sex distribution of the patients treated by the physicians involved in the study and does not differ substantially from the sex distribution (62 of 66 female) of the patients to whom the study was presented. There was no selection bias in offering the trial to patients, as it was offered to all eligible patients during the study period. Because the racial distribution and socioeconomic status of the patients in this cohort are also generally reflective of the overall treatment population of our clinic, we conclude that there were not observable differences across demographic groups in patients’ decision to participate. The demographic characteristics of the patients in this study are substantially different from those in a previously published study,13 most notably in that all patients in that study identified their race as “White/Non-Hispanic” (personal communication with T. Atwood, March 2022). Nevertheless, our study findings are consistent with the results of that study and demonstrate that the intervention may have added benefit for certain patient groups.
Although this study did not show an increase in treatment adherence in the intervention arm, such an improvement would have been highly unlikely given the characteristics of the patient cohort. Specifically, the treatment adherence rate in both the intervention and control arms was 96%. These exceptional adherence rates were observed despite transportation difficulties and winter weather events, and most notably, the completion of the entire trial during the COVID-19 pandemic. As a result, the treatment adherence rate for patients in this cohort left almost no room for improvement.
Although this study evaluated specifically how this intervention affected patients’ anxiety and distress, understanding of and adherence to their treatment, and feelings about the care they received, enlisting patients into active participation in their care may also improve patient safety. A patient who understands exactly what should happen and why may be more likely to identify something out of the ordinary during the treatment process and to verbalize this concern to the care provider. We hope future work can demonstrate that increased patient participation in their care can improve patient safety.
Limitations of this study include a narrow set of treatment sites in which the majority (91%) of patients were receiving breast radiation therapy and a relatively homogeneous study population in terms of sex (98% female). Although it is possible that there was some selection bias with respect to sex in patient preference to participate in the trial, the overall sex distribution of patients who were offered the trial (94%) was very similar to that of the study population, and we do not have sufficient data to demonstrate a difference in trial participation with regard to sex. One of the 4 physicists involved was female; however, we do not have sufficient data to determine whether the sex of the physicist influenced the results. Another limitation of the study is that the physicists did not continue to meet with the patients throughout the course of treatment. This may explain the relative decrease in the observed relationship between the intervention and control arms in Figs. 1 and 2 from the first to the last survey time point. Finally, one may question the relative value of the improvements demonstrated in this intervention in relation to the time and resource effort expended to create them. This is a determination that must be made by each individual institution.
Conclusions
Through the administration of a phase 2 screening randomized trial, we have demonstrated that the establishment of a direct patient-provider relationship with the medical physicist reduces anxiety in patients receiving radiation therapy. An increase in understanding of the technical aspects of care and in overall satisfaction were also observed at the initiation of treatment. These improvements, along with the reduction in anxiety, were shown to decrease over time, suggesting that the value of the intervention diminished over time and/or that patients in the control arm became more comfortable with their treatment process through the course of radiation therapy. This study did not demonstrate an increase in treatment adherence; however, we hope to demonstrate in future studies that improvements in patient-provider communication and patient education may improve treatment adherence in more vulnerable patient cohorts.
Supplementary Material
Acknowledgments
This project was funded by the Karmanos Cancer Institute.
Footnotes
Disclosures: none.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ijrobp.2022.10.011.
References
- 1.Arora NK, Weaver KE, Clayman ML, Oakley-Girvan I, Potosky AL. Physicians’ decision-making style and psychosocial outcomes among cancer survivors. Patient Educ Couns 2009;77:404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: A meta-analysis. Med Care 2009;47:826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyer M, Rohe J, Nicklin PJ, Haynes K. Communication and patient safety. In: Sandars J, Cook G, eds. ABC of Patient Safety. Dekalb, IL: Wiley & Sons; 2007:16–19. [Google Scholar]
- 4.Geinitz H, Marten-Mittag B, Schäfer C, et al. Patient satisfaction during radiation therapy. Strahlenther Onkol 2012;188:492–498. [DOI] [PubMed] [Google Scholar]
- 5.Halkett GK, Short M, Kristjanson LJ. How do radiation oncology health professionals inform breast cancer patients about the medical and technical aspects of their treatment? Radiother Oncol 2009;90:153–159. [DOI] [PubMed] [Google Scholar]
- 6.Rutten LJ, Arora NK, Bakos AD, et al. Information needs and sources of information among cancer patients: A systematic review of research (1980–2003). Patient Educ Couns 2005;57:250–261. [DOI] [PubMed] [Google Scholar]
- 7.Rosenburg SA, Francis DM, Hullet CR, et al. Online patient information from radiation oncology departments is too complex for the general population. Pract Radiat Oncol 2017;7:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habboush Y, Shannon RP, Niazi SK, et al. Patient-reported distress and survival among patients receiving definitive radiation therapy. Adv Radiat Oncol 2017;2:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin LS, Williams SL, Haskard KB, DiMatteo R. The challenge of patient adherence. Ther Clin Risk Manag 2005;1:189–199. [PMC free article] [PubMed] [Google Scholar]
- 10.Bonvicini KA, Perlin MJ, Bylund CL, Carroll G, Rouse RA, Goldstein MG. Impact of communication training on physician expression of empathy in patient encounters. Patient Educ Couns 2009;75:3–10. [DOI] [PubMed] [Google Scholar]
- 11.Levinson W, Lesser CS, Epstein RM. Developing physician communication skills for patient centered care. Health Affairs 2010;29:1310–1318. [DOI] [PubMed] [Google Scholar]
- 12.Keller VF, Carroll JG. A new model for physician-patient communication. Patient Education and Counseling 1994;23:131–140. [DOI] [PubMed] [Google Scholar]
- 13.Atwood TF, Brown DW, Murphy JD, et al. Establishing a new clinical role for medical physicists: A prospective phase II trial. Int J Radiat Oncol Biol Phys 2018;102:635–641. [DOI] [PubMed] [Google Scholar]
- 14.Brown DW, Atwood TF, Juang T, et al. Evaluation of a patient communication skills training program for medical physicists. Int J Radiat Oncol Biol Phys 2020;108:1284–1291. [DOI] [PubMed] [Google Scholar]
- 15.Chew LD, Griffin JM, Partin MR, et al. Validation of screening questions for limited health literacy in a larger VA outpatient population. J Gen Internal Med 2008;23:561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maly RC, Frank JC, Marshall GN, et al. Perceived Efficacy in Patient-Physician Interactions (PEPPI): Validation of an instrument in older persons. J Am Geriatr Soc 1998;46:889–894. [DOI] [PubMed] [Google Scholar]
- 17.Hibbard JH, Mahoney ER, Stockard J, et al. Development and testing of a short form of the Patient Activation Measure. Health Serv Res 2005;40:1918–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psych 1992;31:301–306. [DOI] [PubMed] [Google Scholar]
- 19.Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer 2005;103:1494–1502. [DOI] [PubMed] [Google Scholar]
- 20.Street RL Jr, Gordon H, Haidet P. Physicians’ communication and perceptions of patients: Is it how they look, how they talk, or is it just the doctor? Soc Sci Med 2007;65:586–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bretz F, Maurer W, Brannath W, Posch M. A graphical approach to sequentially rejective multiple test procedures. Stat Med 2009;28:586–604. [DOI] [PubMed] [Google Scholar]
- 22.Eggly S, Harper FW, Penner LA, Gleason MJ, Foster T, Albrecht TL. Variation in question asking during cancer clinical interactions: A potential source of disparities in access to information. Patient Educ Couns 2011;82:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM, Powe NR. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med 2003;139:907–915. [DOI] [PubMed] [Google Scholar]
- 24.Cooper-Patrick L, Gallo JJ, Gonzales JJ, et al. Race, gender, and partnership in the patient-physician relationship. JAMA 1999;282:583–589. [DOI] [PubMed] [Google Scholar]
- 25.Hamel LM, Chapman R, Malloy M, et al. Critical shortage of African American medical oncologists in the United States. J Clin Oncol 2015;33:3697–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson RL, Roter D, Powe NR, Cooper LA. Patient race/ethnicity and quality of patient-physician communication during medical visits. Am J Public Health 2004;94:2084–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eggly S, Barton E, Winckles A, Penner LA, Albrecht TL. A disparity of words: Racial differences in oncologist-patient communication about clinical trials. Health Expect 2015;18:1316–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. [DOI] [PubMed] [Google Scholar]
- 29.Sungur H, Yllmaz NG, Chan BMG, van den Muijsenbergh METC, van Weert JCM, Schouten BC. Development and evaluation of a digital intervention for fulfilling the needs of older migrant patients with cancer: User-centered design approach. J Med Internet Res 2020;22:e21238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eggly S, Hamel LM, Foster TS, et al. Randomized trial of a question prompt list to increase patient active participation during interactions with Black patients and their oncologists. Patient Educ Couns 2017;100:818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


