Abstract
Spinal cord ischemia-reperfusion injury (IR) is a terrible non-traumatic injury that occurs after abdominal aortic occlusion and causes serious damage to neurological function. Several treatment strategies have been suggested for IR, but they were not unable to effectively improve these conditions. Herein we investigated whether exosomes derived from human placental mesenchymal stem cells (hpMSCs-Exos) in combination with hyperbaric oxygen (HBO) could alleviate injury and promote recovery in IR rats. Eighty male Sprague-Dawley rats were randomly allocated into five equal groups. In addition to the control group that only underwent laparotomy, IR animals were planned into four groups as follows: IR group; IR-Exos group; IR-HBO group; and IR-Exos + HBO group. Neurological function evaluated before, 6 h, 12 h, 24 h, and 48 h after injury. After the last neurological evaluation, tissue samples were obtained for stereological, biochemical, and molecular assessments. Our results indicated that the neurological function scores (MDI), the numerical density of neurons, the levels of antioxidative factors (GSH, SOD, and CAT), and anti-inflammatory cytokine (IL-10) were considerably greater in treatment groups than in the IR group, and these changes were more obvious in the IR-Exos + HBO ones. This is while the numerical density of glial cells, the levels of an oxidative factor (MDA) and inflammatory cytokines (IL-1β, TNF-α, and IL-18), as well as the expression of an apoptotic protein (caspase-3) were meaningfully decreased in treatment groups, especially IR-Exos + HBO group, compared to the IR group. Generally, it was found that co-administration of hpMSCs-Exos and HBO has synergistic neuroprotective effects in the rats undergoing IR.
Keywords: Spinal cord ischemia-reperfusion injury, Human placenta, Mesenchymal stem cells, Exosomes, Hyperbaric oxygen, Oxidative stress
Highlights
-
•
We studied the effects of hpMSCs-Exos and HBO treatment following spinal cord ischemia-reperfusion injury.
-
•
Treatment improved rehabilitation of motor function.
-
•
Treatment increased the survival of motor neurons and reduced gliosis.
-
•
Treatment attenuated oxidative stress.
-
•
Treatment inhibited neuroinflammation and apoptosis.
1. Introduction
Paraplegia caused by spinal ischemia-reperfusion injury (IR) during abdominal aortic surgery is one of the most terrible and catastrophic events [1]. The incidence of this destructive complication in patients undergoing surgery is estimated at 30% [2]. This complication causes the patient to have a serious neurological disorder and his life is seriously affected [3]. Oxidative stress, excessive inflammation, and activation of phospholipase-A2 play a major role in the pathogenesis of IR. Meanwhile, oxidative stress is more prominent due to the widespread release of O2 radicals, which causes lipid peroxidation and the death of neurons [4,5]. So far, several treatment methods including hypothermia induction, cerebrospinal fluid drainage, and drug interventions have been proposed, but none of them have had reliable clinical results [4].
Therefore, since several factors are effective in the pathogenesis of IR, the application of therapeutic measures should also be based on multi-factorial approaches.
Recently, several studies have pointed to the great potential of human placenta mesenchymal stem cells (hpMSCs) in the treatment of spinal cord injury (SCI) [[6], [7], [8]]. However, in these studies, the ability of hpMSCs to repair SCI is believed to be primarily due to the secretion of paracrine exosomes from these cells, not to their multi-potential differentiation [9,10]. Exosomes are nano-sized vesicles that are secreted by cells and contain compounds such as lipids, proteins, and nucleic acid. These vesicles play a very important role in cell communication, cell differentiation, and even tissue function [9]. Compared to cells, exosomes are safer, more stable, have a longer half-life in circulation, and can cross the blood-brain barrier [11,12]. Studies have shown that the hpMSCs-derived exosomes (hpMSCs-Exos) have neuroprotective effects and cause modulation of oxidant status, modulate inflammatory reactions, and inhibition of apoptosis [11,13,14].
However, as mentioned, due to the complexity of the IR, the use of other complementary compounds along with the hpMSCs-Exos can have synergistic and effective effects.
Hyperbaric oxygen (HBO) is one of the most effective compounds in recent years in the treatment of some diseases such as central nervous system injuries and chronic wounds [15,16]. Studies have shown that HBO increases the survival of neurons and functional recovery in the SCI by inhibiting inflammation and oxidative stress [17,18]. Cheshmi et al. reported that HBO reduces the level of inflammatory factors such as interleukin 1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α), inhibits oxidative stress, and inhibits the apoptosis of neurons in the SCI rats [11]. Also, it has been reported that HBO stabilizes lysosomal enzymes and promotes nitric oxide production [19].
Considering the advantages mentioned about hpMSCs-Exos and HBO therapy, however, the combination use of these has not been documented in previous experimental studies. Therefore, in the present study, we investigated the effects of hpMSCs-Exos in the IR for the first time. Furthermore, we have investigated whether the administration of hpMSCs-Exos together with HBO therapy could synergistically attenuate injury and improve the neurological function in an experimental animal model of IR.
2. Material and methods
All materials used in the present study were purchased from Sigma-Aldrich (St. Louis, MO), except where assigned otherwise.
2.1. Isolation and culture of hpMSCs
To collect the placenta, written consent was first obtained from the pregnant mothers who underwent cesarean, and then the placenta was collected under aseptic conditions and immediately transferred to the laboratory to isolation the hpMSCs. The hpMSCs were isolated and cultured as previously described [11]. Briefly, after obtaining the placenta, it was washed 3 times with phosphate-buffered saline (PBS; Thermos, US) to separate blood and other contents. Then, the placenta was cut into 2 cm pieces and approximately 15 g of them were transferred to a tube containing 15 ml of digestion solution at room temperature. The solution was centrifuged at 800 rpm for 5 min to separate the cell pellet. Next, the cell pellets were resuspended in Dulbecco modified Eagle medium (DMEM; Gibco) + 20% fetal bovine serum (FBS; Gibco) + 1% penicillin-streptomycin (Gibco). In the end, the cells were seeded in T-75 flasks and incubated. To characterize the hpMSCs, flow cytometry analysis of CD73, CD105, CD45, and CD34 markers were performed [11].
2.2. Isolation and identification of hpMSCs-Exos
Isolation and identification of hpMSCs-Exos were performed according to Cheshmi et al. [11]. When the cells reached 60–70% confluence at passage 3–5, they were washed twice with sterile PBS and incubated for 48 h in serum-free low glucose-DMEM (Gibco). Then, the supernatant was collected, centrifuged at 3000 rpm for 20 min, and passed through a 0.22 μm filter to remove cell debris. Subsequently, 5 ml of sterile PBS was added to the isolated supernatant and ultracentrifuged at 100,000 rpm for 70 min. Finally, for exosome extraction, the second round of ultracentrifugation was repeated at 100,000 rpm for 70 min. The purified exosomes were filtered by a 0.22 μm pore filter (Millipore) and transferred to a −80 freezer for further assessment. The morphology and size were analyzed using transmission electron microscopy (TEM; JEM-1400, Japan Electronics Co., Ltd.) and dynamic light scattering (DLS), respectively [11].
In addition, to evaluate the presence of typical exosome markers (CD9, CD63, and CD81), Western blot method was performed [14]. The protein concentration was determined using the BCA assay kit (Kalazist, Tehran, Iran). Equal concentrations of total protein were separated by electrophoresis in SDS-PAGE gel. After electrophoresis, proteins were transferred to 0.25 μm polyvinylidene difluoride (PVDF) membranes for 60 min. Next, the PVDF membranes were washed by tris-buffered-saline-tween (TBST) to remove non-specific binding. After that, CD9 (1:1000; Abcam ab223052), CD63 (1:1000; Abcam ab134045), and CD81 (1:1000; Abcam ab79559) were added and incubated for 24 h at 4 °C. The membrane was then washed with TBST before adding secondary antibody (1:30,000; Abcam ab97047) and incubating for 2 h at room temperature. To detection of positive reactions, enhanced chemiluminescence was used.
2.3. Animals
Healthy adult male Sprague-Dawley rats weighing 220–260 g were recruited. All animals were kept under standard conditions of temperature, lighting, and access to rodent chow and drinking water. Methods were performed according to ARRIVE guidelines.
2.4. Study groups and experimental IR model
Eighty rats were randomly planned into five groups (n = 16). In addition to the control group that only underwent laparotomy, IR animals were planned into four groups as follows: IR group; IR-Exos group; IR-HBO group; and IR-Exos + HBO group.
IR induction was performed as previously described [2]. Briefly, the rats were anesthetized by intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). Under aseptic conditions, a midline incision was made on the abdomen and the aorta was exposed. Before the aortic clamp and induction of ischemia, to prevent coagulation, intravenous heparin was administered. The location of the aortic clamp was about 1 cm below the left renal artery, which was performed using a microvascular clamp. IR was created via occlusion of the aorta for 60 min. In the end, the clamps were removed, and the abdomen wall was closed in layers with silk sutures, and then immediately gentamicin (Abidi Co, Tehran, Iran) was administered (40,000 U; im) to prevent infection [2]. To reduce the pain, all animals were treated with ibuprofen (20 mg/kg) every 8–12 h before surgery, which was continued to the end of the experiment. After IR induction, animals were subjected to behavioral assessment before surgery, 6 h, 12 h, 24 h, and 48 h after surgery. After the last evaluation was performed, all rats were sampled for histological, biochemical, and molecular evaluations.
2.5. hpMSCs-Exos and HBO administration
To the administration of hpMSCs-Exos, 30 min after IR, 0.1 μg/μL of exosomes precipitated in 200 μl of PBS was injected through the tail vein [11,20,21]. For HBO administration, a standard hyperbaric oxygen chamber was used. Oxygen therapy was performed two times 60 min during ischemia induction and then during reperfusion. Each administration was done in three steps. At first, the oxygen pressure was gradually increased to 2.5 atm for 5 min to avoid the damage caused by a sudden increase in pressure. Then the oxygen pressure was maintained at 2.5 atm for 50 min. In the last 5 min, to prevent injury, the oxygen pressure was gradually reduced to 1 atm [4].
2.6. Histological and stereological assessments
In the end, tissue samples were obtained and fixed in 4% paraformaldehyde. After going through routine histological procedures, the samples were embedded in paraffin. Ten evenly spaced sections were selected from each rat and stained with hematoxylin–eosin (H&E). For the evaluation of the numerical densities (Nv) of neurons and glial cells, the stereological method was performed using the below formula [22].
ΣQ: cell number (nucleus); h: height of the dissector; Σp: the total number of each cells that counted inside the probe; ; BA: block advance of the microtome; t: section thickness [23].
2.7. Biochemical evaluation
To evaluate how hpMSCs-Exos and HBO exerts neuroprotective effects, the antioxidative (glutathione: GSH; superoxide dismutase: SOD; and catalase: CAT) activities and oxidative (malondialdehyde; MDA) levels in spinal cord homogenates were detected simultaneously. Briefly, the MDA level was evaluated by a method based on thiobarbituric acid. The GSH, SOD, and CAT activities were determined based on the trichloroacetic, pyrogallol, and hydrogen peroxide, respectively [11]. All procedures were performed under the guidance of the manufacturer's instructions (Yekta Tajhiz Co., Tehran, Iran).
2.8. Determination of inflammatory cytokines
In the present study, we measured the levels of three cytokines effective in inflammation (IL-1β, TNF-α, and IL-18) and an anti-inflammatory cytokine (IL-10) using the ELISA method. For this purpose, 100 mg of spinal cord tissue was homogenized in a lysis buffer solution. The contents of this solution included phenylmethanesulfonyl fluoride (1 mM), Triton X-100 (1%), EDTA (2 mM), sodium pyrophosphate (2.5 mM), Tris (20 mM), and leupeptin (0.5 μg/ml). The solution was centrifuged at 15.000 rpm for 15 min to separate the cell pellet. Finally, the cytokines levels were determined using ELISA kits according to the manufacturer's instructions (Invitrogen, USA). Data were expressed as picogram per milligram protein [24].
2.9. Western blot analysis
To evaluate the expression of the apoptotic protein, the Western blot method was performed for caspase-3 antibody [24]. Homogenized spinal cord samples were lysed using lysis buffer (containing of 62.5 mM Tris–HCl, 1 g of sodium deoxycholate, 0.2 g of sodium dodecyl sulfate, 150 mM NaCl, 2 mM EDTA, and 1% Triton X-100) while placed on ice. The protein concentrations were determined using the BCA assay kit (Kalazist, Tehran, Iran). Equal concentrations of total protein were separated by electrophoresis in SDS-PAGE gel. After electrophoresis, proteins were transferred to 0.25 μm PVDF membranes for 60 min. Next, the PVDF membrane was washed by TBST to remove non-specific binding. After that, caspase-3 (1:200; Abcam ab4051) was added and incubated for 24 h at 4 °C. The membrane was then washed with TBST before adding a secondary antibody (1:30,000; Abcam ab97047) and incubating for 2 h at room temperature. To detection of positive reactions, enhanced chemiluminescence was used [11]. Additionally, β-actin (1:5000; Abcam ab119716) was used as an internal control.
2.10. Neurological evaluation
To evaluate neurological function, a motor deficit index (MDI) test was performed before and 6 h, 12 h, 24 h, and 48 h after the IR. The maximum MDI score was 6 (score of 2 for placing or stepping reflex and score of 4 for ambulation). Ambulation of hind limb was graded as follows: 0: normal; 1: toes flat under the body when walking but ataxia present; 2: knuckle walking; 3: unable to knuckle walk but some movement of the hind limbs; and 4: no movement or drags lower extremities. The placing or stepping reflex was evaluated by the dragging movements and responses of the hind paw dorsum when touching the floor surface. Hind paw placing or stepping reflex was graded as follows: 0: normal; 1: weak; and 2: no stepping [2].
2.11. Statistical analysis
Quantitative and qualitative variables were reported as Mean ± SD and frequency (%). Data normality was confirmed using the Kolmogorov-Smirnov test. Our results revealed that all quantitative variables had a normal distribution. Comparison the mean of quantitative variables was performed using one-way analysis of variance (ANOVA). Then, Tukey's post hoc test was used to test the differences between the pairs of groups. Differences were considered significant at P < 0.05. Statistical analyses were performed using the GraphPad Prism 6 software package (GraphPad Prism Software Inc., San Diego, CA, USA).
3. Results
3.1. hpMSCs marker analysis
Flow cytometry outcomes showed that the hpMSCs almost completely expressed CD73 (98.8%) and CD105 (91.3%). In contrast, CD45 (0.78%) and CD34 (0.42%) were slightly expressed (see Fig. 1).
Fig. 1.
Characterization of human placenta mesenchymal stem cells. (A) hpMSCs grew as spindle-shaped, fibroblast cell colonies. Flow cytometry analysis of the hpMSCs revealed that they expressed a high cluster of CD73 (B) and CD105 (C), but they expressed a few CD45 (D), and CD34 (E).
3.2. Characterization of hpMSC-Exos
The obtained exosomes from hpMSCs were round (Fig. 2A). The mean diameter size of exosomes was 80 nm (Fig. 2B). In addition, exosome surface proteins (CD9, CD63, and CD81) were detected in Western blot analysis (Fig. 2C) (Supplementary Fig. 1).
Fig. 2.
Characterization of hpMSCs-Exos. (A) Morphology of hpMSCs-Exos obtained by transmission electron microscopy. (B) The distribution of hpMSCs-Exos size by DLS analysis. (C) Exosome specific surface proteins (CD9, CD63, and CD81) were examined by Western blot analysis.
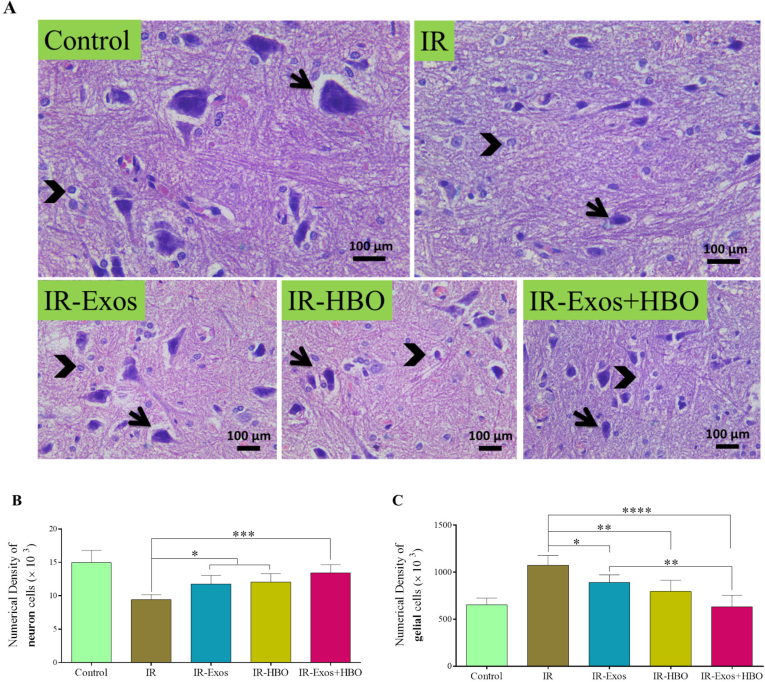
3.3. Combination of hpMSCs-Exos and HBO increased neuronal survival and decreased gliosis
Fig. 3A shows H&E staining on tissue sections. Also, Fig. 3B and C shows the result graphs for the determined stereological parameters.
Fig. 3.
Effects of hpMSCs-Exos in combination with HBO on histological and stereological changes. (A) The photomicrographs of H&E staining from the anterior horn of the spinal cord. Numerical densities of (B) neurons and (C) glial cells which were determined by the optical dissector method. Values are mean ± SD. ∗p < 0.05; ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Considering the numerical density of neurons, we found that the IR-Exos, IR-HBO, and IR-Exos + HBO groups were meaningfully higher compared to the IR group (p < 0.05, p < 0.05, and p < 0.001) (Fig. 3B).
In the assessment of the numerical density of glial cells, we observed that the IR-Exos, IR-HBO, and IR-Exos + HBO groups had significantly lower cells compared to the IR group (p < 0.05, p < 0.01, and p < 0.0001). Furthermore, in comparing the outcomes between treatment groups, the IR-Exos + HBO group had considerably lower cells compared to the IR-Exos group (p < 0.01) (Fig. 3C).
3.4. Combination of hpMSCs-Exos and HBO inhibited IR-induced oxidative stress
Our finding revealed that the IR-Exos, IR-HBO, and IR-Exos + HBO groups had considerably higher activities in the GSH (p < 0.05, p < 0.01, and p < 0.001), SOD (p < 0.01, p < 0.01, and p < 0.001), and CAT (p < 0.05, p < 0.01, and p < 0.0001) than in the IR group. Furthermore, we observed that the IR-Exos + HBO group compared to the IR-Exos and IR-HBO groups was meaningfully higher in the GSH (p < 0.01 and p < 0.05), SOD (both, p < 0.05), and CAT (p < 0.01 and p < 0.05) (Fig. 4A).
Fig. 4.
Effects of hpMSCs-Exos in combination with HBO on oxidative-stress related biomarkers and inflammatory cytokines levels. (A) Concentrations of antioxidative (GSH, SOD, and CAT) activities and oxidative (MDA) levels were determined by biochemistry method. (B) The levels for anti-inflammatory (IL-10) and inflammatory (IL-1β, TNF-α, and IL-18) cytokines were determined using ELISA method. Values are mean ± SD. ∗p < 0.05; ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Considering the oxidative (MDA) levels, we found that the IR-Exos, IR-HBO, and IR-Exos + HBO groups were meaningfully lower compared to the IR group (p < 0.05, p < 0.01, and p < 0.0001). In addition, MDA level in the IR-Exos + HBO group was meaningfully low than in the IR-Exos and IR-HBO groups (p < 0.01 and p < 0.05) (Fig. 4A).
3.5. Combination of hpMSCs-Exos and HBO inhibited IR-induced inflammation
The determination the level of anti-inflammatory cytokine (IL-10) indicated that the IR-Exos, IR-HBO, and IR-Exos + HBO groups compared to the IR group had considerably higher levels (p < 0.01, p < 0.01, and p < 0.0001). Meanwhile, comparing the level of IL-10 cytokine between the treatment groups indicated that the IR-Exos + HBO group had significantly higher level compared to the IR-Exos and IR-HBO groups (both, p < 0.05) (Fig. 4B).
Considering the proinflammatory cytokines, the outcomes indicated that the IR-Exos, IR-HBO, and IR-Exos + HBO groups compared to the IR group had considerably lower levels of the IL-1β (p < 0.01, p < 0.001, and p < 0.0001), TNF-α (p < 0.05, p < 0.01, and p < 0.001), and IL-18 (p < 0.05, p < 0.01, and p < 0.0001). Moreover, the IR-Exos + HBO group compared to the IR-Exos and IR-HBO groups had meaningfully lower levels of the TNF-α and IL-18 (both, p < 0.01 and p < 0.05). In addition, the level of the IL-1β cytokine in the IR-Exos + HBO group was significantly lower compared to the IR-Exos group (p < 0.05) (Fig. 4B).
3.6. Combination of hpMSCs-Exos and HBO decreased caspase-3 levels
To assess the protective effect of hpMSCs-Exos and HBO against neurons apoptosis, caspase-3 levels in the spinal cord were detected using western blotting. The finding showed that the IR-Exos, IR-HBO, and IR-Exos + HBO groups had considerably lower caspase-3 levels than the IR group (p < 0.05, p < 0.01, and p < 0.0001). In addition, the IR-Exos + HBO group compared to the IR-Exos group had noteworthy lower caspase-3 levels (p < 0.05) (Fig. 5) (Supplementary Fig. 2).
Fig. 5.
Effects of hpMSCs-Exos in combination with HBO on the expression of the apoptotic protein. (A) Western-blotting analysis of apoptotic (caspase-3) protein isolated from the spinal cord. (B) Data were analyzed using densitometry. Values are mean ± SD. ∗p < 0.05; ∗∗p < 0.01, ∗∗∗∗p < 0.0001.
3.7. Combination of hpMSCs-Exos and HBO improved IR-induced neurological function deficit
To evaluate the effect of combination of hpMSCs-Exos and HBO on functional recovery in IR-induced rats, the MDI test was used (Fig. 6). Regarding the MDI scores, we observed that the IR-Exos, IR-HBO, and IR-Exos + HBO groups compared to the IR group had significantly lower scores in 6 h (all, p < 0.001), 12 h (p < 0.01, p < 0.01, and p < 0.001), 24 h (p < 0.01, p < 0.01, and p < 0.001), and 48 h (all, p < 0.001) after IR-induction. Furthermore, the MDI scores in the IR-Exos + HBO group were meaningfully lower compared to the IR-Exos and IR-HBO groups in 6 h (p < 0.01 and p < 0.05), and compared to the IR-Exos group in 12 h, 24 h, and 48 h (all, p < 0.05), after IR-induction.
Fig. 6.
Effects of hpMSCs-Exos in combination with HBO on neurological functions. Motor deficit index (MDI) test was performed before and 6 h, 12 h, 24 h, and 48 h after IR. Values are mean ± SD. ∗p < 0.05; ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. a p < 0.001 IR-Exos, IR-HBO, and IR-Exos + HBO groups vs IR group; b p < 0.01 IR-Exos + HBO group vs IR-Exos group; c p < 0.05 IR-Exos + HBO group vs IR-HBO group; d p < 0.001 IR-Exos + HBO group vs IR group; e p < 0.01 IR-Exos and IR-HBO groups vs IR group; f p < 0.01 IR-Exos + HBO group vs IR-Exos group; g p < 0.05 IR-Exos + HBO group vs IR-Exos group.
4. Discussion
The spinal cord is more susceptible to damage caused by ischemia due to poor segmental blood supply [2]. Studies have shown that the main mechanisms in spinal cord injury following ischemia-reperfusion are the occurrence of oxidative stress, excessive inflammation, and as a result, apoptosis of cells in the ischemia site [1]. Finally, all these cases can disrupt the function of the spinal cord and cause serious disturbance in nerve exchanges in the damaged spinal cord segments as well as the lower segments of the injured area. Therefore, according to the pathogenesis of IR, the best treatment should include three parts in addition to quick action in damage control [25]. First, oxidative stress should be inhibited. Second, the spread of inflammation should be prevented. And the third is to prevent the apoptosis of cells in the damaged area. Therefore, since several factors are effective in the pathogenesis of IR, the application of therapeutic measures should also be based on multi-factorial approaches [4]. In the current study, we found that the co-administration of hpMSCs-Exos and HBO effectively improved neurological function and synergically alleviated histopathological damages by reserving neuronal survival and inhibited gliosis in the spinal cord. Moreover, combination use of the hpMSCs-Exos and HBO considerably inhibited oxidative stress and inflammation, and attenuated apoptosis following the IR.
Oxidative stress is one of the main destructive factors in IR [26]. Studies have shown that after IR, lipid peroxidation is significantly activated, and then the membranes of neurons are seriously damaged and can cause the destruction of cells. Meanwhile, following this pathological event, the activity of antioxidant enzymes also decreases dramatically [25,27]. Therefore, studies have shown that if MDA levels can be reduced and the activity of antioxidant enzymes such as GSH, SOD, and CAT can be increased, the damage caused by IR can be significantly inhibited [2,28]. In the present study, all four biochemical indices related to oxidative stress were evaluated. Our results showed that the levels of the MDA and the activities of the GSH, SOD, and CAT enzymes in all treatment groups were decreased and increased, respectively, compared to the IR group, and these changes were more pronounced in the combined group. In this regard, Cheshmi et al. documented that the hpMSCs-Exos can significantly inhibit the level of oxidative stress in the injured spinal cord by reducing the levels of the MDA and increasing the activity of antioxidant enzymes (GSH, SOD, and CAT) [11]. Also, Lu et al. reported that the hpMSCs-Exos with paracrine activity increase the activity of antioxidant enzymes and can also reduce the level of lipid peroxidation [29]. On the other hand, Ilhan et al. reported that the HBO administration increased the MDA concentration and decreased the SOD, GSH-Px, and CAT levels in animals subjected to IR [4]. Also, Ahmadi et al. reported that HBO can significantly prevent the occurrence of oxidative stress in spinal cord injury by inhibiting lipid peroxidation and increasing the activity of antioxidant enzymes [17]. Therefore, in the present study, we found that each combination of hpMSCs-Exos and HBO can inhibit oxidative stress in rats subjected to the IR, however, their combined use had these effects synergistically, and even compared to single treatment groups, the situation was better.
On the other hand, the severe inflammatory response is another destructive factor in the pathogenesis of IR. This event occurs within a few hours after the injury and is associated with the release of inflammatory cytokines such as IL-1β, TNF-α, and IL-18 [30]. Studies have shown that two factors play a very important role in controlling this situation, quick treatment action and the use of an effective combination [4,11,14,17]. In addition, experimental studies have shown that inhibiting and stimulating the expression of IL-1β and IL-10 cytokines, respectively, increases the survival of neurons and prevents the occurrence of gliosis at the injury site [11,31]. In the present study, we found that the combined administration of the hpMSCs-Exos and HBO during ischemia induction and immediately after that in the reperfusion stage synergically reduced the levels of inflammatory cytokines and the numerical density of glial cells. In addition, we observed that in the treatment groups, especially the IR-Exos + HBO group, the levels of IL-10 cytokine as an anti-inflammatory agent and the numerical density of neurons also increased. In this regard, it has been documented that the hpMSCs-Exos have immunosuppressive and immunomodulatory properties [9]. Cheshmi et al. documented that the hpMSCs-Exos have considerable anti-inflammatory effects and can significantly reduce the expression levels of inflammatory cytokines (TNF-α and IL-1β) and the numerical density of glial cells, and instead increase the IL-10 cytokine levels and the neurons survival in the SCI. They stated that these effects are probably due to the overexpression of Nrf2, which inhibits oxidative stress and the expression of pro-inflammatory genes [11,32]. On the other hand, studies have shown that HBO, by stimulating the production of prostacyclin, causes cell protection and inhibits inflammation in the spinal cord during aortic occlusion [4,33]. Also, Ahmadi et al. and Cheshmi et al. reported that HBO therapy in the SCI significantly reduces the level of inflammation and gliosis and increases the survival of neurons in the injury site [11,17].
Apoptosis of nerve cells is the last step in the IR and is irreversible, and as a result, it causes a serious disturbance in the motor function of the spinal cord in the damaged segment and areas below the injury site [34]. In the present study, we found that the expression level of caspase-3 protein as an indicator of cell apoptosis in the treatment groups, especially the IR-Exos + HBO group, was significantly reduced. In this regard, Hu et al. reported that the hpMSCs-Exos reduced inflammation and inhibited apoptosis by modulating NF-κB/MAPK and PI3K signaling pathways [35]. Also, Cheshmi et al. reported that the hpMSCs-Exos have neuroprotective effects and improved neurological function in the SCI rats by inhibiting apoptosis [11]. Regarding the anti-apoptotic effects of HBO, Zhang et al. reported that HBO administration inhibits the expression of apoptotic factors and ultimately prevents the death of neurons [18].
5. Conclusion
Generally, our finding revealed that the separate administration of hpMSCs-Exos and HBO had neuroprotective effects in the rats undergoing IR. However, we found that the co-administration of both compounds had more effects and synergically alleviates spinal cord ischemia-reperfusion injury in rats. However, the neuroprotective effects of hpMSCs-Exos and HBO in the treatment of spinal cord ischemia-reperfusion injury patients require more clinical studies.
Data availability statement
The original contributions presented in this study are included in the article material, further inquiries can be directed to the corresponding author.
Ethics statement
All experimental protocols were approved by the Ethics Committee of Mazandaran University of Medical Sciences (Ethic code: IR.MAZUMS.4.REC.1400.13785).
Authors contributions
A.J. and A.R.K. contributed to the study design, data acquisition and analysis, as well as drafting of the manuscript. S.T. designed molecular assessments and analyses. M.P., and M.As. isolation and characterization of hpMSCs-Exos. M.S.M, and I.N. interpretation of data, editing and final approval of the manuscript. E.A. designed behavioral assessment and analysis. A.R. designed stereological assessments and analyses. D.N. supervised the study, provided financial support, and contributed to the study concept and design, interpretation of data, and editing and final approval of the manuscript. All authors reviewed and commented on the manuscript and approved the final manuscript.
Funding
The current project was financially supported by grants to D. N. from Mazandaran University of Medical Sciences (Grant No. 13785), Sari, Iran.
Declaration of competing interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2023.09.003.
Contributor Information
Aref Jafari, Email: Arefjafari120@yahoo.com.
Ali Reza Khalatbary, Email: Khalat90@yahoo.com.
Saeid Taghiloo, Email: Saeid.taghiloo@yahoo.com.
Mohamad Sedigh Mirzaie, Email: Msmirzaie1@gmail.com.
Eisa Nazar, Email: Isa.nazar89@gmail.com.
Mahnaz Poorhassan, Email: Porhassan729@gmail.com.
Esmaeil Akbari, Email: Akbari_esmaeil@yahoo.Com.
Mahdiyeh Asadzadeh, Email: Mahdiyehasadzadeh1@gmail.com.
Amir Raoofi, Email: Amirrezaraoofi@yahoo.com.
Davood Nasiry, Email: Davood1990nasiry@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Gao M., Zhang Z., Lai K., Deng Y., Zhao C., Lu Z., et al. Puerarin: a protective drug against ischemia-reperfusion injury. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahadi S., Zargari M., Khalatbary A.R. Assessment of the neuroprotective effects of (-)-epigallocatechin-3-gallate on spinal cord ischemia-reperfusion injury in rats. J Spinal Cord Med. 2019:1–8. doi: 10.1080/10790268.2019.1691862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen F.-S., Tong X.-Y., Fang B., Wang D., Li X.-Q., Zhang Z.-L. The roles of microRNAs in spinal cord ischemia-reperfusion injury. Neural Regen Res. 2022;17(12):2593. doi: 10.4103/1673-5374.339471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ilhan G., Aksun M., Ozpak B., Gunes T., Bozok S., Durakoglugil M.E., et al. The effect of combined hyperbaric oxygen and iloprost treatment on the prevention of spinal cord ischaemia–reperfusion injury: an experimental study. Eur J Cardio Thorac Surg. 2013;44(5):e332–e340. doi: 10.1093/ejcts/ezt398. [DOI] [PubMed] [Google Scholar]
- 5.Gökce E.C., Kahveci R., Gökce A., Cemil B., Aksoy N., Sargon M.F., et al. Neuroprotective effects of thymoquinone against spinal cord ischemia-reperfusion injury by attenuation of inflammation, oxidative stress, and apoptosis. J Neurosurg. 2016;24(6):949–959. doi: 10.3171/2015.10.SPINE15612. [DOI] [PubMed] [Google Scholar]
- 6.Deng J., Li M., Meng F., Liu Z., Wang S., Zhang Y., et al. 3D spheroids of human placenta-derived mesenchymal stem cells attenuate spinal cord injury in mice. Cell Death Dis. 2021;12(12):1096. doi: 10.1038/s41419-021-04398-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alishahi M., Anbiyaiee A., Farzaneh M., Khoshnam S.E. Human mesenchymal stem cells for spinal cord injury. Curr Stem Cell Res Ther. 2020;15(4):340–348. doi: 10.2174/1574888X15666200316164051. [DOI] [PubMed] [Google Scholar]
- 8.Kulubya E.S., Clark K., Hao D., Lazar S., Ghaffari-Rafi A., Karnati T., et al. The unique properties of placental mesenchymal stromal cells: a novel source of therapy for congenital and acquired spinal cord injury. Cells. 2021;10(11):2837. doi: 10.3390/cells10112837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasiry D., Khalatbary A.R. Stem cell-derived extracellular vesicle-based therapy for nerve injury: a review of the molecular mechanisms. World Neurosurg: X. 2023 doi: 10.1016/j.wnsx.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou Y., Liao L., Dai J., Mazhar M., Yang G., Wang H., et al. Mesenchymal stem cell-derived extracellular vesicles/exosome: a promising therapeutic strategy for intracerebral hemorrhage. Regen Ther. 2023;22:181–190. doi: 10.1016/j.reth.2023.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheshmi H., Mohammadi H., Akbari M., Nasiry D., Rezapour-Nasrabad R., Bagheri M., et al. Human placental mesenchymal stem cell-derived exosomes in combination with hyperbaric oxygen synergistically promote recovery after spinal cord injury in rats. Neurotox Res. 2023:1–15. doi: 10.1007/s12640-023-00649-0. 0.1007/s12640-023-00649-0. [DOI] [PubMed] [Google Scholar]
- 12.Fan Y., Chen Z., Zhang M. Role of exosomes in the pathogenesis, diagnosis, and treatment of central nervous system diseases. J Transl Med. 2022;20(1):291. doi: 10.1186/s12967-022-03493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C., Zhang C., Xu Y., Li C., Cao Y., Li P. Exosomes derived from human placenta-derived mesenchymal stem cells improve neurologic function by promoting angiogenesis after spinal cord injury. Neurosci Lett. 2020;739 doi: 10.1016/j.neulet.2020. [DOI] [PubMed] [Google Scholar]
- 14.Zhou W., Silva M., Feng C., Zhao S., Liu L., Li S., et al. Exosomes derived from human placental mesenchymal stem cells enhanced the recovery of spinal cord injury by activating endogenous neurogenesis. Stem Cell Res Ther. 2021;12(1):1–14. doi: 10.1186/s13287-021-02248-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Z.-C., Liang F., Yang J., Hai Y., Su Q.-J., Liu X.-H. The mechanism by which hyperbaric oxygen treatment alleviates spinal cord injury: genome-wide transcriptome analysis. Neural Regen Res. 2022;17(12):2737. doi: 10.4103/1673-5374.339498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nasiry D., Khalatbary A.R., Abdollahifar M.-A., Bayat M., Amini A., Ashtiani M.K., et al. SDF-1α loaded bioengineered human amniotic membrane-derived scaffold transplantation in combination with hyperbaric oxygen improved diabetic wound healing. J Biosci Bioeng. 2022;133(5):489–501. doi: 10.1016/j.jbiosc.2022.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Ahmadi F., Zargari M., Nasiry D., Khalatbary A.R. Synergistic neuroprotective effects of hyperbaric oxygen and methylprednisolone following contusive spinal cord injury in rat. J Spinal Cord Med. 2021:1–10. doi: 10.1080/10790268.2021.1896275. 1080/10790268.2021.1896275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z., Li Q., Yang X., Li B., Zhou Y., Hu T., et al. Effects of hyperbaric oxygen therapy on postoperative recovery after incomplete cervical spinal cord injury. Spinal Cord. 2022;60(2):129–134. doi: 10.1038/s41393-021-00674-w. [DOI] [PubMed] [Google Scholar]
- 19.Dayan K., Keser A., Konyalioglu S., Erturk M., Aydin F., Sengul G., et al. The effect of hyperbaric oxygen on neuroregeneration following acute thoracic spinal cord injury. Life Sci. 2012;90(9–10):360–364. doi: 10.1016/j.lfs.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Liu W., Wang Y., Gong F., Rong Y., Luo Y., Tang P., et al. Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of A1 neurotoxic reactive astrocytes. J Neurotrauma. 2019;36(3):469–484. doi: 10.1089/neu.2018.5835. [DOI] [PubMed] [Google Scholar]
- 21.Sun G., Li G., Li D., Huang W., Zhang R., Zhang H., et al. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater Sci Eng, C. 2018;89:194–204. doi: 10.1016/j.msec.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Howard V., Reed M. Garland Science; 2004. Unbiased stereology: three-dimensional measurement in microscopy. [Google Scholar]
- 23.Seyed Sharifi S.H., Nasiry D., Mahmoudi F., Etezadpour M., Ebrahimzadeh M.A. Evaluation of sambucus ebulus fruit extract in full-thickness diabetic wound healing in rats. J Maz Univ Med. 2021;31(200):11–25. [Google Scholar]
- 24.Fan L., Li X., Liu T. Asiaticoside inhibits neuronal apoptosis and promotes functional recovery after spinal cord injury in rats. J Mol Neurosci. 2020;70(12):1988–1996. doi: 10.1007/s12031-020-01601-z. 10.007/s12031-020-01601-z. [DOI] [PubMed] [Google Scholar]
- 25.Fu J., Sun H., Wei H., Dong M., Zhang Y., Xu W., et al. Astaxanthin alleviates spinal cord ischemia-reperfusion injury via activation of PI3K/Akt/GSK-3β pathway in rats. J Orthop Surg Res. 2020;15(1):1–11. doi: 10.1186/s13018-020-01790-8. 0.1186/s13018-020-01790-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun F., Zhang H., Shi J., Huang T., Wang Y. Astragalin protects against spinal cord ischemia reperfusion injury through attenuating oxidative stress-induced necroptosis. BioMed Res Int. 2021;2021:1–8. doi: 10.1016/10.155/2021/7254708. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Xie L., Yu S., Yang K., Li C., Liang Y. Hydrogen sulfide inhibits autophagic neuronal cell death by reducing oxidative stress in spinal cord ischemia reperfusion injury. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurt G., Yildirim Z., Cemil B., Celtikci E., Kaplanoglu G.T. Effects of curcumin on acute spinal cord ischemia-reperfusion injury in rabbits. J Neurosurg Spine. 2014;20(4):464–470. doi: 10.3171/2013.12.SPINE1312. [DOI] [PubMed] [Google Scholar]
- 29.Lu J., Liu Z., Shu M., Zhang L., Xia W., Tang L., et al. Human placental mesenchymal stem cells ameliorate chemotherapy-induced damage in the testis by reducing apoptosis/oxidative stress and promoting autophagy. Stem Cell Res Ther. 2021;12(1):1–10. doi: 10.1186/s13287-021-02275-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin X.-L., Zhu J., Wang L.-M., Yan F., Sha W.-P., Yang H.-L. MiR-92b-5p inhibitor suppresses IL-18 mediated inflammatory amplification after spinal cord injury via IL-18BP up-regulation. Eur Rev Med Pharmacol Sci. 2019;23(5):1891–1898. doi: 10.26355/eurrev_201903_17226. [DOI] [PubMed] [Google Scholar]
- 31.Schizas N., Andersson B., Hilborn J., Hailer N.P. Interleukin-1 receptor antagonist promotes survival of ventral horn neurons and suppresses microglial activation in mouse spinal cord slice cultures. J Neurosci Res. 2014;92(11):1457–1465. doi: 10.1002/jnr.23429. [DOI] [PubMed] [Google Scholar]
- 32.Nilforoushzadeh M.A., Raoofi A., Afzali H., Gholami O., Zare S., Nasiry D., et al. Promotion of cutaneous diabetic wound healing by subcutaneous administration of Wharton's jelly mesenchymal stem cells derived from umbilical cord. Arch Dermatol Res. 2023;315(2):147–159. doi: 10.1007/s00403-022-2326-2. [DOI] [PubMed] [Google Scholar]
- 33.Carbajal D., Arruzazabala M., Noa M., Molina V., Más R., Arango E., et al. Protective effect of D-003 on experimental spinal cord ischemia in rabbits. Prostaglandins Leukot Essent Fatty Acids. 2004;70(1):1–6. doi: 10.1016/S0952-3278(03)00082-6. [DOI] [PubMed] [Google Scholar]
- 34.Gökce E.C., Kahveci R., Gökce A., Cemil B., Aksoy N., Sargon M.F., et al. Neuroprotective effects of thymoquinone against spinal cord ischemia-reperfusion injury by attenuation of inflammation, oxidative stress, and apoptosis. J Neurosurg Spine. 2016;24(6):949–959. doi: 10.3171/2015.10.SPINE15612. [DOI] [PubMed] [Google Scholar]
- 35.Hu Y., Qu H., He J., Zhong H., He S., Zhao P., et al. Human placental mesenchymal stem cell derived exosomes exhibit anti-inflammatory effects via TLR4-mediated NF-κB/MAPK and PI3K signaling pathways. Pharmazie. 2022;77(3–4):112–117. doi: 10.1691/ph.2022.11082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in the article material, further inquiries can be directed to the corresponding author.