Abstract
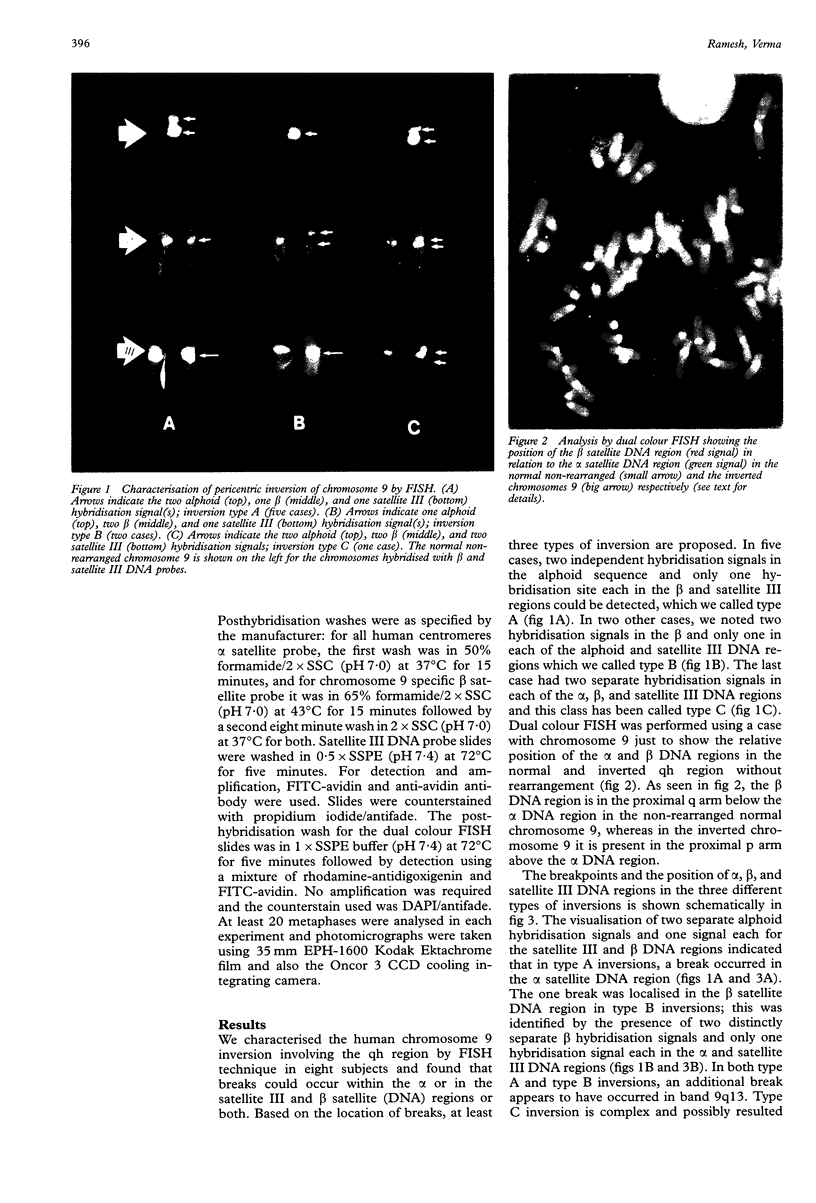
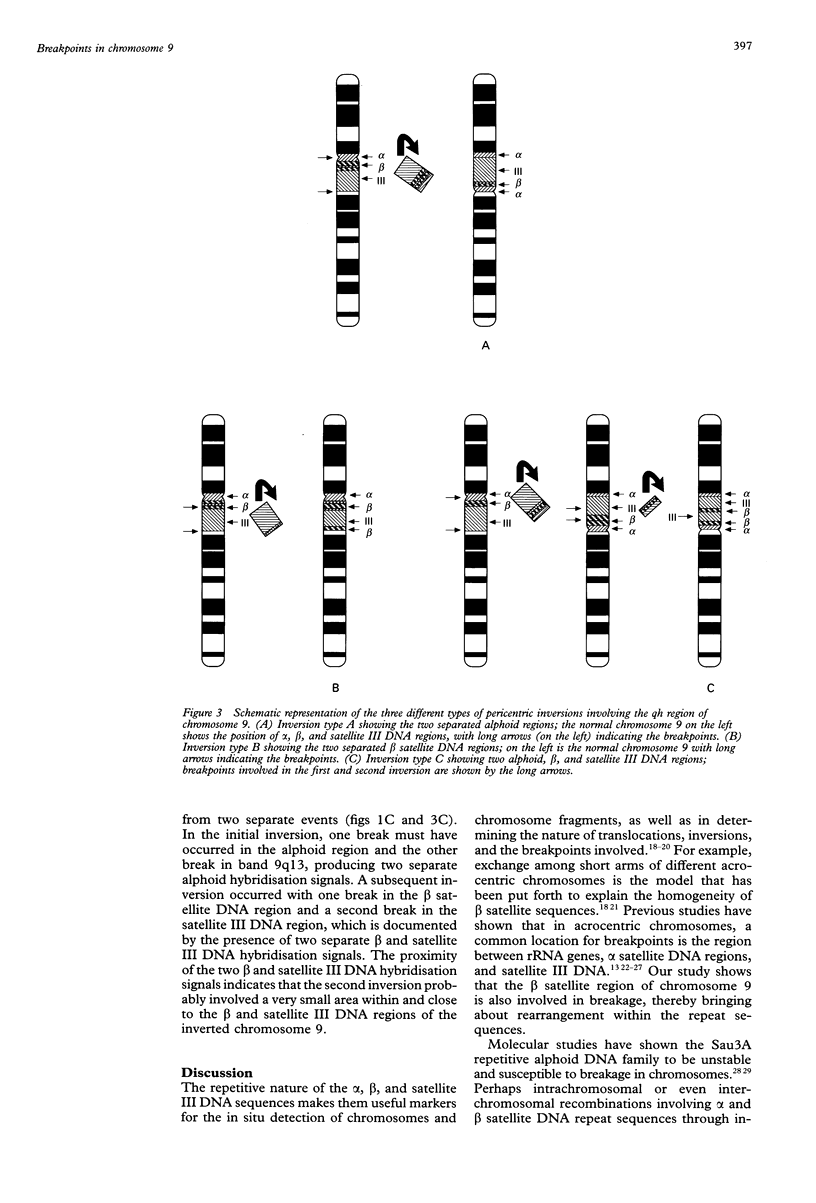
Human chromosome 9 with a pericentric inversion involving the qh region is considered normal. It has probably evolved through breakage and reunion and is retained through mendelian inheritance without any apparent phenotypic consequences. Fluorescent in situ hybridisation (FISH) technique using alpha, beta, and satellite III DNA probes showed that the breakpoints are variable and can be localised in the alpha or in the satellite III and beta DNA regions or both. Three types of inversions are proposed which appear similar by CBG banding: pericentric inversions with two alphoid, one beta, and one satellite III hybridisation signals were classified as type A. Type B were those with two beta, one alpha, and one satellite III hybridisation signals, while type C was complex, and most likely involved two inversions, since two separate hybridisation signals were detected in each of the alphoid, beta satellite, and satellite III DNA regions. Based on eight cases, type A is likely to be the most frequent, but the frequencies, which at present appear non-random for these different types of inversions in the population, can only be estimated by studying a larger sample size. Inversion heteromorphisms may promote reshuffling of tandem arrays of DNA repeat sequences, thereby giving rise to new heteromorphic domains. Alternatively, the repetitive nature of the sequences lends to the structural variations observed within the inv(9) chromosomes (or any other abnormal chromosome that is the result of recombination between, or breakage within, repetitive DNA).
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agresti A., Meneveri R., Siccardi A. G., Marozzi A., Corneo G., Gaudi S., Ginelli E. Linkage in human heterochromatin between highly divergent Sau3A repeats and a new family of repeated DNA sequences (HaeIII family). J Mol Biol. 1989 Feb 20;205(4):625–631. doi: 10.1016/0022-2836(89)90308-2. [DOI] [PubMed] [Google Scholar]
- Babu A., Verma R. S. Characterization of human chromosomal constitutive heterochromatin. Can J Genet Cytol. 1986 Oct;28(5):631–644. doi: 10.1139/g86-093. [DOI] [PubMed] [Google Scholar]
- Babu A., Verma R. S. Chromosome structure: euchromatin and heterochromatin. Int Rev Cytol. 1987;108:1–60. doi: 10.1016/s0074-7696(08)61435-7. [DOI] [PubMed] [Google Scholar]
- Charlesworth B., Sniegowski P., Stephan W. The evolutionary dynamics of repetitive DNA in eukaryotes. Nature. 1994 Sep 15;371(6494):215–220. doi: 10.1038/371215a0. [DOI] [PubMed] [Google Scholar]
- Cheung S. W., Sun L., Featherstone T. Molecular cytogenetic evidence to characterize breakpoint regions in Robertsonian translocations. Cytogenet Cell Genet. 1990;54(3-4):97–102. doi: 10.1159/000132970. [DOI] [PubMed] [Google Scholar]
- Choo K. H. Role of acrocentric cen-pter satellite DNA in Robertsonian translocation and chromosomal non-disjunction. Mol Biol Med. 1990 Oct;7(5):437–449. [PubMed] [Google Scholar]
- Cook K. R., Karpen G. H. A rosy future for heterochromatin. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5219–5221. doi: 10.1073/pnas.91.12.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earle E., Shaffer L. G., Kalitsis P., McQuillan C., Dale S., Choo K. H. Identification of DNA sequences flanking the breakpoint of human t(14q21q) Robertsonian translocations. Am J Hum Genet. 1992 Apr;50(4):717–724. [PMC free article] [PubMed] [Google Scholar]
- Fernández J. L., Goyanes V., Pereira S., López-Fernández C., Gosálvez J. 5-azacytidine produces differential undercondensation of alpha, beta and classical human satellite DNAs. Chromosome Res. 1994 Jan;2(1):29–35. doi: 10.1007/BF01539451. [DOI] [PubMed] [Google Scholar]
- Gravholt C. H., Friedrich U., Caprani M., Jørgensen A. L. Breakpoints in Robertsonian translocations are localized to satellite III DNA by fluorescence in situ hybridization. Genomics. 1992 Dec;14(4):924–930. doi: 10.1016/s0888-7543(05)80113-2. [DOI] [PubMed] [Google Scholar]
- Greig G. M., Willard H. F. Beta satellite DNA: characterization and localization of two subfamilies from the distal and proximal short arms of the human acrocentric chromosomes. Genomics. 1992 Mar;12(3):573–580. doi: 10.1016/0888-7543(92)90450-7. [DOI] [PubMed] [Google Scholar]
- Haaf T., Ward D. C. Structural analysis of alpha-satellite DNA and centromere proteins using extended chromatin and chromosomes. Hum Mol Genet. 1994 May;3(5):697–709. doi: 10.1093/hmg/3.5.697. [DOI] [PubMed] [Google Scholar]
- Han J. Y., Choo K. H., Shaffer L. G. Molecular cytogenetic characterization of 17 rob(13q14q) Robertsonian translocations by FISH, narrowing the region containing the breakpoints. Am J Hum Genet. 1994 Nov;55(5):960–967. [PMC free article] [PubMed] [Google Scholar]
- Holmquist G. P. Chromatin self-organization by mutation bias. J Mol Evol. 1994 Nov;39(5):436–438. doi: 10.1007/BF00173411. [DOI] [PubMed] [Google Scholar]
- Irick H. A new function for heterochromatin. Chromosoma. 1994 Mar;103(1):1–3. doi: 10.1007/BF00364720. [DOI] [PubMed] [Google Scholar]
- Kiyama R., Matsui H., Oishi M. A repetitive DNA family (Sau3A family) in human chromosomes: extrachromosomal DNA and DNA polymorphism. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4665–4669. doi: 10.1073/pnas.83.13.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyama R., Okumura K., Matsui H., Bruns G. A., Kanda N., Oishi M. Nature of recombination involved in excision and rearrangement of human repetitive DNA. J Mol Biol. 1987 Dec 20;198(4):589–598. doi: 10.1016/0022-2836(87)90202-6. [DOI] [PubMed] [Google Scholar]
- Luke S., Aggarwal G., Stetka D. G., Verma R. S. Alphoid DNA diversity of a so-called monocentric Robertsonian fusion. Chromosome Res. 1994 Jan;2(1):73–75. doi: 10.1007/BF01539457. [DOI] [PubMed] [Google Scholar]
- Luke S., Verma R. S., Conte R. A., Mathews T. Molecular characterization of the secondary constriction region (qh) of human chromosome 9 with pericentric inversion. J Cell Sci. 1992 Dec;103(Pt 4):919–923. doi: 10.1242/jcs.103.4.919. [DOI] [PubMed] [Google Scholar]
- Macera M. J., Verma R. S., Conte R. A., Bialer M. G., Klein V. R. Mechanisms of the origin of a G-positive band within the secondary constriction region of human chromosome 9. Cytogenet Cell Genet. 1995;69(3-4):235–239. doi: 10.1159/000133972. [DOI] [PubMed] [Google Scholar]
- Rocchi M., Archidiacono N., Ward D. C., Baldini A. A human chromosome 9-specific alphoid DNA repeat spatially resolvable from satellite 3 DNA by fluorescent in situ hybridization. Genomics. 1991 Mar;9(3):517–523. doi: 10.1016/0888-7543(91)90419-f. [DOI] [PubMed] [Google Scholar]
- Verma R. S., Luke S., Brennan J. P., Mathews T., Conte R. A., Macera M. J. Molecular topography of the secondary constriction region (qh) of human chromosome 9 with an unusual euchromatic band. Am J Hum Genet. 1993 May;52(5):981–986. [PMC free article] [PubMed] [Google Scholar]
- Wang J. C., Miller W. A. Molecular cytogenetic characterization of two types of chromosome 9 variants. Cytogenet Cell Genet. 1994;67(3):190–192. doi: 10.1159/000133820. [DOI] [PubMed] [Google Scholar]
- Warburton P. E., Willard H. F. Genomic analysis of sequence variation in tandemly repeated DNA. Evidence for localized homogeneous sequence domains within arrays of alpha-satellite DNA. J Mol Biol. 1990 Nov 5;216(1):3–16. doi: 10.1016/s0022-2836(05)80056-7. [DOI] [PubMed] [Google Scholar]
- Waye J. S., Willard H. F. Human beta satellite DNA: genomic organization and sequence definition of a class of highly repetitive tandem DNA. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6250–6254. doi: 10.1073/pnas.86.16.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff D. J., Schwartz S. Characterization of Robertsonian translocations by using fluorescence in situ hybridization. Am J Hum Genet. 1992 Jan;50(1):174–181. [PMC free article] [PubMed] [Google Scholar]
- Wolff D. J., Schwartz S. The effect of Robertsonian translocation on recombination on chromosome 21. Hum Mol Genet. 1993 Jun;2(6):693–699. doi: 10.1093/hmg/2.6.693. [DOI] [PubMed] [Google Scholar]