Abstract
In animals, adaptation to changes in cellular oxygen levels is coordinated largely by 2-oxoglutarate-dependent prolyl-hydroxylase domain (PHD) dioxygenase family members, which regulate the stability of their hypoxia-inducible factor (HIF) substrates to promote expression of genes that adapt cells to hypoxia. Recently, 2-aminoethanethiol dioxygenase (ADO) was identified as a novel O2-sensing enzyme in animals. Through N-terminal cysteine dioxygenation and the N-degron pathway, ADO regulates the stability of a set of non-transcription factor substrates; the regulators of G-protein signaling 4, 5. and 16 and interleukin-32. Here, we set out to compare and contrast the in cellulo characteristics of ADO and PHD enzymes in an attempt to better understand their co-evolution in animals. We find that ADO operates to regulate the stability of its substrates rapidly and with similar O2-sensitivity to the PHD/HIF pathway. ADO appeared less sensitive to iron chelating agents or transition metal exposure than the PHD enzymes, possibly due to tighter catalytic-site Fe2+ coordination. Unlike the PHD/HIF pathway, the ADO/N-degron pathway was not subject to feedback by hypoxic induction of ADO, and induction of ADO substrates was well sustained in response to prolonged hypoxia. The data also reveal strong interactions between proteolytic regulation of targets by ADO and transcriptional induction of those targets, that shape integrated cellular responses to hypoxia. Collectively, our comparative analysis provides further insight into ADO/N-degron-mediated oxygen sensing and its integration into established mechanisms of oxygen homeostasis.
Keywords: hypoxia, sensing, dioxygenase, hydroxylase, ADO, HIF, PHD
Systems that sense, adapt, and alleviate exposure to hypoxia (low O2) are observed throughout evolution, reflecting the fundamental importance of O2 in biology and its obligate role in multicellular life forms. In mammals, a sensing system comprised of a set of 2-oxoglutarate-dependent dioxygenase enzymes (Prolyl Hydroxylase Domain, PHD1, 2, and 3, otherwise known as EGLN 2,1, 3) and their Hypoxia-Inducible factor (HIF-1, -2, and 3α) target substrates is widespread and likely responsible for the majority of adaptive transcriptional responses to hypoxia (reviewed in (1, 2)). Similarly, oxygen sensing in plants is orchestrated by plant cysteine dioxygenases (PCOs) which regulate the stability of ethylene response factor VII transcription factors (3, 4, 5). Both systems respond to changes in environmental O2 by inducing a pattern of gene expression capable of reducing cellular O2 usage, while simultaneously increasing O2 delivery at the tissue level.
It was recently demonstrated that enzymatic N-cysteine dioxygenation also occurs in mammals through an orthologue of PCO, 2-aminoethanethiol dioxygenase (ADO) (6). ADO was first identified as an enzyme that catalyzes the oxidation of cysteamine to hypotaurine (7). However, recent work indicates that it also functions as an N-terminal cysteine dioxygenase that regulates protein stability via the Arg/Cys branch of the N-degron pathway (8) in a manner similar to the PCOs (6). Unlike PCO and PHD enzymes, the known substrates of ADO are not transcription factors but rather regulators of G-protein signaling (RGS4, 5, and 16) and the atypical cytokine interleukin-32 (IL-32). These RGS proteins interact with Gαq and i/o subunits of the heterotrimeric G-protein complex to increase the rate of GTP hydrolysis, thereby repressing downstream signaling through the associated G-protein coupled receptor (as reviewed in (9)). The physiological role of IL-32 remains to be elucidated, but strong associations have been made between its expression and the severity of non-alcoholic fatty liver disease (10, 11). IL-32 has also been implicated in the host response to hepatitis B and C infections (12, 13). Under normoxic conditions, ADO substrates are degraded via N-degron-mediated proteolysis, but reductions in cellular O2 levels lead to a reduction in ADO activity and thus to substrate stabilization. Much like the PHD enzymes, recombinant ADO and PCO are highly sensitive to O2 in vitro (6, 14) and so could potentially transduce signals under physiological and pathophysiological levels of hypoxia (e.g., 0–10% O2). However, in contrast to the detailed characterization of PHD/HIF, little is known about the analogous ADO/RGS system or how the two systems interface.
Here we compare and contrast the operation of these O2 sensing pathways in mammalian cells. We show that both pathways operate with similar in cellulo sensitivity with respect to O2 but have important biochemical and pharmacological distinctions. Understanding the constraints within which each pathway operates in cells will be important in deciphering their shared and distinct roles in mammalian hypoxia physiology and potentially in the therapeutic modulation of these systems.
Results
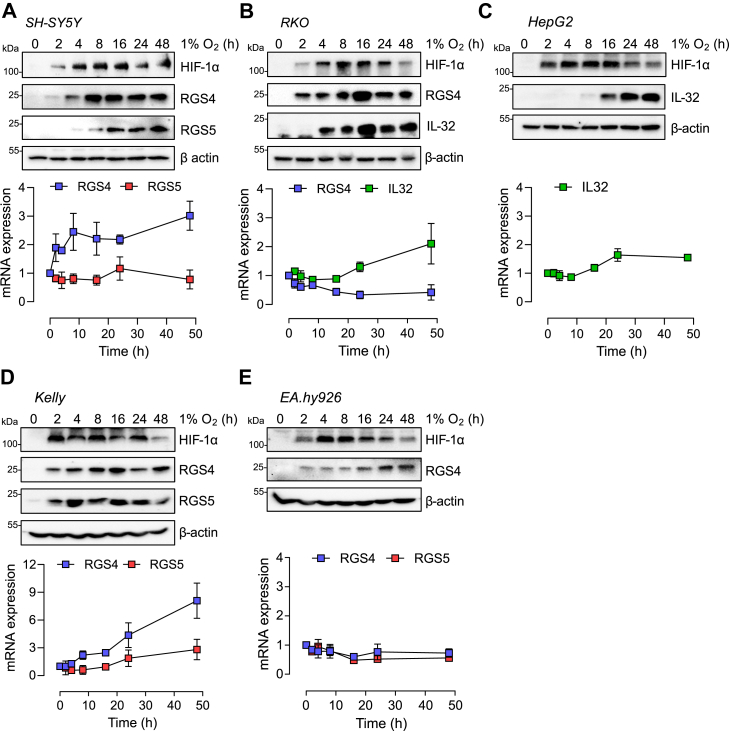
A defining feature of physiological O2-sensing mechanisms, exemplified by the HIF/PHD system, is a graded response to changes in cellular oxygenation within the physiological range. In in vitro studies, both the PHD enzymes and the ADO have been reported to be similarly sensitive to O2, with apparent KmO2 values 200 to 500 μM O2 (6, 15, 16). We have previously reported limited studies of the sensitivity of the ADO substrates, RGS4, and 5 to hypoxia in cell lines. In the present analysis, we first sought to extend this work by comparing the oxygen sensitivity of known ADO substrates to that of HIF over a wide range of physiological to pathophysiological oxygen concentrations. Since cellular oxygenation in culture is defined by a complex interaction between the ambient O2 levels, monolayer confluency, and cellular O2 consumption (17), these experiments were conducted under similar conditions across a range of cell lines (SH-SY5Y, RKO, HepG2, Kelly, and EA.hy926). To guide these analyses, RNA extracts from each cell line were initially screened for the presence of transcripts encoding the four known ADO substrates; RGS4, RGS5, RGS16, and IL32 (Fig. 1A). This revealed that, among the cell lines studied, RGS4 is the most widely expressed substrate, with RGS16 and IL32 being confined to the neuroblastomal (SH-SY5Y and Kelly) and the gastrointestinal cell lines (HepG2 and RKO), respectively. We then exposed the cells to different O2 levels (18, 7.5, 3, 1, and 0.1% v/v O2) for 4 h (for RGS4/5) or 18 h (for IL-32, for the time course of induction see Fig. 6) and measured the abundance of HIF-1α and expressed ADO substrates. These experiments revealed strikingly similar sensitivity of HIF-1α and RGS4/5 and/or IL-32 to hypoxia in all cell types studied. Data are illustrated for all proteins studied in each cell line in Figure 1B and quantified using non-linear regression analysis in Figure 1C and demonstrates a closely similar relationship between HIF-1α and ADO substrate protein levels and applied pO2. We were unable to detect RGS16 protein in Kelly cells nor was RGS5 protein detectable in EA.hy926 cells, despite the presence of the corresponding mRNA transcript in these cells.
Figure 1.
Response to graded hypoxia in mammalian cell lines.A, the expression pattern of ADO substrate mRNA transcripts in five cell lines. Color scale (white to blue) represents the Log10 fold change in substrate mRNA level relative to HPRT. B, SH-SY5Y, RKO, HepG2, Kelly, and EA.hy926 cells were subjected to 4-h hypoxia (18 h for IL-32 in HepG2) at the indicated level of hypoxia and expression of ADO substrates present in each cell line assessed by immunoblotting. HIF-1α protein levels were analyzed in parallel for comparison. C, immunoblots from (B) were quantified and subjected to nonlinear regression analysis. In all but HepG2 cells, all data points were better represented statistically using one curve than individual curves, with p values 0.15 to 0.86. Data represent the mean ± SD from three independent experiments.
Figure 6.
Time course of induction of known hypoxia-inducible proteins. Five cell lines; SH-SY5Y (A), RKO (B), HepG2 (C), Kelly (D), and EA.hy926 (E) were exposed to 1% O2 for the indicated periods of time. Representative immunoblots (top) and mRNA levels of the ADO substrates (RGS5, RGS4, and IL-32) are shown below. All data represent the mean ± SD from three independent experiments.
In previous studies, the destabilizing action of N-terminal cysteine dioxygenation in human cells was demonstrated by measuring the half-life of fusion proteins bearing the N-terminus of the plant ERF-VII transcription factor fused to a green fluorescent protein-V5 reporter protein, thereby targeting the reporter protein for N-degron dependent proteolysis in the presence of O2 (6). HIF-1α has been reported as having a remarkably short half-life in oxygenated cells (18). Given similarities between HIF-1α, RGS4, and 5 with respect to protein accumulation in hypoxic cells, we next wanted to compare the relative level of these proteins in cells using assays of endogenous protein levels following re-oxygenation of hypoxic cells (Fig. 2). Whilst levels of RGS4 and RGS5 proteins remained stable under continued hypoxic exposure, reoxygenation resulted in rapid degradation of RGS4 and 5 with a T1/2 (time for disappearance of half of the species) of approximately 4 min. These kinetics were very similar to those of HIF-1α (T1/2 5 min), assayed under the same conditions. Notably, these experiments were performed in the absence of the protein synthesis inhibitor, cycloheximide, as this was found to reduce RGS4 and 5 expressions beyond reliable detection at all time points. Therefore, the figures for T1/2 represent maximum values.
Figure 2.
Degradation of RGS4, 5, and HIF-1α in the presence of oxygen. SH-SY5Y cells were cultured under hypoxic conditions (1% O2) for 16 h then either maintained under hypoxia or reoxygenated by exposure to atmospheric levels of oxygen for 5, 15, or 45 min. Levels of RGS4, 5, and HIF-1α protein were assessed by immunoblotting, quantified, and subjected to nonlinear exponential decay regression to quantify the degradation. Data plotted are mean ± SD from three independent experiments.
The HIF Prolyl-hydroxylases and Nt-Cysteine dioxygenases coordinate their catalytic Fe2+ via His-Asp-His and His-His-His triads, respectively (19, 20, 21), and in previous studies, ADO appeared sensitive to a narrower range of Fe2+ chelating small molecules than the PHDs (6). To investigate this further, we tested a range of iron chelating compounds for their ability to inhibit ADO using RGS41–11GFP fusion protein that has been shown to report ADO-dependent proteolysis in cells (22). RKO cells stably expressing RGS41–11GFP were treated with six different iron chelators (2,2DIP −2,2′-dipyridyl, DFO – deferoxamine, Dp44mT - di-2-pyridyl ketone 4,4-dimethyl-3-thiosemicarbazone, ICL670A - desferasirox, L1 – deferiprone, SIH - salicylaldehyde isonicotinoyl hydrazone) at a range of concentrations, and accumulation of the reporter assayed by monitoring GFP fluorescence. Of the compounds tested only 2,2DIP, Dp44 mT and SIH manifest inhibition of ADO at concentrations that did not elicit cytotoxicity (Fig. 3A). Similar results were obtained when the accumulation of endogenous RGS4 or 5 was analyzed in RKO or SH-SY5Y cells, respectively (Fig. 3B). In contrast, all compounds were broadly capable of stabilizing HIF-1α (Fig. 3B). Analysis of intracellular free Fe2+ in RKO cells using a fluorescent indicator demonstrated that all compounds except L1 reduced intracellular Fe2+ levels within 4 h of treatment (Fig. 3C). Whilst this was consistent with the presence or absence of HIF-1α stabilization in this cell type (Fig. 3B), the efficacy of ADO inhibition (as inferred from RGS4 expression) did not correlate with intracellular free Fe2+ chelation, suggesting a more complex interface between chelators and the catalytic iron center. To investigate this further, we next analyzed the total Fe content of FLAG-tagged ADO and PHD2 proteins immunoprecipitated from SH-SY5Y cells treated with either 2,2DIP or DFO. In line with observations on RGS4/5 stability, 2,2DIP was potent at removing iron from both ADO and PHD2, whereas DFO had very little impact on ADO iron content despite effectively chelating all the iron from PHD2 (Fig. 3D). Interestingly, ADO consistently contained a higher relative iron content than PHD2 following immunoprecipitation, corroborating earlier observations noting a remarkably high iron occupancy of purified ADO relative to CDO1 (7). Finally, there was no further stabilization of RGS4 observed in ADO-deficient cells following treatment with 2,2DIP, Dp44mT, or SIH (Fig. 3E), consistent with action through ADO.
Figure 3.
Differential sensitivity of ADO and PHD enzymes to iron chelators.A, RKO cells stably expressing an RGS41–11GFP fusion reporter protein were treated with various iron chelator compounds at the concentrations indicated for 24 h. Fluorescence (expressed as arbitrary units and closed squares) was measured and then cellular viability (open squares) was assessed using an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. B, RKO and SH-SY5Y cells were treated with the same six iron chelating compounds for 4 h at a maximal non-toxic concentration (all 100 μM except Dp44mT–3 μM and ICL670A–30 μM) and levels of the endogenous substrates RGS4 or RGS5 assessed by immunoblotting. Expression of HIF-1α was assayed in parallel for comparison. C, intracellular Fe2+ levels were measured using FerroOrange in RKO cells treated with the indicated iron chelators for 4 h. D, measurement of total iron content in FLAG-ADO and FLAG-PHD2 immunoprecipitates from cells treated with DFO or 2,2DIP for 4 h, determined using ICP-MS. E, ADO-deficient RKO cells were treated with the Fe2+ chelators shown to stabilize RGS4 (2,2DIP, Dp44mT, and SIH) for 4 h and levels of RGS4 protein assayed. Data in (A), (C), and (D) represent the mean ± SD from three independent experiments; all immunoblots are representative of at least three independent experiments.
In addition to Fe2+, the PHDs and other 2-OG dependent dioxygenases are inhibited by transition metal ions such as Co2+ (23, 24, 25) and activation of HIF target genes such as erythropoietin is an important feature of the medical toxicology of such metals. The proposed explanation for the inhibition of PHD enzymes by Co2+ is the displacement of Fe2+ within the catalytic domain, thus rendering the enzyme inactive (25). In view of the differences in the efficacy of Fe2+ chelators observed earlier, we hypothesized that ADO might be differentially sensitive to Co2+, or other divalent cations, as compared to the PHD enzymes. Treatment of RGS41–11GFP reporter cells with increasing concentrations of either CoCl2, MnCl2 or NiCl2 resulted in dose-dependent cytotoxicity, with minimal accumulation of GFP (Fig. 4A). However, CoCl2 treatment did elicit striking induction of endogenous RGS4 in RKO cells and RGS5 in SH-SY5Y cells (Fig. 4B). Note, RGS4 protein level was not assessed in SH-SY5Y cells due to the contending influence of Co2+ on HIF-mediated transcription in this cell line. Surprisingly, another ADO target, IL-32, was unaffected and induction of RGS4/5 was also clearly evident in ADO-deficient cells. Induction of RGS4 was not accompanied by detectable changes in RGS4 mRNA levels, as might be expected if it reflected a transcriptional response to HIF (Fig. 4C).
Figure 4.
Regulation of RGS4 and 5 by Co2+.A, RKO cells stably expressing an RGS41–11GFP fusion reporter gene were treated with increasing concentrations of CoCl2, MnCl2 or NiCl2 for 24 h. Accumulation of the reporter gene was monitored by fluorescence, and viability was assessed in parallel. B, Co2+ but not Mn2+ or Ni2+ induced RGS4 or 5 in RKO and SH-SH5Y cells, respectively. 2,2DIP and HIF-1α immunoblots were used as positive controls. All chemicals were applied at 100 μM. C, ADO-competent or -deficient RKO cells were treated with CoCl2 for 4 or 24 h, and accumulation of RGS4 protein or mRNA transcript was assessed. D, RKO cells were treated for 24 h with Co2+ alone or in the presence of ascorbate (asc, 100 μM), N-acetyl-L-cysteine (NAC, 1 mM), PEGylated superoxide dismutase (SOD, 50 U/ml) or the vitamin E analogue Trolox (200 μM). E, SH-SY5Y cells stably expressing a C-terminal HA-tagged WT or C2A mutant RGS4 were treated with CoCl2 for 24 h as before. Endogenous RGS5 levels are shown for comparison. F, RGS4 precipitation from ADO-deficient SH-SY5Y cell extracts using His-beads pre-charged with CoCl2 and successive elutions of increasing strength to assess the affinity of binding. β-actin was used as a negative control. All data are representative of at least three independent experiments.
It was recently proposed that Co2+ can cause “over-oxidation” of Nt-Cysteine residues, targeting them for lysosomal degradation (26). As was reported (26), we also observed an inhibitory effect of N-acetyl cysteine (NAC) on Co2+ stimulated RGS4 and also on HIF-1α induction (Fig. 4D). However other compounds known to scavenge reactive oxygen species (ROS) such as ascorbate, the vitamin E analog Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), or PEGylated superoxide dismutase had no effect on RGS4 levels. In contrast ascorbate did substantially reduce the level of HIF-1α, consistent with its known function in maintaining the activity of the catalytic Fe2+ center of PHD enzymes (27). A persistent action of Co2+ in the absence of ADO, in combination with the lack of effect on the accumulation of the RGS41–11GFP reporter protein, suggested that Co2+ does not interfere with N-terminal cysteine dioxygenation. In line with this hypothesis, Co2+ induced protein levels of both RGS4:HA and a mutant that ablated the target cysteine residue RGS4(C2A):HA proteins in SH-SY5Y cells (Fig. 4E), with the latter being more abundant at baseline, as expected from a lack of action of ADO (6). It was previously reported that Co2+ could directly bind to HIFα polypeptides and inhibit degradation irrespective of any effect on the PHD enzymes or HIF prolyl hydroxylation (28, 29). Interestingly, we found significant Co2+-dependent enrichment of RGS4 in His-immunoprecipates from ADO-deficient SH-SY5Y lysates (Fig. 4F), strongly suggesting that RGS4 protein also binds Co2+. Taken together, this data indicates a hitherto undescribed effect of Co2+ on RGS4 and RGS5 that is apparently independent of ADO and the action of reactive oxygen species.
Another characteristic of the HIF/PHD system is the operation of a feedback loop by which HIF-dependent upregulation of the HIF prolyl hydroxylases PHD2 and PHD3 serves to limit HIF activity over a period of hours (30). We observed that neither ADO protein nor mRNA levels were affected by periods of up to 48 h of hypoxia in SH-SY5Y cells, directly contrasting with a robust upregulation of PHD3 under those conditions (Fig. 5, A and B). Moreover, we found no evidence that other known components of the Arg/Cys N-degron pathway were altered at the mRNA level by hypoxia (Fig. 5C). To address the effects on the time-dependent accumulation of ADO substrate proteins, cell lines were subjected to hypoxia (1% O2), over periods ranging from 2 to 48 h, and the levels of substrate mRNAs and proteins were assessed (Fig. 6). These experiments revealed rapid accumulation of RGS4 and 5 in all cell lines tested, which was well-sustained even after 48 h continuous exposure to hypoxia, in contrast with HIF-1α. Most of these responses to hypoxia occurred without a change in the corresponding mRNA, consistent with an action of ADO on protein stability. In some cases, a modest induction of the corresponding mRNA was also observed (Fig. 6, A, B, and D) although this was nearly always delayed relative to the accumulation of protein.
Figure 5.
Lack of apparent feedback regulation in the ADO pathway during hypoxic exposure.A, levels of ADO, arginyl transferase 1 (ATE1), and HIF prolyl-hydroxylase 3 (PHD3) protein in SH-SY5Y exposed to hypoxia for 4, 24, or 48 h. B, hypoxic upregulation of mRNA transcript encoding PHD3, but not ADO, in SH-SY5Y cells. C, no effect of hypoxia was observed on the transcript levels of other components of the Arg/Cys N-degron pathway (METAP1, methionine aminotransferase 1, UBR1/2, Ubiquitin Protein Ligase E3 Component N-Recognin 1/2). All data represent the mean ± SD from three independent experiments.
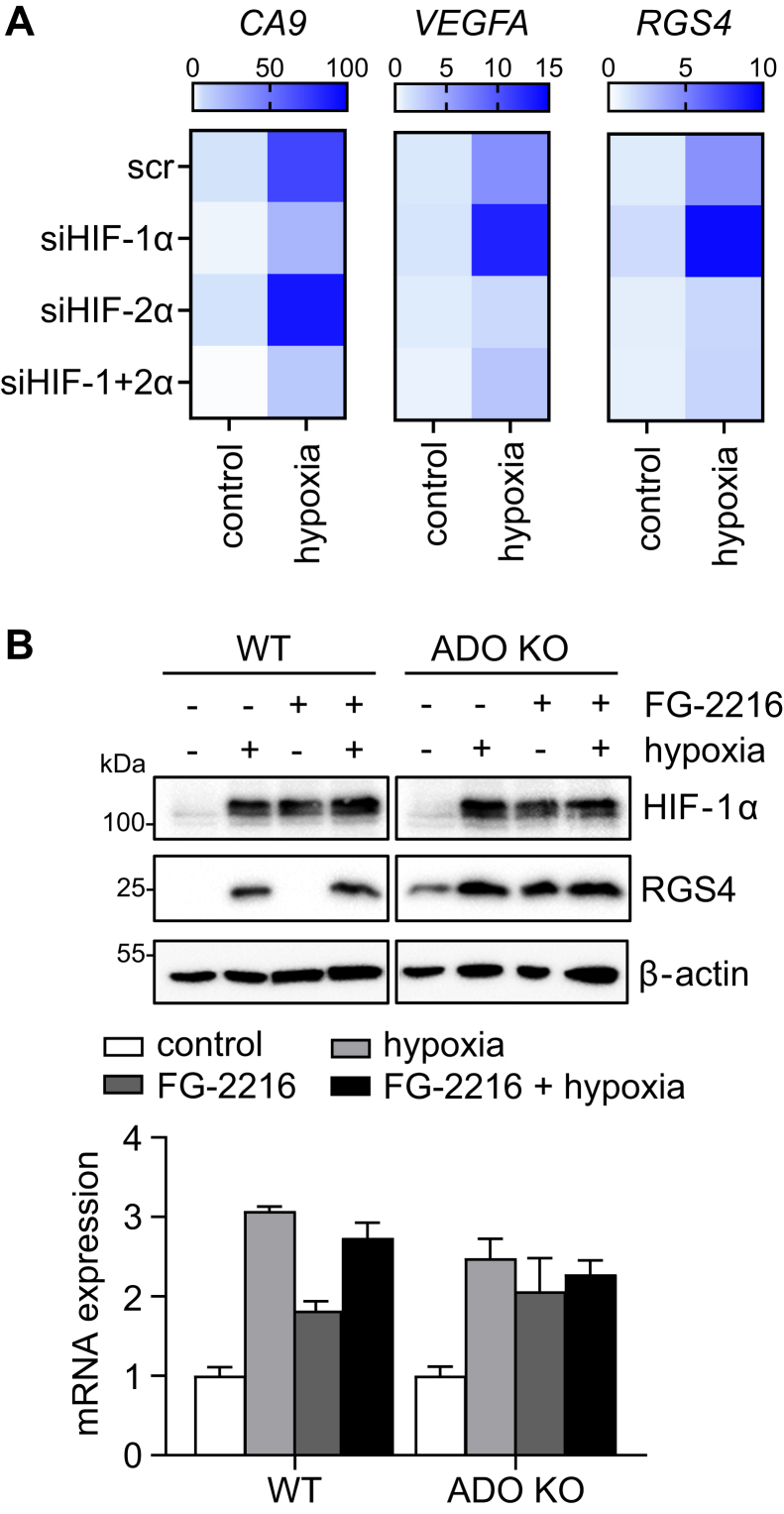
Since all the ADO targets analyzed here have been reported to respond directly or indirectly to the HIF/PHD system in specific settings (31, 32, 33), we went on to investigate interactions between these systems. We first examined the expression of RGS4 mRNA in SH-SY5Y cells which it is been reported to be a target of HIF as well as ADO (31). siRNA targeting either HIF-1α or -2α was used to confirm the HIF dependency of this response, with successful knock-down confirmed by attenuation of hypoxia-induced CA9 and VEGFA transcripts, which were selected to report specific HIF-α isoform activity (Fig. 7A). Notably, increased hypoxic induction was observed when cells were treated with siRNA targeting the alternative HIF-α isoform (HIF-2α for CA9 and HIF-1α for VEGFA) which was repressed when both isoforms were targeted. Importantly, hypoxic induction of RGS4 mRNA was reduced in the presence of siRNA targeting HIF-2α and HIF-1+2α, whilst increased when cells were treated with HIF-1α siRNA alone (Fig. 7A), confirming an action of HIF-2α on RGS4 transcripts in SH-SY5Y cells. We then proceeded to address the relative contributions of ADO and HIF pathways to the accumulation of RGS4 protein in hypoxia. To this end, ADO-competent or -deficient SH-SY5Y cells were exposed to hypoxia (1% O2) in the presence or absence of a HIF-prolyl hydroxylase inhibitor ([(1-chloro-4-hydroxy-isoquinoline-3-carbonyl)-amino]-acetic acid, FG-2216 (34), Fig. 7B), a molecule previously shown not to inhibit ADO (6). As expected, hypoxia resulted in the accumulation of RGS4 in ADO-competent cells. Interestingly however, FG-2216 had no effect on RGS4 protein in normoxic cells whereas in ADO-deficient cells, in which RGS4 was constitutively stable, transcriptional upregulation of mRNA by either hypoxia or FG-2216 resulted in a substantial additional increase in RGS4 protein levels. Thus, combined inhibition of ADO and PHD amplified the induction of RGS4 protein levels. Nevertheless, proteolytic control via ADO likely remains the dominant regulatory mechanism under many conditions, as witnessed by the absence of an effect of FG-2216 in ADO-competent normoxic cells.
Figure 7.
Coordinated regulation of RGS4 by HIF-2α and ADO under hypoxia.A, SH-SY5Y cells were transfected with siRNA targeting HIF-1α and/or HIF-2α, or scrambled control (scr), and subjected to 24 h of hypoxia (1% O2). RGS4 mRNA levels were assessed alongside canonical HIF-1α (CA9) or HIF-2α (VEGF) target genes as positive controls. The color scale represents mean fold change relative to cells treated with scr control (normoxia) from three independent experiments. B, ADO-competent or -deficient SH-SY5Y cells were exposed to hypoxia (1% O2) or treated with the PHD inhibitor FG-2216 (100 μM), or both, for 4 h and samples blotted for RGS4 and HIF-1α protein. RGS4 mRNA levels were assessed in parallel, confirming transcript upregulation. Immunoblots are representative of three separate experiments, and data in the histogram are the mean ± SD, n = 3. All treatments significantly induced RGS4 mRNA, 2-way ANOVA with Holm-Sidak post-hoc analysis, p < 0.001.
Conversely, when proteolytic degradation is prevented, the accumulation of a given protein will be largely determined by the rate of de novo transcription/translation. We, therefore, wished to explore other interactions between transcriptional regulation and ADO-dependent proteolysis. IL32 has been shown to be regulated at the transcript level by inflammatory mediators such as TNFα (35, 36). We, therefore, hypothesized that TNFα pre-treatment might influence the rate of IL-32 protein accumulation upon exposure to hypoxia, which was slower in HepG2 cells relative to other substrates in other cell lines (Fig. 6). To test this, HepG2 cells were pre-treated with TNFα for 16 h to elicit a robust induction of IL32 mRNA (Fig. 8A), and the effects of timed exposure to hypoxia (1% O2, 0.5–8 h) compared with and without this TNFα pre-treatment. These experiments revealed a marked interaction. Cells pre-treated with TNFα demonstrated a greatly enhanced response of IL-32 protein levels to hypoxia (Fig. 8B) consistent with TNFα amplifying the effects of O2-regulated proteolysis transduced by ADO. To confirm this, ADO was inactivated in HepG2 cells by CRISPR/Cas9 gene editing, and these cells were treated with TNFα for 16 h, before a brief exposure to hypoxia (1% O2, 2 h). As ADO has been reported to influence TNFα signaling (37), cells were also treated with IL-1β (38). As shown in Figure 8C, induction of IL-32 protein by TNFα and IL-1β was much higher in ADO-deficient than parental HepG2 cells (Fig. 8C, compare lanes 1–3 and 7–9). As before, induction of IL-32 by hypoxia was greatly exaggerated in cytokine-treated cells, and observed even after brief (2 h) exposure. However, in ADO-deficient cells the very high levels of IL-32 were not further increased by hypoxia, consistent with ablation of ADO-dependent proteolysis. Taken together these results illustrate strong interactions in the multi-level control of substrates of the ADO pathway, with transcriptional induction amplifying O2-dependent regulation of ADO substrates at the protein level.
Figure 8.
Interplay between transcriptional and proteolytic regulation of IL-32.A, induction of IL32 mRNA in HepG2 cells treated for 16 h with TNFα (20 ng/μl). B, Accumulation of IL-32 protein in HepG2 cells treated with TNFα prior to exposure to hypoxia for the times indicated. C, ADO-competent or -deficient HepG2 cells were treated with TNFα or IL-1β (20 ng/μl) for 16 h then exposed to hypoxia for a further 2 h, and levels of IL-32 protein were analyzed. HIF-1α protein levels were assayed for comparison. All data represent the mean ± SD from three independent experiments, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, Mann–Whitney t test (A) or 2-way ANOVA with Holm-Sidak post hoc analysis (B and C).
Discussion
Despite a common and fundamental requirement to maintain oxygen homeostasis, many different systems for sensing and responding to hypoxia have been defined in different species (39). Somewhat surprisingly, several of these different systems deploy a common strategy of enzymatic protein oxidation coupled with proteolysis as the core signaling process. Of particular interest are the plant and animal systems that deploy N-terminal cysteine dioxygenation and prolyl hydroxylation to regulate transcriptional responses mediated by ERF-VII transcription factors and HIF respectively. Remarkably, the plant system of N-terminal cysteine dioxygenation is also represented in humans and other animal species by the N-terminal cysteine dioxygenase, ADO, which therefore has the potential to work alongside PHD/HIF. Since oxygen homeostasis must be maintained across different tissues, operating at different levels of oxygen and over different time scales, we sought to compare and contrast the characteristics of these pathways in human cells.
Our findings indicate that the two systems are similarly sensitive to oxygen concentrations, but that their temporal responses and pharmacological interactions display different characteristics. Importantly, the known action of RGS4 and 5 on Gαi/q activity is likely immediate and therefore the physiological impact directly reflects the rapid accumulation of RGS proteins upon exposure to hypoxia (Fig. 6), and also the very rapid degradation when cells are re-oxygenated (Fig. 2). This contrasts with the HIF-PHD pathway in which, although the HIFα subunits are rapidly stabilized, accumulation of the target proteins is typically delayed and, once expressed, are not inherently O2-sensitive and therefore function on a time-scale dependent on their own half-lives. This distinction may be predicted to be important during rapid cycling of oxygen levels such as occurs in disorders of breathing control including obstructive sleep apnea, in ischemia reperfusion, and in the tumor microenvironment. Similar sensitivity to oxygen also suggested that the PHD/HIF and ADO systems might interact should they share common targets. Remarkably, a number of reports have described transcriptional responses to HIF among the limited range of ADO substrates defined to date. Our findings confirmed this and revealed that in specific settings the two systems do indeed interact to amplify responses to hypoxia. These interactions may be important both physiologically and therapeutically where, for instance, HIF prolyl hydroxylase inhibition may be more effective in hypoxic regions where ADO activity is also reduced. Moreover, the interaction between inflammation and hypoxia in the regulation of IL-32 further support the established bidirectional relationship between the two (40, 41, 42), manifesting in diseases such as inflammatory bowel disease.
Although both PHD and ADO enzymes share similar kinetic properties relating to O2, the reactions they catalyze are quite distinct. HIF prolyl-hydroxylation requires 2-oxoglutarate as co-substrate and hence is potentially affected by metabolic signals that modulate cellular levels of this metabolite. The N-terminal cysteine dioxygenase ADO incorporates both oxygen atoms into the cysteine residue and has no such co-substrate requirement or reaction by-products. Moreover, both classes of enzymes coordinate their catalytic iron center using distinct motifs (His-Asp-His for PHDs, 3xHis for ADO). Although our studies revealed that ADO, like the PHDs, is sensitive to inhibition by iron chelating agents, these effects differed substantially between the two types of enzyme. Notably, while inhibition of the PHDs by chelating agents was broadly concordant with their action to reduce intracellular iron, this was not the case with inhibition of ADO, for which only selected chelators were effective and inhibitory activity did not correlate with simple depletion of intracellular iron. This implies that the catalytic Fe2+ in ADO is not freely exchanging with cytosolic Fe2+ and that the inhibitory chelators have a more specific interaction with the catalytic iron center of ADO. The Fe2+ in ADO may also be better protected from oxidation to Fe3+, inactivating oxidation. This is supported by our observations that neither Co2+ nor ascorbate, thought to promote (43) and prevent (27) this reaction respectively, influenced ADO activity. Moreover, those chelators demonstrated to be ineffective against ADO (DFO, ICL670A, and L1) are reported to preferentially bind Fe3+ (44, 45). However, it must be stated that the iron chelating compounds used vary considerably in their physiochemical properties (size, cell permeability, binding capacity, and affinity) and may also contribute to differential action on ADO versus PHDs.
Interestingly, although Co2+ ions appeared incapable of inhibiting ADO activity with only minimal induction of an ADO-dependent reporter gene observed, a striking upregulation of RGS4 and RGS5 proteins was observed in response to Co2+ treatment, as has recently been reported by others (26). It was proposed that Co2+-induced tri-oxidation of the N-terminal cysteine of RGS4/5 leads to lysosomal degradation rather than via the canonical 26S proteasome, with the former presumably being much less efficient to account for the observed large increase in accumulated RGS4/5 in cells exposed to Co2+. If true, a reduction in the steady-state RGS4/5 protein level in ADO-deficient cells treated with Co2+ would be expected, as a portion of the constitutively stabilized RGS4/5 would now be targeted for autophagy and a new equilibrium achieved. We have presented several lines of evidence in contradiction to this hypothesis (Fig. 4). The near absence of action of Co2+ on the RGS41–11GFP reporter protein and the persistence of an increase in endogenous RGS4/5 in ADO-deficient cells and a C2A mutant of RGS4 all suggest that the action of Co2+ on RGS4 and RGS5 are not, in the main, mediated by an effect on the oxidation status of N-terminal cysteine. We suggest that the action of N-acetyl cysteine to inhibit this proposed oxidation likely results from direct chelation of Co2+ ions in solution (46, 47). Indeed, NAC is clinically approved for the treatment of acute Co2+ poisoning (48, 49). Further work is needed to define the precise mechanism through which Co2+ acts to increase RGS4/5 stabilization, and its relevance to the toxicology of cobalt.
In summary, our data provide evidence that ADO acts as a physiological oxygen sensor operating in cells alongside, and interacting with, HIF. The ADO system responds at oxygen concentrations similar to those that induce HIF, but its direct action to promote degradation of RGS proteins over a time scale of minutes enables the transduction of responses to altered oxygen levels on a shorter time scale. In cellulo characterization of the ADO pathway revealed marked interactions with the transcriptional induction of specific targets and distinct divalent metal pharmacology that offers new insight into the biochemistry of cellular dioxygenases.
Experimental procedures
Cell culture
The human cell lines SH-SY5Y, RKO and EA.hy926 were cultured as described previously (6). HepG2 was kindly gifted by Jane McKeating (University of Oxford, UK), and Kelly cells were purchased from the European Collection of Authenticated Cell Cultures (ECACC 92110411). RKO, EA.hy926, and HepG2 cells were cultured in DMEM, and SH-SY5Y and Kelly cells in DMEM/F12, all supplemented with 10% fetal bovine serum, 2 mM L-Glutamine and 100 U/ml penicillin/10 μg/ml streptomycin. All cell lines were maintained at 37 °C incubator containing 5% CO2, and hypoxic exposure was performed using an atmosphere-regulated workstation set to 0.1 to 7.5% O2: 5% CO2: balance N2 (Invivo 400, Baker-Ruskinn Technologies).
Immunoblotting
Protein samples were collected in lysis buffer (10 mM Tris pH 7.5, 0.25 M NaCl, 0.5% Igepal) supplemented with complete protease inhibitor cocktail (Sigma Aldrich) and centrifuged at 13,000 rpm for 3 min at 4 °C. The supernatant was mixed with Laemmli sample buffer and proteins were separated via SDS-PAGE electrophoresis. Membranes were blocked in 4% milk for 1 h, then incubated in primary antibody overnight: HIF-1α (610959, BD Biosciences), RGS5 (sc-514184, SCBT), CA9 (5648, CST), RGS4 (15129, CST), IL-32 (sc-517408, SCBT), ADO (ab134102, Abcam), ATE1 (HPA038444, Human Protein Atlas), PHD3 (188e (50)) GFP (11814460001, Sigma Aldrich), and HA (3F10, Roche). HRP- conjugated secondary antibodies were sourced from DAKO and used in conjunction with chemiluminescence substrate (West Dura, 34076; Thermo Fisher Scientific) to visualize protein expression using a ChemiDoc XRS+ imaging system (BioRad). β-actin primary antibody was conjugated directly to HRP (ab49900, Abcam). Densitometric analysis was performed using ImageJ software (NIH) and values were presented relative to β-actin.
RT-qPCR
mRNA was extracted from Tri-Reagent lysates by phase separation and equal amounts were used for cDNA synthesis using the High-Capacity cDNA Kit (Applied Biosystems). qPCR analysis was performed using Fast SYBR Green Master Mix on a StepOne thermocycler (Thermo Fisher Scientific) using the ΔΔCt method. Levels of the housekeeping gene Hypoxanthine-guanine phosphoribosyl transferase (HPRT) were used as a reference. Sequences for the primers used are as follows;
RGS4 (F_GCAAAGGGCTTGCAGGTCT, R_CAGCAGGAAACCTAGCCGAT),
RGS5 (F_TGGTGACCTTGTCATTCCG, R_TTGTTCTGCAGGAGTTTGT),
IL32 (F_CTTCCCGAAGGTCCTCTCTGAT, R_GTCCTCAGTGTCACACGCT),
HPRT (F_GACCAGTCAACAGGGGACAT, R_AACACTTCGTGGGGTCCTTTTC),
ADO (F_GCCGGGACTGCCACTATTAC, R_ACCAGAAGTCATCGGCCTGT)’,
ATE1 (F_CTGATTTGCTGTGCCCTGAG, R_GGTTCCGTACTGCGATCCTC,
EGLN1 (F_GCAGCATGGACGACCTGATA, R_CCATTGCCCGGATAACAAGC),
EGLN3 (F_CACGAAGTGCAGCCCTCTTA), R_TTGGCTTCTGCCCTTTCTTCA),
METAP1 (F_CATCAAGCTGGGCATCCAGG, R_GCTTCGCCTTTTCATCTTTTGC),
UBR1 (F_TATGGAGGAAGAGAGCACCCC, R_GATGGACCCCGTTTAGGACC)
UBR2 (F_ACCAGCAGTTGCAGAGAGAT, R_GTGATGAGCATTCGAGCCAGA),
CA9 (F_CTTGGAAGAAATCGCTGAGG, R_ TGGAAGTAGCGGCTGAAGTC)
VEGFA (F_TGTCTAATGCCCTGGAGCCT, R_GCTTGTCACATCTGCAAGTACG)
siRNA-mediated gene knockdown
SH-SY5Y cells were seeded into 12-well plates and transfected twice on subsequent days with 40 nM of either scrambled siRNA (4390843) or siRNA sequences targeting HIF-1α (s6539), HIF-2α (s4700, Ambion) or a combination of both, diluted in OptiMEM and Lipofectamine RNAiMAX (Thermo Fisher Scientific).
Monitoring ADO activity using RGS41–11GFP
The design of the ADO reporter construct was based on a derivative previously published (RGS41–20GFP) (6, 22). The first 11 amino acids of human RGS4 (Uniprot: P49798-1) were fused to the N-terminus of eGFP. This construct was then inserted into the pcDNA3+ vector and 2 μg of plasmid DNA transfected into a 6 cm plate of subconfluent RKO cells using GeneJuice transfection reagent. Transfected cells were selected for using 1.4 mg/ml G418 for 2 weeks, producing a polyclonal pool of reporter cells. Cells were seeded at 10,000 cells/well in black 96-well plates and allowed to grow to confluence, before being treated with increasing concentrations of Fe2+ chelator (2,2DIP −2,2′-dipyridyl, DFO – deferoxamine, Dp44 mT - di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone, ICL670A - desferasirox, L1 – deferiprone, SIH - salicylaldehyde isonicotinoyl hydrazone) or divalent metal ion for 24 h, after which time fluorescence was read at 480nmex/520nmem using a plate reader (FLUOstar, BMG Labtech). Cells were then incubated with MTT (0.5 mg.ml−1) for 4 h to monitor viability (51).
Measurement of intracellular and ADO/PHD2-bound Fe2+
Intracellular labile Fe2+ levels were measured using FerroOrange, a fluorescent probe specific to Fe2+ (52). RKO cells were seeded in black 96-well plates and treated with Fe2+ chelators for 4 h in standard medium, which was subsequently replaced with imaging buffer (in mM; 117 NaCl, 4.5 KCl, 25 NaHCO3, 11 Glucose, 1 MgCl2, 1 NaH2PO4, 1 CaCl2) containing 1 μM FerroOrange. Cells were incubated in a probe containing medium for 30 min at 37 °C then fluorescence was measured at 544nmex/590nmem using a FLUOstar Omega plate reader (BMG Labtech, UK). Fluorescence from a well containing medium alone (no FerroOrange) was used as a blank. To measure the total iron content of ADO and PHD2, N-terminally FLAG-tagged enzymes were immunoprecipitated from cells treated with either 2,2-dipyridyl or DFO (both 100 μM), and total Fe content was analyzed by Inductively coupled plasma mass spectrometry (ICP-MS). SH-SY5Y cells stably expressing an N-terminally FLAG-tagged HsADO or HsPHD2 were cultured to confluence in 15 cm plates, then treated for 4 h with 100 μM of either DFO or 2,2DIP and lysed in lysis buffer (see above). Enzymes were immunoprecipitated using anti-FLAG(M2) conjugated agarose beads overnight at 4 °C, washed in PBS five times then eluted in 2% nitric acid. Beads incubated with lysis buffer (without cellular extract) were subjected to the same procedure as background readings for correction. Elutions were further diluted (2×) in 2% nitric acid and analyzed on a PerkinElmer NexION 2000B ICP-MS, calibrated using external calibration analysis with QMX standard dilutions, and spiked with 1 ng/g Rhodium to normalize any instrument drift. Values in immunoprecipitates were corrected for non-specific backgrounds by subtracting the iron detected in equivalent samples from cells that did not express any FLAG-tagged construct.
Cobalt-pull down
To measure the Co2+ binding of RGS4/5, ADO-deficient SH-SY5Y cell lysates were incubated with HisBind resin (Millipore 69,670) pre-charged with Co2+ ions, allowing the precipitation of Co2+-binding proteins as described previously (29). 25 μl (packed volume) of HisBind resin was washed in PBS and then incubated with 50 mM CoCl2 at room temperature for 1 h, then washed five times with lysis buffer. Cell lysate (15 cm dish per condition) was then incubated with Co2+ (or control) loaded resin for 1 h under constant rotation at 4 °C. Following this, the resin was pelleted (2000 rpm, 2 min) and a sample was taken and mixed with sample buffer to represent an unbound fraction. The resin was then further washed and subjected to the following elutions; 60 μl 60 mM imidazole for 10 min, 60 μl 1 M imidazole, then finally 70 μl SDS sample buffer. After each elution, the supernatant was taken and heated at 95 °C for 5 min, then subjected to SDS-PAGE and immunoblotted as described above.
Data availability
All data are contained within the manuscript.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Author contributions
Y.-M. T., P. H., T. Q. T., and T. P. K. investigation; Y.-M. T. methodology; P. J. R. and T. P. K. conceptualization; P. J. R. and T. P. K. writing–review & editing; P. J. R. and T. P. K. supervision; P. J. R. funding acquisition; T. P. K. formal analysis; T. P. K. writing–original draft; T. P. K. visualization.
Funding and additional information
This work was funded by the Wellcome Trust (grant no. 106241/Z/14/Z) and the Ludwig Institute for Cancer Research (P. J. R., Distinguished Scholar). This work was also supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001501), the UK Medical Research Council (FC001501), and the Wellcome Trust (FC001501).
Reviewed by members of the JBC Editorial Board. Edited by Donita C. Brady
Contributor Information
Peter J. Ratcliffe, Email: peter.ratcliffe@ndm.ox.ac.uk.
Thomas P. Keeley, Email: thomas.keeley@ndm.ox.ac.uk.
References
- 1.Pugh C.W., Ratcliffe P.J. New horizons in hypoxia signaling pathways. Exp. Cell Res. 2017;356:116–121. doi: 10.1016/j.yexcr.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myllyharju J. Prolyl 4-hydroxylases, master regulators of the hypoxia response. Acta Physiol. (Oxf.) 2013;208:148–165. doi: 10.1111/apha.12096. [DOI] [PubMed] [Google Scholar]
- 3.Gibbs D.J., Lee S.C., Isa N.M., Gramuglia S., Fukao T., Bassel G.W., et al. Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature. 2011;479:415–418. doi: 10.1038/nature10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Licausi F., Kosmacz M., Weits D.A., Giuntoli B., Giorgi F.M., Voesenek L.A., et al. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature. 2011;479:419–422. doi: 10.1038/nature10536. [DOI] [PubMed] [Google Scholar]
- 5.Weits D.A., Giuntoli B., Kosmacz M., Parlanti S., Hubberten H.M., Riegler H., et al. Plant cysteine oxidases control the oxygen-dependent branch of the N-end-rule pathway. Nat. Commun. 2014;5:3425. doi: 10.1038/ncomms4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masson N., Keeley T., Giuntoli B., White M.D., Puerta M., Perata P., et al. Conserved N-terminal cysteine dioxygenases transduce responses to hypoxia in animals and plants. Science. 2019;365:65–69. doi: 10.1126/science.aaw0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominy J.E., Jr., Simmons C.R., Hirschberger L.L., Hwang J., Coloso R.M., Stipanuk M.H. Discovery and characterization of a second mammalian thiol dioxygenase, cysteamine dioxygenase. J. Biol. Chem. 2007;282:25189–25198. doi: 10.1074/jbc.M703089200. [DOI] [PubMed] [Google Scholar]
- 8.Varshavsky A. N-degron and C-degron pathways of protein degradation. Proc. Natl. Acad. Sci. U. S. A. 2019;116:358–366. doi: 10.1073/pnas.1816596116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bansal G., Druey K.M., Xie Z. R4 RGS proteins: regulation of G-protein signaling and beyond. Pharmacol. Ther. 2007;116:473–495. doi: 10.1016/j.pharmthera.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dali-Youcef N., Vix M., Costantino F., El-Saghire H., Lhermitte B., Callari C., et al. Interleukin-32 contributes to human nonalcoholic fatty liver disease and insulin resistance. Hepatol. Commun. 2019;3:1205–1220. doi: 10.1002/hep4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baselli G.A., Dongiovanni P., Rametta R., Meroni M., Pelusi S., Maggioni M., et al. Liver transcriptomics highlights interleukin-32 as novel NAFLD-related cytokine and candidate biomarker. Gut. 2020;69:1855–1866. doi: 10.1136/gutjnl-2019-319226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moschen A.R., Fritz T., Clouston A.D., Rebhan I., Bauhofer O., Barrie H.D., et al. Interleukin-32: a new proinflammatory cytokine involved in hepatitis C virus-related liver inflammation and fibrosis. Hepatology. 2011;53:1819–1829. doi: 10.1002/hep.24285. [DOI] [PubMed] [Google Scholar]
- 13.Pan X., Cao H., Lu J., Shu X., Xiong X., Hong X., et al. Interleukin-32 expression induced by hepatitis B virus protein X is mediated through activation of NF-kappaB. Mol. Immunol. 2011;48:1573–1577. doi: 10.1016/j.molimm.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 14.White M.D., Kamps J., East S., Taylor Kearney L.J., Flashman E. The plant cysteine oxidases from Arabidopsis thaliana are kinetically tailored to act as oxygen sensors. J. Biol. Chem. 2018;293:11786–11795. doi: 10.1074/jbc.RA118.003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsila M., Koivunen P., Gunzler V., Kivirikko K.I., Myllyharju J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J. Biol. Chem. 2003;278:30772–30780. doi: 10.1074/jbc.M304982200. [DOI] [PubMed] [Google Scholar]
- 16.Tarhonskaya H., Hardy A.P., Howe E.A., Loik N.D., Kramer H.B., McCullagh J.S., et al. Kinetic investigations of the role of factor inhibiting hypoxia-inducible factor (FIH) as an oxygen sensor. J. Biol. Chem. 2015;290:19726–19742. doi: 10.1074/jbc.M115.653014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keeley T.P., Mann G.E. Defining physiological normoxia for improved translation of cell physiology to animal models and humans. Physiol. Rev. 2019;99:161–234. doi: 10.1152/physrev.00041.2017. [DOI] [PubMed] [Google Scholar]
- 18.Huang L.E., Gu J., Schau M., Bunn H.F. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. U. S. A. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonough M.A., Li V., Flashman E., Chowdhury R., Mohr C., Lienard B.M., et al. Cellular oxygen sensing: crystal structure of hypoxia-inducible factor prolyl hydroxylase (PHD2) Proc. Natl. Acad. Sci. U. S. A. 2006;103:9814–9819. doi: 10.1073/pnas.0601283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y.A., Shin I., Li J., Liu A. Crystal structure of human cysteamine dioxygenase provides a structural rationale for its function as an oxygen sensor. J. Biol. Chem. 2021;297 doi: 10.1016/j.jbc.2021.101176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White M.D., Dalle Carbonare L., Lavilla Puerta M., Iacopino S., Edwards M., Dunne K., et al. Structures of Arabidopsis thaliana oxygen-sensing plant cysteine oxidases 4 and 5 enable targeted manipulation of their activity. Proc. Natl. Acad. Sci. U. S. A. 2020;117:23140–23147. doi: 10.1073/pnas.2000206117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith E., Keeley T.P. Monitoring ADO dependent proteolysis in cells using fluorescent reporter proteins. Methods Enzymol. 2023;686:267–295. doi: 10.1016/bs.mie.2023.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Epstein A.C.R., Gleadle J.M., McNeill L.A., Hewitson K.S., O'Rourke J., Mole D.R., et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 24.Bruick R.K., McKnight S.L. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 25.Hirsila M., Koivunen P., Xu L., Seeley T., Kivirikko K.I., Myllyharju J. Effect of desferrioxamine and metals on the hydroxylases in the oxygen sensing pathway. FASEB J. 2005;19:1308–1310. doi: 10.1096/fj.04-3399fje. [DOI] [PubMed] [Google Scholar]
- 26.Heo A.J., Kim S.B., Ji C.H., Han D., Lee S.J., Lee S.H., et al. The N-terminal cysteine is a dual sensor of oxygen and oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2107993118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knowles H.J., Raval R.R., Harris A.L., Ratcliffe P.J. Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res. 2003;63:1764–1768. [PubMed] [Google Scholar]
- 28.Kanaya K., Kamitani T. pVHL-independent ubiquitination of HIF1alpha and its stabilization by cobalt ion. Biochem. Biophys. Res. Commun. 2003;306:750–755. doi: 10.1016/s0006-291x(03)01041-6. [DOI] [PubMed] [Google Scholar]
- 29.Yuan Y., Hilliard G., Ferguson T., Millhorn D.E. Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-alpha. J. Biol. Chem. 2003;278:15911–15916. doi: 10.1074/jbc.M300463200. [DOI] [PubMed] [Google Scholar]
- 30.Berra E., Benizri E., Ginouvès A., Volmat V., Roux D., Pouysségur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003;22:4082–4090. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olechnowicz S.W., Fedele A.O., Peet D.J. Hypoxic induction of the regulator of G-protein signalling 4 gene is mediated by the hypoxia-inducible factor pathway. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zahoor M., Westhrin M., Aass K.R., Moen S.H., Misund K., Psonka-Antonczyk K.M., et al. Hypoxia promotes IL-32 expression in myeloma cells, and high expression is associated with poor survival and bone loss. Blood Adv. 2017;1:2656–2666. doi: 10.1182/bloodadvances.2017010801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin Y., An X., Ye Z., Cully B., Wu J., Li J. RGS5, a hypoxia-inducible apoptotic stimulator in endothelial cells. J. Biol. Chem. 2009;284:23436–23443. doi: 10.1074/jbc.M109.032664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsieh M.M., Linde N.S., Wynter A., Metzger M., Wong C., Langsetmo I., et al. HIF prolyl hydroxylase inhibition results in endogenous erythropoietin induction, erythrocytosis, and modest fetal hemoglobin expression in rhesus macaques. Blood. 2007;110:2140–2147. doi: 10.1182/blood-2007-02-073254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shioya M., Nishida A., Yagi Y., Ogawa A., Tsujikawa T., Kim-Mitsuyama S., et al. Epithelial overexpression of interleukin-32alpha in inflammatory bowel disease. Clin. Exp. Immunol. 2007;149:480–486. doi: 10.1111/j.1365-2249.2007.03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcondes A.M., Mhyre A.J., Stirewalt D.L., Kim S.H., Dinarello C.A., Deeg H.J. Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelomonocytic leukemia modulates apoptosis and impairs NK function. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2865–2870. doi: 10.1073/pnas.0712391105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kearney C.J., Vervoort S.J., Hogg S.J., Ramsbottom K.M., Freeman A.J., Lalaoui N., et al. Tumor immune evasion arises through loss of TNF sensitivity. Sci. Immunol. 2018;3 doi: 10.1126/sciimmunol.aar3451. [DOI] [PubMed] [Google Scholar]
- 38.Nold-Petry C.A., Nold M.F., Zepp J.A., Kim S.H., Voelkel N.F., Dinarello C.A. IL-32-dependent effects of IL-1beta on endothelial cell functions. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3883–3888. doi: 10.1073/pnas.0813334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammarlund E.U., Flashman E., Mohlin S., Licausi F. Oxygen-sensing mechanisms across eukaryotic kingdoms and their roles in complex multicellularity. Science. 2020;370 doi: 10.1126/science.aba3512. [DOI] [PubMed] [Google Scholar]
- 40.Campbell E.L., Bruyninckx W.J., Kelly C.J., Glover L.E., McNamee E.N., Bowers B.E., et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40:66–77. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartmann G., Tschop M., Fischer R., Bidlingmaier C., Riepl R., Tschop K., et al. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine. 2000;12:246–252. doi: 10.1006/cyto.1999.0533. [DOI] [PubMed] [Google Scholar]
- 42.Hackett P.H., Roach R.C. High-altitude illness. N. Engl. J. Med. 2001;345:107–114. doi: 10.1056/NEJM200107123450206. [DOI] [PubMed] [Google Scholar]
- 43.Salnikow K., Donald S.P., Bruick R.K., Zhitkovich A., Phang J.M., Kasprzak K.S. Depletion of intracellular ascorbate by the carcinogenic metals nickel and cobalt results in the induction of hypoxic stress. J. Biol. Chem. 2004;279:40337–40344. doi: 10.1074/jbc.M403057200. [DOI] [PubMed] [Google Scholar]
- 44.Yuan J., Lovejoy D.B., Richardson D.R. Novel di-2-pyridyl-derived iron chelators with marked and selective antitumor activity: in vitro and in vivo assessment. Blood. 2004;104:1450–1458. doi: 10.1182/blood-2004-03-0868. [DOI] [PubMed] [Google Scholar]
- 45.Kalinowski D.S., Richardson D.R. The evolution of iron chelators for the treatment of iron overload disease and cancer. Pharmacol. Rev. 2005;57:547–583. doi: 10.1124/pr.57.4.2. [DOI] [PubMed] [Google Scholar]
- 46.Luczak M.W., Zhitkovich A. Role of direct reactivity with metals in chemoprotection by N-acetylcysteine against chromium(VI), cadmium(II), and cobalt(II) Free Radic. Biol. Med. 2013;65:262–269. doi: 10.1016/j.freeradbiomed.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mlejnek P. Direct interaction between N-acetylcysteine and cytotoxic electrophile-an overlooked in vitro mechanism of protection. Antioxidants (Basel) 2022;11:1485. doi: 10.3390/antiox11081485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giampreti A., Lonati D., Ragghianti B., Ronchi A., Petrolini V.M., Vecchio S., et al. N-Acetyl-Cysteine as effective and safe chelating agent in metal-on-metal hip-implanted patients: two cases. Case Rep. Orthop. 2016;2016 doi: 10.1155/2016/8682737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D'Ambrosi R., Ursino N. N-Acetyl-Cysteine reduces blood chromium and cobalt levels in metal-on-metal hip arthroplasty. Arthroplast. Today. 2020;6:149–152. doi: 10.1016/j.artd.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Appelhoff R.J., Tian Y.M., Raval R.R., Turley H., Harris A.L., Pugh C.W., et al. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J. Biol. Chem. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- 51.Keeley T.P., Siow R.C.M., Jacob R., Mann G.E. Reduced SERCA activity underlies dysregulation of Ca(2+) homeostasis under atmospheric O2 levels. FASEB J. 2018;32:2531–2538. doi: 10.1096/fj.201700685RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirayama T., Niwa M., Hirosawa S., Nagasawa H. High-throughput screening for the discovery of iron homeostasis modulators using an extremely sensitive fluorescent probe. ACS Sens. 2020;5:2950–2958. doi: 10.1021/acssensors.0c01445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are contained within the manuscript.