Abstract
Metallic cellular solids, made of biocompatible alloys like titanium, stainless steel, or cobalt-chromium, have gained attention for their mechanical strength, reliability, and biocompatibility. These three-dimensional structures provide support and aid tissue regeneration in orthopedic implants, cardiovascular stents, and other tissue engineering cellular solids. The design and material chemistry of metallic cellular solids play crucial roles in their performance: factors such as porosity, pore size, and surface roughness influence nutrient transport, cell attachment, and mechanical stability, while their microstructure imparts strength, durability and flexibility. Various techniques, including additive manufacturing and conventional fabrication methods, are utilized for producing metallic biomedical cellular solids, each offering distinct advantages and drawbacks that must be considered for optimal design and manufacturing. The combination of mechanical properties and biocompatibility makes metallic cellular solids superior to their ceramic and polymeric counterparts in most load bearing applications, in particular under cyclic fatigue conditions, and more in general in application that require long term reliability. Although challenges remain, such as reducing the production times and the associated costs or increasing the array of available materials, metallic cellular solids showed excellent long-term reliability, with high survival rates even in long term follow-ups.
Keywords: Biomedical cellular solids, Metallic materials, Tissue engineering, Regenerative medicine, Fatigue resistance, Biocompatibility
Graphical abstract
1. Introduction
A biomedical cellular solid is an artificial three-dimensional frame structure that serves as a mimic of extracellular matrix for cell adhesion, migration, proliferation, and tissue regeneration in three dimensions. In particular, metallic biomedical cellular solids are three-dimensional structures that are designed to provide mechanical support and facilitate tissue regeneration in the body [1]. These cellular solids are used in a variety of biomedical applications, including orthopedic implants, cardiovascular stents, and tissue engineering [2].
Most metallic cellular solids are used in orthopedic surgery to improve the fixation of articular implants [3], to provide mechanical support [4], to stimulate bone growth and to repair damaged bone [5]. They are designed to mimic the structure and mechanical properties of bone, while also providing a support structure for new bone growth. This can help improve implant integration in the surrounding tissues, reduce the risk of implant failure, and promote faster healing [5].
Despite being often used as synonyms [[6], [7], [8], [9], [10]]; “scaffolds” are more specifically a sub-category of “cellular solids” utilized for the regeneration or restoration of impaired or absent tissues within the human body. These cellular solids serve as frameworks to facilitate cell attachment, proliferation, and tissue formation and are typically designed to be temporary structures.
There are several different metallic alloys that can be used in orthopedic surgery, including titanium [11,12], stainless steel [13,14], cobalt-chromium [15,16], tantalum [17,18], zinc [19,20], iron [21,22] and magnesium [23,24] alloys. Each of these materials has different properties, advantages and limitations, which can make them suitable for specific applications that will be discussed more in detail in a following paragraph.
The main factors affecting the performance of metallic cellular solids include morphological characteristics, such as porosity and pore size, chemo-physical structure and surface properties. Cellular solids can allow for better nutrient and oxygen transport, while also providing a larger surface area for cell attachment. However, cellular solids with larger pore sizes and overall higher porosity may be weaker and less mechanically stable, which can limit their use in load-bearing applications. To control the structure and chemical composition of cellular solids can be a complex task, as most metallic cellular solids cannot be obtained by conventional machining techniques. For these reasons, for each class of metals there is only a limited number of compatible alloys that can be used for production and few post-treatments that can be applied to improve their microstructure afterwards [25].
In addition to these design and material chemistry-related considerations, researchers are also exploring new techniques for improving bone integration with metallic cellular solids. For example, some studies have investigated the use of stem cells or growth factors to promote bone regeneration [26], while many others have explored the possibility of applying biocompatible coatings [27,28].
There are several advantages to using cellular solids in biomedical applications, in particular in the field of orthopedic surgery:
-
-
Tissue regeneration [29]: cellular solids can promote tissue regeneration by providing a 3D structure for cells to attach to and grow on. This can be especially useful in cases where the body's natural healing processes are insufficient, such as in large bone defects;
-
-
Customization [30]: cellular solids can be customized to fit the specific needs of a patient, allowing for a personalized approach to treatment. This can improve the overall effectiveness of the implant and reduce the risk of complications;
-
-
Improved mechanical properties [5]: counter-intuitively, despite reducing the effective cross-sectional area the porous structure of metallic cellular solids can favorably change some of their mechanical properties, in particular reduce the elastic modulus, making it closer to that of the surrounding bone tissue thus preventing stress-shielding effects;
-
-
Potential for drug delivery [31]: Metallic cellular solids can be designed to incorporate drug delivery mechanisms, allowing for the controlled release of drugs to the site of the implant. This can help to prevent infection, promote tissue regeneration, and improve the overall success of the implant.
Overall, metallic cellular solids are a promising area of research for improving bone integration in orthopedic surgery, where both biocompatibility and fatigue resistance are requisites, or more in general in application that require components to be reliable and stable over time.
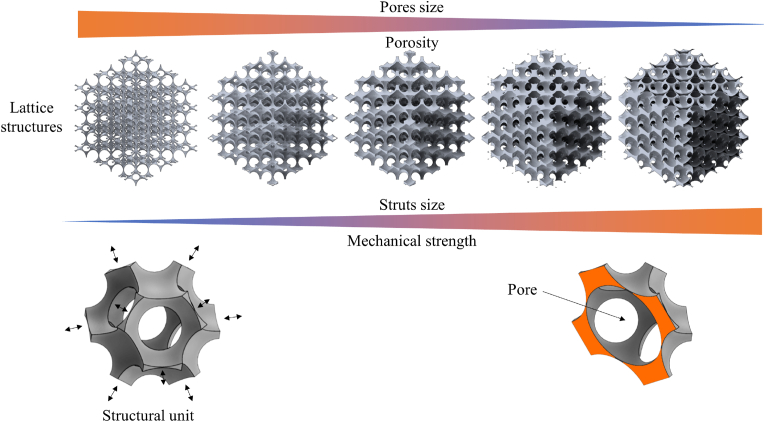
An example of ordered cellular solids based on rhombic dodecahedron structural units with pores at the vertices and in the center of the solids is presented in Fig. 1. The structures have decreasing pore diameters going from left to right, but the size of the structural unit is kept constant.
Fig. 1.
Lattice cellular solids based on a rhombic dodecahedron with porosities at the vertex and in the center of the structural units All structures have the same structural unit size and increasing pore diameters going from left to right. The rhombic dodecahedron is the dual polyhedron of the cuboctahedron.
2. Definitions
For the purposes of this review, the following definitions apply:
-
-
Cellular solids: Cellular solids are a class of materials characterized by a three-dimensional structure composed of interconnected cells or pores within a solid matrix. These cells can have various shapes and sizes, and their arrangement can be ordered or stochastic. Cellular solids exhibit a combination of properties, such as low density, high specific surface area, and unique mechanical behaviors, making them suitable for diverse engineering applications, including aerospace, automotive, biomedical, and energy-related fields. The porosity and cellular architecture of these materials can be engineered to tailor their mechanical, thermal, and acoustic properties for specific functional requirements;
-
-
Foam: the term “foam” is usually associated with stochastic porous structures where the pore (and usually also struts) sizes vary locally. Most foams are anisotropic, but this is not a prerequisite by definition. For the sake of this review, the term will refer only to bend-dominated stochastic structures, as described in section 7;
-
-
Lattice: in general, the term "lattice" refers to a repeating and interconnected framework of struts or beams that form the structure of the material. The lattice provides a pattern of interconnected pores, resulting in a porous material with specific mechanical properties determined by the arrangement of the struts. For the sake of this review, the term will refer to only ordered, stretch- or bend-dominated ordered structures, as described in section 7;
-
-
Ordered structure: an "ordered structure" refers to a regular and predictable arrangement of struts or beams forming a lattice. The ordered structure exhibits a repetitive pattern that can be precisely defined and controlled. This organization contributes to consistent mechanical properties and allows for tailored designs with specific functionalities in cellular solids;
-
-
Pores: the term “pores” refers to the empty spaces or cavities within the structure of a material, which are surrounded by solid struts or walls and are responsible for the morphology of the cellular solid;
-
-
Pore connectivity: “pore connectivity” refers to the degree of interconnection between the pores or pores within the material's structure. It impacts properties like permeability, stiffness, and strength, where higher connectivity enhances fluid flow, and overall functionality of the cellular material;
-
-
Porosity: the term “porosity” might refer to either the volume fraction of pore spaces or pores within the material's structure or to the pore present inside a singular structural unit. For the sake of clarity, in this review the former definition will be used;
-
-
Scaffolds: scaffolds are cellular solids designed to support and guide tissue regeneration and repair. These scaffolds serve as temporary templates that mimic the extracellular matrix of the target tissue, providing a supportive environment for cell attachment, proliferation, and differentiation. Scaffolds are typically made from biocompatible and biodegradable materials to avoid adverse reactions within the body and facilitate gradual integration with the newly formed tissue;
-
-
Stochastic structure: a "stochastic structure" refers to a random or probabilistic arrangement of struts or beams lacks a strict regular pattern, leading to a local variability and unpredictability in the material's properties;
-
-
Structural units: “structural units” refer to the fundamental building blocks or basic elements that constitute the overall microstructure of the material. They represent the individual cells or pores with the surrounding struts that repeat in a regular or semi-regular pattern to create the overall material structure. In some cases, cellular solids may have a single type of structural unit throughout the material, resulting in a homogeneous microstructure. In other cases, they may have different types of structural units, leading to a heterogeneous microstructure;
-
-
Struts: struts refer to the slender, beam-like elements that form the interconnected framework or lattice structure of the cellular solids;
-
-
Topology: “topology”, when speaking about cellular solids, refers to the arrangement of cells or unit elements within the solid material. Topology describes how these cells are connected and arranged in the material, which significantly influences its mechanical, thermal, and acoustic properties. The topology of cellular solids can be engineered to achieve specific performance characteristics, such as high strength-to-weight ratio, low thermal conductivity, or excellent energy absorption capabilities.
3. Advantages of metallic cellular solids
Biomedical cellular solids are used to support the growth of new tissue or to replace damaged tissue in the body and thus require adequate mechanical properties. Depending on the type of tissue and the anatomical location, the strength requisites of biomedical cellular solids can vary greatly and in particular for orthopedic applications most polymeric cellular solids are not up to the task [32]. Metals used in structural biomedical cellular solids, such as Ti6Al4V and CoCrMo, possess greater mechanical and overall comparable biocompatibility, despite being heavier and more stiff [5]. Ceramic, on the other hand, possess superior compressive strength, but are relatively weak at flexural loads and not as reliable in cyclic fatigue conditions as they can fracture catastrophically because of their brittleness [33]. Moreover, ceramic cellular solids production techniques require either reaching higher temperatures during production or an additional firing step to reach full density and remove binders [34].
Fatigue resistance refers to the ability of a material to withstand repeated cycles of stress and strain without breaking or degrading. This property is particularly important for biomedical cellular solids, which must be able to withstand the stresses and strains of daily use within the human body without requiring periodic maintenance. The cellular solid's porosities act as stress intensifier, thus causing the mechanical collapse of the structure at only a fraction of the ultimate load of the dense material [35]. Metals can elastically and plastically deform to absorb energy, plus they have a high resistance to fatigue crack propagation, meaning that small cracks that develop over time are less likely to grow and cause failure of the material. Fatigue resistance in cellular solids will be further discussed in a dedicated paragraph.
There are several different classes of metals that can be used for biomedical cellular solids, with titanium alloys being the most common. Overall, they can be divided in two main categories:
-
-
Structural, used in applications that require high mechanical strength, in particular load-bearing devices such as in orthopedic prosthesis. This category comprises materials such as titanium alloys, cobalt alloys, stainless steels and tantalum. Structural biomedical cellular solids have been produced for decades, and the follow-up showed relatively high survival rates for most materials;
-
-
Bioresorbable, where the main focus is on bioactivity and the capability to be resorbed by the surrounding tissues over time. Bioresorbable metals are mechanically weaker than structural alloys such as titanium or cobalt-chromium, but maintain sufficient strength to support the body throughout the healing process. Magnesium and zinc alloys are two examples of this category. Most bioresorbable metallic cellular solids are still under development or in early stage pre-clinical trials;
While both "structural" and "bioresorbable" cellular solids serve as types mechanical supports during tissue regeneration, there exists a significant disparity in their maturity levels, as structural cellular solids have been established in the market for decades, whereas bioresorbable cellular solids still encounter unresolved challenges that require attention and solutions.
Fig. 2 summarizes the mechanical strength versus the qualitative biocompatibility of the most common classes of metals that are used – or could be used – to produce biomedical cellular solids, based on various literature references [[36], [37], [38], [39], [40], [41], [42]]. The biological performance is primarily influenced by potential cytotoxicity and ion release, and secondarily by the alloys ability to promote osseointegration. Despite stainless steels being the stronger and most mechanically reliable class of metallic alloys, their application to biomedical cellular solids is limited due to the lack of proper biocompatibility and the tendency to corrode over time in biological environments. Biomedical grade cobalt chromium alloys (often written just as CoCr, CrCo or CoCrMo) have lower mechanical strength, but their superior resistance to corrosion and wear makes them better candidates for biomedical applications, in particular in articulations. Despite being heavy and stiff, cobalt alloys proved themselves as excellent biomedical cellular solid materials, with positive clinical follow-ups of 20 of more years. Titanium and tantalum have comparable mechanical properties, but while titanium is used to produce various alloys, some of which, like Ti–6Al–4V (Grade 5) have mechanical strength comparable with stainless steels, in the biomedical field tantalum is mainly applied unalloyed, but the lower mechanical strength is balanced by a superior biocompatibility. Both titanium and tantalum are biocompatible thanks to the high chemical stability of their outermost native TiO2 and Ta2O5 oxide layers, more resistant to chemical attack than the complex oxides formed on stainless steel.
Fig. 2.
Mechanical strength versus qualitative biocompatibility for the most common classes of metallic biomaterials that are even used commercially or studied to produce metallic cellular solids.
Alloys based on magnesium, zinc, and iron are not intended for long-term structural applications: they should retain sufficient strength to support the body throughout healing and after are able to be resorbed via by the body's tissues. In contrast to titanium, chromium, and tantalum alloys, which are designed to be bio-stable (more precisely bio-tolerated, as they actually trigger an inflammatory reaction that in the end results in the encapsulation of the implant by fibrous tissue [43,44]), magnesium-zinc-iron alloys exhibit an high biocompatibility and during degradation their ions can actually contribute to the bone healing processes by stimulating the osteoblast proliferation and differentiation, by activating the signaling pathway related to the osteogenesis or by improving cell adhesion [45].
In Fig. 2 iron-based bioresorbable alloys have a qualitative biocompatibility index between “good” and “very good”. This is due to their slow degradation rate in vivo, which still represent a challenge and a potential limitation to their applicability. Additionally, the biocompatibility of these alloys didn't yet reach a full scientific consensus in the community. While these problems have still not been fully resolved, many studies have been published that propose different approaches to the issues [46].
The use of metals for biomedical cellular solids offers several advantages on ceramic and polymeric alternatives, in particular under cyclic loading conditions. They also exhibit lower elastic moduli than their ceramic counterparts and higher resistance to degradation when compared to polymers. While ceramics and polymers may also have their own unique advantages, metals are often the preferred choice for applications that require high strength and durability.
4. Fatigue resistance in metallic cellular solids
Cellular solids are particularly susceptible to fatigue failure, because of the reduction in resisting area and the stress concentrations promoted by the presence of micro-architectures [47,48]. More specifically, fatigue of cellular materials depends on cyclic stress intensity range, cell size, relative density, presence of imperfections and the fatigue parameters of the material from which they are made [[49], [50], [51], [52]]. For homogeneous structural units, the crack propagation in cellular solids can be approximated by a modified Paris’ Law [53], but a comprehensive detailed model is still lacking and hierarchically organized porous materials can show fatigue resistance results higher than what would be normally be expected for their porosity.
Typically, cellular solids made of high performance metals such as titanium and cobalt-chromium are considered to have superior fatigue resistance when compared to the same structures obtained from high performance ceramics such as alumina or zirconia thanks to their ability to absorb energy thorough deformation, in particular under bending conditions.
A method used to increase fatigue resistance in all types of cellular solids is the application of hierarchical porosities [54,55]. Hierarchical porosities in materials refer to the presence of multiple levels or scales of pores within the structure. These hierarchical arrangements can offer several advantages, such as deflecting cracks or dissipating energy, but if correctly designed hierarchical porosities can also effectively redistribute stresses and suppress strain localization, thus improving the fatigue resistance of the component.
Despite their lower biocompatibility when compared to bioceramics, metallic cellular solids are commonly used in biomedical applications because of their high mechanical reliability due to their intrinsic ductility, which promotes energy dissipation and slower crack propagation. In ceramics, cracks tend to propagate more rapidly because of their brittle nature, which makes cracks of ceramic components more unpredictable and harder to detect in advance [56]. Moreover, the lack of plastic deformation mechanisms in ceramics limits their ability to dissipate energy and arrest crack growth. Some biocompatible ceramic materials, like for example zirconia, zirconia toughened alumina and silicon nitride, can be engineered to stop (or at least slow down) crack propagation, improving their reliability under fatigue conditions.
Zirconia undergoes a phase transformation from a tetragonal to a monoclinic crystal structure under stress [57,58]. When a crack propagates through the material, the stress concentration at the crack tip triggers the transformation of the surrounding tetragonal grains into monoclinic grains. This transformation generates a volume expansion, causing a compressive stress field around the crack tip. The compressive stress counters the tensile stress associated with the crack, effectively stopping crack propagation. This phenomenon is known as transformation toughening [59] and significantly enhances zirconia's fracture resistance. Silicon nitride, on the other hand, has a unique microstructure, comprising elongated grains with a high aspect ratio [60]. When a crack propagates through the material, it encounters these elongated grains and grain boundaries. The elongated grains restrict crack propagation by deflecting and blunting the crack tip, leading to crack bridging. The grain boundaries also provide a barrier for crack advancement, enhancing the material's toughness and crack resistance.
When designing cellular solids for fatigue-sensitive applications, the choice of material becomes crucial, and metallic structures may be preferred to ensure longer-lasting and more durable performance. On the other hand, for bulk materials subjected to tribological wear [61,62] or when biocompatibility is prioritized [63,64], ceramic materials are often considered the safer choice.
5. Production techniques
There are several techniques used to create metallic cellular solids for biomedical applications. These techniques can be broadly categorized into conventional and additive manufacturing methods.
Some of the most common conventional production methods include:
-
-
Casting [65]: casting is a traditional method used to fabricate metallic components. In this method, molten metal is poured into a mold and allowed to cool and solidify. After solidification, the cellular solid is removed from the mold and post-processed to achieve the desired shape and size. Casting of a cellular solid requires a template, usually made of plastic materials such as polystyrene or polyurethane, and the template is lost when the molten metal is poured inside the mold. Casting of cellular solids has been mainly used to improve endoprosthetic fixation [66];
-
-
Foaming [11]: an array of different techniques can be used to generate “bubbles” (or dispersions of secondary phases) in melt and semisolid metals, resulting in stochastic closed pore morphologies with an overall poor control on structural units size and distribution. Foaming has been tested mainly for bone implants and endoprosthetics fixation [11];
-
-
Powder Metallurgy [67]: In this method, metallic powders are compacted into a desired shape by the use of space holders and then sintered to form a solid structure. The sintering process involves heating the compacted powders to a high temperature below the melting point of the metal, causing them to fuse together. Space holders are particles with different chemical composition that are mixed together with metal and act as temporary “voids” within the powder mixture. Space holders are generally either burned out during sintering (in particular when polymeric) or dissolved after sintering (salts), creating a porous cellular solid with the desired topography [10]. Powder metallurgy has been mainly used for the production of cellular solids for dental and endoprosthetic fixation [68], spinal fusion cages [69], cranial implants [70] orthopedic screws [71];
-
-
Spraying [72]: melt droplets of metals can be assembled into stochastic porous cellular solids by using an high energy source, for example a plasma torch. This technique is often used just to increase the surface roughness of metal implants, but it also can produce porous materials by carefully controlling the process parameters. As spraying produces a layer of cellular solid material on the top of bulk substrate, these techniques have been mainly applied for dental and endoprosthetic fixation [3,73];
-
-
Deposition on a replica [3]: by using chemical vapor deposition or other similar technologies on an adequate template, it is possible to obtain porous metallic structures. In this case the template is covered by metal but not lost during the process. One of such examples is trabecular metal from Zimmer Biomet, where using chemical vapor deposition metallic tantalum is deposited on a foam of vitreous carbon obtained by pyrolysis of polyurethane [74]. These processes have been used to produce cellular solids for spinal fusion cages [75], endoprosthetic fixation [76], bone scaffolds [77] and dental implant abutments [78].
Additively manufacturing methods include:
-
-
SLM, Selective Laser Melting [79]: also known as Direct Metal Printing (DMP) and Laser Powder Bed Fusion (LPBF), is the most common and mature powder bed fusion technology for the additive manufacturing of metal parts. In SLM a high powdered laser is used to locally fuse layers of metal powders, with extremely high spatial resolution. The process then proceeds layer-by-layer. Biomedical metallic cellular solids obtained by SLM include bone implants [80], endoprosthetic fixation [81], orthopedic screws [82], dental implant abutments [83] and cranial implants [84];
-
-
EBM, Electron Beam Melting [85,86]: electron beam melting is a powder bed fusion method that uses a high-energy electron beam to melt and fuse metallic powders together, creating a solid cellular solid structure. EBM can produce highly complex geometries and intricate structures with high precision and accuracy, making it suitable for the fabrication of customized cellular solids. EBM is less precise than SLM as the spot size is larger, but it also results in lesser internal stresses and overall better fatigue resistance. During processing the material is in vacuum, strongly reducing the risk for contamination and, in the case of titanium alloys, preventing the formation of the mechanically weaker sub-superficial layer known as “alpha case”. EBM cellular solids have been applied in dental implant abutments [87], bone implants [88] and endoprosthetic fixation [85];
-
-
DED, Directed Energy Deposition [89]: in directed energy deposition the energy source (either a laser or an electron beam) is used to melt and fuse metal wire or powder that is directly fed into the melt pool. When compared to powder bed fusion methods, DED is usually faster and cheaper, but less precise and for this reason DED is mainly applied for the production of larger parts that require lower resolution. Cellular solids obtained by DED are mainly used to improve endoprosthetic fixation [90];
-
-
Binder jetting [91]: in binder jetting a binder material is selectively deposited onto a layer of powder using inkjet print heads. The binder then solidifies and bonds the powder particles together. After the printing is complete, the part is sintered, leaving behind a final additively manufactured metallic object. Binder jetting has been mainly tested for bone implants [92] and dental implants abutments [93];
-
-
Robocasting [94]: also known as direct ink writing, is an advanced additive manufacturing technique used to fabricate complex three-dimensional structures with precise control over their architecture. The process involves the deposition of a printable material, often referred to as a bioink, through a computer-controlled robotic arm equipped with a dispensing nozzle or a syringe. The robotic arm follows a predetermined pattern to extrude the material layer by layer, progressively building the desired 3D structure. Robocasting showed great potential for biomedical applications, in particular for bone implants [94].
Additive manufacturing methods based on powder beds, such as SLM and EBM, can enable production of more complex open 3D lattice structures than other methods and without the need for additional support structures as the powder bed itself acts as a support for the part during manufacture.
A qualitative comparison of the different production techniques for what concerns typical production time and spatial resolution, is presented in Fig. 3. Casting can be used as a reference, as it consists of various common techniques that can reach a broad range of resolution and can produce pieces in a matter of minutes or hours, mainly depending on the size. Foaming techniques are somehow similar to casting, with the difference that gas bubbles (or other phases) are introduced into the molten metal in order to produce the porosities. As the process is more complex, pieces produced with these techniques are usually smaller and require less time to be produced. The accuracy is also relatively low. Thermal spraying techniques occupy a similar area of the graph, but for different reasons. The growth of the sprayed layer is relatively slow, and the accuracy is usually very poor. Still, thermal spraying is used to produce porous coatings on biomedical components so the production process only takes a few hours. Binder jetting has comparable characteristics, but unlike spraying it is used to produce larger volumes at a time. Powder metallurgy occupies the central area of the graph, as it is usually slower than most of the techniques described so far, but not as slow as metal additive manufacturing technologies. It can be used to produce components within a wide range of spatial resolution, depending on the methods used. SLS, EBM and DED are all time-consuming production technologies which can be used to obtain cellular solids with intermediate spatial resolution.
Fig. 3.
Qualitative comparison of typical printing time and spatial resolution for techniques that can be used to produce metallic cellular solids.
6. Topological and morphological control of cellular solids
"Topological control " refers to the deliberate and systematic manipulation of the structure and arrangement of structural units within a solid matrix to achieve specific desired properties and functionalities. In other words, it is the intentional design and control of the geometric configuration and connectivity of the pores in cellular solids.
By controlling the topology of cellular solids, mechanical, thermal, acoustic and biological properties can be tailored on the application. The topology can be modified through adjusting parameters like pore size, shape, distribution, and connectivity, as well as altering the overall architecture, such as lattice designs and foam structures.
Topological control is particularly important in biomaterials science and engineering, as it allows for the creation of advanced materials with optimized mechanical strength, stiffness, energy absorption and biocompatibility.
In cellular solids we can identify structures on three different scales, going from the surface roughness and micro-roughness that can be observed on the metallic struts, to the shape of the singular structural unit and ultimately the overall design of the cellular solid, based on the interconnection between different structural units.
6.1. The role of surface roughness/micro-roughness in bone ingrowth
When compared to conventional machining techniques, most metal cellular solids production methods result in a relatively high surface roughness. Such irregular morphologies, which would be considered excessively rough in most industrial products, play an important role in cell adhesion and bone ingrowth and are without a doubt one of the most important morphological features when it comes to metallic cellular solids’ clinical success.
Perfectly smooth surfaces lack micro and nano-scale features that cells can use to attach themselves, slowing down both adhesion and colonization [95,96]. The pores of the structural units used in biomedical cellular solids (200–600 μm) are usually sensibly larger than single osteoblasts (5–15 μm), so cells can easily slip through the porosities between the struts without mechanical interference. An otherwise optimized biomedical cellular solid would not be easily bio-integrated if its surface was perfectly smooth, as cells would not be able to efficiently use their cytoplasmic extensions to attach to the surface of the material. Cell adhesion is the first step in colonization and colonization eventually leads to tissue ingrowth and ultimately osseointegration, but rough metal surfaces are already capable of promoting cell adhesion, colonization and even osseointegration on bulk components and not just on cellular solids.
Optimal ranges for surface roughness have been previously reported in literature multiple times. Most sources seem to agree that an average roughness in the range of microns is adequate for osseointegration, but values differ depending on the material, the morphology of the surface and the type of cell, while studies performed on materials other than titanium alloys are relatively scarce. For sanded titanium treated and osteoblast cells, for example, cell adhesion was reported to peak at Ra between 0.15 and 0.33 μm [95].
6.2. The role of structural units in bone ingrowth
What defines cellular solids is the presence of matrices of structural units containing one or more “pores”. Structural units vary greatly in shape and complexity, making comprehensive reviews an impossible task. Still, some general information on the most common unit designs and their mechanical properties has been previously summarized in literature [51].
For each type of structural unit, two parameters are usually sufficient to roughly estimate both the mechanical and the biological properties: the diameter of the struts (or, alternatively, the thickness of the sheets for triply periodical minimal surfaces) and the pore radius. While the stud diameter and the sheet thickness can be measured directly, if the pores in the cellular solid are not spherical, which is often the case in real-world structures, defining the pore radius becomes more complex. In such scenarios, the pore shape can vary, and simple measurements like diameter may not be sufficient. Several approaches can be used to estimate the value, one of which is the use of the covering spheres. In this approach, each non-spherical pore is approximated with a set of tangent spheres, known as covering spheres, which enclose the pores while maintaining contact with their surfaces.
In general, a pore radius of 100–500 μm has been shown to be optimal for promoting bone ingrowth in metallic cellular solids [[97], [98], [99]], but both smaller and larger pores have also shown the capability to promote bone ingrowth in vivo, either in animal models [100] or humans [101]. Cellular solids with pores in this range provide a large surface area for cell attachment and proliferation, which can facilitate the formation of new bone tissue. Second, pores within this range allow for the diffusion of nutrients and waste products, which is essential for promoting cell survival and proliferation. Third, they are large enough to accommodate the migration of bone cells and support the ingrowth of one or more fully functional Haversian systems, which constitute the basic unit of structure to compact bone tissue and usually have a diameter between 100 and 200 μm [102].
Another pore-related parameter is the so called “accessible pore radius”, defined as the largest sphere radius that can invade from the cellular solid's edge into that particular pore within the cellular solid. It has been demonstrated that bone ingrowth into pore exhibits a strong correlation to the accessible pore radius [103], and pores with an accessible radius smaller than 100 μm have shown poor – if any – bone ingrowth [[103], [104], [105]]. For ordered structures, the accessible pore radius is constant over the whole structure and is controlled by the pore radius of a single structural unit and the thickness of the struts, but for stochastic or gradient distributions the values can vary greatly within the structure.
The size of the cells involved in adhesion is also an essential factor to consider. The stud diameter should be compatible to facilitate effective attachment and spreading. If the stud diameter is too small compared to the cell size, it may limit the area available for adhesion, potentially leading to weak and unstable attachments. Larger stud diameters also generally provide more surface area for cell adhesion. This increased surface area allows for more cells to attach, which can enhance cell adhesion strength and promote the formation of a stable cell layer on the cellular solid. In most cellular solids on market, the diameter of the struts is in the same order of magnitude of the pore radius [85,101,106,107].
Apart from pore and struts sizes [108], the overall structural unit shape also has an effect on both mechanical and biological properties [109]. In particular, depending on the arrangement of the struts, the mechanical response of the structure can shift from stretch-dominated to bend-dominated (confront section 7 for a detailed description of the two types of structures), drastically affecting elastic modulus, ultimate strength and fatigue resistance [[110], [111], [112]]. Moreover, mechanical strength grows with the number of struts connecting to a single junction and depending on the connection angle the struts can be subject to more intense shear stresses, resulting in a lower mechanical strength and a lower elastic modulus [[113], [114], [115]]. Similar considerations apply in the case of triply periodical minimal surfaces, where junctions are technically absent, but mechanical strength is influenced by the surface's orientation [116].
The shape of the structural units also affect how the cells react to the presence of the cellular solid. The number of struts for unit is approximately proportional to the surface area exposed to the biological environment and thus affects how many cells can adhere and colonize the surface. This is particularly important for osseointegration, and it is one of the major advantages that cellular solids have on bulk materials with “rough” surfaces. On the other hand, not all shapes are equally easy to adhere to and colonize from the cell point of view: sharp edges that create narrow gaps and crevices are colonized more slowly when compared to round fillets [[117], [118], [119]].
6.3. Overall porosity optimization for bone ingrowth
The ideal porosity of metallic biomedical cellular solids can vary greatly depending on the specific application and the material used. In general, however, porous metallic cellular solids with porosities ranging from 50% to 90% have been shown to promote fast bone ingrowth and integration [120].
However, the ideal porosity of a metallic cellular solid also depends on the material used and the mechanical requirements of the implant. For example, highly porous cellular solids may be weaker and less mechanically stable, which can limit their use in load-bearing applications [120]. On the other hand, cellular solids with lower porosities may be more mechanically stable, but may not provide the necessary surface area for cell attachment and tissue ingrowth.
Therefore, the ideal porosity of a metallic cellular solid for bone integration is often a balance between mechanical stability and bone ingrowth. In general, cellular solids with porosities between 60% and 80% have been shown to provide a good compromise between mechanical stability and bone ingrowth [14,121,122]. However, the specific porosity and pore size greatly vary depending on the application and the material used, and usually require further optimization through experimental testing and analysis.
In load bearing applications, the relationship between cellular solid porosity and fatigue resistance can be particularly complex and depend on several factors, including the materials used, the pore size and topographical distribution, and the mechanical loading conditions.
In general, highly porous cellular solids, in particular when large pores are present, may have reduced fatigue resistance due to stress concentration effects and the reduced cross-sectional area of the material. The larger pores in these cellular solids can act as stress risers and can initiate cracks or defects in the material under cyclic loading conditions [123]. Additionally, the reduced cross-sectional area of the material can result in lower mechanical strength and stiffness, which can further decrease the cellular solid's fatigue resistance [124].
On the other hand, cellular solids with lower porosities may have improved fatigue resistance due to their higher mechanical strength and stiffness, as they are less susceptible to stress concentration effects and may have a more homogeneous distribution of the mechanical load even under cyclic loading conditions. This is in particular true for ordered porosities, where mechanical resistance is dependent on the orientation [125].
It is important to note that the correlation between cellular solid porosity and fatigue resistance is not always straightforward, in particular in vivo where mechanical loading conditions are more complex, time dependent and overall unpredictable.
7. Stretch-dominated and bend-dominated cellular solids
To understand the mechanical behavior of cellular solids it is necessary to distinguish between stretch-dominated and bend-dominated structures. The mechanical properties of these cellular solids are primarily determined by the architecture and connectivity of the cells [126].
A stretch-dominated cellular solid refers to a structure where the load-bearing struts or beams mainly experience axial or tensile forces when subjected to mechanical stress. In such structures, the load is efficiently transferred along the length of the struts, resulting in high stiffness and strength relative to its mass. These stretch-dominated cellular solids can withstand substantial loads without significant deformation.
A bend-dominated cellular solid, on the other hand, is a structure where the load-bearing elements experience bending stresses rather than primarily axial stresses. When mechanical stress is applied, the struts or beams of these structures tend to flex or bend. Although bend-dominated cellular solids are less stiff and strong compared to stretch-dominated ones, they exhibit higher compliance and can efficiently absorb energy during compression or impact.
The interdependence of properties, structure, and performance in cellular solids has driven the development of diverse structures, including stochastic or non-ordered foams and periodic or ordered lattices, each possessing customizable properties to suit various applications. A prevalent challenge in designing and developing cellular solids, particularly for load-bearing applications, lies in balancing specific strength and energy absorption properties, often leading to trade-offs between these key attributes [127].
The stochastic cellular configuration present in foams leads to bending-dominated deformation of the ligaments, causing a swift reduction in both strength and stiffness with increasing porosity. Conversely, specific ordered lattice-type cellular configurations can exhibit nearly ideal stretch-dominated properties, yielding materials wherein strength and stiffness scale linearly with the solid volume fraction of the material [128].
The structure of a metallic biomedical cellular solid can significantly affect its mechanical properties, biocompatibility, and potential for tissue regeneration. There are three main possible design approaches: to use a ordered design, such as in lattice structures [129], a stochastic design, such as in foam structures [130], or a mix of the two.
Ordered structures offer several technological advantages, including:
-
-
Predictable mechanical properties [131]: The ordered structure of the cellular solid allows for more predictable mechanical properties, such as stiffness and strength, which can be important for load-bearing applications;
-
-
Enhanced cell proliferation and migration [129]: The ordered structure can provide directional guidance to cells, enhancing their proliferation and migration into the cellular solid.
However, there are also some disadvantages to using a ordered structure for a metallic cellular solid, including:
-
-
Limited adaptability: The ordered structure of the cellular solid may limit its ability to conform to irregularly shaped defects or match the complex structure of surrounding tissues. When cut in specific directions, ordered structures can be locally weaker because of the reduction in effective cross-section. This effect can be prevented by carefully designing the edges of the implant;
-
-
Increased risk of stress shielding as a function of orientation: ordered structures can lead to stress shielding as they usually have higher mechanical strength and stiffness when oriented in specific directions. This effect can be prevented by carefully modeling the cellular solid so that even the stiffest orientation has an adequate elastic modulus.
In contrast, a stochastic structure refers to a cellular solid with a less-defined and disorganized pattern of pores, typically with a varied shape, size, and spacing. Stochastic structures increase adaptability and reduce risk of stress shielding, but have two major drawbacks:
-
-
Less predictable mechanical properties [132]: as the topographic orientation and the local density regulate the mechanical response, completely stochastic structures can lead to less predictable macroscopic and/or local mechanical properties, making it more challenging to design cellular solids for load-bearing applications. For example, a portion of the cellular solid with larger, more numerous or more aligned porosities will inevitably have a lower mechanical resistance, at least along one specific loading direction, when compared to the average of the whole structure. In ordered cellular solids these “weak areas” can be predicted and modeled in advanced;
-
-
Variable cell proliferation and migration [133]: stochastic structures can reduce the directional guidance for cells, potentially leading to different cell proliferation and migration into the cellular solid, depending on the size of the structural units at that location. For example, uniform porosities will have consistent and predictable biological properties, such as rate of tissue ingrowth, which will greatly vary locally in completely stochastic structures.
In most stochastic porous structures, such as sponges [134], the average structural unit diameter and distribution are more or less consistent between different specimen, but mechanical properties greatly vary, locally. This unpredictable behavior makes purely stochastic topographies less reliable than ordered ones, in particular when applied in load bearing applications, but depending on the technology and the process parameters, the average pore size and its distribution can be controlled in order to match a specific requirement, to a certain extent.
Recently, computer modeling techniques were used to produce “hybrid” porous structures, where despite porosities being casual in both size and position, they are constrained by boundaries conditions that greatly limit their variability, thus improving reliability [[135], [136], [137]]. When additive manufacturing technologies are utilized, the topography can be intentionally randomized at the design stage by incorporating stochastic seed generation techniques, meaning that their mechanical response can be modeled by altering the boundary conditions [138].
Structural gradients also play a crucial role in tailoring the mechanical properties of cellular solids, whether they possess stochastic or ordered structures. In both cases, the controlled variation of certain design parameters allows for the precise modulation of cellular solid properties to suit specific tissue engineering applications and provide more mechanical support where necessary. A lower porosity in the core of the cellular solid can provide mechanical stability and support to the overall structure. This allows the cellular solid to maintain its structural integrity during the tissue healing process, while the higher porosity on the outer surface facilitate cell adhesion and proliferation, as well as the ingress of nutrients from the surrounding environment [139,140].
In summary, the choice of ordered or stochastic structure for a metallic biomedical cellular solid depends on the specific application and requirements. From the mechanical point of view, an ordered structure may be more suitable for applications where the loading distribution is simple and well known, so that it can be matched with the strongest structural orientation. A stochastic structure, on the other hand, will be more suitable for regions where the loading distribution is unknown, or multi-directional. From the biological point of view, ordered structures are more suitable for applications in which a directional cell growth is preferable, while stochastic structures will more likely result in an isotropic growth. It is essential to carefully consider the advantages and disadvantages of each approach and select the most appropriate structure for the intended application.
8. Clinical applications of metallic cellular solids
Metallic cellular solids have a wide range of clinical applications in bone tissue engineering and orthopedic surgery, in particular:
-
-
Bone fillers for bone defects [5,141,142]: Metallic cellular solids can be used to treat bone defects resulting from trauma, tumors, or degenerative diseases. In this context, the cellular solids serve as a temporary or permanent support structure for the damaged or missing bone tissue, providing mechanical reinforcement to an otherwise weakened area and facilitating the ingrowth of new bone tissue from the healthy surrounding areas. Depending on the anatomical location and the size of the defect, bone substitute cellular solids might require not just opportunely designed porosities to stimulate bone ingrowth, but also high mechanical strength and fatigue resistance, which are difficult to achieve when using polymeric or ceramic cellular solids, as they either lack mechanical strength or fatigue resistance. Bone substitute cellular solids are usually highly porous, and their elastic modulus can reach values comparable to those of the surrounding bone [85,86], thus preventing stress-shielding effects even for weakened tissues as in presence of osteoporosis. Typically, the modulus of human trabecular bone ranges between 10 and 3000 MPa while titanium alloys range from 55 to 114 GPa and magnesium alloys between 40 and 60 GPa. Cellular solids, on the other hand, can reach elastic moduli in the range of few GPa [44,45], and results as low as 2.87 GPa have been reported for titanium [143];
-
-
Spinal cage implants [5,144,145]: spinal fusion is a surgical procedure used to treat a range of spinal conditions, including degenerative disc disease, spinal fractures, and spinal deformities. The goal of spinal fusion is to stabilize the spine and promote fusion of the two vertebrae, reducing pain and restoring function. As for bone defects, metallic cellular solids can be used in spinal fusion surgery as a support structure for new bone growth, but spinal fusion devices are usually not completely porous, containing pores only close to the surfaces in contact with bone, as they only require a stable bonding with the surface of the two vertebras. Moreover, unlike bone filling cellular solids, spinal devices are constantly under high mechanical stress in fatigue conditions that could be detrimental for completely porous components. Another peculiarity of spinal fusion devices is that they connect compact bone which, when compared to spongy bone, has less strict requisites for the elastic modulus in order to prevent stress shielding effects. Still, excessively “stiff” cages can cause the fracture of the adjacent vertebrae, so a relatively low elastic modulus is recommended in medical practice [146]. Both ceramic and polymeric spinal fusion devices are available on the market, but metallic components are considered the most mechanically reliable. Ultimately, it is up to the surgeon to determine the best material for each individual case depending on anatomical location, surgical procedure and patient's health status;
-
-
Porous coatings for joint replacement [[147], [148], [149]]: Joint replacement surgery is a common procedure used to treat severe joint pain and disability caused by degenerative joint diseases such as osteoarthritis, rheumatoid arthritis, or trauma. During joint replacement surgery, the damaged joint is removed and replaced with an artificial joint made from various materials, including metallic cellular solids. Artificial joints can be roughly divided in three parts: there is the articulating region in the center, where two components made with high wear resistance materials are sliding one against the other (alumina, zirconia, zirconia toughened alumina, cobalt chrome and ultra-high molecular weight polyethylene), and two bone interfaces at the sides that provide mechanical support and stability while also binding with the surrounding bone tissue. Similarly to spinal fusion devices, artificial joints don't need a complete bone ingrowth, but only fixation, so the cellular solids with a total thickness of up to a few millimeters are applied as the outermost layer of the implant. Still, unlike spinal fusion devices, they are mainly in contact with spongy bone, so they require an adequate elastic modulus in order to prevent stress shielding. Metallic cellular solids made of cobalt chrome, tantalum or titanium are among the most common choices for this application;
-
-
Support structures for maxillofacial reconstruction [[150], [151], [152]]: Maxillofacial reconstruction is a surgical procedure that restores form and function to the head, neck, and facial region, often following trauma or cancer treatment. Metallic cellular solids can be used in maxillofacial reconstruction as a support structure for bone regeneration and to restore normal facial contours and function. While the basic principles of metallic cellular solid design and fabrication for maxillofacial reconstruction are similar to those for bone defects, there are several key differences. One major difference is the complex geometry of the facial bones and the need to restore the normal contours and appearance of the face, which requires a high degree of precision and customization in cellular solid design and fabrication. Another key difference is the importance of soft tissue support and reconstruction in maxillofacial surgery. In addition to providing support for bone regeneration, metallic cellular solids used in maxillofacial reconstruction must also be designed to support soft tissue regeneration and maintain the integrity of the facial features. This requires careful consideration of factors such as pore size, pore shape, and cellular solids surface characteristics to promote soft tissue attachment and integration;
-
-
Dental implants [[153], [154], [155]]: Dental implant surgery involves placing artificial tooth roots into the jawbone to support dental prostheses, such as crowns or dentures, and restore missing teeth. Metallic cellular solids used in dental implants are typically made from biocompatible materials such as titanium or its alloys, which have excellent mechanical properties and a low risk of implant rejection. Just like in artificial joints, the metallic cellular solid is designed to provide a stable support structure for the dental implant, which is typically a small titanium post that is surgically implanted into the jawbone. The cellular solid is often coated with a layer of hydroxyapatite or other bone-forming materials to promote bone growth and enhance implant integration. One advantage of metallic cellular solids for dental implants is their ability to withstand the mechanical stresses of chewing and biting, which can be significant in the jawbone;
-
-
Osteoporosis treatment [[156], [157], [158]]: Metallic cellular solids have been proposed as a potential treatment for osteoporosis, as they can provide mechanical support and enhance bone regeneration. Despite the obvious similarities, the major difference between metallic cellular solids for osteoporosis treatment and those used for bone defects is the design of the cellular solid. In the case of osteoporosis treatment, the cellular solid is designed to provide mechanical support to the weakened bone, in order to reduce the risk of fractures, more than promote the regeneration of new bone tissue, so the two key properties of biomedical metallic cellular solids bare a different weight in the treatment of osteoporosis than they do in the treatment of bone defects. Osteoporotic bone is slow to regenerate and brittle, so the newly formed bone tissue is not expected to regenerate completely to the point to be able to sustain the same mechanical load of the original, healthy tissue. Just as a walking cane, the metallic cellular solids will compensate for the lack of strength.
9. Surface treatment of biomedical metallic cellular solids
When compared to ceramics, and organic substances, metals usually considered to be bio-tolerated more than bio-active. Surface treatments can be applied to metallic alloys to further improve their bioactivity, but not all conventional treatments are suitable for metallic cellular solids, as their surface is complex and internal areas can be hard to reach for most coating technologies.
Some of the most common solutions are:
-
-
Application of bioactive coatings [[159], [160], [161]]: One of the most common and effective surface treatments to increase bioactivity is to use a more bioactive material to produce a coating on the surface of the cellular solid. In the field of orthopedics, the most common materials for such applications are hydroxyapatite (HA), calcium phosphates, or bioglass. These materials have been shown to improve the biological response of metallic cellular solids by promoting cell attachment, proliferation, and differentiation. In addition, the use of bioactive coatings can enhance cellular solid integration with the surrounding tissue, leading to better overall performance. Only a limited numbers of techniques are currently available to coat cellular solids with bioactive materials, including physical vapor deposition [162,163], plasma spray [164,165], atomic layer deposition [166,167], sol-gel processes [168,169] and electrochemical methods [170,171] Biocompatibility is mainly regulated at a surface level, so the contribution of these treatments to the overall bioactivity of metals is independent on the morphology of the cellular solid. Nevertheless, cellular solids with narrow access points can be difficult to coat completely using some of these methods, plasma spray in particular, and might require the use of techniques with greater penetration capability, such as Atomic Layer Deposition (ALD) [172]. ALD has two advantages over other techniques: reactants are in gaseous form, meaning that they can reach any point of the cellular solid, and the deposition is surface controlled, meaning that all the surface will be uniformly coated by one molecular layer at a time;
-
-
Anodization of the base material [[173], [174], [175]]: anodization is conventionally considered a coating technology, as it results in the formation of a coating with different chemical composition on the substrate material. On the other hand, it is also quite different from other coating technologies, as it involves subjecting the material to an electrochemical process in an electrolyte solution which, unlike the techniques listed in the previous paragraph, uses the substrate itself as one of the constituents. This creates a controlled oxide layer on the surface of the cellular structure, enhancing biocompatibility by promoting osseointegration and reducing the risk of inflammation, making it suitable for medical implants and tissue engineering applications. Despite being technically applicable also to other biocompatible metals such as zirconium and tantalium, anodization has been prevalently applied to titanium alloys. By controlling the composition of the bath, specific ions can also be introduced in the oxide layer to further improve biocompatibility. Due to their topography, anodizing cellular solids is a challenging task, as the electric field cannot be properly controlled;
-
-
Use of physisorption and chemisorption processes [[176], [177], [178]]: Growth factors or other biomolecules can be immobilized on the cellular solids surface to promote cell attachment, proliferation, and differentiation. This can be achieved through covalent bonding or physical adsorption, and can improve the biological response of the cellular solid in hundreds of different ways. Such treatments are so different in nature, chemical composition and application that will not be discussed in detail in this review, but the key concept is that the high surface area-to-volume ratio of metallic cellular solids makes them ideal substrates for such a functionalization;
-
-
Increase the surface roughness [[179], [180], [181]]: A “roughened” surface can have micro and nano-scale features, such as porosities, spikes, and ridges, which can promote the adhesion and proliferation of cells and enhance the deposition of calcium and phosphorous ions. These surface features can also create topographical cues that can guide the behavior of cells and promote their differentiation into bone-forming cells. The “roughening effect”, in cellular solids, is usually limited to the morphology of the struts and surfaces, and it doesn't lead to alterations in the shape and dimension of the structural units. These processes are usually limited to is usually obtained either plasma etching [182,183] or acid etching [[184], [185], [186]], and each of the techniques offers advantages and limitations. Acid etching is a chemical process that involves applying an acid or acidic solution to the material's surface, which selectively removes or dissolves a thin layer of material or one (or more) phases. This results in a change in the surface texture, leading to an increase in surface roughness. In powder based additive manufacturing processes the acid treatment may also reduce surface roughness inherent to these processes and assist to remove poorly bound powder particles [185]. Achieving consistent and uniform etching across a large and complex area, such as in the case of cellular solids, can be challenging: variations in acid concentration, temperature, and etching time can lead to uneven surface textures. If not properly controlled, aggressive etching can lead to surface damage, such as over-etching, pitting, or loss of structural integrity. Plasma etching on the other hand is a process that involves using ionized gases (plasma) to selectively remove material from a surface. The positively charged ions in the plasma are accelerated by the electrical field and directed toward the metal surface. Upon collision, the high-energy ions cause the metal atoms to become dislodged from the surface in a process known as sputtering. The sputtered metal atoms are then ejected from the surface. As the metal atoms are sputtered away, surface irregularities and microstructures are gradually exposed. The continuous ion bombardment leads to the formation of pits, grooves, and surface defects. Plasma etching takes longer and is more expensive than acid etching, but involves lower risks of surface contamination and can be controlled more easily, in particular over time.
10. Clinical outcomes of metallic cellular solids
The clinical outcomes of metallic cellular solids for bone regeneration and tissue engineering have been generally positive, with many studies reporting successful integration of the cellular solid with the surrounding tissue and satisfactory functional outcomes. However, the expected life of different metallic cellular solid materials and their failure rates can vary depending on several factors, including the specific application, the patient's condition, the surgical technique, and the type of cellular solid material used.
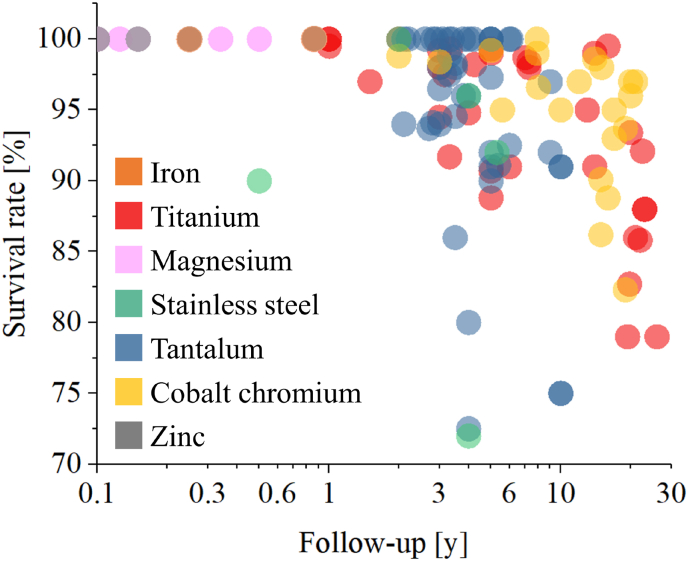
Titanium and its alloys are among the most commonly used metallic materials for bone cellular solids due to their excellent biocompatibility, mechanical strength, and corrosion resistance. The expected life of titanium cellular solids is generally long, with studies reporting successful outcomes and stable implants for over 10 years [[187], [188], [189]]. The failure rate of titanium cellular solids is also relatively low, with reported failure rates ranging from 1 to 5% [172,190]. New bone tissue can form on titanium cellular solids after just a couple of weeks [191] and bone ingrowth can be reached after a few months [192]. Spinal cages coated with titanium cellular solids can result in fusion after about 6 months from the application [193]. Clinical outcomes for 30 published research papers [188,172,190,[194], [195], [196], [197], [198], [199], [200], [201], [202], [203], [204], [205], [206], [207], [208], [209], [210], [211], [212], [213], [214], [215], [216], [217], [218], [219], [220], [221]] as a function of follow-up year are listed in Fig. 4. As titanium cellular solids have been on the market for a long time, we also have follow-ups of 20 years or more, showing a sharp decrease in survival rates. It must be noted that those follow-ups were based on technological solutions that are likely not on the market anymore, while modern cellular solids are supposed to be able to outlast those of previous generations both from the point of view of mechanical strength and biocompatibility.
Fig. 4.
Clinical outcomes of metallic cellular solids expressed as survival rate as a function of follow-up. For magnesium and zinc, clinical follow-ups are substituted by in vivo animal model follow-ups.
Stainless steel could potentially be used to produced biomedical metallic cellular solids due to its low cost and high mechanical strength [222]. It has, however, lower biocompatibility than titanium and is more prone to corrosion in the aggressive biological environment, which can lead to premature implant failure. It is also stiffer, which makes stainless steel cellular solids more prone to stress shielding when compared to titanium alloys [223]. The expected life of stainless steel cellular solids is shorter than titanium, but very few tests were conducted in vivo, often limited to animal models and coated in order to improve biocompatibility [[224], [225], [226]]. Despite being available for almost one century, just a few follow ups can be found in literature for porous stainless steel cellular solids, which are usually intended for very specific applications or based on animal models [[227], [228], [229], [230]].
Cobalt-chromium alloys are also used for metallic cellular solids due to their excellent mechanical strength [9]. These alloys are well tolerated by human cells, with no cytotoxic effect reported on osteoblasts [231]. Spongiosa metal [3], one of the first commercially available porous cobalt-chromium biomedical products, was reported to have a survival rate between 88.8% [232] and 98% [233] in follow ups of 15, 20 or more years, with variable degree of stress shielding phenomena occurring in 21% of cases [232]. The clinical survival rate of cobalt-chromium cellular solids from 20 scientific papers [101,215,[232], [233], [234], [235], [236], [237], [238], [239], [240], [241]] has been presented in Fig. 4. As for titanium, cobalt-chromium also have a long history of in vivo applications, with follow ups up to about 20 years being relatively common.
Like titanium and cobalt-chrome, numerous and detailed clinical follow ups were published on the clinical outcomes of the few types of commercially available tantalum orthopedic cellular solid materials, Trabecular Metal in particular. When implanted as metaphyseal cones, tantalum cellular solids showed an average improvement of 33 points in the Knee Society clinical scores after 34 months [242]. For tantalum plugs applied to osteonecrotic hips the success rate at 12 months was 77.8% postoperatively and 44.5% overall at Steinberg stage III and IV[243], while the survival rate was 86% at an average of 39 months [244] and 72.5% at 48 months for early-stage hip osteonecrosis [245]. In the treatment of severe acetabular defects, the survival rate was reported to be 96.5% at 3 years [246], with a mean implant survivorship of 8.99 years and with osseointegration in 97% of cases [247]. Clinical outcomes for 30 published research papers [77,[248], [249], [250], [251], [252], [253], [254], [255], [256], [257], [258], [259], [260], [261], [262], [263], [264], [265], [266], [267], [268], [269], [270], [271], [272], [273], [274], [275], [276], [277]] as a function of follow-up year are listed in Fig. 4.
Unlike the other materials listed in this section, magnesium cellular solids are intended to be bio resorbed in within a few months from application, which makes their clinical outcomes difficult to compare with permanent implants. Nevertheless, on animal models and preliminary trials [[278], [279], [280], [281], [282]], magnesium cellular solids proved to be reliable (on the short-term) and to lead to a good integration with the surrounding tissue (Fig. 4). Magnesium stimulates expression of multiple intrinsic biological factors, such as promotion of osteoblast differentiation [283]. It also affects the expression of genes related to bone formation and resorption, as well as the activity of various enzymes and transcription factors that control bone cell function [284]. Hydrogen evolution in biomedical magnesium cellular solids can pose potential risks, not just because hydrogen may lead to local embrittlement and reduced mechanical integrity of the cellular solid, but also because its release in the surrounding tissue can create undesirable effects, such as inflammation reactions and acidification [285]. Moreover, hydrogen bubbles may occupy the space of callus growth, affecting tissue regeneration [286]. It must be also noted that, when compared to usual clinical trial, these results are based on fewer specimen and are less statistically significant.
Just like magnesium, also zinc cellular solids have yet to be tested in vivo on humans. Of the few available articles based on in vivo animal models [[287], [288], [289]], most are with really short follow-ups, even when compared to magnesium. Nevertheless, preliminary results show good and complete integration with the surrounding tissue and a fast resorption rate over time. As for magnesium, these results are based on few specimen and would require careful evaluation and further characterization before being applied in the medical field.
When compared to magnesium and zinc, iron-based bioresorbable cellular solids have an even more limited amount of supporting literature available, in particular concerning in vivo testing and relative follow ups, but test on Fe–Mn scaffolds implanted on rabbits shown an excellent biological response up to 48 weeks [290].
The failure of metallic cellular solids can occur due to a variety of factors, including mechanical stress, corrosion, infection and implant loosening. In some cases, revision surgery may be required to address implant failure. Similar cellular solids can perform differently depending on the anatomical location: for example, porous coatings on femoral implants have usually better clinical outcomes at long term when compared to the same applied to acetabular cups. Long-term clinical studies are needed to better understand the expected life and failure rates of different metallic cellular solid materials, but Fig. 4 shows common trends between the three main permanent cellular solid materials (titanium, tantalum and cobalt-chromium) despite the clear morphological differences. Spongiosa metal, for example, is a porous material with very large porosities, over the optimal equivalent diameter for fast osseointegration, but its long-term follow ups show survival rates comparable to more optimized titanium and tantalum cellular solids, meaning that, on the long term, all three materials are biocompatible and durable and will eventually integrate with the surrounding tissues. The rate at this integration happens is, on the other hand, greatly dependent on pore size and surface roughness, as discussed in section 6.
11. Customization of biomedical metallic cellular solids
Despite having a complex geometry, depending on the production technology used, metallic cellular solids can be custom-made to meet patient-specific requirements, such as shape, morphology and density. While conventional techniques would require a complete revision of the production process, additive manufacturing technologies can obtain customized parts in almost the same amount of time as serial components, with a relatively small additional effort during the design phase. Overall, the process of creating patient-specific implants typically involves the following steps:
-
-
Imaging: The first step is to obtain imaging data of the patient's anatomy using techniques such as CT (computed tomography) or MRI (magnetic resonance imaging). This imaging data is used to create a 3D digital model of the patient's anatomy;
-
-
Design: The digital model is then used to design a cellular solid that matches the shape and size of the defect. The design process may involve selecting the appropriate material, pore size, and pore structure based on the patient's specific needs. Moreover, the cellular solid can be further reinforced with additional supports or require additional screw holes for primary fixation. The cellular solid can be automatically generated from 3D arrays of ordered porosities or stochastic. When porosities are stochastic the nodes locations can be either casually generated or based on finite element calculations of the load distribution;
-
-
Additive manufacturing: Once the design is complete, the cellular solid can be additively manufactured using all the techniques listed in section 5, depending on the material, the application and the resolution required. The additive manufacturing process allows for precise control over the shape and structure of the cellular solid, but the final result can differ greatly depending on the technique used;
-
-
Support removal and machining: when additively manufactured, complex structures often require additional supports such as rafts, skirts and brims, that need to be removed after printing. Depending on the application, the parts might also need additional machining to reach the desired shape and surface roughness;
-
-
Sterilization: due to their complex design, sterilization is considered to be more challenging in cellular solids when compared to bulk materials. Various sterilization techniques have been reported in literature, going from UV [291] to β-[292] and γ-[293] irradiation, autoclaving [294] and ethylene oxide [295] sterilization processes, but it should be noted that, depending on the morphology of the cellular solid, irradiation techniques are less likely to penetrate the whole structure and might result in an inadequate sterilization;
-
-
Quality control: custom-made biomedical implants, and additively manufactured cellular solids in particular, need to be carefully checked for internal and external defects. When compared to conventional techniques, additively manufacturing technologies have been found to result in higher rejection rates during inspection.
There are a few, crucial advantages in using custom-made cellular solid implants over mass produced components:
-
-
Precise fit: because the cellular solid is designed to match the patient's anatomy exactly, it can provide a better fit than off-the-shelf implants;
-
-
Improved mechanical properties: the cellular solid can be designed to provide the ideal mechanical properties distribution for a specific patient, in particular load distribution and elastic modulus, which can improve its durability and stability over time;
-
-
Reduced risk of infection: because the cellular solid fits the patient's anatomy precisely, there is less chance of movement or displacement, which reduces the amount of manipulation required during surgery, consequently reducing the risk of infection;
-
-
Faster recovery time: custom-made implants can speed up recovery when compared to off-the-shelf alternatives as they can be designed to be anatomically more similar to the original bone tissue and even display a gradient of porosity (structural unit size) to accelerate the process;
-
-
Cost-effective: despite the additional time and effort required to design and produce a patient-specific implant, the use of additive manufacturing technology can make this process more cost-effective than traditional implant manufacturing techniques as it will not require a complete redesign of the production process.
In summary, the use of cellular solids to produce patient-specific implants is an exciting development in the field of regenerative medicine. This approach allows for greater precision, improved outcomes, and reduced risk of complications compared to traditional implants, and it has the potential to revolutionize the way that a wide range of medical conditions are treated.
Additive manufacturing technologies are not the only way to achieve customization. Modular prosthesis can be adapted to the anatomy of the patient during the surgery by selecting the more suitable components from a set of standardized modules with different geometries. Modular prostheses are cost-competitive with additive manufacturing of personalized implants, and in applications such as hip surgery they provide enough variability to adapt to most anatomies.
12. Limitations and problems for metallic cellular solids
Despite the decades of positive clinical outcomes, the use of cellular solids in the biomedical fields still faces drawbacks and limitations.
The complex design of cellular solids requires advanced manufacturing techniques and methods. When compared to conventional production techniques, cellular solids have more likely to be rejected after production because they don't meet the quality standards. This is in particular true for components obtained using additive manufacturing which in some cases present residual porosities [296].
While titanium and cobalt-chromium in particular are widely used and considered biocompatible, there can still be instances of adverse reactions in some patients due to individual sensitivities or allergies to certain alloying elements. These effects can be further exacerbated by the high surface-to-volume ratio of cellular solids. Ensuring comprehensive biocompatibility testing and proper patient assessment is essential to minimize such risks [297].
Additionally, metallic cellular solids, like most other cellular solids for biomedical applications, have complex shapes that can be hard to properly clean and sterilize, both after production and before implantation, and this might lead to higher chances of post-operatory infection [298].
Another major concern related to the application of metallic biomedical cellular solids is the risk of mechanical failure: the real resisting section is reduced due to the presence of porosity and the stress intensification factor can lead to the formation of cracks, in particular under cyclic fatigue conditions. Some articles suggest that a properly structured hierarchical porosity could result in an increase in mechanical properties, in particular under fatigue conditions, but these studies are still preliminary and require further experimental confirmation [299]. At this time, the long term outcome of the implant stability and integrity is difficult to predict and model.
Additionally, controlling the microstructure of metallic cellular solids is often a challenging task, as mechanical properties can greatly vary from location to location due to the complex morphology [300].
13. Conclusions and future perspectives
In conclusion, metallic cellular solids hold tremendous promise in the field of tissue engineering, offering a unique combination of mechanical strength, biocompatibility, and versatility. This review has provided a comprehensive analysis of the influence of cellular solid characteristics, such as porosity, structural unit size, shape, and surface roughness, on mechanical strength and biocompatibility. The findings highlight the critical role of these factors in cellular solid performance and tissue integration.
With advancements in production technologies and an increased understanding of cellular solid-host interactions, metallic cellular solids are poised to revolutionize the field. However, further research is needed to optimize cellular solid designs, refine fabrication techniques, and evaluate long-term clinical outcomes.
Ultimately, the translation of metallic cellular solids from the laboratory to clinical settings has the potential to transform patient care, enabling the regeneration of damaged or diseased bones. As we forge ahead, it is imperative to prioritize safety, efficacy, and patient-centered outcomes to ensure the successful translation of these remarkable cellular solids into practical and impactful clinical applications.
In summary, the future of bone repair lies in the hands of these forged metallic cellular solids, and their journey towards healing continues to hold immense promise for improving human health and well-being.
CRediT authorship contribution statement
Elia Marin: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Project administration.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Elia Marin reports financial support was provided by Japan Society for the Promotion of Science.
Data availability
No data was used for the research described in the article.
References
- 1.Kumar A., Nune K.C., Murr L.E., Misra R.D.K. Biocompatibility and mechanical behaviour of three-dimensional scaffolds for biomedical devices: process–structure–property paradigm. Int. Mater. Rev. 2016 Jan 2;61(1):20–45. [Google Scholar]
- 2.Ni J., Ling H., Zhang S., Wang Z., Peng Z., Benyshek C., et al. Three-dimensional printing of metals for biomedical applications. Mater Today Bio. 2019 Jun;3(100024) doi: 10.1016/j.mtbio.2019.100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marin E., Fedrizzi L., Zagra L. Porous metallic structures for orthopaedic applications: a short review of materials and technologies. Eur Orthop Traumatol. 2010 Dec;1(3–4):103–109. [Google Scholar]
- 4.Heinl P., Müller L., Körner C., Singer R.F., Müller F.A. Cellular Ti-6Al-4V structures with interconnected macro porosity for bone implants fabricated by selective electron beam melting. Acta Biomater. 2008 Sep;4(5):1536–1544. doi: 10.1016/j.actbio.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez K., Nakajima H. Metallic scaffolds for bone regeneration. Materials. 2009 Jul 23;2(3):790–832. [Google Scholar]
- 6.Vasconcellos LMR de, Oliveira MV de, Graça ML. de A., Vasconcellos LGO de, Carvalho Y.R., Cairo C.A.A. Porous titanium scaffolds produced by powder metallurgy for biomedical applications. Mater. Res. 2008 Sep;11(3):275–280. [Google Scholar]
- 7.Wang X., Li Y., Xiong J., Hodgson P.D., Wen C. Porous TiNbZr alloy scaffolds for biomedical applications. Acta Biomater. 2009 Nov;5(9):3616–3624. doi: 10.1016/j.actbio.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Shimko D.A., Shimko V.F., Sander E.A., Dickson K.F., Nauman E.A. Effect of porosity on the fluid flow characteristics and mechanical properties of tantalum scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2005 May;73(2):315–324. doi: 10.1002/jbm.b.30229. [DOI] [PubMed] [Google Scholar]
- 9.Wanniarachchi C.T., Arjunan A., Baroutaji A., Singh M. Mechanical performance of additively manufactured cobalt-chromium-molybdenum auxetic meta-biomaterial bone scaffolds. J. Mech. Behav. Biomed. Mater. 2022 Oct;134(105409) doi: 10.1016/j.jmbbm.2022.105409. [DOI] [PubMed] [Google Scholar]
- 10.Arifvianto B., Zhou J. Fabrication of metallic biomedical scaffolds with the space holder method: a review. Materials. 2014 May 6;7(5):3588–3622. doi: 10.3390/ma7053588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh R., Lee P.D., Dashwood R.J., Lindley T.C. Titanium foams for biomedical applications: a review. Mater. Technol. 2010 Sep;25(3–4):127–136. [Google Scholar]
- 12.Hao Y.-L., Li S.-J., Yang R. Biomedical titanium alloys and their additive manufacturing. Rare Met. 2016 Sep;35(9):661–671. [Google Scholar]
- 13.Munir K., Biesiekierski A., Wen C., Li Y. Metallic Biomaterials Processing and Medical Device Manufacturing. Elsevier; 2020. Selective laser melting in biomedical manufacturing; pp. 235–269. [Google Scholar]
- 14.Lv Y., Wang B., Liu G., Tang Y., Lu E., Xie K., et al. Metal material, properties and design methods of porous biomedical scaffolds for additive manufacturing: a review. Front. Bioeng. Biotechnol. 2021 Mar 26;9 doi: 10.3389/fbioe.2021.641130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liverani E., Rogati G., Pagani S., Brogini S., Fortunato A., Caravaggi P. Mechanical interaction between additive-manufactured metal lattice structures and bone in compression: implications for stress shielding of orthopaedic implants. J. Mech. Behav. Biomed. Mater. 2021 Sep;121(104608) doi: 10.1016/j.jmbbm.2021.104608. [DOI] [PubMed] [Google Scholar]
- 16.Harun W.S.W., Kamariah M.S.I.N., Muhamad N., Ghani S.A.C., Ahmad F., Mohamed Z. A review of powder additive manufacturing processes for metallic biomaterials. Powder Technol. 2018 Mar;327:128–151. [Google Scholar]
- 17.Wang H., Su K., Su L., Liang P., Ji P., Wang C. Comparison of 3D-printed porous tantalum and titanium scaffolds on osseointegration and osteogenesis. Mater. Sci. Eng., C. 2019 Nov;104 doi: 10.1016/j.msec.2019.109908. [DOI] [PubMed] [Google Scholar]
- 18.Wang X., Zhou K., Li Y., Xie H., Wang B. Preparation, modification, and clinical application of porous tantalum scaffolds. Front. Bioeng. Biotechnol. 2023 Apr 4;11 doi: 10.3389/fbioe.2023.1127939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao L., Zhang Z., Song Y., Liu S., Qi Y., Wang X., et al. Mechanical properties and in vitro biodegradation of newly developed porous Zn scaffolds for biomedical applications. Mater. Des. 2016 Oct;108:136–144. [Google Scholar]
- 20.Hehrlein C., Schorch B., Kress N., Arab A., von Zur Mühlen C., Bode C., et al. Zn-alloy provides a novel platform for mechanically stable bioresorbable vascular stents. PLoS One. 2019 Jan 2;14(1) doi: 10.1371/journal.pone.0209111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heiden M., Walker E., Stanciu L. Magnesium, iron and zinc alloys, the trifecta of bioresorbable orthopaedic and vascular implantation-a review. J. Biotechnol. Biomater. 2015;5(2):1. [Google Scholar]
- 22.Toong D.W.Y., Ng J.C.K., Huang Y., Wong P.E.H., Leo H.L., Venkatraman S.S., et al. Bioresorbable metals in cardiovascular stents: material insights and progress. Materialia. 2020 Aug;12(100727) [Google Scholar]
- 23.Yazdimamaghani M., Razavi M., Vashaee D., Moharamzadeh K., Boccaccini A.R., Tayebi L. Porous magnesium-based scaffolds for tissue engineering. Mater. Sci. Eng., C. 2017 Feb 1;71:1253–1266. doi: 10.1016/j.msec.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 24.Aljihmani L., Alic L., Boudjemline Y., Hijazi Z.M., Mansoor B., Serpedin E., et al. Magnesium-based bioresorbable Stent materials: review of reviews. J Bio- Tribo-Corros [Internet] 2019 Mar;5(1) doi: 10.1007/s40735-019-0216-x. [DOI] [Google Scholar]
- 25.Murr L.E. A metallographic review of 3D printing/additive manufacturing of metal and alloy products and components. Metallogr Microstruct Anal. 2018 Apr;7(2):103–132. [Google Scholar]
- 26.Yelick P.C., Sharpe P.T. Tooth bioengineering and regenerative dentistry. J. Dent. Res. 2019 Oct;98(11):1173–1182. doi: 10.1177/0022034519861903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baino F., Verné E. Glass-based coatings on biomedical implants: a state-of-the-art review. Biomed Glasses. 2017 Apr 25;3(1):1–17. [Google Scholar]
- 28.Smith J.R., Lamprou D.A., Larson C., Upson S.J. Biomedical applications of polymer and ceramic coatings: a review of recent developments. Trans. Inst. Met. Finish. 2022 Jan 2;100(1):25–35. [Google Scholar]
- 29.Do A.-V., Khorsand B., Geary S.M., Salem A.K. 3D printing of scaffolds for tissue regeneration applications. Adv. Healthcare Mater. 2015 Aug 26;4(12):1742–1762. doi: 10.1002/adhm.201500168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirkhalaf M., Men Y., Wang R., No Y., Zreiqat H. Personalized 3D printed bone scaffolds: a review. Acta Biomater. 2023 Jan 15;156:110–124. doi: 10.1016/j.actbio.2022.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Tesfamariam B. Bioresorbable vascular scaffolds: biodegradation, drug delivery and vascular remodeling. Pharmacol. Res. 2016 May;107:163–171. doi: 10.1016/j.phrs.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 32.Szymczyk-Ziółkowska P., Łabowska M.B., Detyna J., Michalak I., Gruber P. A review of fabrication polymer scaffolds for biomedical applications using additive manufacturing techniques. Biocybern. Biomed. Eng. 2020 Apr;40(2):624–638. [Google Scholar]
- 33.Barrack R.L., Burak C., Skinner H.B. Concerns about ceramics in THA. Clin. Orthop. Relat. Res. 2004 Dec;429(429):73–79. doi: 10.1097/01.blo.0000150132.11142.d2. [DOI] [PubMed] [Google Scholar]
- 34.Marques A., Miranda G., Silva F., Pinto P., Carvalho Ó. Review on current limits and potentialities of technologies for biomedical ceramic scaffolds production. J. Biomed. Mater. Res. B Appl. Biomater. 2021 Mar;109(3):377–393. doi: 10.1002/jbm.b.34706. [DOI] [PubMed] [Google Scholar]
- 35.Arjunan A., Demetriou M., Baroutaji A., Wang C. Mechanical performance of highly permeable laser melted Ti6Al4V bone scaffolds. J. Mech. Behav. Biomed. Mater. 2020 Feb;102(103517) doi: 10.1016/j.jmbbm.2019.103517. [DOI] [PubMed] [Google Scholar]
- 36.Hussein M., Mohammed A., Al-Aqeeli N. Wear characteristics of metallic biomaterials: a review. Materials. 2015 May 21;8(5):2749–2768. [Google Scholar]
- 37.Prasad K., Bazaka O., Chua M., Rochford M., Fedrick L., Spoor J., et al. Metallic biomaterials: current challenges and opportunities. Materials. 2017 Jul 31;10(8):884. doi: 10.3390/ma10080884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y.-L., Niinomi M., Akahori T., Nakai M., Fukui H. Comparison of various properties between titanium-tantalum alloy and pure titanium for biomedical applications. Mater. Trans. 2007;48(3):380–384. [Google Scholar]
- 39.Eliades T., Pratsinis H., Kletsas D., Eliades G., Makou M. Characterization and cytotoxicity of ions released from stainless steel and nickel-titanium orthodontic alloys. Am. J. Orthod. Dentofacial Orthop. 2004 Jan;125(1):24–29. doi: 10.1016/j.ajodo.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Liu Q., Li A., Liu S., Fu Q., Xu Y., Dai J., et al. Cytotoxicity of biodegradable zinc and its alloys: a systematic review. J. Funct. Biomater. 2023 Apr 7;14(4):206. doi: 10.3390/jfb14040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhen Z., Liu X., Huang T., Xi T., Zheng Y. Hemolysis and cytotoxicity mechanisms of biodegradable magnesium and its alloys. Mater. Sci. Eng., C. 2015 Jan;46:202–206. doi: 10.1016/j.msec.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 42.Scarcello E., Lison D. Are Fe-based stenting materials biocompatible? A critical review of in vitro and in vivo studies. J. Funct. Biomater. 2019 Dec 21;11(1):2. doi: 10.3390/jfb11010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vitkov L., Hartl D., Hannig M. Is osseointegration inflammation-triggered? Med. Hypotheses. 2016 Aug;93:1–4. doi: 10.1016/j.mehy.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Ehashi T., Takemura T., Hanagata N., Minowa T., Kobayashi H., Ishihara K., et al. Comprehensive genetic analysis of early host body reactions to the bioactive and bio-inert porous scaffolds. PLoS One. 2014 Jan 14;9(1) doi: 10.1371/journal.pone.0085132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Z., Zhang W., Wang M., Backman L.J., Chen J. Effects of zinc, magnesium, and iron ions on bone tissue engineering. ACS Biomater. Sci. Eng. 2022 Jun 13;8(6):2321–2335. doi: 10.1021/acsbiomaterials.2c00368. [DOI] [PubMed] [Google Scholar]
- 46.Gąsior G., Szczepański J., Radtke A. Biodegradable iron-based materials-what was done and what more can be done? Materials. 2021 Jun 18;14(12):3381. doi: 10.3390/ma14123381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiong J., Mines R., Ghosh R., Vaziri A., Ma L., Ohrndorf A., et al. Advanced micro-lattice materials. Adv. Eng. Mater. 2015 Sep;17(9):1253–1264. [Google Scholar]
- 48.Jang D., Meza L.R., Greer F., Greer J.R. Fabrication and deformation of three-dimensional hollow ceramic nanostructures. Nat. Mater. 2013 Oct;12(10):893–898. doi: 10.1038/nmat3738. [DOI] [PubMed] [Google Scholar]
- 49.Huang J.S., Lin J.Y. Fatigue of cellular materials. Acta Mater. 1996 Jan;44(1):289–296. [Google Scholar]
- 50.Huang J.S., Lin J.Y. Modeling of fatigue for cellular materials. Mater Res Soc Symp Proc [Internet] 1998;521(27) doi: 10.1557/proc-521-27. [DOI] [Google Scholar]
- 51.Benedetti M., du Plessis A., Ritchie R.O., Dallago M., Razavi N., Berto F. Architected cellular materials: a review on their mechanical properties towards fatigue-tolerant design and fabrication. Mater. Sci. Eng. R Rep. 2021 Apr;144(100606) [Google Scholar]
- 52.Peng C., Tran P., Nguyen-Xuan H., Ferreira A.J.M. Mechanical performance and fatigue life prediction of lattice structures: parametric computational approach. Compos. Struct. 2020 Mar;235(111821) [Google Scholar]
- 53.Noble F.W., Lilley J. Fatigue crack growth in polyurethane foam. J. Mater. Sci. 1981 Jul;16(7):1801–1808. [Google Scholar]
- 54.Tan C., Zou J., Li S., Jamshidi P., Abena A., Forsey A., et al. Additive manufacturing of bio-inspired multi-scale hierarchically strengthened lattice structures. Int. J. Mach. Tool Manufact. 2021 Aug;167 [Google Scholar]
- 55.Fratzl P., Weinkamer R. Nature's hierarchical materials. Prog. Mater. Sci. 2007 Nov;52(8):1263–1334. [Google Scholar]
- 56.Le Ferrand H., Athanasiou C.E. A materials perspective on the design of damage-resilient bone implants through additive/advanced manufacturing. JOM. 2020 Mar;72(3):1195–1210. 1989. [Google Scholar]
- 57.Bal B.S., Zhu W., Zanocco M., Marin E., Sugano N., McEntire B.J., et al. Reconciling in vivo and in vitro kinetics of the polymorphic transformation in zirconia-toughened alumina for hip joints: I. Phenomenology. Mater. Sci. Eng., C. 2017 Mar;72:252–258. doi: 10.1016/j.msec.2016.11.059. [DOI] [PubMed] [Google Scholar]
- 58.Pezzotti G., Zhu W., Zanocco M., Marin E., Sugano N., McEntire B.J., et al. Reconciling in vivo and in vitro kinetics of the polymorphic transformation in zirconia-toughened alumina for hip joints: II. Theory. Mater Sci Eng C Mater Biol Appl. 2017 Feb 1;71:446–451. doi: 10.1016/j.msec.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 59.De Aza A.H., Chevalier J., Fantozzi G., Schehl M., Torrecillas R. Crack growth resistance of alumina, zirconia and zirconia toughened alumina ceramics for joint prostheses. Biomaterials. 2002 Feb;23(3):937–945. doi: 10.1016/s0142-9612(01)00206-x. [DOI] [PubMed] [Google Scholar]
- 60.McEntire B.J., Enomoto Y., Zhu W., Boffelli M., Marin E., Pezzotti G. Surface toughness of silicon nitride bioceramics: II, Comparison with commercial oxide materials. J. Mech. Behav. Biomed. Mater. 2016 Feb;54:346–359. doi: 10.1016/j.jmbbm.2015.08.044. [DOI] [PubMed] [Google Scholar]
- 61.Saikko V., Ahlroos T., Calonius O., Keränen J. Wear simulation of total hip prostheses with polyethylene against CoCr, alumina and diamond-like carbon. Biomaterials. 2001 Jun;22(12):1507–1514. doi: 10.1016/s0142-9612(00)00306-9. [DOI] [PubMed] [Google Scholar]
- 62.Dahl J., Snorrason F., Nordsletten L., Röhrl S.M. More than 50% reduction of wear in polyethylene liners with alumina heads compared to cobalt-chrome heads in hip replacements: a 10-year follow-up with radiostereometry in 43 hips. Acta Orthop. 2013 Aug;84(4):360–364. doi: 10.3109/17453674.2013.810516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Germain M.A., Hatton A., Williams S., Matthews J.B., Stone M.H., Fisher J., et al. Comparison of the cytotoxicity of clinically relevant cobalt-chromium and alumina ceramic wear particles in vitro. Biomaterials. 2003 Feb;24(3):469–479. doi: 10.1016/s0142-9612(02)00360-5. [DOI] [PubMed] [Google Scholar]
- 64.Plecko M., Sievert C., Andermatt D., Frigg R., Kronen P., Klein K., et al. Osseointegration and biocompatibility of different metal implants--a comparative experimental investigation in sheep. BMC Muscoskel. Disord. 2012 Mar 8;13:32. doi: 10.1186/1471-2474-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trinidad J., Marco I., Arruebarrena G., Wendt J., Letzig D., Sáenz de Argandoña E., et al. Processing of magnesium porous structures by infiltration casting for biomedical applications. Adv. Eng. Mater. 2014 Feb;16(2):241–247. [Google Scholar]
- 66.Grundei H. Ossäre Integration. Springer Berlin Heidelberg; 2006. Geschichtliche Entwicklung der Endoprothetik und der Fixation durch Spongiosa-Metal; pp. 2–13. [Google Scholar]
- 67.Wang C., Chen H., Zhu X., Xiao Z., Zhang K., Zhang X. An improved polymeric sponge replication method for biomedical porous titanium scaffolds. Mater. Sci. Eng., C. 2017 Jan 1;70(Pt 2):1192–1199. doi: 10.1016/j.msec.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 68.Dewidar M.M., Yoon H.-C., Lim J.K. Mechanical properties of metals for biomedical applications using powder metallurgy process: a review. Met. Mater. Int. 2006 Jun;12(3):193–206. [Google Scholar]
- 69.Rodriguez-Contreras A., Punset M., Calero J.A., Gil F.J., Ruperez E., Manero J.M. Powder metallurgy with space holder for porous titanium implants: a review. J. Mater. Sci. Technol. 2021 Jun;76:129–149. [Google Scholar]
- 70.Applications PM. PM Applications: Powder Metallurgy Technology in Production of Medical Implants..
- 71.Munir K., Biesiekierski A., Wen C., Li Y. Metallic Biomaterials Processing and Medical Device Manufacturing. Elsevier; 2020. Powder metallurgy in manufacturing of medical devices; pp. 159–190. [Google Scholar]
- 72.Čapek J., Pinc J., Msallamová Š., Jablonská E., Veřtát P., Kubásek J., et al. Thermal plasma spraying as a new approach for preparation of zinc biodegradable scaffolds: a complex material characterization. J. Therm. Spray Technol. 2019 Apr;28(4):826–841. [Google Scholar]
- 73.Kupp D., Claar D., Flemmig Fraunhofer Usa K. 2002. Processing of Controlled Porosity Titanium-Based Materials.https://tu-dresden.de/ing/maschinenwesen/ifww/psv/ressourcen/dateien/veroeffentlichung/2002/2002_flemmig1.pdf [Internet]. tu-dresden.de; [cited 2023 Aug 1]. Available from: [Google Scholar]
- 74.Metallurgical Characterization of a Porous Tantalum Biomaterial (Trabecular Metal) for Orthopaedic Implant Applications.
- 75.Lequin M.B., Verbaan D., Bouma G.J. Posterior lumbar interbody fusion with stand-alone Trabecular Metal cages for repeatedly recurrent lumbar disc herniation and back pain. J. Neurosurg. Spine. 2014 Jun;20(6):617–622. doi: 10.3171/2014.2.SPINE13548. [DOI] [PubMed] [Google Scholar]
- 76.Gross A.E., Goodman S.B. Rebuilding the skeleton: the intraoperative use of trabecular metal in revision total hip arthroplasty. J. Arthroplasty. 2005 Jun;20(4 Suppl 2):91–93. doi: 10.1016/j.arth.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 77.Hasart O., Perka C., Lehnigk R., Tohtz S. Rekonstruktion größerer Pfannendefekte mit metallischen Augmentaten – trabecular Metal Technology. Operat. Orthop. Traumatol. 2010 Jul;22(3):268–277. doi: 10.1007/s00064-010-8026-9. [DOI] [PubMed] [Google Scholar]
- 78.Bencharit S., Byrd W.C., Altarawneh S., Hosseini B., Leong A., Reside G., et al. Development and applications of porous tantalum trabecular metal-enhanced titanium dental implants. Clin. Implant Dent. Relat. Res. 2014 Dec;16(6):817–826. doi: 10.1111/cid.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Munir K.S., Li Y., Wen C. Metallic Foam Bone. Elsevier; 2017. Metallic scaffolds manufactured by selective laser melting for biomedical applications; pp. 1–23. [Google Scholar]
- 80.Yang Y., Wang G., Liang H., Gao C., Peng S., Shen L., et al. Additive manufacturing of bone scaffolds. Int J Bioprinting. 2019;5(1):148. doi: 10.18063/IJB.v5i1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang C., Sun B., Zhang Y., Wang C., Yang G. Design of a novel trabecular acetabular cup and selective laser melting fabrication. Materials. 2022 Sep 4;15(17):6142. doi: 10.3390/ma15176142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dhandapani R., Krishnan P.D., Zennifer A., Kannan V., Manigandan A., Arul M.R., et al. Additive manufacturing of biodegradable porous orthopaedic screw. Bioact. Mater. 2020 Sep;5(3):458–467. doi: 10.1016/j.bioactmat.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ji F., Zhang C., Chen X. Structure optimization of porous dental implant based on 3D printing. IOP Conf. Ser. Mater. Sci. Eng. 2018 Mar 1;324(1) [Google Scholar]
- 84.Selvaraj S., Dorairaj J., Shivasankar N. 3d cranial reconstruction using titanium implant - a case report. Afr. Health Sci. 2022 Sep 28;22(3):383–390. doi: 10.4314/ahs.v22i3.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marin E., Fusi S., Pressacco M., Paussa L., Fedrizzi L. Characterization of cellular solids in Ti6Al4V for orthopaedic implant applications: trabecular titanium. J. Mech. Behav. Biomed. Mater. 2010 Jul;3(5):373–381. doi: 10.1016/j.jmbbm.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 86.Marin E., Pressacco M., Fusi S., Lanzutti A., Turchet S., Fedrizzi L. Characterization of grade 2 commercially pure Trabecular Titanium structures. Mater. Sci. Eng., C. 2013 Jul 1;33(5):2648–2656. doi: 10.1016/j.msec.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 87.The Bio-Compatible Dental Implant Designed by Using Non-stochastic Porosity Produced by Electron Beam Melting®(EBM).
- 88.Crovace A.M., Lacitignola L., Forleo D.M., Staffieri F., Francioso E., Di Meo A., et al. 3D biomimetic porous titanium (Ti6Al4V ELI) scaffolds for large bone critical defect reconstruction: an experimental study in sheep. Animals (Basel) 2020 Aug 11;10(8):1389. doi: 10.3390/ani10081389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carroll B.E., Palmer T.A., Beese A.M. Anisotropic tensile behavior of Ti–6Al–4V components fabricated with directed energy deposition additive manufacturing. Acta Mater. 2015 Apr;87:309–320. [Google Scholar]
- 90.Ahn D.-G. Directed energy deposition (DED) process: state of the art. Int J Precis Eng Manuf-Green Technol. 2021 Mar;8(2):703–742. [Google Scholar]
- 91.Ke D., Bose S. Effects of pore distribution and chemistry on physical, mechanical, and biological properties of tricalcium phosphate scaffolds by binder-jet 3D printing. Addit. Manuf. 2018 Aug;22:111–117. [Google Scholar]
- 92.Meenashisundaram G.K., Xu Z., Nai M.L.S., Lu S., Ten J.S., Wei J. Binder jetting additive manufacturing of high porosity 316L stainless steel metal foams. Materials. 2020 Aug 24;13(17):3744. doi: 10.3390/ma13173744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mostafaei A., Stevens E.L., Ference J.J., Schmidt D.E., Chmielus M. Binder jetting of a complex-shaped metal partial denture framework. Addit. Manuf. 2018 May;21:63–68. [Google Scholar]
- 94.Oliver-Urrutia C., Kashimbetova A., Slámečka K., Čelko L., Montufar E.B. Robocasting additive manufacturing of titanium and titanium alloys: a review. Trans. Indian Inst. Met. 2023 Feb;76(2):389–402. [Google Scholar]
- 95.Huang H.-H., Ho C.-T., Lee T.-H., Lee T.-L., Liao K.-K., Chen F.-L. Effect of surface roughness of ground titanium on initial cell adhesion. Biomol. Eng. 2004 Nov;21(3–5):93–97. doi: 10.1016/j.bioeng.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 96.Anselme K., Bigerelle M., Noel B., Dufresne E., Judas D., Iost A., et al. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J. Biomed. Mater. Res. 2000 Feb;49(2):155–166. doi: 10.1002/(sici)1097-4636(200002)49:2<155::aid-jbm2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 97.Taniguchi N., Fujibayashi S., Takemoto M., Sasaki K., Otsuki B., Nakamura T., et al. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: an in vivo experiment. Mater. Sci. Eng., C. 2016 Feb;59:690–701. doi: 10.1016/j.msec.2015.10.069. [DOI] [PubMed] [Google Scholar]
- 98.Chen Z., Yan X., Yin S., Liu L., Liu X., Zhao G., et al. Influence of the pore size and porosity of selective laser melted Ti6Al4V ELI porous scaffold on cell proliferation, osteogenesis and bone ingrowth. Mater. Sci. Eng., C. 2020 Jan;106(110289) doi: 10.1016/j.msec.2019.110289. [DOI] [PubMed] [Google Scholar]
- 99.Kapat K., Srivas P.K., Rameshbabu A.P., Maity P.P., Jana S., Dutta J., et al. Influence of porosity and pore-size distribution in Ti6Al4 V foam on physicomechanical properties, osteogenesis, and quantitative validation of bone ingrowth by micro-computed tomography. ACS Appl. Mater. Interfaces. 2017 Nov 15;9(45):39235–39248. doi: 10.1021/acsami.7b13960. [DOI] [PubMed] [Google Scholar]
- 100.Itälä A.I., Ylänen H.O., Ekholm C., Karlsson K.H., Aro H.T. Pore diameter of more than 100 μm is not requisite for bone ingrowth in rabbits. J. Biomed. Mater. Res. 2001;58(6):679–683. doi: 10.1002/jbm.1069. [DOI] [PubMed] [Google Scholar]
- 101.Al Muderis M., Bohling U., Grittner U., Gerdesmeyer L., Scholz J. Cementless total hip arthroplasty using the spongiosa-I fully coated cancellous metal surface. J Bone Joint Surg Am. 2011 Jun;93(11):1039–1044. doi: 10.2106/JBJS.I.01757. [DOI] [PubMed] [Google Scholar]
- 102.Ayers R.A., Simske S.J., Nunes C.R., Wolford L.M. Long-term bone ingrowth and residual microhardness of porous block hydroxyapatite implants in humans. J. Oral Maxillofac. Surg. 1998 Nov;56(11):1297–1301. doi: 10.1016/s0278-2391(98)90613-9. ; discussion 1302. [DOI] [PubMed] [Google Scholar]
- 103.Otsuki B., Takemoto M., Fujibayashi S., Neo M., Kokubo T., Nakamura T. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials. 2006 Dec;27(35):5892–5900. doi: 10.1016/j.biomaterials.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 104.Jones A.C., Arns C.H., Sheppard A.P., Hutmacher D.W., Milthorpe B.K., Knackstedt M.A. Assessment of bone ingrowth into porous biomaterials using MICRO-CT. Biomaterials. 2007 May;28(15):2491–2504. doi: 10.1016/j.biomaterials.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 105.Jones J.R., Poologasundarampillai G., Atwood R.C., Bernard D., Lee P.D. Non-destructive quantitative 3D analysis for the optimisation of tissue scaffolds. Biomaterials. 2007 Mar;28(7):1404–1413. doi: 10.1016/j.biomaterials.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 106.Lütten C., Lorenz H., Thomas W. Metallspongiöse Endoprothesen für Revisionseingriffe am Hüftgelenk. Z. Orthop. Ihre Grenzgeb. 1990 Mar;128(2):153–159. doi: 10.1055/s-2008-1039492. [DOI] [PubMed] [Google Scholar]
- 107.Wen C.E., Yamada Y., Shimojima K., Chino Y., Hosokawa H., Mabuchi M. Novel titanium foam for bone tissue engineering. J. Mater. Res. 2002 Oct;17(10):2633–2639. [Google Scholar]
- 108.Brezny R., Green D.J. The effect of cell size on the mechanical behavior of cellular materials. Acta Metall. Mater. 1990 Dec;38(12):2517–2526. [Google Scholar]
- 109.Woesz A., Stampfl J., Fratzl P. Cellular solids beyond the apparent density– an experimental assessment of mechanical properties. Adv. Eng. Mater. 2004 Mar;6(3):134–138. [Google Scholar]
- 110.Li S.J., Xu Q.S., Wang Z., Hou W.T., Hao Y.L., Yang R., et al. Influence of cell shape on mechanical properties of Ti-6Al-4V meshes fabricated by electron beam melting method. Acta Biomater. 2014 Oct;10(10):4537–4547. doi: 10.1016/j.actbio.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 111.Yin S., Wu L., Nutt S. Stretch–bend-hybrid hierarchical composite pyramidal lattice cores. Compos. Struct. 2013 Apr;98:153–159. [Google Scholar]
- 112.Taylor S.V., Moustafa A.R., Cordero Z.C. Interpenetrating lattices with tailorable energy absorption in tension. Acta Mater. 2021 Sep;216(117115) [Google Scholar]
- 113.Yan C., Hao L., Hussein A., Young P., Raymont D. Advanced lightweight 316L stainless steel cellular lattice structures fabricated via selective laser melting. Mater Eng. 2014 Mar;55:533–541. [Google Scholar]
- 114.Ford C.M., Gibson L.J. Uniaxial strength asymmetry in cellular materials: an analytical model. Int. J. Mech. Sci. 1998 Jun;40(6):521–531. [Google Scholar]
- 115.Roberts A.P., Garboczi E.J. Elastic properties of model random three-dimensional open-cell solids. J. Mech. Phys. Solid. 2002 Jan;50(1):33–55. [Google Scholar]
- 116.DeValk T., Lakes R. Poisson's ratio and modulus of gyroid lattices. Phys. Status Solidi B. 2021 Dec;258(12) [Google Scholar]
- 117.Chang H.-I., Wang Y. Regenerative Medicine and Tissue Engineering - Cells and Biomaterials. InTech; 2011. Cell responses to surface and architecture of tissue engineering scaffolds. [Google Scholar]
- 118.Stachewicz U., Szewczyk P.K., Kruk A., Barber A.H., Czyrska-Filemonowicz A. Pore shape and size dependence on cell growth into electrospun fiber scaffolds for tissue engineering: 2D and 3D analyses using SEM and FIB-SEM tomography. Mater. Sci. Eng., C. 2019 Feb;95:397–408. doi: 10.1016/j.msec.2017.08.076. [DOI] [PubMed] [Google Scholar]
- 119.Bidan C.M., Kommareddy K.P., Rumpler M., Kollmannsberger P., Fratzl P., Dunlop J.W.C. Geometry as a factor for tissue growth: towards shape optimization of tissue engineering scaffolds. Adv. Healthcare Mater. 2013 Jan;2(1):186–194. doi: 10.1002/adhm.201200159. [DOI] [PubMed] [Google Scholar]
- 120.Karageorgiou V., Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005 Sep;26(27):5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 121.Wang X., Xu S., Zhou S., Xu W., Leary M., Choong P., et al. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: a review. Biomaterials. 2016 Mar;83:127–141. doi: 10.1016/j.biomaterials.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 122.Mitra I., Bose S., Dernell W.S., Dasgupta N., Eckstrand C., Herrick J., et al. 3D Printing in alloy design to improve biocompatibility in metallic implants. Mater. Today. 2021 May;45:20–34. doi: 10.1016/j.mattod.2020.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tammas-Williams S., Withers P.J., Todd I., Prangnell P.B. The influence of porosity on fatigue crack initiation in additively manufactured titanium components. Sci. Rep. 2017 Aug 4;7(1):7308. doi: 10.1038/s41598-017-06504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bandyopadhyay A., Espana F., Balla V.K., Bose S., Ohgami Y., Davies N.M. Influence of porosity on mechanical properties and in vivo response of Ti6Al4V implants. Acta Biomater. 2010 Apr;6(4):1640–1648. doi: 10.1016/j.actbio.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Weißmann V., Bader R., Hansmann H., Laufer N. Influence of the structural orientation on the mechanical properties of selective laser melted Ti6Al4V open-porous scaffolds. Mater. Des. 2016 Apr;95:188–197. [Google Scholar]
- 126.Ashby M.F. The properties of foams and lattices. Philos Trans A Math Phys Eng Sci. 2006 Jan 15;364(1838):15–30. doi: 10.1098/rsta.2005.1678. [DOI] [PubMed] [Google Scholar]
- 127.Hunt J.A. Regenerative medicine: materials in a cellular world. Nat. Mater. 2008 Aug;7(8):617–618. doi: 10.1038/nmat2242. [DOI] [PubMed] [Google Scholar]
- 128.Deshpande V.S., Ashby M.F., Fleck N.A. Foam topology: bending versus stretching dominated architectures. Acta Mater. 2001 Apr;49(6):1035–1040. [Google Scholar]
- 129.Truscello S., Kerckhofs G., Van Bael S., Pyka G., Schrooten J., Van Oosterwyck H. Prediction of permeability of regular scaffolds for skeletal tissue engineering: a combined computational and experimental study. Acta Biomater. 2012 Apr;8(4):1648–1658. doi: 10.1016/j.actbio.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 130.Savio G., Rosso S., Meneghello R., Concheri G. Geometric modeling of cellular materials for additive manufacturing in biomedical field: a review. Appl. Bionics Biomech. 2018:1–14. doi: 10.1155/2018/1654782. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Giannitelli S.M., Accoto D., Trombetta M., Rainer A. Current trends in the design of scaffolds for computer-aided tissue engineering. Acta Biomater. 2014 Feb;10(2):580–594. doi: 10.1016/j.actbio.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 132.Carletti E., Motta A., Migliaresi C. Scaffolds for tissue engineering and 3D cell culture. Methods Mol. Biol. 2011;695:17–39. doi: 10.1007/978-1-60761-984-0_2. [DOI] [PubMed] [Google Scholar]
- 133.Chi J., Wang M., Chen J., Hu L., Chen Z., Backman L.J., et al. Topographic orientation of scaffolds for tissue regeneration: recent advances in biomaterial design and applications. Biomimetics. 2022 Sep 12;7(3):131. doi: 10.3390/biomimetics7030131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Davies G.J., Zhen S. Metallic foams: their production, properties and applications. J. Mater. Sci. 1983 Jul;18(7):1899–1911. [Google Scholar]
- 135.Yang N., Gao L., Zhou K. Simple method to generate and fabricate stochastic porous scaffolds. Mater. Sci. Eng., C. 2015 Nov 1;56:444–450. doi: 10.1016/j.msec.2015.06.039. [DOI] [PubMed] [Google Scholar]
- 136.Kanwar S., Al-Ketan O., Vijayavenkataraman S. A novel method to design biomimetic, 3D printable stochastic scaffolds with controlled porosity for bone tissue engineering. Mater. Des. 2022 Aug;220 [Google Scholar]
- 137.Kechagias S., Oosterbeek R.N., Munford M.J., Ghouse S., Jeffers J.R.T. Controlling the mechanical behaviour of stochastic lattice structures: the key role of nodal connectivity. Addit. Manuf. 2022 Jun;54 [Google Scholar]
- 138.Pei X., Wang L., Zhou C., Wu L., Lei H., Fan S., et al. Ti6Al4V orthopedic implant with biomimetic heterogeneous structure via 3D printing for improving osteogenesis. Mater. Des. 2022 Sep;221 [Google Scholar]
- 139.Montazerian H., Mohamed M.G.A., Montazeri M.M., Kheiri S., Milani A.S., Kim K., et al. Permeability and mechanical properties of gradient porous PDMS scaffolds fabricated by 3D-printed sacrificial templates designed with minimal surfaces. Acta Biomater. 2019 Sep 15;96:149–160. doi: 10.1016/j.actbio.2019.06.040. [DOI] [PubMed] [Google Scholar]
- 140.Davoodi E., Montazerian H., Mirhakimi A.S., Zhianmanesh M., Ibhadode O., Shahabad S.I., et al. Additively manufactured metallic biomaterials. Bioact. Mater. 2022 Sep;15:214–249. doi: 10.1016/j.bioactmat.2021.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alonzo M., Primo F.A., Kumar S.A., Mudloff J.A., Dominguez E., Fregoso G., et al. Bone tissue engineering techniques, advances and scaffolds for treatment of bone defects. Curr Opin Biomed Eng. 2021 Mar;17 doi: 10.1016/j.cobme.2020.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lin H., Shi S., Lan X., Quan X., Xu Q., Yao G., et al. Scaffold 3D-printed from metallic nanoparticles-containing ink simultaneously eradicates tumor and repairs tumor-associated bone defects. Small Methods. 2021 Sep;5(9) doi: 10.1002/smtd.202100536. [DOI] [PubMed] [Google Scholar]
- 143.Wen C.E., Yamada Y., Shimojima K., Chino Y., Asahina T., Mabuchi M. Processing and mechanical properties of autogenous titanium implant materials. J. Mater. Sci. Mater. Med. 2002 Apr;13(4):397–401. doi: 10.1023/a:1014344819558. [DOI] [PubMed] [Google Scholar]
- 144.Blanco J.F., Sánchez-Guijo F.M., Carrancio S., Muntion S., García-Briñon J., del Cañizo M.-C. Titanium and tantalum as mesenchymal stem cell scaffolds for spinal fusion: an in vitro comparative study. Eur. Spine J. 2011 Aug;20(Suppl 3):353–360. doi: 10.1007/s00586-011-1901-8. (S3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Elhattab K., Hefzy M.S., Hanf Z., Crosby B., Enders A., Smiczek T., et al. Biomechanics of additively manufactured metallic scaffolds-A review. Materials. 2021 Nov 12;14(22):6833. doi: 10.3390/ma14226833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Smit T.H., Müller R., van Dijk M., Wuisman P.I.J.M. Changes in bone architecture during spinal fusion: three years follow-up and the role of cage stiffness. Spine. 2003 Aug 15;28(16):1802–1808. doi: 10.1097/01.BRS.0000083285.09184.7A. ; discussion 1809. [DOI] [PubMed] [Google Scholar]
- 147.Milošev I., Levašič V., Kovač S., Sillat T., Virtanen S., Tiainen V.-M., et al. Joint Replacement Technology. Elsevier; 2021. Metals for joint replacement; pp. 65–122. [Google Scholar]
- 148.Wong K.-C., Scheinemann P. Additive manufactured metallic implants for orthopaedic applications. Sci. China Mater. 2018 Apr;61(4):440–454. [Google Scholar]
- 149.Tan X.P., Tan Y.J., Chow C.S.L., Tor S.B., Yeong W.Y. Metallic powder-bed based 3D printing of cellular scaffolds for orthopaedic implants: a state-of-the-art review on manufacturing, topological design, mechanical properties and biocompatibility. Mater. Sci. Eng. C. 2017 Jul 1;76:1328–1343. doi: 10.1016/j.msec.2017.02.094. [DOI] [PubMed] [Google Scholar]
- 150.Ângelo D.F., Gil F.M. Tissue engineering in temporomandibular joint reconstruction. Atlas Oral Maxillofac Surg Clin North Am. 2022 Sep;30(2):235–246. doi: 10.1016/j.cxom.2022.06.003. [DOI] [PubMed] [Google Scholar]
- 151.Saijo H., Igawa K., Kanno Y., Mori Y., Kondo K., Shimizu K., et al. Maxillofacial reconstruction using custom-made artificial bones fabricated by inkjet printing technology. J. Artif. Organs. 2009 Sep 19;12(3):200–205. doi: 10.1007/s10047-009-0462-7. [DOI] [PubMed] [Google Scholar]
- 152.Zhang Q., Wu W., Qian C., Xiao W., Zhu H., Guo J., et al. Advanced biomaterials for repairing and reconstruction of mandibular defects. Mater. Sci. Eng., C. 2019 Oct;103 doi: 10.1016/j.msec.2019.109858. [DOI] [PubMed] [Google Scholar]
- 153.Abraham A.M., Venkatesan S. A review on application of biomaterials for medical and dental implants. Proc Inst Mech Eng L J Mater Des Appl. 2023 Feb;237(2):249–273. [Google Scholar]
- 154.Al-Zubaidi S.M., Madfa A.A., Mufadhal A.A., Aldawla M.A., Hameed O.S., Yue X.-G. Improvements in clinical durability from functional biomimetic metallic dental implants. Front Mater [Internet. 2020 May 22;7 doi: 10.3389/fmats.2020.00106. [DOI] [Google Scholar]
- 155.Nouri A. Metallic Foam Bone. Elsevier; 2017. Titanium foam scaffolds for dental applications; pp. 131–160. [Google Scholar]
- 156.He X.-Y., Yu H.-M., Lin S., Li Y.-Z. Advances in the application of mesenchymal stem cells, exosomes, biomimetic materials, and 3D printing in osteoporosis treatment. Cell. Mol. Biol. Lett. 2021 Nov 14;26(1):47. doi: 10.1186/s11658-021-00291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lei C., Song J.-H., Li S., Zhu Y.-N., Liu M.-Y., Wan M.-C., et al. Advances in materials-based therapeutic strategies against osteoporosis. Biomaterials. 2023 May;296 doi: 10.1016/j.biomaterials.2023.122066. [DOI] [PubMed] [Google Scholar]
- 158.Wang B., Feng C., Pan J., Zhou S., Sun Z., Shao Y., et al. The effect of 3D printing metal materials on osteoporosis treatment. BioMed Res. Int. 2021 Jun 17 doi: 10.1155/2021/9972867. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Priyadarshini B., Rama M., Chetan Vijayalakshmi U. Bioactive coating as a surface modification technique for biocompatible metallic implants: a review. J Asian Ceram Soc. 2019 Oct 2;7(4):397–406. [Google Scholar]
- 160.Sarian M.N., Iqbal N., Sotoudehbagha P., Razavi M., Ahmed Q.U., Sukotjo C., et al. Potential bioactive coating system for high-performance absorbable magnesium bone implants. Bioact. Mater. 2022 Jun;12:42–63. doi: 10.1016/j.bioactmat.2021.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang B.G.X., Myers D.E., Wallace G.G., Brandt M., Choong P.F.M. Bioactive coatings for orthopaedic implants-recent trends in development of implant coatings. Int. J. Mol. Sci. 2014 Jul 4;15(7):11878–11921. doi: 10.3390/ijms150711878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Geyao L., Yang D., Wanglin C., Chengyong W. Development and application of physical vapor deposited coatings for medical devices: a review. Procedia CIRP. 2020;89:250–262. [Google Scholar]
- 163.Kaliaraj G.S., Siva T., Ramadoss A. Surface functionalized bioceramics coated on metallic implants for biomedical and anticorrosion performance - a review. J. Mater. Chem. B. 2021 Dec 1;9(46):9433–9460. doi: 10.1039/d1tb01301g. [DOI] [PubMed] [Google Scholar]
- 164.Sun Y., Zhang X., Luo M., Hu W., Zheng L., Huang R., et al. Plasma spray vs. Electrochemical deposition: toward a better osteogenic effect of hydroxyapatite coatings on 3D-printed titanium scaffolds. Front. Bioeng. Biotechnol. 2021 Jul 26;9 doi: 10.3389/fbioe.2021.705774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zieliński A., Sobieszczyk S., Serbiński W., Seramak T., Ossowska A. Materials design for the titanium scaffold based implant. Solid State Phenom. 2011 Dec;183:225–232. [Google Scholar]
- 166.Dobrzański L.A., Dobrzańska-Danikiewicz A.D., Szindler M., Achtelik-Franczak A., Pakieła W. Atomic layer deposition of TiO2 onto porous biomaterials. Archives of Materials Science and Engineering. 2015;75(1):5–11. [Google Scholar]
- 167.Zhang X., Guan S., Qiu J., Qiao Y., Qian S., Tan J., et al. Atomic layer deposition of tantalum oxide films on 3D-printed Ti6Al4V scaffolds with enhanced osteogenic property for orthopedic implants. ACS Biomater. Sci. Eng. 2023 Jul 10;9(7):4197–4207. doi: 10.1021/acsbiomaterials.3c00217. [DOI] [PubMed] [Google Scholar]
- 168.Cachinho S.C.P., Correia R.N. Titanium scaffolds for osseointegration: mechanical, in vitro and corrosion behaviour. J. Mater. Sci. Mater. Med. 2008 Jan;19(1):451–457. doi: 10.1007/s10856-006-0052-7. [DOI] [PubMed] [Google Scholar]
- 169.Xu W., Hu W.Y., Li M.H., Ma Q.Q., Hodgson P.D., Wen C.E. Sol-gel derived HA/TiO2 double coatings on Ti scaffolds for orthopaedic applications. Trans Nonferrous Met Soc China. 2006 Jun;16:s209–s216. [Google Scholar]
- 170.Lopez-Heredia M.A., Sohier J., Gaillard C., Quillard S., Dorget M., Layrolle P. Rapid prototyped porous titanium coated with calcium phosphate as a scaffold for bone tissue engineering. Biomaterials. 2008 Jun;29(17):2608–2615. doi: 10.1016/j.biomaterials.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 171.Tamaddon M., Samizadeh S., Wang L., Blunn G., Liu C. Intrinsic osteoinductivity of porous titanium scaffold for bone tissue engineering. Int J Biomater. 2017 Jul 26;2017 doi: 10.1155/2017/5093063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tunchel S., Blay A., Kolerman R., Mijiritsky E., Shibli J.A. 3D printing/additive manufacturing single titanium dental implants: a prospective multicenter study with 3 years of follow-up. Int J Dent. 2016 May 29;2016 doi: 10.1155/2016/8590971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Longhitano G.A., Conde A., Arenas M.A., Jardini A.L., Zavaglia CA. de C., Maciel Filho R., et al. Corrosion resistance improvement of additive manufactured scaffolds by anodizing. Electrochim. Acta. 2021 Jan;366 [Google Scholar]
- 174.Lee U.-L., Yun S., Lee H., Cao H.-L., Woo S.-H., Jeong Y.-H., et al. Osseointegration of 3D-printed titanium implants with surface and structure modifications. Dent. Mater. 2022 Oct;38(10):1648–1660. doi: 10.1016/j.dental.2022.08.003. [DOI] [PubMed] [Google Scholar]
- 175.Fan X., Feng B., Liu Z., Tan J., Zhi W., Lu X., et al. Fabrication of TiO2 nanotubes on porous titanium scaffold and biocompatibility evaluation in vitro and in vivo. J. Biomed. Mater. Res. 2012 Dec;100(12):3422–3427. doi: 10.1002/jbm.a.34268. [DOI] [PubMed] [Google Scholar]
- 176.Oliveira W.F., Arruda I.R.S., Silva G.M.M., Machado G., Coelho L.C.B.B., Correia M.T.S. Functionalization of titanium dioxide nanotubes with biomolecules for biomedical applications. Mater. Sci. Eng., C. 2017 Dec;81:597–606. doi: 10.1016/j.msec.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 177.Schliephake H., Scharnweber D. Chemical and biological functionalization of titanium for dental implants. J. Mater. Chem. 2008;18(21):2404. [Google Scholar]
- 178.Kashiwagi K., Tsuji T., Shiba K. Directional BMP-2 for functionalization of titanium surfaces. Biomaterials. 2009 Feb;30(6):1166–1175. doi: 10.1016/j.biomaterials.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 179.Lin C.-C., Cheng H.-C., Huang C.-F., Lin C.-T., Lee S.-Y., Chen C.-S., et al. Enhancement of biocompatibility on bioactive titanium surface by low-temperature plasma treatment. Jpn. J. Appl. Phys. 2008;44(12R):8590. 2005 Dec 1. [Google Scholar]
- 180.Kumar S., Simpson D., Smart R.S.C. Plasma processing for inducing bioactivity in stainless steel orthopaedic screws. Surf. Coat. Technol. 2007 Dec;202(4–7):1242–1246. [Google Scholar]
- 181.Ou K.-L., Shih Y.-H., Huang C.-F., Chen C.-C., Liu C.-M. Preparation of bioactive amorphous-like titanium oxide layer on titanium by plasma oxidation treatment. Appl. Surf. Sci. 2008 Dec;255(5):2046–2051. [Google Scholar]
- 182.Grabarczyk J., Jastrzębski K., Wrotniak M. Post-processing of titanium 3D printouts with radio frequency plasma. Engineering of Biomaterials [Internet] 2021;24(160) https://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-f23d8194-9ffa-4704-adf7-1e77c6547f93 Available from: [Google Scholar]
- 183.Mallaiah M., Kumar Gupta R. Surface engineering of titanium using anodization and plasma treatment. IOP Conf. Ser. Mater. Sci. Eng. 2020 Oct 1;943(1) [Google Scholar]
- 184.Yao Y.-T., Liu S., Swain M.V., Zhang X.-P., Zhao K., Jian Y.-T. Effects of acid-alkali treatment on bioactivity and osteoinduction of porous titanium: an in vitro study. Mater. Sci. Eng., C. 2019 Jan 1;94:200–210. doi: 10.1016/j.msec.2018.08.056. [DOI] [PubMed] [Google Scholar]
- 185.Vanzillotta P.S., Sader M.S., Bastos I.N., Soares G. de A. Improvement of in vitro titanium bioactivity by three different surface treatments. Dent. Mater. 2006 Mar;22(3):275–282. doi: 10.1016/j.dental.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 186.Hsu H.-C., Wu S.-C., Hsu S.-K., Liao Y.-H., Ho W.-F. Bioactivity of hybrid micro/nano-textured Ti-5Si surface by acid etching and heat treatment. Mater. Des. 2016 Aug;104:205–210. [Google Scholar]
- 187.Wegrzyn J., Kaufman K.R., Hanssen A.D., Lewallen D.G. Performance of porous tantalum vs. Titanium cup in total hip arthroplasty: randomized trial with minimum 10-year follow-up. J. Arthroplasty. 2015 Jun;30(6):1008–1013. doi: 10.1016/j.arth.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 188.Perticarini L., Zanon G., Rossi S.M.P., Benazzo F.M. Clinical and radiographic outcomes of a trabecular titaniumTM acetabular component in hip arthroplasty: results at minimum 5 years follow-up. BMC Muscoskel. Disord. 2015 Dec 3;16(1):375. doi: 10.1186/s12891-015-0822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Huang Y., Zhou Y.-X., Tian H., Wang J.-W., Liu W.-G., Li H. Minimum 7-year follow-up of A porous coated trabecular titanium cup manufactured with electron beam melting technique in primary total hip arthroplasty. Orthop. Surg. 2021 May;13(3):817–824. doi: 10.1111/os.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gallart X., Fernández-Valencia J.A., Riba J., Bori G., García S., Tornero E., et al. Trabecular TitaniumTM cups and augments in revision total hip arthroplasty: clinical results, radiology and survival outcomes. Hip Int. 2016 Sep;26(5):486–491. doi: 10.5301/hipint.5000378. [DOI] [PubMed] [Google Scholar]
- 191.Matsuzaka K., Yoshinari M., Kokubu E., Shimono M., Yamada Y., Mabuchi M., et al. Bone formation in titanium porous scaffold with immobilization of BMP-2. J. Oral Tissue Eng. 2005;2(2):60–65. [Google Scholar]
- 192.Ponader S., von Wilmowsky C., Widenmayer M., Lutz R., Heinl P., Körner C., et al. In vivo performance of selective electron beam-melted Ti-6Al-4V structures. J. Biomed. Mater. Res. 2010 Jan;92(1):56–62. doi: 10.1002/jbm.a.32337. [DOI] [PubMed] [Google Scholar]
- 193.van Jonbergen H.-P.W., Spruit M., Anderson P.G., Pavlov P.W. Anterior cervical interbody fusion with a titanium box cage: early radiological assessment of fusion and subsidence. Spine J. 2005 Nov;5(6):645–649. doi: 10.1016/j.spinee.2005.07.007. ; discussion 649. [DOI] [PubMed] [Google Scholar]
- 194.Yoshioka S., Nakano S., Kinoshita Y., Nakamura M., Goto T., Hamada D., et al. Comparison of a highly porous titanium cup (Tritanium) and a conventional hydroxyapatite-coated porous titanium cup: a retrospective analysis of clinical and radiological outcomes in hip arthroplasty among Japanese patients. J. Orthop. Sci. 2018 Nov;23(6):967–972. doi: 10.1016/j.jos.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 195.De Meo F., Cacciola G., Bellotti V., Bruschetta A., Cavaliere P. Trabecular Titanium acetabular cups in hip revision surgery: mid-term clinical and radiological outcomes. Hip Int. 2018 Nov;28(2_suppl):61–65. doi: 10.1177/1120700018812992. [DOI] [PubMed] [Google Scholar]
- 196.Delanois R.E., Gwam C.U., Mohamed N., Khlopas A., Chughtai M., Malkani A.L., et al. Midterm outcomes of revision total hip arthroplasty with the use of a multihole highly-porous titanium shell. J. Arthroplasty. 2017 Sep;32(9):2806–2809. doi: 10.1016/j.arth.2017.03.065. [DOI] [PubMed] [Google Scholar]
- 197.Ramappa M., Bajwa A., Kulkarni A., McMurtry I., Port A. Early results of a new highly porous modular acetabular cup in revision arthroplasty. Hip Int. 2009 Jul;19(3):239–244. doi: 10.1177/112070000901900309. [DOI] [PubMed] [Google Scholar]
- 198.Munegato D., Bigoni M., Sotiri R., Bruschetta A., Omeljaniuk R.J., Turati M., et al. Clinical and radiological outcomes of acetabular revision with the Delta Revision TT cup. Hip Int. 2018 Nov;28(2_suppl):54–60. doi: 10.1177/1120700018813224. [DOI] [PubMed] [Google Scholar]
- 199.Steno B., Kokavec M., Necas L. Acetabular revision arthroplasty using trabecular titanium implants. Int. Orthop. 2015 Mar;39(3):389–395. doi: 10.1007/s00264-014-2509-5. [DOI] [PubMed] [Google Scholar]
- 200.Mangano C., Mangano F., Shibli J.A., Luongo G., De Franco M., Briguglio F., et al. Prospective clinical evaluation of 201 direct laser metal forming implants: results from a 1-year multicenter study. Laser Med. Sci. 2012 Jan;27(1):181–189. doi: 10.1007/s10103-011-0904-3. [DOI] [PubMed] [Google Scholar]
- 201.Liu C., Huang S., Guo F., Li Y., Zhao B., Luo A., et al. Immediate, non-submerged, three-dimensionally printed, one-piece mandibular molar porous root-analogue titanium implants: a 2-year prospective study involving 18 patients. J Stomatol Oral Maxillofac Surg. 2022 Nov;123(6):e770–e776. doi: 10.1016/j.jormas.2022.05.019. [DOI] [PubMed] [Google Scholar]
- 202.Fan H., Fu J., Li X., Pei Y., Li X., Pei G., et al. Implantation of customized 3-D printed titanium prosthesis in limb salvage surgery: a case series and review of the literature. World J. Surg. Oncol. 2015 Nov 4;13(1):308. doi: 10.1186/s12957-015-0723-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hosny H.A.H., El-Bakoury A., Srinivasan S.C.M., Yarlagadda R., Keenan J. Tritanium acetabular cup in revision hip replacement: a six to ten years of follow-up study. J. Arthroplasty. 2018 Aug;33(8):2566–2570. doi: 10.1016/j.arth.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 204.Sagheb K., Schiegnitz E., Moergel M., Walter C., Al-Nawas B., Wagner W. Clinical outcome of alveolar ridge augmentation with individualized CAD-CAM-produced titanium mesh. Int. J. Implant Dent. 2017 Dec;3(1):36. doi: 10.1186/s40729-017-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Carli A.V., Warth L.C., de Mesy Bentley K.L., Nestor B.J. Short to midterm follow-up of the Tritanium primary acetabular component: a cause for concern. J. Arthroplasty. 2017 Feb;32(2):463–469. doi: 10.1016/j.arth.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 206.Castagnini F., Bordini B., Stea S., Calderoni P.P., Masetti C., Busanelli L. Highly porous titanium cup in cementless total hip arthroplasty: registry results at eight years. Int. Orthop. 2019 Aug;43(8):1815–1821. doi: 10.1007/s00264-018-4102-9. [DOI] [PubMed] [Google Scholar]
- 207.Massari L., Bistolfi A., Grillo P.P., Borré A., Gigliofiorito G., Pari C., et al. Periacetabular bone densitometry after total hip arthroplasty with highly porous titanium cups: a 2-year follow-up prospective study. Hip Int. 2017 Nov;27(6):551–557. doi: 10.5301/hipint.5000509. [DOI] [PubMed] [Google Scholar]
- 208.Sodhi N., Izant T., Diana J., Del Gaizo D., Baratz M., Levine A., Campbell D., Harwin S.F., Mont M.A. Three-Year Outcomes of a Highly Porous Acetabular Shell in Primary Total Hip Arthroplasty Comparison of a highly porous titanium cup (Tritanium) and a conventional hydroxyapatite-coated porous titanium cup: a retrospective analysis of clinical and radiological outcomes in hip arthroplasty among Japanese patients. Orthopedics. 2018;41(1):154–157. doi: 10.3928/01477447-20171102-03. [DOI] [PubMed] [Google Scholar]
- 209.Gwynne-Jones D.P., Garneti N., Wainwright C., Matheson J.A., King R. The morscher press fit acetabular component. J Bone Joint Surg Br. 2009 Jul;91-B(7):859–864. doi: 10.1302/0301-620X.91B7.22013. [DOI] [PubMed] [Google Scholar]
- 210.Gwynne-Jones D.P., Lash H.W.R., James A.W., Iosua E.E., Matheson J.A. The Morscher press-fit acetabular component: an independent long-term review at 18-22 years. J. Arthroplasty. 2017 Aug;32(8):2444–2449. doi: 10.1016/j.arth.2017.02.052. [DOI] [PubMed] [Google Scholar]
- 211.Kim Y.-H., Park J.-W., Kim J.-S., Kim I.-W. Twenty-five- to twenty-seven-year results of a cemented vs a cementless stem in the same patients younger than 50 years of age. J. Arthroplasty. 2016 Mar;31(3):662–667. doi: 10.1016/j.arth.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 212.Kim Y.-H. Long-term results of the cementless porous-coated anatomic total hip prosthesis. J Bone Joint Surg Br. 2005 May;87(5):623–627. doi: 10.1302/0301-620X.87B5.15554. [DOI] [PubMed] [Google Scholar]
- 213.Belmont P.J., Jr., Powers C.C., Beykirch S.E., Hopper Rh Jr, Engh C.A., Jr., Engh C.A. Results of the anatomic medullary locking total hip arthroplasty at a minimum of twenty years. A concise follow-up of previous reports. J Bone Joint Surg Am. 2008 Jul;90(7):1524–1530. doi: 10.2106/JBJS.G.01142. [DOI] [PubMed] [Google Scholar]
- 214.Ihle M., Mai S., Pfluger D., Siebert W. The results of the titanium-coated RM acetabular component at 20 years. J Bone Joint Surg Br. 2008 Oct;90-B(10):1284–1290. doi: 10.1302/0301-620X.90B10.20274. [DOI] [PubMed] [Google Scholar]
- 215.Della Valle C.J., Mesko N.W., Quigley L., Rosenberg A.G., Jacobs J.J., Galante J.O. Primary total hip arthroplasty with a porous-coated acetabular component. A concise follow-up, at a minimum of twenty years, of previous reports. J Bone Joint Surg Am. 2009 May;91(5):1130–1135. doi: 10.2106/JBJS.H.00168. [DOI] [PubMed] [Google Scholar]
- 216.Howard J.L., Kremers H.M., Loechler Y.A., Schleck C.D., Harmsen W.S., Berry D.J., et al. Comparative survival of uncemented acetabular components following primary total hip arthroplasty. J Bone Joint Surg Am. 2011 Sep 7;93(17):1597–1604. doi: 10.2106/JBJS.J.00195. [DOI] [PubMed] [Google Scholar]
- 217.Saito S., Ishii T., Mori S., Hosaka K., Tokuhashi Y. The Harris-Galante cementless THA: a 19- to 25-year follow-up study. Orthopedics. 2011 Jan 3;34(1):12. doi: 10.3928/01477447-20101123-08. [DOI] [PubMed] [Google Scholar]
- 218.Loughead J.M., O'Connor P.A., Charron K., Rorabeck C.H., Bourne R.B. Twenty-three-year outcome of the porous coated anatomic total hip replacement: a concise follow-up of a previous report. J Bone Joint Surg Am. 2012 Jan 18;94(2):151–155. doi: 10.2106/JBJS.J.01553. [DOI] [PubMed] [Google Scholar]
- 219.Stefl M.D., Callaghan J.J., Liu S.S., Perdesen D.R., Goetz D.D., Johnston R.C. Primary cementless acetabular fixation at a minimum of twenty years of follow-up: a concise update of a previous report. JBJS. 2012;94(3):234–239. doi: 10.2106/JBJS.K.00237. [DOI] [PubMed] [Google Scholar]
- 220.Sanz-Reig J., Lizaur-Utrilla A., Llamas-Merino I., Lopez-Prats F. Cementless total hip arthroplasty using titanium, plasma-sprayed implants: a study with 10 to 15 years of follow-up. J. Orthop. Surg. 2011 Aug;19(2):169–173. doi: 10.1177/230949901101900207. [DOI] [PubMed] [Google Scholar]
- 221.Panichkul P., McCalden R.W., MacDonald S.J., Somerville L.E., Naudie D.N. Minimum 15-year results of a dual-offset uncemented femoral stem in total hip arthroplasty. J. Arthroplasty. 2019 Dec;34(12):2992–2998. doi: 10.1016/j.arth.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 222.Luo J., Jia X., Gu R., Zhou P., Huang Y., Sun J., et al. 316L stainless steel manufactured by selective laser melting and its biocompatibility with or without hydroxyapatite coating. Metals. 2018 Jul 18;8(7):548. [Google Scholar]
- 223.Mat Noor F. Universiti Teknologi Malaysia; 2018. Foam Replicated Porous 316l Stainless Steel Based on Taguchi Method for Biomedical Applications.http://eprints.uthm.edu.my/id/eprint/692 [Internet] [doctoral] [cited 2023 May 11]. Available from: [Google Scholar]
- 224.Ducheyne P., Hench L.L., Kagan A., 2nd, Martens M., Bursens A., Mulier J.C. Effect of hydroxyapatite impregnation on skeletal bonding of porous coated implants. J. Biomed. Mater. Res. 1980 May;14(3):225–237. doi: 10.1002/jbm.820140305. [DOI] [PubMed] [Google Scholar]
- 225.Gimeno M., Pinczowski P., Vázquez F.J., Pérez M., Santamaría J., Arruebo M., et al. Porous orthopedic steel implant as an antibiotic eluting device: prevention of post-surgical infection on an ovine model. Int. J. Pharm. 2013 Aug 16;452(1–2):166–172. doi: 10.1016/j.ijpharm.2013.04.076. [DOI] [PubMed] [Google Scholar]
- 226.Pilliar R.M. Powder metal-made orthopedic implants with porous surface for fixation by tissue ingrowth. Clin. Orthop. Relat. Res. 1983 Jun;176(176):42–51. [PubMed] [Google Scholar]
- 227.Datti R., Cavagnaro G., Camici S. Stainless steel wire mesh cranioplasty: ten years' experience with 183 patients (100 followed up) Acta Neurochir. 1985;78(3–4):133–135. doi: 10.1007/BF01808692. [DOI] [PubMed] [Google Scholar]
- 228.Analysis of Reconstruction of Mandibular Defects Using Single Stainless Steel A-O Recons True Tion P/a Tes. [DOI] [PubMed]
- 229.Woolson S.T., Maloney W.J. Cementless total hip arthroplasty using a porous-coated prosthesis for bone ingrowth fixation. 3 1/2-year follow-up. J. Arthroplasty. 1992;7(Suppl):381–388. doi: 10.1016/s0883-5403(07)80028-3. [DOI] [PubMed] [Google Scholar]
- 230.Chakraborty S., Ghosh S., Burman R., Ray A. Seven-year retrospective clinical study evaluating efficacy of stainless steel mesh in mandibular fractures. J. Oral Maxillofac. Surg. 2011 Oct;69(10):2608–2612. doi: 10.1016/j.joms.2011.02.110. [DOI] [PubMed] [Google Scholar]
- 231.Kiradzhiyska D.D., Mantcheva R.D., Mileva D.L., Draganova K.I., Feodorova Y.N., Sarafan V.S., et al. Investigation of chromium-cobalt coated scaffolds on cell cultures in vitro. Folia Med. 2014 Oct;56(4):275–281. doi: 10.1515/folmed-2015-0008. [DOI] [PubMed] [Google Scholar]
- 232.PA31 Fifteen to seventeen year follow-up of the uncemented spongiosa metal surface (SMS) total hip arthroplasty. Osteoarthritis Cartilage. 2001 Sep;9:S28. [Google Scholar]
- 233.Al Muderis M., Bohling U., Grittner U., Gerdesmeyer L., Scholz J. Cementless total hip arthroplasty using the Spongiosa-I fully coated cancellous metal surface: a minimum twenty-year follow-up. J Bone Joint Surg Am. 2011 Jun 1;93(11):1039–1044. doi: 10.2106/JBJS.I.01757. [DOI] [PubMed] [Google Scholar]
- 234.Götze C., Tschugunow A., Wiegelmann F., Osada N., Götze H.G., Böttner F. Langfristiger Einfluss der anatomisch angepassten spongiösen Endoprothese auf den periprothetischen Knochen. Z. Orthop. Ihre Grenzgeb. 2006 Mar;144(2):192–198. doi: 10.1055/s-2006-921573. [DOI] [PubMed] [Google Scholar]
- 235.Maeda A., Hirose I., Kondo S., Kuroki Y., Kusaba A., Nagase K., et al. Long term survival analysis of spongiosa metal I cement less total HIP prosthesis in Japan. Orthopaedic Proceedings. 2010 Oct 1;92-B(SUPP_IV) 525–525. [Google Scholar]
- 236.Gollwitzer H., Gerdesmeyer L., Horn C., Diehl P., Töpfer A., Gradinger R. 8-year follow-up after cementless hip arthroplasty with a second generation spongy metal total hip replacement. Hip Int. 2009 Oct;19(4):359–366. doi: 10.1177/112070000901900410. [DOI] [PubMed] [Google Scholar]
- 237.Gerdesmeyer L., Al Muderis M., Gollwitzer H., Harrasser N., Stukenberg M., Clifford M.-A., et al. 19 years outcome after cementless total hip arthroplasty with spongy metal structured implants in patients younger than 65 years. BMC Muscoskel. Disord. 2016 Oct 18;17(1):429. doi: 10.1186/s12891-016-1285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Love B.R. A femoral component inserted without cement in total hip arthroplasty. A study of the Tri-Lock component with an average ten-year duration of follow-up. J Bone Joint Surg Am. 1999 Jul;81(7):1044–1045. [PubMed] [Google Scholar]
- 239.Survivorship of Total Hip Arthroplasty with a Proximally Coated Tapered- Wedge Femoral Stem: A Retrospective. [DOI] [PMC free article] [PubMed]
- 240.Panichkul P., Parks N.L., Ho H., Hopper R.H., Jr., Hamilton W.G. New approach and stem increased femoral revision rate in total hip arthroplasty. Orthopedics. 2016 Jan;39(1):e86–e92. doi: 10.3928/01477447-20151222-06. [DOI] [PubMed] [Google Scholar]
- 241.Clinical and Radiographic Results of Total Hip Arthroplasty after a Minimum Follow-Up Period of 5 Years after Insertion of a TriLock Bone Preservation Stem.
- 242.Meneghini R.M., Lewallen D.G., Hanssen A.D. Use of porous tantalum metaphyseal cones for severe tibial bone loss during revision total knee replacement. J Bone Joint Surg Am. 2008 Jan;90(1):78–84. doi: 10.2106/JBJS.F.01495. [DOI] [PubMed] [Google Scholar]
- 243.Nadeau M., Séguin C., Theodoropoulos J.S., Harvey E.J. Short term clinical outcome of a porous tantalum implant for the treatment of advanced osteonecrosis of the femoral head. Mcgill J. Med. 2007 Jan;10(1):4–10. [PMC free article] [PubMed] [Google Scholar]
- 244.Shuler M.S., Rooks M.D., Roberson J.R. Porous tantalum implant in early osteonecrosis of the hip: preliminary report on operative, survival, and outcomes results. J. Arthroplasty. 2007 Jan;22(1):26–31. doi: 10.1016/j.arth.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 245.Tsao A.K., Roberson J.R., Christie M.J., Dore D.D., Heck D.A., Robertson D.D., et al. Biomechanical and clinical evaluations of a porous tantalum implant for the treatment of early-stage osteonecrosis. J Bone Joint Surg Am. 2005 Nov;87:22–27. doi: 10.2106/JBJS.E.00490. [DOI] [PubMed] [Google Scholar]
- 246.Sporer S.M., Paprosky W.G. The use of a trabecular metal acetabular component and trabecular metal augment for severe acetabular defects. J. Arthroplasty. 2006 Sep;21(6 Suppl 2):83–86. doi: 10.1016/j.arth.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 247.Russell S.P., O'Neill C.J., Fahey E.J., Guerin S., Gul R., Harty J.A. Trabecular Metal augments for severe acetabular defects in revision hip arthroplasty: a long-term follow-up. J. Arthroplasty. 2021 May;36(5):1740–1745. doi: 10.1016/j.arth.2020.12.033. [DOI] [PubMed] [Google Scholar]
- 248.Divano S., Cavagnaro L., Zanirato A., Basso M., Felli L., Formica M. Porous metal cones: gold standard for massive bone loss in complex revision knee arthroplasty? A systematic review of current literature. Arch. Orthop. Trauma Surg. 2018 Jun;138(6):851–863. doi: 10.1007/s00402-018-2936-7. [DOI] [PubMed] [Google Scholar]
- 249.Abdelaziz H., Jaramillo R., Gehrke T., Ohlmeier M., Citak M. Clinical survivorship of aseptic revision total knee arthroplasty using hinged knees and tantalum cones at minimum 10-year follow-up. J. Arthroplasty. 2019 Dec;34(12):3018–3022. doi: 10.1016/j.arth.2019.06.057. [DOI] [PubMed] [Google Scholar]
- 250.Edelmann A.R., Patel D., Allen R.K., Gibson C.J., Best A.M., Bencharit S. Retrospective analysis of porous tantalum trabecular metal–enhanced titanium dental implants. J. Prosthet. Dent. 2019 Mar 1;121(3):404–410. doi: 10.1016/j.prosdent.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 251.Unger A.S., Lewis R.J., Gruen T. Evaluation of a porous tantalum uncemented acetabular cup in revision total hip arthroplasty: clinical and radiological results of 60 hips. J. Arthroplasty. 2005 Dec;20(8):1002–1009. doi: 10.1016/j.arth.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 252.Trabecular MetalTM Cups for Acetabular Defects with 50% or Less Host Bone Contact. [DOI] [PMC free article] [PubMed]
- 253.Nehme A., Lewallen D.G., Hanssen A.D. Modular porous metal augments for treatment of severe acetabular bone loss during revision hip arthroplasty. Clin. Orthop. Relat. Res. 2004 Dec;429(429):201–208. doi: 10.1097/01.blo.0000150133.88271.80. [DOI] [PubMed] [Google Scholar]
- 254.Sporer S.M., Paprosky W.G. Acetabular revision using a trabecular metal acetabular component for severe acetabular bone loss associated with a pelvic discontinuity. J. Arthroplasty. 2006 Sep;21(6 Suppl 2):87–90. doi: 10.1016/j.arth.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 255.Weeden S.H., Schmidt R.H. The use of tantalum porous metal implants for Paprosky 3A and 3B defects. J. Arthroplasty. 2007 Sep;22(6 Suppl 2):151–155. doi: 10.1016/j.arth.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 256.Flecher X., Sporer S., Paprosky W. Management of severe bone loss in acetabular revision using a trabecular metal shell. J. Arthroplasty. 2008 Oct;23(7):949–955. doi: 10.1016/j.arth.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 257.Kim W.Y., Greidanus N.V., Duncan C.P., Masri B.A., Garbuz D.S. Porous tantalum uncemented acetabular shells in revision total hip replacement: two to four year clinical and radiographic results. Hip Int. 2008 Jan;18(1):17–22. doi: 10.1177/112070000801800104. [DOI] [PubMed] [Google Scholar]
- 258.Van Kleunen J.P., Lee G.-C., Lementowski P.W., Nelson C.L., Garino J.P. Acetabular revisions using trabecular metal cups and augments. J. Arthroplasty. 2009 Sep;24(6 Suppl):64–68. doi: 10.1016/j.arth.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 259.Lakstein D., Backstein D., Safir O., Kosashvili Y., Gross A.E. Trabecular Metal cups for acetabular defects with 50% or less host bone contact. Clin. Orthop. Relat. Res. 2009 Sep;467(9):2318–2324. doi: 10.1007/s11999-009-0772-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Siegmeth A., Duncan C.P., Masri B.A., Kim W.Y., Garbuz D.S. Modular tantalum augments for acetabular defects in revision hip arthroplasty. Clin. Orthop. Relat. Res. 2009 Jan;467(1):199–205. doi: 10.1007/s11999-008-0549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Malkani A.L., Price M.R., Crawford C.H., 3rd, Baker D.L. Acetabular component revision using a porous tantalum biomaterial: a case series. J. Arthroplasty. 2009 Oct;24(7):1068–1073. doi: 10.1016/j.arth.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 262.Simon J.-P., Bellemans J. Clinical and radiological evaluation of modular trabecular metal acetabular cups. Short-term results in 64 hips. Acta Orthop. Belg. 2009 Oct;75(5):623–630. [PubMed] [Google Scholar]
- 263.Lingaraj K., Teo Y.H., Bergman N. The management of severe acetabular bone defects in revision hip arthroplasty using modular porous metal components. J Bone Joint Surg Br. 2009 Dec;91(12):1555–1560. doi: 10.1302/0301-620X.91B12.22517. [DOI] [PubMed] [Google Scholar]
- 264.Flecher X., Paprosky W., Grillo J.-C., Aubaniac J.-M., Argenson J.-N. Do tantalum components provide adequate primary fixation in all acetabular revisions? Orthop Traumatol Surg Res. 2010 May;96(3):235–241. doi: 10.1016/j.otsr.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 265.Kosashvili Y., Safir O., Backstein D., Lakstein D., Gross A.E. Salvage of failed acetabular cages by nonbuttressed trabecular metal cups. Clin. Orthop. Relat. Res. 2010 Feb;468(2):466–471. doi: 10.1007/s11999-009-0935-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Fernández-Fairen M., Murcia A., Blanco A., Meroño A., Murcia A., Jr., Ballester J. Revision of failed total hip arthroplasty acetabular cups to porous tantalum components: a 5-year follow-up study. J. Arthroplasty. 2010 Sep;25(6):865–872. doi: 10.1016/j.arth.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 267.Jafari S.M., Bender B., Coyle C., Parvizi J., Sharkey P.F., Hozack W.J. Do tantalum and titanium cups show similar results in revision hip arthroplasty? Clin. Orthop. Relat. Res. 2010 Feb;468(2):459–465. doi: 10.1007/s11999-009-1090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Alfaro J.J.B., Fernández J.S. Trabecular Metal buttress augment and the Trabecular Metal cup-cage construct in revision hip arthroplasty for severe acetabular bone loss and pelvic discontinuity. Hip Int. 2010 Apr 1;20(7_suppl):119–127. doi: 10.1177/11207000100200s720. [DOI] [PubMed] [Google Scholar]
- 269.Lachiewicz P.F., Soileau E.S. Tantalum components in difficult acetabular revisions. Clin. Orthop. Relat. Res. 2010 Feb;468(2):454–458. doi: 10.1007/s11999-009-0940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Skyttä E.T., Eskelinen A., Paavolainen P.O., Remes V.M. Early results of 827 trabecular metal revision shells in acetabular revision. J. Arthroplasty. 2011 Apr;26(3):342–345. doi: 10.1016/j.arth.2010.01.106. [DOI] [PubMed] [Google Scholar]
- 271.Davies J.H., Laflamme G.Y., Delisle J., Fernandes J. Trabecular metal used for major bone loss in acetabular hip revision. J. Arthroplasty. 2011 Dec;26(8):1245–1250. doi: 10.1016/j.arth.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 272.Del Gaizo D.J., Kancherla V., Sporer S.M., Paprosky W.G. Tantalum augments for Paprosky IIIA defects remain stable at midterm followup. Clin. Orthop. Relat. Res. 2012 Feb;470(2):395–401. doi: 10.1007/s11999-011-2170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sternheim A., Backstein D., Kuzyk P.R.T., Goshua G., Berkovich Y., Safir O., Gross A.E. Porous metal revision shells for management of contained acetabular bone defects at a mean follow-up of six years: a comparison between up to 50% bleeding host bone contact and more than 50% contact. J. Bone Joint Surg. Br. 2012;94(2):158–162. doi: 10.1302/0301-620X.94B2.27871. [DOI] [PubMed] [Google Scholar]
- 274.Borland W.S., Bhattacharya R., Holland J.P., Brewster N.T. Use of porous trabecular metal augments with impaction bone grafting in management of acetabular bone loss. Acta Orthop. 2012 Aug;83(4):347–352. doi: 10.3109/17453674.2012.718518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Abolghasemian M., Tangsataporn S., Sternheim A., Backstein D., Safir O., Gross A.E. Combined trabecular metal acetabular shell and augment for acetabular revision with substantial bone loss: a mid-term review. Bone Joint Lett. J. 2013 Feb;95-B(2):166–172. doi: 10.1302/0301-620X.95B2.30608. [DOI] [PubMed] [Google Scholar]
- 276.Clement R.G.E., Ray A.G., MacDonald D.J., Wade F.A., Burnett R., Moran M. Trabecular metal use in Paprosky type 2 and 3 acetabular defects: 5-year follow-up. J. Arthroplasty. 2016 Apr;31(4):863–867. doi: 10.1016/j.arth.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 277.Lachiewicz P.F., O'Dell J.A. Tantalum components in difficult acetabular revisions have good survival at 5 to 10 years: longer term followup of a previous report. Clin. Orthop. Relat. Res. 2018 Feb;476(2):336–342. doi: 10.1007/s11999.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Witte F., Ulrich H., Rudert M., Willbold E. Biodegradable magnesium scaffolds: Part 1: appropriate inflammatory response. J. Biomed. Mater. Res. 2007 Jun 1;81(3):748–756. doi: 10.1002/jbm.a.31170. [DOI] [PubMed] [Google Scholar]
- 279.Zeng R., Dietzel W., Witte F., Hort N., Blawert C. Progress and challenge for magnesium alloys as biomaterials. Adv. Eng. Mater. 2008 Aug;10(8) B3–14. [Google Scholar]
- 280.Bobe K., Willbold E., Morgenthal I., Andersen O., Studnitzky T., Nellesen J., et al. In vitro and in vivo evaluation of biodegradable, open-porous scaffolds made of sintered magnesium W4 short fibres. Acta Biomater. 2013 Nov;9(10):8611–8623. doi: 10.1016/j.actbio.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 281.Yu W., Zhao H., Ding Z., Zhang Z., Sun B., Shen J., et al. In vitro and in vivo evaluation of MgF2 coated AZ31 magnesium alloy porous scaffolds for bone regeneration. Colloids Surf. B Biointerfaces. 2017 Jan 1;149:330–340. doi: 10.1016/j.colsurfb.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 282.Zhang N., Zhao D., Liu N., Wu Y., Yang J., Wang Y., et al. Assessment of the degradation rates and effectiveness of different coated Mg-Zn-Ca alloy scaffolds for in vivo repair of critical-size bone defects. J. Mater. Sci. Mater. Med. 2018 Aug 17;29(9):138. doi: 10.1007/s10856-018-6145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Li Y., Pan Q., Xu J., He X., Li H.A., Oldridge D.A., et al. Overview of methods for enhancing bone regeneration in distraction osteogenesis: potential roles of biometals. J Orthop Translat. 2021 Mar;27:110–118. doi: 10.1016/j.jot.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Galli S., Stocchero M., Andersson M., Karlsson J., He W., Lilin T., et al. The effect of magnesium on early osseointegration in osteoporotic bone: a histological and gene expression investigation. Osteoporos. Int. 2017 Jul;28(7):2195–2205. doi: 10.1007/s00198-017-4004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Li X., Liu X., Wu S., Yeung K.W.K., Zheng Y., Chu P.K. Design of magnesium alloys with controllable degradation for biomedical implants: from bulk to surface. Acta Biomater. 2016 Nov;45:2–30. doi: 10.1016/j.actbio.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 286.Dong J., Lin T., Shao H., Wang H., Wang X., Song K., et al. Advances in degradation behavior of biomedical magnesium alloys: a review. J. Alloys Compd. 2022 Jul;908 [Google Scholar]
- 287.Jia B., Yang H., Han Y., Zhang Z., Qu X., Zhuang Y., et al. In vitro and in vivo studies of Zn-Mn biodegradable metals designed for orthopedic applications. Acta Biomater. 2020 May;108:358–372. doi: 10.1016/j.actbio.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 288.Jia B., Yang H., Zhang Z., Qu X., Jia X., Wu Q., et al. Biodegradable Zn-Sr alloy for bone regeneration in rat femoral condyle defect model: in vitro and in vivo studies. Bioact. Mater. 2021 Jun;6(6):1588–1604. doi: 10.1016/j.bioactmat.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Qin Y., Liu A., Guo H., Shen Y., Wen P., Lin H., et al. Additive manufacturing of Zn-Mg alloy porous scaffolds with enhanced osseointegration: in vitro and in vivo studies. Acta Biomater. 2022 Jun;145:403–415. doi: 10.1016/j.actbio.2022.03.055. [DOI] [PubMed] [Google Scholar]
- 290.Nie Y., Chen G., Peng H., Tang S., Zhou Z., Pei F., et al. In vitro and 48 weeks in vivo performances of 3D printed porous Fe-30Mn biodegradable scaffolds. Acta Biomater. 2021 Feb;121:724–740. doi: 10.1016/j.actbio.2020.12.028. [DOI] [PubMed] [Google Scholar]
- 291.Cheng A., Humayun A., Cohen D.J., Boyan B.D., Schwartz Z. Additively manufactured 3D porous Ti-6Al-4V constructs mimic trabecular bone structure and regulate osteoblast proliferation, differentiation and local factor production in a porosity and surface roughness dependent manner. Biofabrication. 2014 Oct 7;6(4) doi: 10.1088/1758-5082/6/4/045007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lovati A.B., Lopa S., Bottagisio M., Talò G., Canciani E., Dellavia C., et al. Peptide-enriched silk fibroin sponge and trabecular titanium composites to enhance bone ingrowth of prosthetic implants in an Ovine model of bone gaps. Front. Bioeng. Biotechnol. 2020 Oct 19;8 doi: 10.3389/fbioe.2020.563203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Donati D.M., Frisoni T., Spazzoli B. 3D Printing in Bone Surgery. Springer International Publishing; Cham: 2022. Custom reconstruction around the knee; pp. 65–73. [Google Scholar]
- 294.Garot C., Bettega G., Picart C. Additive manufacturing of material scaffolds for bone regeneration: toward application in the clinics. Adv. Funct. Mater. 2021 Jan 27;31(5) doi: 10.1002/adfm.202006967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Yin B., Xue B., Wu Z., Ma J., Wang K. A novel hybrid 3D-printed titanium scaffold for osteogenesis in a rabbit calvarial defect model. Am J Transl Res. 2018 Feb 15;10(2):474–482. [PMC free article] [PubMed] [Google Scholar]
- 296.Szymczyk-Ziółkowska P., Ziółkowski G., Hoppe V., Rusińska M., Kobiela K., Madeja M., et al. Improved quality and functional properties of Ti-6Al-4V ELI alloy for personalized orthopedic implants fabrication with EBM process. J. Manuf. Process. 2022 Apr;76:175–194. [Google Scholar]
- 297.Hildebrand H., Veron C., Martin P. Nickel, chromium, cobalt dental alloys and allergic reactions: an overview. Biomaterials. 1989 Oct;10(8):545–548. doi: 10.1016/0142-9612(89)90060-4. [DOI] [PubMed] [Google Scholar]
- 298.De Nardo L., Moscatelli M., Silvi F., Tanzi M.C., Yahia L., Farè S. Chemico-physical modifications induced by plasma and ozone sterilizations on shape memory polyurethane foams. J. Mater. Sci. Mater. Med. 2010 Jul;21(7):2067–2078. doi: 10.1007/s10856-010-4082-9. [DOI] [PubMed] [Google Scholar]
- 299.Slámečka K., Kashimbetova A., Pokluda J., Zikmund T., Kaiser J., Montufar E.B., et al. Fatigue behaviour of titanium scaffolds with hierarchical porosity produced by material extrusion additive manufacturing. Mater. Des. 2023 Jan;225 [Google Scholar]
- 300.Alkhader M., Vural M. Mechanical response of cellular solids: role of cellular topology and microstructural irregularity. Int. J. Eng. Sci. 2008 Oct;46(10):1035–1051. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.