Abstract
Background.
The country of Georgia initiated its hepatitis C virus (HCV) elimination program in 2015, at which point a serosurvey showed the adult prevalence of HCV antibody (anti-HCV) and HCV RNA to be 7.7% and 5.4%, respectively. This analysis reports hepatitis C results of a follow-up serosurvey conducted in 2021, and progress towards elimination.
Methods.
The serosurvey used a stratified, multistage cluster design with systematic sampling to include adults and children (aged 5–17 years) providing consent (or assent with parental consent). Blood samples were tested for anti-HCV and if positive, HCV RNA. Weighted proportions and 95% confidence intervals (CI) were compared with 2015 age-adjusted estimates.
Results.
Overall, 7237 adults and 1473 children were surveyed. Among adults, the prevalence of anti-HCV was 6.8% (95% CI, 5.9–7.7). The HCV RNA prevalence was 1.8% (95% CI, 1.3–2.4), representing a 67% reduction since 2015. HCV RNA prevalence decreased among those reporting risk factors of ever injecting drugs (51.1% to 17.8%), and ever receiving a blood transfusion (13.1% to 3.8%; both P < .001). No children tested positive for anti-HCV or HCV RNA.
Conclusions.
These results demonstrate substantial progress made in Georgia since 2015. These findings can inform strategies to meet HCV elimination targets.
Keywords: Georgia, elimination, hepatitis C, prevalence, serosurvey
Globally, in 2019 an estimated 58 million people were living with hepatitis C virus (HCV) infection and 290 000 people died from infection-related causes such as cirrhosis and hepatocellular carcinoma [1]. Due to the global burden, the World Health Organization (WHO) set a goal of eliminating hepatitis C as a public health threat by the year 2030. Georgia, a middle-income country with a population of 3.7 million, is on the forefront of this effort and launched an ambitious national HCV elimination program [2] in 2015.
Georgia provides hepatitis C screening and treatment via highly effective direct-acting antivirals to all citizens free of charge, aiming to achieve WHO elimination targets by 2030 [3]. To establish baseline prevalence, Georgia conducted its first nationally representative seroprevalence survey in 2015 [4], which estimated that 7.7% of the adult population had evidence of exposure to hepatitis C (anti-HCV) and 5.4% had chronic HCV infection (HCV RNA), corresponding to an estimated 150 000 people living with chronic HCV infection. Since then, Georgia’s HCV elimination program has made great progress, and as of December 2021 has treated over 76 000 people, achieving a cure rate of 98.9% [5].
The achievements of the HCV elimination program have been critical in developing Georgia’s public health capacity, which have contributed to laboratory testing capacity, data systems and management, and the ongoing response to 19 corona-virus disease 2019 (COVID-19). However, challenges remain in identifying HCV-infected individuals and linking them to care, especially among middle-aged men [6] and persons who inject drugs (PWID) [7]. Recognizing the need to monitor progress towards HCV elimination, the Government of Georgia, led by the Ministry of Internally Displaced Persons from the Occupied Territories, Health, Labor, and Social Affairs and the National Center for Disease Control and Public Health (NCDC), in partnership with the US Centers for Disease Control and Prevention (CDC), and Abbott, conducted a second nationwide serosurvey on hepatitis C, hepatitis B, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 2021.
The primary objectives of the serosurvey were to estimate exposure to and prevalence of HCV infection among children and adults, assess geographic distribution and risk factors associated with infection, and update information on knowledge and perceptions toward viral hepatitis. This analysis reports on the hepatitis C components of the 2021 serosurvey, and progress towards elimination with comparisons to 2015 serosurvey results.
METHODS
Sample Selection
A cross-sectional, nationwide household survey was conducted in Georgia in 2021. Adults aged ≥18 years and children aged 5–17 years were recruited using a stratified, multistage cluster design with systematic sampling. A sample size of 8010 adults and 2692 children was calculated based on an estimated anti–SARS-CoV-2 prevalence of 10%, a design effect of 2, and an anticipated 70% participation rate to produce 95% confidence intervals (95% CI) with a margin of error of 1.1% as well as expected proportion of households with children of applicable age. This calculated sample size was confirmed to produce estimates of HCV infection among adults with a margin of error of 0.67% based on an estimated prevalence of 2.9%. The country was divided into 10 strata across all regions and the capital city of Tbilisi, excluding the separatist regions of Abkhazia and South Ossetia. To reach the target sample size, 267 clusters were selected across the 10 strata, and 30 households were selected per cluster. A household was defined as a group of persons who reside in the same place and prepare meals together. Households were chosen systematically using a skip pattern, and 1 adult and 1 child of eligible age (in households with ≥ 1 child) were selected per household using a Kish grid [8]. An additional 1880 households from 50 clusters in 4 undersampled strata were included for children to account for low initial enrollment. To maximize comparability between the 2015 and 2021 findings, sampling methods were kept as close as possible to those utilized for the 2015 survey and the same statisticians were consulted in the design of both.
Data collection in the field took place during June to October 2021. Individual interviews were administered face-to-face using a structured questionnaire with responses recorded electronically using tablets and uploaded to a cloud-based server (Open Data Kit software application). Participants were asked about their demographic information, medical and behavioral history, and knowledge of hepatitis C. Each participant’s questionnaire was labeled with a unique identifier (barcoded label) that was linked to their blood sample to maintain confidentiality and allow linking of laboratory results and notification to individuals. The hepatitis questionnaire was kept as similar to 2015 as possible to maximize comparability.
Inclusion/Exclusion Criteria
Randomly selected members of each household were enrolled for participation after obtaining voluntary informed consent (or parental/legal guardian consent for children aged 5–17 years, paired with assent for children ≥ 7 years of age). Persons with altered mental status precluding consent and any participants who could not give blood because of severe illness or hemophilia were excluded.
Laboratory Methods
Whole venous blood was collected from all participants, with serum separated on site and transported to the Serology Laboratory, Lugar Center for Public Health Research, NCDC for testing. All samples were tested for anti-HCV by the anti-HCV chemiluminescent microparticle immunoassay on a fully automated ARCHITECT i2000SR analyzer (Abbott Diagnostics). Positive samples were tested for HCV RNA by the Abbott RealTime HCV Assay (Abbott Molecular, Inc) on the Abbott m2000rt System (Abbott). Samples found to be anti-HCV positive and HCV RNA negative were further tested by immunoassay (INNO-LIA HCV Score, in vitro diagnostics, Innogenetics) to confirm the anti-HCV result. Test results were provided to participants within a maximum 6 months after sample collection, and infected individuals were counseled and referred to a local provider for linkage to care.
Statistical Analysis
All analysis was performed in SAS version 9.4. Data were weighted at cluster, household, and individual levels, and adjusted by sex, age, and geographic distribution using 2014 census data to produce nationally representative estimates. Sample weights were computed by taking the inverse probability of selection and then multiplied by the poststratification adjustment by age, sex, and region. Weighted proportions and 95% CIs were calculated and compared with 2015 survey results using χ2 test with an α of .05. Variance was calculated using Taylor series linearization. For prevalence comparisons by age groups, 2015 data (collected May to August 2015) were age-adjusted by adding 6 years to participants’ ages. Multivariable analysis was performed using weighted estimates to determine independent risk factors for anti-HCV and HCV RNA positivity, with variables associated in bivariate analysis included in the final model to produce adjusted odds ratios (aOR) after assessing for multicollinearity. For model stability, responses of “I don’t remember/know” were treated as a separate category if they were >10% of all responses.
This study was approved by the ethical committee of Georgia’s NCDC and was determined by US CDC’s Human Subjects Research Office to be public health surveillance and therefore judged to not involve human subjects research.
RESULTS
Study Population
In total, 8710 individuals participated in the survey, including 7237 adults (90.3% participation rate) and 1473 children (72.2% participation rate). After weighting the results according to the age, sex, and regional distributions in the 2014 Georgian census, among adults, the median age was 46 years (interquartile range [IQR], 32–61 years), 53.3% (95% CI, 51.3%–55.2%) were female, and 31.8% (95% CI, 30.6%–33.0%) lived in Tbilisi (Table 1). Overall, 90.7% (95% CI, 87.7%–93.1%) of adults were of Georgian ethnicity, a plurality (42.2%; 95% CI, 39.8%–44.6%) completed university or higher education, and 18.9% (95% CI, 17.4%–20.6%) were unemployed. Among children, the median age was 10 years (IQR, 7–13 years), 52.3% (95% CI, 48.8%–55.8%) were male, and 33.0% (95% CI, 30.5%–35.6%) lived in Tbilisi.
Table 1.
Demographic characteristics of all adults and children enrolled in nationwide hepatitis C serosurve — Georgia, 2021
| Variables | Adults, overall | Children, overall | ||
|---|---|---|---|---|
| Participants, No. | Weighted % (95% CI) |
Participants, No. | Weighted % (95% CI) |
|
| Age | ||||
| 5–9 | ‒– | ‒– | 493 | 42.3 (38.8–45.9) |
| 10–14 | ‒– | ‒– | 660 | 38.2 (35.1–41.5) |
| 15–17 | ‒– | ‒– | 320 | 19.4 (16.9–22.3) |
| 18–29 | 762 | 19.2 (17.7–20.8) | ‒– | ‒– |
| 30–39 | 1,249 | 19.0 (17.6–20.5) | ‒– | ‒– |
| 40–49 | 1,233 | 16.8 (15.6–18.1) | ‒– | ‒– |
| 50–59 | 1,517 | 16.9 (15.8–18.0) | ‒– | ‒– |
| ≥60 | 2,476 | 28.1 (26.6–29.7) | ‒– | ‒– |
| Sex | ||||
| Male | 2,409 | 46.7 (44.8–48.7) | 773 | 47.7 (44.2–51.2) |
| Female | 4,828 | 53.3 (51.3–55.2) | 700 | 52.3 (48.8–55.8) |
| Region a | ||||
| Tbilisi | 1,282 | 31.8 (30.6–33.0) | 400 | 33.0 (30.5–35.6) |
| Eastern Georgia | 3,121 | 33.1 (31.9–34.4) | 604 | 34.6 (31.7–37.6) |
| Western Georgia | 2,834 | 35.0 (33.9–36.2) | 469 | 32.4 (29.9–35.1) |
| Ethnicity | ||||
| Georgian | 6,407 | 90.7 (87.7–93.1) | 1359 | 93.4 (90.7–95.3) |
| Armenian | 353 | 2.9 (2.0–4.2) | 32 | 1.9 (1.1–3.4) |
| Azerbaijani | 280 | 4.7 (2.7–8.1) | 48 | 4.0 (2.4–6.6) |
| Other | 133 | 1.6 (1.2–2.3) | 13 | 0.7 (0.4–1.2) |
| Missing | 64 | |||
| Highest level of education completed | ||||
| ≤ Elementary/primary school | 645 | 7.9 (6.7–9.4) | ‒– | ‒– |
| Secondary school | 2,355 | 32.2 (30.3–34.3) | ‒– | ‒– |
| Professional/technical school | 1,465 | 17.6 (16.3–19.0) | ‒– | ‒– |
| ≥ University/college | 2,704 | 42.2 (39.8–44.6) | ‒– | ‒– |
| Missing | 68 | |||
| Employment Status | ||||
| Employed, student, homemaker, retired | 5,904 | 81.1 (79.4–82.6) | ‒– | ‒– |
| Unemployed | 1,263 | 18.9 (17.4–20.6) | ‒– | ‒– |
| Missing | 70 | |||
Eastern Georgia = Kakheti, Mtskheta-Mtaineti, Samtkhe-Javakheti, Kvemo Katrtli, Shida Kartli
Western Georgia = Adjara, Guria, Imereti, Racha-Lechkhumi and Kvemo Svaneti, Samegrelo-Zemo SvanetiAbbreviations: CI = confidence interval
Prevalence of Hepatitis C
The overall adult prevalence of anti-HCV in 2021 was 6.8% (95% CI, 5.9%–7.7%). Anti-HCV positivity differed by age, with the highest prevalence among those aged 40–49 years (11.5%; 95% CI, 8.8%–14.8%) and 50–59 years (10.7%; 95% CI, 8.5%–13.3%) and lowest among those aged 18–29 years (1.7%; 95% CI, .7%–3.8%) (P < .001) (Table 2). Anti-HCV prevalence was higher among men (11.1%; 95% CI, 9.4%–13.1%) than women (3.0%; 95% CI, 2.4%–3.7%) (P < .001) and varied by region with a high of 8.9% (95% CI, 6.2%–12.7%) in Guria (Western Georgia) and a low of 2.9% (95% CI, 1.7%–4.9%) in Mtskheta-Mtianeti (Eastern Georgia) (P = .02). Unemployed persons had a higher prevalence (13.4%; 95% CI, 10.8%–16.3%) than those who were employed, retired, a student, or homemaker (5.3%; 95% CI, 4.5%–6.2%).
Table 2.
Hepatitis C prevalence by demographics and risk behaviors from all adults enrolled in nationwide hepatitis C serosurvey in Georgia — 2021
| Variables | Total No. | Anti-HCV Positive | HCV RNA Positive | ||||
|---|---|---|---|---|---|---|---|
| No. | Weighted Row % (95% CI) |
Chi-square P-value |
No. | Weighted Row % (95% CI) |
Chi-square P-value |
||
| Overall | |||||||
| All adults | 7,237 | 418 | 6.8 (5.9–7.7) | -- | 87 | 1.8 (1.3–2.4) | -- |
| Age | |||||||
| 18–29 | 762 | 8 | 1.7 (0.7–3.8) | <0.001 | 4 | 0.9 (0.3–2.7) | 0.34 |
| 30–39 | 1,249 | 44 | 5.7 (3.9–8.4) | 15 | 1.6 (0.7–3.5) | ||
| 40–49 | 1,233 | 104 | 11.5 (8.8–14.8) | 20 | 2.7 (1.6–4.7) | ||
| 50–59 | 1,517 | 133 | 10.7 (8.5–13.3) | 18 | 1.6 (0.9–3.0) | ||
| ≥60 | 2,476 | 129 | 5.8 (4.6–7.4) | 30 | 2.0 (1.1–3.4) | ||
| Sex | |||||||
| Male | 2,409 | 273 | 11.1 (9.4–13.1) | <0.001 | 64 | 3.1 (2.1–4.4) | <0.001 |
| Female | 4,828 | 145 | 3.0 (2.4–3.7) | 23 | 0.6 (0.4–1.0) | ||
| Region a | |||||||
| Tbilisi | 1,282 | 88 | 8.0 (6.3–10.2) | 0.002 | 17 | 1.8 (1.0–3.2) | 0.14 |
| Eastern Georgia | 3,121 | 29 | 4.6 (3.7–5.6) | 26 | 1.1 (0.7–1.8) | ||
| Western Georgia | 2,834 | 55 | 7.7 (6.2–9.6) | 6 | 2.4 (1.5–3.8) | ||
| Ethnicity | |||||||
| Georgian | 6,407 | 389 | 7.0 (6.1–8.1) | 0.05 | 81 | 1.9 (1.4–2.6) | 0.06 |
| Armenian | 353 | 7 | 3.7 (1.6–8.3) | 1 | 0.5 (0.1–2.6) | ||
| Azerbaijani | 280 | 9 | 3.3 (1.7–6.4) | 3 | 0.5 (0.2–1.3) | ||
| Other | 133 | 10 | 9.7 (4.7–18.9) | 2 | 1.6 (0.3–6.7) | ||
| Missing | 64 | ||||||
| Highest level of education completed | |||||||
| ≤ Elementary/primary | 645 | 36 | 5.8 (3.7–9.0) | 0.15 | 7 | 1.5 (0.6–3.7) | 0.84 |
| Secondary school | 2,355 | 127 | 5.8 (4.7–7.2) | 34 | 1.6 (1.0–2.3) | ||
| Professional/technical | 1,465 | 100 | 8.4 (6.5–10.9) | 20 | 2.0 (1.2–3.5) | ||
| ≥ University/college | 2,704 | 152 | 7.1 (5.7–8.7) | 26 | 1.9 (1.1–3.1) | ||
| Missing | 68 | ||||||
| Employment Status | |||||||
| Employed, student, homemaker, retired | 5,904 | 266 | 5.3 (4.5–6.2) | <0.001 | 52 | 1.5 (1.0–2.2) | 0.005 |
| Unemployed | 1,263 | 148 | 13.4 (10.8–16.3) | 35 | 3.0 (2.0–4.5) | ||
| Missing | 70 | ||||||
| Health care occupation, ever | |||||||
| Yes | 455 | 19 | 4.1 (2.3–7.3) | 0.08 | 3 | 1.1 (0.2–4.6) | 0.48 |
| No | 6,720 | 396 | 7.0 (6.1–8.0) | 84 | 1.8 (1.3–2.5) | ||
| Missing | 62 | ||||||
| Injection drug use, ever | |||||||
| Yes | 120 | 90 | 70.6 (57.3–81.1) | <0.001 | 21 | 17.8 (10.5–28.6) | <0.001 |
| No | 6,987 | 310 | 4.6 (4.0–5.4) | 62 | 1.2 (0.9–1.8) | ||
| Missing | 130 | ||||||
| Incarceration, ever | |||||||
| Yes | 169 | 67 | 39.6 (29.1–51.1) | <0.001 | 21 | 14.6 (7.9–25.2) | <0.001 |
| No | 6,993 | 346 | 5.4 (4.7–6.2) | 66 | 1.3 (0.9–1.8) | ||
| Missing | 75 | ||||||
| Invasive dental procedure, ever | |||||||
| Yes | 6,713 | 387 | 6.7 (5.9–7.7) | 0.23 | 83 | 1.8 (1.3–2.4) | 0.8 |
| No | 462 | 28 | 7.6 (4.7–12.1) | 4 | 1.5 (0.4–5.3) | ||
| Missing | 62 | ||||||
| Blood transfusion, ever | |||||||
| Yes | 9 | 58 | 18.6 (13.5–25.2) | <0.001 | 13 | 3.8 (2.0–7.4) | 0.03 |
| No | 7,129 | 350 | 6.1 (5.3–7.1) | 72 | 1.6 (1.2–2.3) | ||
| Missing | 114 | ||||||
| Surgery, ever | |||||||
| Yes | 347 | 266 | 7.9 (6.8–9.2) | 0.006 | 55 | 2.2 (1.5–3.1) | 0.09 |
| No | 6,776 | 149 | 5.5 (4.4–6.9) | 32 | 1.3 (0.8–2.1) | ||
| Missing | 77 | ||||||
| Who administered last injection | |||||||
| Healthcare worker | 4,802 | 293 | 7.2 (6.1. 8.4) | 0.0003 | 55 | 1.6 (1.1–2.4) | <0.001 |
| Non-healthcare worker | 694 | 32 | 5.7 (3.7–8.4) | 7 | 1.6 (0.7–3.5) | ||
| Self | 204 | 21 | 18.0 (9.8–30.8) | 8 | 10.0 (3.6–25.6) | ||
| Don’t know/remember | 1,218 | 59 | 4.9 (3.4–7.0) | 14 | 1.2 (0.6–2.3) | ||
| Missing | 62 | ||||||
| Receipt of permanent tattoo | |||||||
| Yes | 561 | 83 | 15.6 (12.1–19.8) | <0.001 | 21 | 4.2 (2.5–6.9) | 0.0005 |
| No | 6,607 | 332 | 5.8 (5.0–6.7) | 66 | 1.5 (1.0–2.1) | ||
| Missing | 69 | ||||||
| Sex with a commercial sex worker | |||||||
| Yes | 302 | 38 | 12.3 (8.2–18.1) | 0.002 | 8 | 3.2 (1.3–7.5) | 0.15 |
| No | 6,626 | 352 | 6.1 (5.3–7.1) | 72 | 1.6 (1.1–2.2) | ||
| Missing | 309 | ||||||
| Sex partners, lifetime | |||||||
| 0 | 477 | 9 | 0.8 (0.3–1.9) | <0.001 | 2 | 0.2 (0.0–0.8) | <0.001 |
| 1–5 | 4,824 | 168 | 3.8 (3.2–4.5) | 27 | 0.7 (0.5–1.1) | ||
| >5 | 417 | 238 | 13.1 (9.5–17.8) | 58 | 4.2 (2.3–7.5) | ||
| Don’t know/remember | 938 | 108 | 11.1 (8.6–14.3) | 23 | 2.6 (1.4–4.6) | ||
| Missing | 581 | ||||||
| MSM, ever | |||||||
| Yes | 2 | 1 | 60.7 (8.7–96.2) | 0.03 | 0 | -- | -- |
| No | 2,262 | 257 | 11.0 (9.2–13.1) | 61 | 3.2 (2.2–4.6) | ||
| Missing | 145 | ||||||
Eastern Georgia = Kakheti, Mtskheta-Mtaineti, Samtkhe-Javakheti, Kvemo Katrtli, Shida Kartli
Western Georgia = Adjara, Guria, Imereti, Racha-Lechkhumi and Kvemo Svaneti, Samegrelo-Zemo SvanetiAbbreviations: HCV = hepatitis C virus; MSM = men who have sex with men
Among adults, the overall HCV RNA prevalence in 2021 was 1.8% (95% CI, 1.3%–2.4%), which differed among men (3.1%; 95% CI, 2.1%–4.4%) and women (0.6%; 95% CI, .4%–1.0%) (P < .001). Among all infected persons, injection drug use (IDU) was reported by 29.9% (95% CI, 17.5%–46.2%), an additional 8.5% (95% CI, 3.9%–17.7%) received a blood transfusion, and 13.4% (95% CI, 6.2%–26.5%) had neither exposure but reported > 5 sex partners. The remaining 48.2% (95% CI, 33.3%–63.4%) reported none of these risk factors for HCV infection.
In bivariate analysis, HCV RNA positivity was associated with unemployment (P = .005), history of IDU (P < .001), past incarceration (P < .001), receipt of a blood transfusion (P = .03) or permanent tattoo (P < .001), number of lifetime sex partners (P < .001), and provider of last therapeutic injection (P < .001). After adjusting for all covariates associated with HCV RNA in bivariate analysis, ever injecting drugs (aOR, 3.09; 95% CI, 1.11–8.56), receipt of a blood transfusion (aOR, 3.10; 95% CI, 1.29–7.48), and having > 5 versus 1–5 lifetime sex partners (aOR, 3.14; 95% CI, 1.05–9.46) remained significantly associated with HCV infection (Table 3). Anti-HCV positivity was associated in a multivariable model with being unemployed (aOR, 1.7; 95% CI, 1.2–2.5), ever injecting drugs (aOR, 26.8; 95% CI, 12.5–57.4), receipt of a blood transfusion (aOR, 4.5; 95% CI, 2.9–7.1), and having 0 versus 1–5 lifetime sex partners (aOR, 0.3; 95% CI, .1–.9).
Table 3.
Association of selected risk factors with hepatitis C virus infection reported by adults enrolled in nationwide hepatitis C serosurvey — Georgia, 2021
| Variables | HCV RNA Positive Weighted Row % (95% CI) |
Unadjusted Odds Ratio (95% CI) |
Adjusteda Odds Ratio (95% CI) |
|---|---|---|---|
| Injection drug use, ever | |||
| Yes | 17.8 (10.5–28.6) | 17.28 (8.36–35.72) | 3.09 (1.11–8.56) |
| No | 1.2 (0.9–1.8) | 1 | 1 |
| Incarceration, ever | |||
| Yes | 14.6 (7.9–25.2) | 13.46 (6.17–29.38) | 1.95 (0.82–4.65) |
| No | 1.3 (0.9–1.8) | 1 | 1 |
| Blood transfusion, ever | |||
| Yes | 3.8 (2.0–7.4) | 2.39 (1.09–5.28) | 3.10 (1.29–7.48) |
| No | 1.6 (1.2–2.3) | 1 | 1 |
| Who administered last injection | |||
| Healthcare worker | 1.6 (1.1–2.4) | 1 | 1 |
| Non-healthcare worker | 1.6 (0.7–3.5) | 1.00 (0.41–2.42) | 1.24 (0.47–3.26) |
| Myself | 10.0 (3.6–25.6) | 6.78 (2.12–21.74) | 3.78 (0.97–14.71) |
| Don’t remember/know | 1.2 (0.6–2.3) | 0.75 (0.36–1.54) | 0.85 (0.35–2.08) |
| Receipt of permanent tattoo | |||
| Yes | 4.2 (2.5–6.9) | 2.92 (1.54–5.53) | 1.47 (0.56–3.85) |
| No | 1.5 (1.0–2.1) | 1 | 1 |
| Sex partners, lifetime | |||
| ’0 | 0.2 (0.0–0.8) | 0.27 (0.06–1.15) | 0.14 (0.02–0.99) |
| 1–5 | 0.7 (0.5–1.1) | 1 | 1 |
| >5 | 4.2 (2.3–7.5) | 6.16 (2.84–13.38) | 3.14 (1.05–9.46) |
| Don’t remember/know | 2.6 (1.4–4.6) | 3.72 (1.79–7.75) | 2.46 (0.95–6.38) |
Weighted estimates adjusted for sex, employment status, and all variables shown here
Abbreviations: HCV = hepatitis C virus; CI = confidence interval
No children in the sample (n = 1473) tested positive for anti-HCV or HCV RNA.
Progress Towards Elimination
The prevalence of anti-HCV was not significantly different from 2015 (7.7%; 95% CI, 6.6%–8.8%; P = .20). However, HCV RNA prevalence decreased substantially (from 5.4% [95% CI, 4.5%–6.3%] to 1.8% [95% CI, 1.3%–2.4%]; P < .001). This represents a 67% (95% CI, 46.7%–79.4%) reduction in persons with chronic HCV infection, after the HCV elimination program had treated 51% (n = 76 644) of the 2015 estimate of 150 000 (95% CI, 128 000–173 000) infected. These findings were also observed at the regional level, with no significant changes in anti-HCV prevalence, but HCV RNA prevalence reductions from 47% to 85% (Supplementary Table 1). Prevalence of chronic HCV infection decreased significantly among adults aged 40–49 years (from 9.8% [95% CI, 7.4%–12.9%] in 2015 to 2.7% [95% CI, 1.6%–4.7%] in 2021; P < .001), 50–59 years (from 8.7% [95% CI, 6.5%–11.7%] to 1.6% [95% CI, .9%–3.0%]; P < .001), and ≥60 years (from 3.8% [95% CI, 2.9%–5.2%] to 2.0% [95% CI, 1.1%–3.4%]; P = .02) (Figure 1). Substantial decreases were observed for both men (from 9.0% [95% CI, 7.5%–10.9%] to 3.1% [95% CI, 2.1%–4.4%]) and women (from 2.2% [95% CI, 1.6%–3.0%] to 0.6% [95% CI, .4%–1.0%]) (both P < .001).
Figure 1.
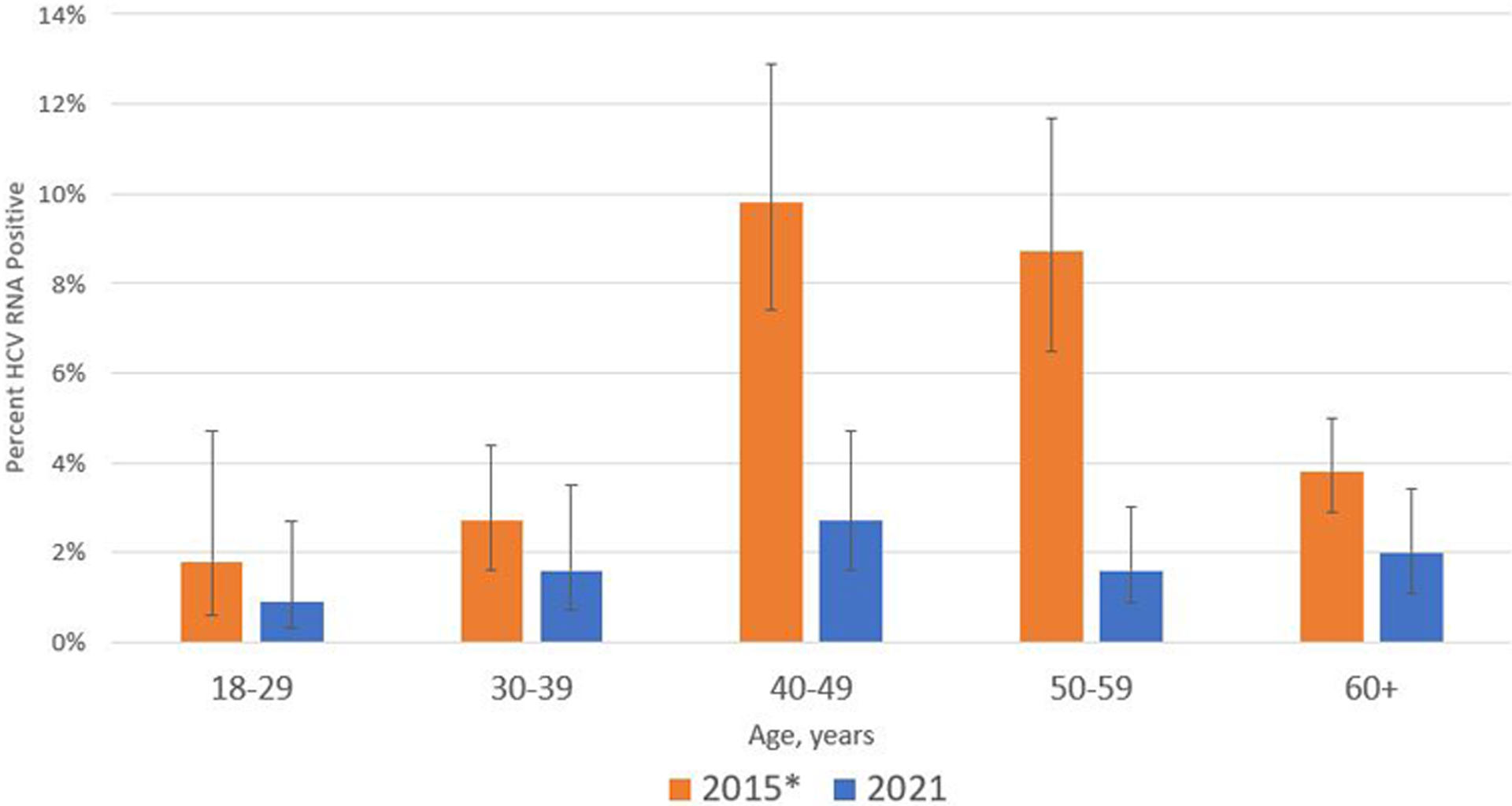
Prevalence of hepatitis C virus (HCV) RNA positivity with 95% confidence intervals by age group among adults in nationwide hepatitis C serosurveys—Georgia, 2015 and 2021. The 2015 data were age-adjusted by adding 6 years to participants’ ages.
Independent risk factors for exposure to hepatitis C in 2015 included history of IDU and receipt of a blood transfusion. Both risk factors were reported less frequently in 2021: the proportion reporting ever injecting drugs decreased from 4.2% (95% CI, 3.4%–5.1%) in 2015% to 3.0% (95% CI, 2.3%–3.9%) in 2021 (P = .03), and blood transfusions decreased from 7.0% (95% CI, 6.1%–7.8%) to 4.7% (95% CI, 3.9%–5.5%) (P < .001). Among those reporting these risk factors, the proportion with chronic HCV infection also decreased substantially, from 51.1% (95% CI, 41.8%–60.3%) to 17.8% (95% CI, 10.5%–28.6%) among persons who ever injected drugs and 13.1% (95% CI, 8.9%–18.9%) to 3.8% (95% CI, 2.0%–7.4%) among those who received a blood transfusion (Figure 2). Although not independent risk factors in multivariable analysis, a significant prevalence decrease was observed in bivariate analysis for those with other established risk factors of past incarceration (32.2% [95% CI, 23.8%–42.0%] to 14.6% [95% CI, 7.9%–25.2%]; P = .01) and surgery (5.3% [95% CI, 4.2%–6.6%] to 2.2% [95% CI, 1.5%–3.1%]; P < .001).
Figure 2.
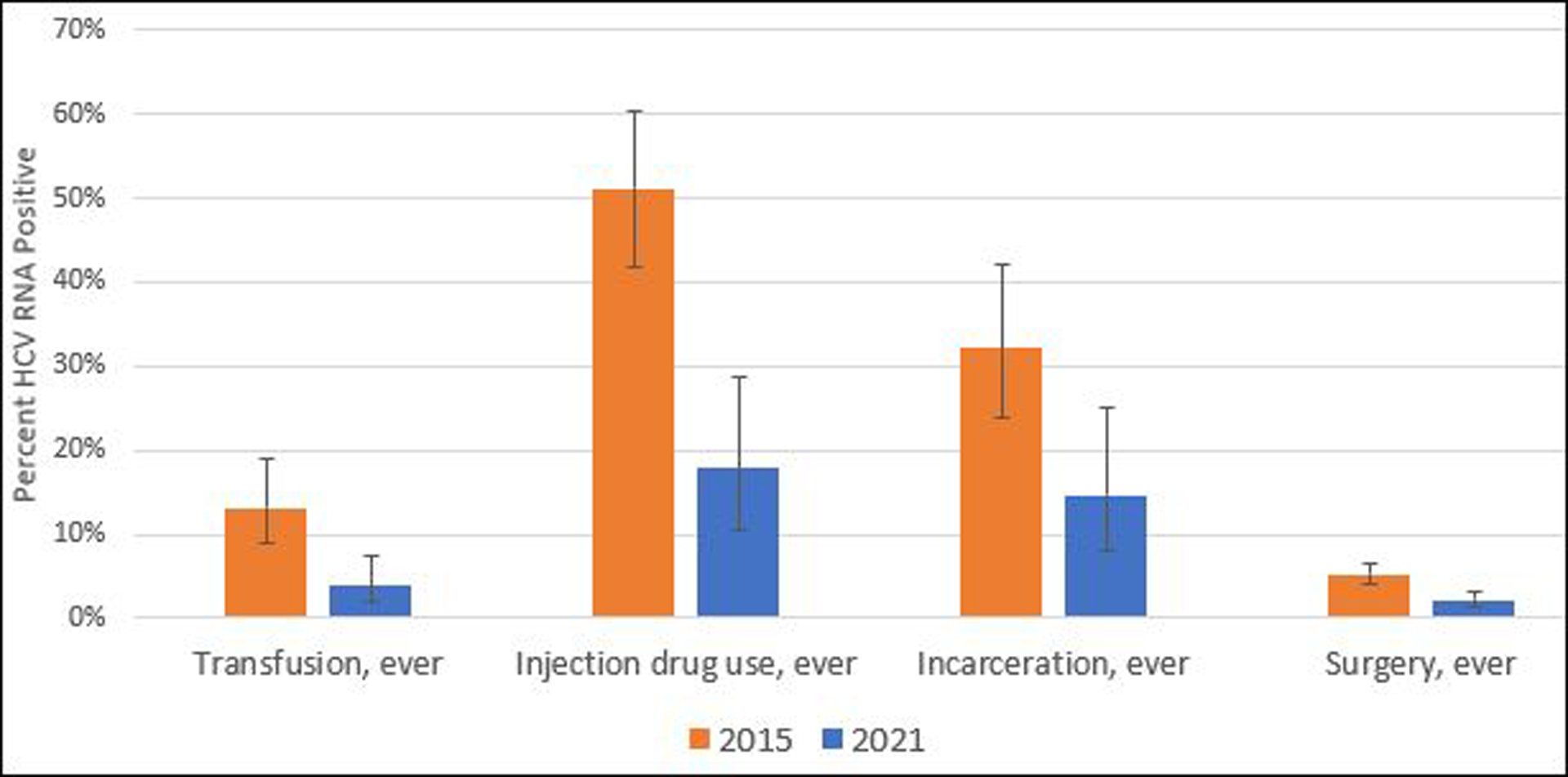
Prevalence of hepatitis C virus (HCV) RNA positivity with 95% confidence intervals by risk factors among adults in nationwide hepatitis C serosurveys—Georgia, 2015 and 2021.
Hepatitis C Knowledge
Overall, 66.1% (95% CI, 63.9%–68.2%) reported ever having heard of the hepatitis C virus in 2021, a decline from 73.0% (95% CI, 71.1%–74.9%) in 2015 (P < .001). Among those who had heard of the virus, a higher proportion in 2021 knew it could be cured with medications (77.2% [95% CI, 75.1%–79.2%] vs 70.5% [95% CI, 68.5%–72.6%]; P < .001) and could be transmitted through blood (91.6% [95% CI, 90.2%–92.9%) vs 77.0% [95% CI, 74.8%–79.1%]; P < .001). However, a lower proportion in 2021 correctly identified sharing needles or syringes as a transmission route for HCV infection (54.3% [95% CI, 50.5%–58.0%] vs 71.7% [95% CI, 69.5%–74.0%]; P < .001). Among those who tested HCV RNA positive, 33.1% (95% CI, 21.8%–46.8%) reported having previously been told by a health care provider of their HCV infection.
DISCUSSION
This analysis is among the first to present results of a follow-up serosurvey after implementation of a national HCV elimination program. Overall, the prevalence of anti-HCV and HCV RNA in Georgia in 2021 was 6.8% and 1.8%, respectively, representing a 67% decline in chronic infections among adults. Given the country’s adult population of 2.7 million [9], this corresponds to 48 600 (95% CI, 35 100–64 800) persons remaining with chronic HCV infection. Proportions reporting IDU and blood transfusions, both significant risk factors for exposure in 2015, were significantly lower in 2021, and HCV RNA prevalence among those groups also decreased substantially. However, this analysis still found IDU and receipt of a blood transfusion to be risk factors for HCV infection.
The significant decline in HCV RNA positivity 6 years into a national HCV elimination program is commendable. As of December 2021, Georgia had treated 51% of the 2015 estimate of 150 000 persons chronically infected with HCV in the country [5]. Nevertheless, prevalence dropped 67% by 2021, the reasons for which are likely multifactorial. Uncertainty around point estimates in both 2015 and 2021 must be considered; there is a possibility of overestimation in baseline prevalence and/or underestimation in the current survey. However, anti-HCV prevalence, which is unaffected by treatment, did not change significantly which suggests those estimates are reliable. Mortality, including from COVID-19, and migration within the population could also influence prevalence estimates. Georgia’s population is likely also experiencing the effects of treatment as a means of prevention, whereby the smaller pool of infected persons reduces transmission in the greater population. The benefits of hepatitis C treatment as prevention have previously been demonstrated in prison settings [10] and hypothesized for PWID [11]. This likely contributed to the substantial decrease in HCV infection among those who ever injected drugs, from 51% in 2015 to 18% in 2021. In 2018, Georgia implemented a decentralized approach to hepatitis C care and treatment in 4 sites that offer syringe services [12], which increased access for PWID and served as a model for further expansion to enhance the preventative effects of treatment in this vulnerable population. Georgia has also been providing free screening and treatment for hepatitis C in prisons since 2013 [13]. Although past incarceration was not an independent risk factor for infection in multivariable analysis, HCV RNA prevalence among those with a history of incarceration was 15% in 2021. Additional studies among those currently incarcerated could confirm whether a similar reduction in prevalence has been achieved in prison settings.
Receipt of a blood transfusion was a risk factor for exposure to hepatitis C in 2015 and was associated with active infection in the 2021 multivariable model. The proportion of people reporting a past blood transfusion reduced from 7% to 5%, and among them the prevalence of HCV infection decreased from 13% to under 4%, although still capturing historic transfusion. Although screening for hepatitis C has been mandatory for all blood donations in Georgia since 1997, increasing blood safety has been a focus of the HCV elimination program through encouraging voluntary versus paid donations [14], and implementing nucleic acid amplification testing for HCV, hepatitis B, and human immunodeficiency virus (HIV) for all donations since January 2020 [5]. A separate analysis has also shown a significant decrease in anti-HCV positivity in blood donors since the beginning of the elimination program [14]. Additionally, the fact that no children in the survey tested positive for exposure to hepatitis C is suggestive that improved infection control practices and blood safety are limiting exposure in these younger age groups, in addition to their shorter cumulative exposure to other risk factors. Although mother-to-child transmission is not a primary mode of HCV transmission in Georgia, screening during antenatal care has also been implemented through the program. Since November 2015, HCV screening during the first trimester has been mandatory for pregnant women [15], and reflex confirmatory HCV core antigen testing was implemented by antenatal clinics in 2018 [12]. Continued commitment to blood safety and infection control and prevention is essential to mitigate the risks of iatrogenic HCV infections.
The findings of this analysis show the impact of a widescale, national campaign to eliminate hepatitis C, and are a testament to the efforts made by the Georgian program. Despite substantial progress thus far, approximately 50 000 people still need treatment, and many may be unaware of their infection and therefore more difficult to reach. Treatment numbers have declined since peaking in 2016, prompting Georgia to make all diagnostics free of charge and simplify treatment guidelines [12]. However, the program has since suffered setbacks from the COVID-19 pandemic, which further reduced screening and treatment initiation [16]. To meet elimination goals, both testing and treatment numbers will need to rebound. Two-thirds of HCV RNA-positive persons did not know their infection status, meaning more needs to be done to link these persons to screening and viremia testing. Increased awareness of the HCV elimination program could also help bolster enrollment in the treatment program. A smaller percentage had heard of the hepatitis C virus in 2021 than in 2015, likely due to promotional media efforts being more prominent during the launch of the program. However, among those who had heard of the virus, a greater percentage knew it could be cured with medication. Interventions to target those remaining with HCV infection are needed to link them to testing and treatment to ensure Georgia meets its HCV elimination goals.
Nearly half of HCV RNA-positive persons in this analysis had no identified risk factor for infection, similar to the 2015 survey findings [4]. As previously described, Georgia has made significant headway in reducing the burden of HCV infection among PWID and prison populations and has implemented effective blood safety measures, but ensuring all possible transmission routes are accounted for is essential. Number of lifetime sex partners was also associated with HCV RNA positivity. Future studies could be considered to elucidate the role of sex behaviors in HCV infections, and to better understand the underlying implications. Very few participants (2 of 2409 men) identified themselves as men who have sex with men (MSM), which is likely underestimated. Given that recent pooled estimates showed that prevalence of HCV infection was higher among MSM than the general population [17], this may be a hidden risk for HCV transmission in Georgia. Previous studies have shown a concerning increase in HIV prevalence among MSM in Georgia since 2010 [18], and HCV infection has been shown to be disproportionately higher among MSM living with HIV [19]. Fortunately, a 2018 biobehavioral surveillance study showed a 63% reduction in hepatitis C infections among MSM in Tbilisi between 2015 and 2018, similar to our findings in the general population [20]. Finally, elucidating risk factors related to substandard infection control practices is difficult, given a wide range of service quality and changes over time. Understanding the etiology of these infections with an unknown or ambiguous transmission route could help guide future prevention efforts.
This study was subject to limitations. The cross-sectional design of the study prohibits inference of causal associations; HCV infection could have been acquired at any time prior to the survey and could have preceded the risk behaviors evaluated. Risk factor data were self-reported during face-to-face interviews, and therefore could be subject to recall and/or social desirability biases leading to an underrepresentation of exposures such as IDU and sexual behavior. As in 2015, the current household survey did not include currently incarcerated persons, which could lead to an underestimation of hepatitis C prevalence. The relatively low number of HCV-infected persons likely affected risk factor analysis and regional prevalence estimates. Although every effort was made to adhere to methodology of the 2015 serosurvey, slight differences in sampling strategies may have resulted in findings that are not directly comparable.
This survey demonstrated the substantial progress made since Georgia launched its HCV elimination program in 2015, resulting in decline of HCV RNA prevalence among adults to 1.8%, corresponding to approximately 48 600 people with chronic HCV infection. Findings from this survey can guide future strategies to meet elimination targets and should be encouraging for other jurisdictions and countries seeking to achieve hepatitis C elimination.
Supplementary Material
Financial support.
This work was supported by the US Centers for Disease Control and Prevention and Georgia National Center for Disease Control and Public Health under cooperative agreement “Expanding Efforts and Strategies to Improve and Protect Public Health in Georgia.” Abbott Diagnostics provided the reagents for testing samples.
Footnotes
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copy-edited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Presented in part: EASL International Liver Congress, June 2022, London, UK; Poster No. THU347.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention, the Public Health Service, or the US Department of Health and Human Services.
Potential conflicts of interest. F. A. and G. C. are employed by and own stock in Abbott Diagnostics. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization (WHO). Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. Geneva, Switzerland: WHO, 2021. [Google Scholar]
- 2.Mitruka K, Tsertsvadze T, Butsashvili M, et al. Launch of a nationwide hepatitis C elimination program—Georgia, April 2015. MMWR Morb Mortal Wkly Rep 2015; 64:753–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsertsvadze T, Gamkrelidze A, Chkhartishvili N, et al. Three years of progress toward achieving hepatitis C elimination in the country of Georgia, April 2015-March 2018. Clin Infect Dis 2020; 71:1263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagan LM, Kasradze A, Salyer SJ, et al. Hepatitis C prevalence and risk factors in Georgia, 2015: setting a baseline for elimination. BMC Public Health 2019; 19:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Center for Disease Control and Public Health. Georgia hepatitis elimination program progress report, 2020–2021, 2022. https://www.cdc.gov/hepatitis/global/GeorgiaHepEliminationProgressReport2020-2021.pdf
- 6.Shadaker S, Nasrullah M, Gamkrelidze A, et al. Screening and linkage to care for hepatitis C among inpatients in Georgia’s national hospital screening program. Prev Med 2020; 138:106153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stvilia K, Spradling PR, Asatiani A, et al. Progress in testing for and treatment of hepatitis C virus infection among persons who inject drugs—Georgia, 2018. MMWR Morb Mortal Wkly Rep 2019; 68:637–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kish L A procedure for objective respondent selection within a household. J Am Stat Assoc 1949; 44:380–7. [Google Scholar]
- 9.National Statistics Office of Georgia. Main statistics: population. Tbilisi, Georgia: National Statistics Office of Georgia, 2016. http://geostat.ge/index.php?action=page&p_id=152&lang=eng. Accessed January 13, 2022. [Google Scholar]
- 10.Lim AG, Stone J, Hajarizadeh B, et al. Evaluating the prevention benefit of HCV treatment: modeling the SToP-C treatment as prevention study in prisons. Hepatology 2021; 74:2366–79. [DOI] [PubMed] [Google Scholar]
- 11.Hickman M, Dillon JF, Elliott L, et al. Evaluating the population impact of hepatitis C direct acting antiviral treatment as prevention for people who inject drugs (EPIToPe)—a natural experiment (protocol). BMJ Open 2019; 9:e029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Averhoff F, Shadaker S, Gamkrelidze A, et al. Progress and challenges of a pioneering hepatitis C elimination program in the country of Georgia. J Hepatol 2020; 72:680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris AM, Chokoshvili O, Biddle J, et al. An evaluation of the hepatitis C testing, care and treatment program in the country of Georgia’s corrections system, December 2013—April 2015. BMC Public Health 2019; 19:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloch EM, Kipiani E, Shadaker S, et al. Blood transfusion safety in the country of Georgia: collateral benefit from a national hepatitis C elimination program. Transfusion 2020; 60:1243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ministry of Health Georgia. National Screening Guideline and Strategy [in Georgian]. 2017. https://www.moh.gov.ge/uploads/guidelines/2017/05/08/01e820305307903f4a0e082fae8e9b48.pdf. Accessed March 15, 2022.
- 16.Gamkrelidze A, Handanagic S, Shadaker S, et al. The impact of COVID-19 pandemic on the 2020 hepatitis C cascade of care in the Republic of Georgia. Public Health 2022; 205:182–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin F, Dore GJ, Matthews G, et al. Prevalence and incidence of hepatitis C virus infection in men who have sex with men: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2021; 6:39–56. [DOI] [PubMed] [Google Scholar]
- 18.Mirzazadeh A, Noori A, Shengelia N, Chikovani I. HIV continues to spread among men who have sex with men in Georgia; time for action. PLoS One 2019; 14:e0214785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016; 16:797–808. [DOI] [PubMed] [Google Scholar]
- 20.Curatio International Foundation Center for Information and Counselling on Reproductive Health. HIV risk and prevention behaviors among men who have sex with men in Tbilisi, Batumi and Kutaisi, Georgia, 2018. http://curatiofoundation.org/wp-content/uploads/2022/03/MSM-2018-IBBS-Report_FV_Eng.pdf. Accessed February 10, 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


