Abstract
Background
Telomeres are repetitive DNA sequences located at the ends of chromosomes, playing a vital role in maintaining chromosomal integrity and stability. Dysregulation of telomeres has been implicated in the development of various cancers, including non-small cell lung cancer (NSCLC), which is the most common type of lung cancer. Genetic variations within telomere maintenance genes may influence the risk of developing NSCLC. The present study aimed to evaluate the genetic associations of select variants within telomere maintenance genes in a population from Jammu and Kashmir, North India, and to investigate the relationship between telomere length and NSCLC risk.
Methods
We employed the cost-effective and high-throughput MassARRAY MALDI-TOF platform to assess the genetic associations of select variants within telomere maintenance genes in a population from Jammu and Kashmir, North India. Additionally, we used TaqMan genotyping to validate our results. Furthermore, we investigated telomere length variation and its relation to NSCLC risk in the same population using dual-labeled fluorescence-based qPCR.
Results
Our findings revealed significant associations of TERT rs10069690 and POT1 rs10228682 with NSCLC risk (adjusted p-values = 0.019 and 0.002, respectively), while TERF2 rs251796 and rs2975843 showed no significant associations. The TaqMan genotyping validation further substantiated the associations of TERT rs10069690 and rs2242652 with NSCLC risk (adjusted p-values = 0.02 and 0.003, respectively). Our results also demonstrated significantly shorter telomere lengths in NSCLC patients compared to controls (p = 0.0004).
Conclusion
This study highlights the crucial interplay between genetic variation in telomere maintenance genes, telomere attrition, and NSCLC risk in the Jammu and Kashmir population of North India. Our findings suggest that TERT and POT1 gene variants, along with telomere length, may serve as potential biomarkers and therapeutic targets for NSCLC in this population. Further research is warranted to elucidate the underlying mechanisms and to explore the potential clinical applications of these findings.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-023-11387-z.
Keywords: Biomarker, Gene variants; Telomere maintenance gene; Non-small cell lung cancer; Telomere length
Introduction
Lung cancer, a leading cause of cancer-related mortality worldwide, is a multifactorial disease influenced by both genetic and non-genetic factors [1, 2]. The increasing prevalence of lung cancer in the Indian population, particularly in the Jammu and Kashmir (J&K) region of North India, necessitates the investigation of pathways involved in its etiology. Genome-wide association studies (GWAS) have uncovered numerous genetic variants in telomere maintenance genes that are associated with non-small cell lung cancer (NSCLC) risk [3]. Understanding the role of these genetic variations and their relationship with telomere dynamics could provide valuable insights into the pathogenesis of NSCLC and inform the development of novel diagnostic and therapeutic strategies.Telomeres are critical for maintaining chromosomal integrity and stability. They comprise tandem hexanucleotide repeats (TTAGGG) at the ends of eukaryotic chromosomes and terminate in a 3' single-strand guanine overhang [4]. The length of telomeres is regulated by telomere maintenance genes, which primarily belong to three complexes: Shelterin, CTC1–STN1–TEN1 (CST), and Telomerase [5–7]. Several case–control association studies have reported an association between shortened telomere length and lung cancer risk [8–10].
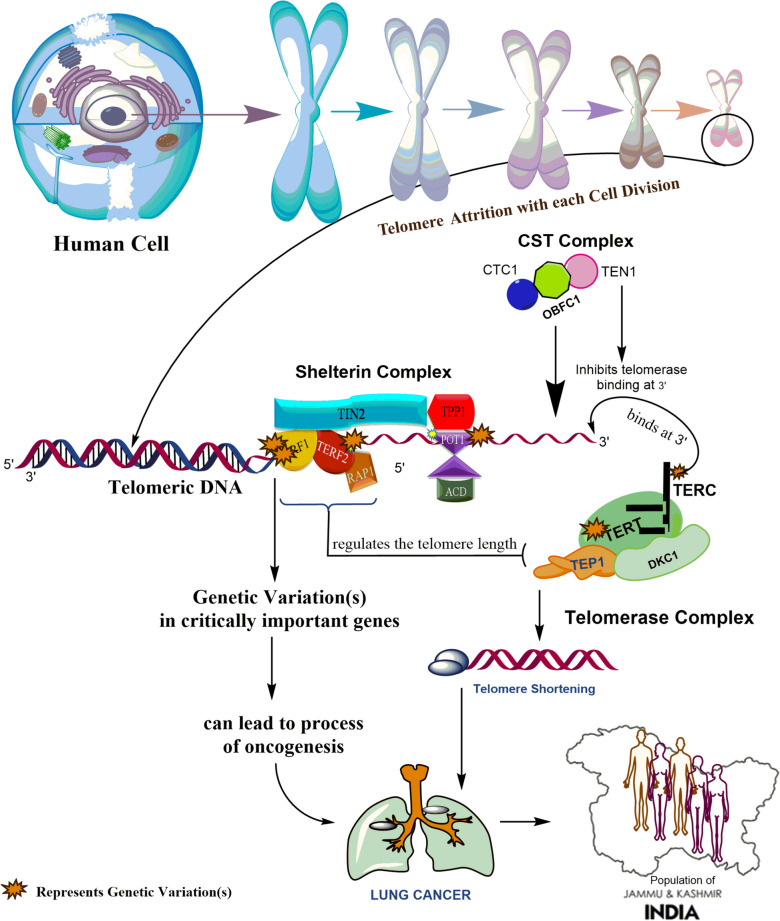
The Shelterin complex is composed of six integral genes: Telomeric Repeat Binding Factor 1 (TERF1), Telomeric Repeat Binding Factor 2 (TERF2), Protection of Telomeres 1 (POT1), TERF1 Interacting Nuclear Factor 2 (TIN2), Tripeptidyl Peptidase 1 (TPP1), and Repressor/activator protein 1 (RAP1) (Fig. 1). The Shelterin complex, along with the CST and Telomerase complexes, play a critical role in preserving telomere integrity [11, 12]. Emerging evidence suggests that genetic variants in these complexes may contribute to telomere dysfunction, which has been implicated in cancer development and progression [13, 14]. For instance, aberrant telomere lengthening, due to telomerase reactivation, has been observed in approximately 90% of human cancers, including NSCLC [15–17]. Conversely, telomere shortening has been associated with increased chromosomal instability, which may contribute to the initiation and progression of malignancies [18].
Fig. 1.
Illustration of genes vital for the maintenance of telomere ends and the regulation of telomere length. The genetic variants targeted from the telomere maintenance genes in the study are designated by (*) asterisk mark like rs10069690 of TERT, rs10228682 of POT1, rs251796 and rs2975843 of TERF2 of shelterin complex
In the context of lung cancer, previous studies have reported associations between genetic variations in TERT and the risk of lung cancer [7, 19]. TERT encodes the catalytic subunit of the telomerase enzyme, which plays a crucial role in maintaining telomere length and protecting against telomere attrition [20]. Moreover, POT1 and TERF2, other components of the Shelterin complex, have also been implicated in lung cancer susceptibility [21, 22]. POT1 is involved in the regulation of telomere length and protection against telomere dysfunction-induced DNA damage, while TERF2 is responsible for the stabilization of the telomeric loop structure and the prevention of DNA damage response activation [23, 24].
Besides genetic variations, epigenetic modifications and environmental factors, such as smoking and exposure to air pollution, have also been shown to influence telomere length and contribute to lung cancer risk [25, 26]. For example, oxidative stress from cigarette smoke exposure can lead to DNA damage and telomere attrition, which may subsequently increase the risk of lung cancer [27]. Therefore, understanding the complex interplay between genetic, epigenetic, and environmental factors in telomere length regulation is crucial for comprehending the pathogenesis of lung cancer and identifying potential targets for intervention.
The present study targets the variants rs10069690, rs10228682, rs251796, and rs2975843 in genes – TERT, POT1, and TERF2, respectively, from the shelterin complex among 723 individuals (162 non-small cell lung cancer cases and 561 healthy control) in the ethnic population of Jammu and Kashmir, North India for NSCLC risk. Furthermore, the telomere length variation among 216 individuals (108 non-small cell lung cancer cases and 108 healthy controls) in the ethnic population of Jammu and Kashmir, North India, was explored using a dual labeled fluorescence probe-based assay [28]. Moreover, another subset of samples of the same population group was evaluated for NSCLC risk, targeting shelterin complex genes by Taqman probe-based methodology.
In summary, this study aims to provide a comprehensive investigation of the associations between genetic variants in telomere maintenance genes and NSCLC risk in the Jammu and Kashmir population of North India. By examining the potential role of telomere length as a prognostic biomarker for NSCLC diagnosis, we hope to contribute valuable insights to the growing body of knowledge on lung cancer etiology and inform the development of novel diagnostic and therapeutic strategies.
Materials & methods
Ethical statement
The study had been approved by the Institutional Ethics Review Board (IERB) of Shri Mata Vaishno Devi University vide IERB Serial No: SMVDU/IERB/16/41. Written informed consent was acquired from all the participants enrolled in the present study.
Sampling
Seven hundred twenty-three individuals (162 non-small cell lung cancer cases and 561 healthy control) recruited for the study were evaluated by MassARRAY, and TaqMan Genotyping evaluated 254 non-small cell lung cancer cases and 405 healthy controls. Furthermore, we also examined the telomere length variation among 216 individuals (108 non-small cell lung cancer cases and 108 healthy controls) after approval from the Institutional Ethical Review Board (IERB). All cancer cases were histopathologically confirmed. Two milliliters of venous blood samples were collected from each participant in an EDTA vial.
DNA isolation
FlexiGene® DNA kit (Qiagen, catalog No. 51206) method was used to extract genomic DNA from the blood samples. The quantity and quality control analysis of genomic DNA was performed by carrying out UV spectrophotometer (Eppendorf Biospectrometer®, Hamburg, Germany) analysis and Gel electrophoresis, respectively. The purified DNA was kept at 4ºC till further use at a concentration of 10 ng/µl.
Selection of variants and genotyping
This study selected the genetic variants of telomere maintenance genes, which have a critical role in non-small cell lung cancer through GWAS and replication studies using the candidate gene approach (CGA)). Finally, 4 genetic variants of 3 vital genes were shortlisted. The details of genetic variants are discussed in Supplementary Table 1. Genotyping was performed on a high-throughput Agena Mass ARRAY platform and Taqman genotyping using Taqman Chemistry. To carry out genotyping, the central Mass ARRAY Analyzer facility at Shri Mata Vaishno Devi University was used, and recommended protocol(s) were followed. AgenaCxV.2.0 was used to design forward, reverse, and single base extension primers (customized) for MassARRAY (Supplementary Table 2). Sequenom Typer 4.0. Software was used to analyze genotype calls. In order to exclude the call errors via spectrograms, all genotype calls were cross-checked. The subjects were excluded from the study if the missing genotypes were higher than 10%. Those that don’t follow the Hardy–Weinberg Equilibrium (HWE) (p-value < 0.05) were also omitted from the study. The genotyping results were replicated in 10% of random samples, and the concordance rate was 98.5%. In the reaction of 384 well plates, one positive and one negative control were added for quality check. Mass ARRAY Cluster Plots were obtained after genotyping.. Another high throughput technique, TaqMan genotyping using TaqMan Chemistry, was used to evaluate the genetic association of rs10069690, rs2242652 of TERT & rs251796 of TERF2 with non-small cell lung cancer risk. Moreover, Leucocyte Telomere Length was assessed by using dual labeled fluorescence probe-based assay. The data was exported in Excel format from the MxPro Software. The triplicates were averaged, and then the averaged Ct (Threshold) value was used to calculate relative telomere length (RTL). RTL was calculated based on Ct values, as [2Ct (telomeres)/2Ct (scg)]–1 = 2–ΔCt [28].
Statistical analysis
A statistical t-test was used to compare the clinical characteristics between cases and controls. Genotype data were analyzed using PLINK v. 1.07 [29] and IBM SPSS statistics 20 software [30]. All the genetic variants were tested for Hardy–Weinberg equilibrium using the chi-square test. The association of variants with non-small lung cancer risk was validated by binary logistic regression analysis adjusted for confounding factors like age, gender, and Body Mass Index (BMI). The odds ratios (ORs) were calculated based on the risk allele observed in this study. One-way ANOVA was employed to compare the clinical characteristics of different genotypes for each variant, adjusted for age and gender (Supplementary table 3). To estimate telomere length student t-test was used to check the significant difference between cases and controls.
Potential role of the variants
Expression Quantitative Trait Loci (eQTL), UCSC Genome Browser, Encyclopedia of DNA Elements (ENCODE) (V3), and HaploReg v4.1 database [31, 32] tools were employed for analyzing the transcriptional regulatory role like histone modifications, DNase hypersensitivity and binding sites for the transcription factor. Besides that, the effect of the variant on splicing was evaluated by using the web tool Human Spicing Finder (HSF) 3.1 and ESE Finder (3.0) [33, 34].
Results
The present study targeted the genetic variations in telomere maintenance genes like TERT, POT1, and TERF2 in the ethnic population of Jammu and Kashmir, North India, for NSCLC risk using MassARRAY.With this perspective, the association between genetic variant rs10069690 of TERT (Telomere Reverse Transcriptase), rs10228682 of POT1 (Protection of Telomeres), rs251796, rs2975843 of TERF2 (Telomere Repeating Factor 2) and non-small cell lung cancer was evaluated. It was observed that rs10069690 of TERT was significantly associated with non-small lung cancer risk with an odds ratio = 1.65 (1.08–2.52 at 95% CI); p-value (adjusted) = 0.019, as shown in Table 1. Genetic variant rs10228682 of POT1 was also found to be significantly associated with non-small cell lung cancer risk with an odds ratio = 1.88 (1.25–2.83 at 95% of CI); p-value (adjusted) = 0.002 as shown in Table 1. The study also targeted the genetic variations in telomere maintenance genes, rs10069690 & rs2242652 of TERT using TaqMan probe-based genotyping on the same population group. It was observed that rs10069690 & rs2242652 were found to be significantly associated with NSCLC risk, with an odds Ratio (OR) of 1.47 (1.06–2.05 at 95% CI); p-value (adjusted) = 0.02; 1.65 (1.18–2.32 at 95% CI); p-value (adjusted) = 0.003 respectively as shown in Table 2.
Table 1.
Allelic, Genotypic distribution and logistic regression analysis of significant variants of genes in our study using the MassARRAY platform
| Variant | rs10228682 | rs10069690 | ||
|---|---|---|---|---|
| Nearest gene w.r.t variant | POT1 | TERT | ||
| Polymorphism | C/T | C/T | ||
| Ancestral allele | C | C | ||
| Allele distribution | C | T | C | T |
| Cases | 0.625 | 0.705 | 0.866 | 0.817 |
| Controls | 0.375 | 0.295 | 0.134 | 0.183 |
| Odds ratio at 95% CI | 1.43 (1.09–1.88) | 0.7(0.49–1.00) | ||
| Total HWE | 0.111 | 0.951 | ||
| Genotypic model | Dominant | Recessive | ||
| (CC + CT vs TT) | (CC vs TT + CT) | |||
| p-Value* | 0.002 | 0.019 | ||
| Odds ratio at 95% CI | 1.88 (1.25–2.83) | 1.65 (1.08–2.52) | ||
*Adjusted for Age, Gender, and BMI
Table 2.
Allelic, Genotypic distribution and logistic regression analysis of significant variants of genes in our study using Taqman Chemistry
| Variant | rs10069690 | rs2242652 | ||
|---|---|---|---|---|
| Nearest gene w.r.t variant | TERT | TERT | ||
| Polymorphism | C/T | A/G | ||
| Ancestral allele | C | A | ||
| Allele distribution | C | T | A | G |
| Cases | 0.67 | 0.33 | 0.74 | 0.26 |
| Controls | 0.73 | 0.27 | 0.81 | 0.19 |
| Odds ratio at 95% CI | 1.29 (1.01–1.64) | 1.46 (1.12–1.89) | ||
| Total HWE | 0.67 | 0.48 | ||
| Genotypic model | Dominant model | Dominant model | ||
| TT/CT vs CC | GG/AG vs AA | |||
| p-Value* | 0.02 | 0.003 | ||
| Odds ratio at 95% CI | 1.47 (1.06–2.05) | 1.65 (1.18–2.32) | ||
*Adjusted for Age, Gender, and BMI
Furthermore, we deemed it essential to assess the results concerning adenocarcinoma and squamous cell carcinoma independently. This distinction stems from the inherent heterogeneity of NSCLC, recognizing that varied underlying biological mechanisms and risk factors may govern divergent subtypes. Our observations pointed towards a meaningful association between the polymorphisms rs10069690 and rs2242652 in both Adenocarcinoma and Squamous cell carcinoma subtypes and the prevalence of non-small cell lung cancer within the population of Jammu & Kashmir, North India. The statistical significance of these associations is underscored by p-values of 0.04 and 0.002 for Adenocarcinoma and 0.046 and 0.002 for Squamous cell carcinoma, respectively. These findings are illustrated in detail in Supplementary Table 4.
However, the variants rs251796 and rs2975843 of TERF2 were evaluated using the MassARRAY platform and Taqman genotyping. No statistically significant association was observed with non-small cell lung cancer risk in the studied population with an odds ratio = 0.82 (0.62–1.08 at 95% of CI); p-value = 0.16; Odds ratio = 0.90 (0.70–1.17 at 95% of CI); p-value = 0.46 (MassARRAY Platform); Odds ratio (OR) = 0.94 (0.75–1.19 at 95% CI); p-value = 0.61 (Taqman Chemistry) respectively as shown in Table 3. Moreover, exploring the bioinformatic approach, cis-eQTL analysis suggested that risk allele (T) of rs10228682 of POT1 is linked with upregulation of the expression of the gene in lungs (p-value = 2.2E-18 and normalized effect size (NES) = 0.45). Since the gene is critical in telomere maintenance, the upregulation of the gene might affect telomere physiology.
Table 3.
Allelic and Genotypic distribution of the variants, which did not show significant association with NSCLC in population of J&K, North India using MassARRAY and Taqman Chemistry
| Variant | rs251796 | rs2975843 | rs251796 | |||
|---|---|---|---|---|---|---|
| Genotyping technique | MassARRAY | MassARRAY | Taqman Chemistry | |||
| Nearest gene W.R.T variant | TERF2 | TERF2 | TERF2 | |||
| Polymorphism | A/G | A/G | A/G | |||
| Ancestral allele | A | T | A | |||
| Allele distribution | A | G | A | G | A | G |
| Cases | 0.70 | 0.30 | 0.58 | 0.42 | 0.68 | 0.32 |
| Controls | 0.66 | 0.34 | 0.56 | 0.44 | 0.66 | 0.34 |
| Odds ratio at 95% CI | 0.82 (0.62–1.08) | 0.90 (0.70–1.17) | 0.94 (0.75–1.19) | |||
| p Value | 0.1623 | 0.4685 | 0.617 | |||
| Total HWE | 0.629 | 0.126 | 0.921 | |||
Besides that, to examine the consequence of this genetic variant on POT1, an in silico approach by Human Splicing Finder (HSF) and exonic splicing enhancers (ESE) was explored. The widely used algorithms for predicting enhancer/silencer motifs by HSF demonstrated that rs10228682 results in a broken site for 9G8. Mutant Motif Value = 79.8 (Reference value 59.24) (Table 4).
Table 4.
Putative Role of the associated variants with non-small cell lung cancer in J&K, North- India utilizing the information from the various databases, including GTEX, UCSC genome browser and HSF
| Variant | Allele Ref/Alt |
eQTL gene | eQTL Tissue | eQTL sample size | eQTL NES | eQTL p-value |
eQTL m-value |
Putative role (cis- eQTL) of variant | Regulatory role of variant (ENCODE and Haploreg data) | Splicing effect |
|---|---|---|---|---|---|---|---|---|---|---|
| rs10228682 | C/T* | POT1-AS1 | Lung | 515 | 0.45 | 2.2E-18 | 1 | Significant and Up regulation | NA | Broken site for 9G8 |
| rs10069690 | *T/C | TERT | Lung | NA | NA | NA | NA | NA | NA |
Creation of new site/ broken site for SF2/ASF (IgM/BRCA1), SF2/ASF and SRp40 |
| rs251796 | *A/G | TERF2 | Lung | 515 | -0.470 | 1.3E-19 | 1 | Significant and down regulation | NA | NA |
| rs2975843 | *A/G | TERF2 | Lung | 515 | -0.067 | 0.07 | 0.988 | Down regulated and non-significant | H3K4me3_Pro, H3K4me1_Enh | NA |
*represents risk allele in this study, NES Normalized Effect Size in Eqtl, m-value Posterior probability that effect exists in each tissue, ranges between 0 and 1, ESE Exonic Splicing Enhancers, SR Serine-Arginine rich proteins, 9G8, SC35 SR splicing factor, SF2/ASF (IgM-BRCA1) Serine-Arginine rich proteins. H3K4me3 Chemical modification (methylation) of the histone proteins (H3) at lysine 4, marks promoters that are active or poised to be activated, Enh Enhancers, Pro Promoters
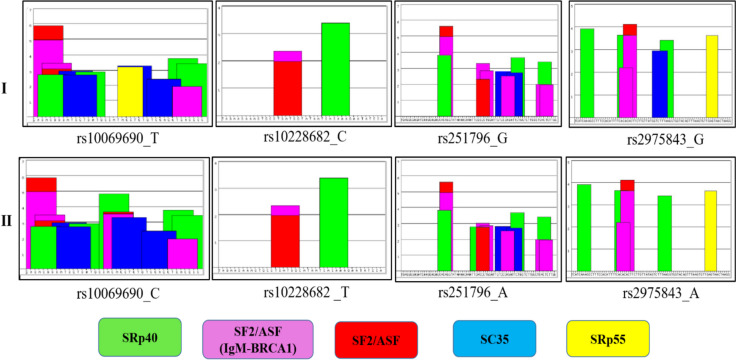
Additionally, an assessment of the impact of the genetic variant rs10069690 on the gene through a computational analysis by Human Splicing Finder (HSF) was carried out. The study revealed a disruption in the exonic splicing enhancer (ESE) site. Most of the algorithms employed by the HSF (v.3.1) tool for the prediction of enhancer/silencer motifs, as illustrated in Table 4 and Fig. 2, suggested that rs10069690 leads to the formation of new binding sites for the (serine/arginine-rich protein) SR40(90.90), SF2/ASF (82.99), and SF2/ASF (IgM-BRCA1) (83.31). The results showed that modifications in the binding of the splicing factor of the exonic splicing enhancer (ESE) intronic site hint at its influence on splice site binding. Moreover, cis-eQTL analysis implied that the risk allele (A) of rs2251796 of TERF2 is associated with a decrease in the gene's expression in the lungs (p-value = 1.3E-19 and normalized effect size (NES) =—0.470). Given the gene's critical role in maintaining telomeres, this reduced expression may influence telomere physiology. Simultaneously, the risk allele (A) of rs2975843 of TERF2 is linked with a decrease in the regulation of gene expression in the lungs, with the site displaying histone marks like (H3K4me3_Pro, H3K4me1_Enh), suggesting involvement in epigenetic regulatory activities.
Fig. 2.
Effect of genetic variation on the Exonic Splicing Enhancers (ESEs) according to the ESE prediction tool. ESE finder enables the recognition of potential ESE sites. The elevation of the colored bars represents the motif scores, and the bars' circumference indicates the motif's length. Bars in red, yellow, blue, purple, and green indicate potential binding sites for Serine-Arginine (SR) proteins SF2/ASF, SRp55, SC35, SF2/ASF (IgM-BRCA1) and SRp40, respectively. Panel-I signifies the ESE sequence with the allele not posing a risk in the population under study, and panel –II denotes the ESE sequence with the risk allele in the studied population
The relative telomere length in peripheral blood lymphocytes was measured by using dual labeled fluorescence probe-based assay in 108 non-small cell lung cancer patients and 108 healthy controls (triplicates). The cases and controls were frequency-matched for age, sex, alcohol, and smoking status. Telomere length was found to be significantly shorter in non-small cell lung cancer patients than in controls (p-value = 4 × 10–3). In our quest for deeper insight, we sub-categorized the NSCLC dataset by categorizing it into distinct subtypes—AC, SCC, and LUAD. This meticulous subcategorization allowed us to investigate the association between telomere length and these respective NSCLC subtypes.
Our findings underscored a significant reduction in telomere length across all cancer subtypes compared to the control group. Specifically, patients with AC, SCC, and LUAD exhibited considerably shorter telomeres, backed by statistically significant p-values of 0.04002, 0.0367, and 0.00042, respectively. This sheds new light on the intricate dynamics between different NSCLC subtypes and telomere attrition.
The regression analysis was performed using the IBM SPSS Statistical software tool (V-20), which showed the effect of covariates on telomere length when adjusted with age, gender, alcohol, smoking status, and gutkha intake. It was observed that smoking is significantly associated with telomere attrition with an Odds ratio = 3.48 (1.7–7.16 at 95% of CI); p-value = 001 (Tables 5 and 6).
Table 5.
Logistic regression analysis adjusted with Age, Gender and BMI
| Variables | p-value** | 95% CI |
|---|---|---|
| TLa | 0.002 | 2.31(1.90–3.74) |
| Smoking | 0.01 | 3.41(1.70–6.10) |
| Alchol intake | 0.04 | 0.93 (0.40–1.90) |
| Guthka intake | 0.04 | 0.13 (0.03–1.02) |
aTelomere Length; **Age, Gender and BMI
Table 6.
Logistic regression analysis adjusted with Age, Gender and BMI
| Variables | p-value* | 95% CI |
|---|---|---|
| Smoking | 0.02 | 3.90(1.8–6.3) |
| Alchol intake | 0.05 | 0.86 (0.6–2.2) |
| Guthka intake | 0.48 | 1.70(1.0–3.02) |
*Age, Gender and BMI
Discussion and conclusion
The success of identifying many genetic variants and susceptibility loci in critically important genes was achieved through genome-wide association studies (GWAS) and transcriptome-wide association studies (TWAS). Worldwide, more than 60 loci have been linked with lung cancer by GWAS and the candidate gene approach. These genes are linked with diverse pathways regulating cell growth, cell survival, apoptosis, and telomere attrition.
Therefore, the present study explored the association between telomere-associated pathway genes (TERF1, TERF2, POT1, TERT) (Fig. 1) and non-small cell lung cancer risk in the less genetically explored population group.
So, we targeted to screen genetic variations in these genes for non-small cell lung cancer risk in an ethnic population of Jammu and Kashmir, North India. We previously conducted a case–control association study of genetic variant rs2853677 of TERT on susceptibility to non-small cell lung Carcinoma in the population of Jammu and Kashmir (p = 0.03) [7].
The present study observed that rs10069690 of TERT was significantly associated with non-small lung cancer risk using the MassARRAY platform with an Odds ratio = 1.65 (1.08–2.52 at 95% CI); p-value (adjusted) = 0.019. Moreover, rs10069690 & rs2242652 of TERT evaluated using Taqman-based genotyping were also found to be significantly associated with NSCLC risk, with an odds Ratio (OR) of 1.47 (1.06–2.05 at 95% CI); p-value (adjusted) = 0.02; 1.65 (1.18–2.32 at 95% CI); p-value (adjusted) = 0.003 respectively. These findings are consistent with the lung cancer risk in the Chinese Han population [35, 36]. Furthermore, genetic variant rs10228682 of POT1 evaluated by MassARRAY was also found to be significantly associated with non-small cell lung cancer risk with an Odds ratio = 1.88 (1.25–2.83 at 95% CI); p-value (adjusted) = 0.002. These observations have been associated with Chinese lung cancer risk [37]. However, genetic variants rs251796 and rs2975843 of TERF2 did not significantly affect non-small cell lung cancer risk with p value = 0.162, 0.61 & 0.468, respectively.
Furthermore, to evaluate the effect of these polymorphisms on the physiology of the telomere maintenance gene, the bioinformatic in silico analysis of these variants was performed by (eQTL), UCSC, ENCODE (V3), HaploReg v4.1, (HSF) 3.1and ESE finder (3.0). It was observed that either some are involved in epigenetic regulation, some result in broken sites, and some result in the creation of new sites, which eventually have a significant effect on the physiology of shelterin complex and telomere length regulation. Moreover, the splicing/epigenetic effect of the variants needs to be confirmed in in vitro analysis. The main aim of identifying these associations in telomere mantainence genes using dual high throughput techniques was to understand the genetic heterogeneity of non-small cell lung cancer and the possibility of using the identified genetic variant of telomere maintenance gene (TERT, POT1, TERF2) as a prognostic biomarker for diagnosis of non-small cell lung cancer.
The limitations of the present case–control association study include the relatively small sample size, and secondly, more data is needed on how the actual genetic polymorphic mechanism of the TERT, TERF2 and POTI genes, which remains conserved among species and different ethnic groups contributes to lung cancer risk in the J&K population, North India. Moreover, we also quest to evaluate these variants with large sample sizes in different cancers in the future with functional validations.
Supplementary Information
Additional file 1: Supplementary Table 1. Details of the variants selected for the study of non-small cell lung cancer in the Jammu and Kashmir population.
Additional file 2: Supplementary Table 2. List of variants and their primer and probe (UEP) sequence.
Additional file 3: Supplementary Table 3. One-way ANOVA of significant Variants with quantitative traits.
Additional file 4: Supplementary Table 4. Allelic and Genotypic distribution of the variants and their association with two main subtypes of NSCLC.
Acknowledgements
We acknowledge all the participants for their contribution to this study. G.R.B. acknowledges Dr. Swarkar Sharma for his guidance and support. G.R.B. would also like to acknowledge Dr. Varun Sharma for his help in data analysis. Open Access funding is provided by the Qatar National Library.R. K. acknowledges Indian Council of Medical Research (5/10/15/CAR-SMVDU/2018-RBMCH) and Department of Science and Technology (DST/SSTP/ J&K/459), & JKST&IC/SRE/J/321-23 Govt. of India for financial assistance.
Authors’ contributions
G.R.B., A.A.B and R.K. designed this study. R.K and A.A.B. supervised the research and coordinated the project. G.R.B., R.S.J., I.S. and M.S. conducted the main experiments. G.R.B.,R.S.J., I.S., H.Q.S. and S.A.M. analyzed the results. A.B., S.V., R.S., M.S. and R.S.J. helped in the sample processing. G.R.B., I.S., H.Q.S. and S.A.M. prepared the figures. G.R.B.,R.S.J., A.A.B and R.K. wrote the manuscript. T.M., S.M., M.H., M.A.M., and A.S.A.A. performed critical revision and editing of the scientific content. The author(s) read and approved the final manuscript.
Funding
This study was funded, in part, by Sidra Medicine Precision Program (Ajaz A. Bhat [SDR400105] and Ammira Al-Shabeeb Akil [SDR400175]); Department of Science and Technology, Govt. of India for Grant (DST/SSTP/J&K/459) to Rakesh Kumar.
Availability of data and materials
The datasets generated or analyzed during the current study are not publicly available but are available with the corresponding author and can be provided upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Institutional Ethics Review Board (IERB) of Shri Mata Vaishno Devi University (SMVDU) vide IERB Serial No: SMVDU/IERB/16/41 and SMVDU/IERB/16/47. The consent form was designed in three languages (English, Hindi, Urdu) and informed and written consent was taken from the patient and control before the sample collection. All the methods included in this study are in accordance with the declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ajaz A. Bhat, Email: abhat@sidra.org
Rakesh Kumar, Email: kumar.rakesh@smvdu.ac.in.
References
- 1.Inamura K, Ishikawa Y. MicroRNA In lung cancer: novel biomarkers and potential tools for treatment. J Clin Med. 2016;5(3):36. doi: 10.3390/jcm5030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shields PG. Molecular epidemiology of smoking and lung cancer. Oncogene. 2002;21(45):6870. doi: 10.1038/sj.onc.1205832. [DOI] [PubMed] [Google Scholar]
- 3.Choi JE, et al. Polymorphisms in telomere maintenance genes and risk of lung cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2773. doi: 10.1158/1055-9965.EPI-09-0323. [DOI] [PubMed] [Google Scholar]
- 4.Deng Y, Chan SS, Chang S. Telomere dysfunction and tumour suppression: the senescence connection. Nat Rev Cancer. 2008;8(6):450–458. doi: 10.1038/nrc2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sethi I, et al. Role of telomeres and associated maintenance genes in Type 2 Diabetes Mellitus: A review. Diabetes Res Clin Pract. 2016;122:92–100. doi: 10.1016/j.diabres.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Bhat GR, et al. Genetic evaluation of the variants using MassARRAY in non-small cell lung cancer among North Indians. Sci Rep. 2021;11(1):11291. doi: 10.1038/s41598-021-90742-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhat GR, et al. Association of newly identified genetic variant rs2853677 of TERT with non-small cell lung cancer and leukemia in population of Jammu and Kashmir, India. BMC Cancer. 2019;19(1):493. doi: 10.1186/s12885-019-5685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu X, et al. Telomere dysfunction: a potential cancer predisposition factor. J National Cancer Inst. 2003;95(16):1211–1218. doi: 10.1093/jnci/djg011. [DOI] [PubMed] [Google Scholar]
- 9.Broberg K, et al. Constitutional short telomeres are strong genetic susceptibility markers for bladder cancer. Carcinogenesis. 2005;26(7):1263–1271. doi: 10.1093/carcin/bgi063. [DOI] [PubMed] [Google Scholar]
- 10.Jang JS, et al. Telomere length and the risk of lung cancer. Cancer Sci. 2008;99(7):1385–1389. doi: 10.1111/j.1349-7006.2008.00831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giraud-Panis MJ, et al. CST meets shelterin to keep telomeres in check. Mol Cell. 2010;39(5):665–676. doi: 10.1016/j.molcel.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Mir SM, et al. Shelterin Complex at Telomeres: Implications in Ageing. Clin Interv Aging. 2020;15:827–839. doi: 10.2147/CIA.S256425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rask-Andersen M, Almen MS, Schioth HB. Trends in the exploitation of novel drug targets. Nat Rev Drug Discov. 2011;10(8):579–590. doi: 10.1038/nrd3478. [DOI] [PubMed] [Google Scholar]
- 14.Ita M, et al. Remarkable enhancement of cytotoxicity of onconase and cepharanthine when used in combination on various tumor cell lines. Cancer Biol Ther. 2008;7(7):1104–1108. doi: 10.4161/cbt.7.7.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanco R, et al. A gene-alteration profile of human lung cancer cell lines. Hum Mutat. 2009;30(8):1199–1206. doi: 10.1002/humu.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janson S, Reed ML. Patients' perceptions of asthma control and impact on attitudes and self-management. J Asthma. 2000;37(7):625–640. doi: 10.3109/02770900009090818. [DOI] [PubMed] [Google Scholar]
- 17.Bailey SM, Murnane JP. Telomeres, chromosome instability and cancer. Nucleic Acids Res. 2006;34(8):2408–2417. doi: 10.1093/nar/gkl303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maciejowski J, de Lange T. Telomeres in cancer: tumour suppression and genome instability. Nat Rev Mol Cell Biol. 2017;18(3):175–186. doi: 10.1038/nrm.2016.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang HM, Zhang XY, Jin B. TERT genetic polymorphism rs2736100 was associated with lung cancer: a meta-analysis based on 14,492 subjects. Genet Test Mol Biomarkers. 2013;17(12):937–941. doi: 10.1089/gtmb.2013.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dratwa M, et al. TERT-Regulation and Roles in Cancer Formation. Front Immunol. 2020;11:589929. doi: 10.3389/fimmu.2020.589929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhari VK, et al. Shelterin complex gene: Prognosis and therapeutic vulnerability in cancer. Biochem Biophys Rep. 2021;26:100937. doi: 10.1016/j.bbrep.2021.100937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Poulos RC, Reddel RR. Role of POT1 in Human Cancer. Cancers (Basel). 2020;12(10):2739. doi: 10.3390/cancers12102739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hockemeyer D, et al. POT1 protects telomeres from a transient DNA damage response and determines how human chromosomes end. EMBO J. 2005;24(14):2667–2678. doi: 10.1038/sj.emboj.7600733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruis P, Boulton SJ. The end protection problem-an unexpected twist in the tail. Genes Dev. 2021;35(1–2):1–21. doi: 10.1101/gad.344044.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Assavanopakun P, et al. Effects of air pollution on telomere length: Evidence from in vitro to clinical studies. Environ Pollut. 2022;312:120096. doi: 10.1016/j.envpol.2022.120096. [DOI] [PubMed] [Google Scholar]
- 26.Wang C, et al. Long-term ozone exposure is positively associated with telomere length in critically ill patients. Environ Int. 2020;141:105780. doi: 10.1016/j.envint.2020.105780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnes RP, Fouquerel E, Opresko PL. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech Ageing Dev. 2019;177:37–45. doi: 10.1016/j.mad.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 29.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee NC, et al. Patterns of failure in high-metastatic node number human papillomavirus-positive oropharyngeal carcinoma. Oral Oncol. 2018;85:35–39. doi: 10.1016/j.oraloncology.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40(Database issue):D930–4. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ward LD, Kellis M. HaploReg v4: systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res. 2016;44(D1):D877–D881. doi: 10.1093/nar/gkv1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desmet F-O, et al. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37(9):e67–e67. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cartegni L, et al. ESEfinder: A web resource to identify exonic splicing enhancers. Nucleic Acids Res. 2003;31(13):3568–3571. doi: 10.1093/nar/gkg616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao L, et al. Polymorphisms in the TERT gene are associated with lung cancer risk in the Chinese Han population. Eur J Cancer Prev. 2014;23(6):497–501. doi: 10.1097/CEJ.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 36.He G, et al. TERT rs10069690 polymorphism and cancers risk: A meta-analysis. Mole Genet Genom Med. 2019;7(10):e00903. doi: 10.1002/mgg3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hosgood HD, 3rd, et al. Genetic variation in telomere maintenance genes, telomere length, and lung cancer susceptibility. Lung Cancer. 2009;66(2):157–161. doi: 10.1016/j.lungcan.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. Details of the variants selected for the study of non-small cell lung cancer in the Jammu and Kashmir population.
Additional file 2: Supplementary Table 2. List of variants and their primer and probe (UEP) sequence.
Additional file 3: Supplementary Table 3. One-way ANOVA of significant Variants with quantitative traits.
Additional file 4: Supplementary Table 4. Allelic and Genotypic distribution of the variants and their association with two main subtypes of NSCLC.
Data Availability Statement
The datasets generated or analyzed during the current study are not publicly available but are available with the corresponding author and can be provided upon reasonable request.